Abstract
Cortisol concentrations of older children in childcare centers have been found to be higher than at home. This study focuses on infant cortisol in childcare centers throughout the first year of life, and aims to investigate whether inter-individual differences can be explained by temperament, the quality of maternal behavior, and the quality of center care. Sixty-four infants were followed for 9 months after entering care at 3 months of age. Salivary samples were taken at 10.00 h and 16.00 h in center care (in post-entry weeks 1, 2, 3, 4, 8, 12, 16, 24, and 36) and at home (in post-entry weeks 1, 24, and 36). Prior to entry, mothers completed a temperament questionnaire and the quality of maternal behavior (sensitivity and cooperation) was observed during routine bathing sessions. Subsequently, the infants were visited three times at center care to observe the quality of infant’s interactive experiences with their professional caregiver. Longitudinal regression models showed that both morning and afternoon cortisol were higher in center care compared to home. Longitudinal regression models showed that infants receiving higher quality of maternal behavior displayed higher morning cortisol in center care, compared to infants receiving lower quality of maternal behavior. Higher quality of maternal behavior was also related to higher afternoon cortisol in center care, but only in infants high in negative emotionality. Center care quality was not related to cortisol. In sum, young infants show higher cortisol concentrations in center care that are related to infant temperament and quality of maternal behavior at home.
Introduction
In many Western countries, infants attend center care from an early age onwards. Attending center care implies separation from the parents and exposure to a novel environment and professional caregivers (Beijers et al., Citation2013; Datler et al., Citation2012). These experiences may be stressful for infants, especially when they are young and have limited capacities to cope (Beijers et al., Citation2013). As a physiological response to stress, cortisol is produced. This reaction is adaptive, but chronic exposure to high cortisol can have negative effects on cognitive, socio-emotional and immune system functioning (Shonkoff et al., Citation2009).
Studies report higher cortisol in center care as compared with home days (for a review, see Sumner et al., Citation2010; Vermeer & van IJzendoorn, Citation2006; Watamura et al., Citation2010). Only a few studies focused on infants, also reporting higher cortisol in center care compared to home (Ahnert et al., Citation2004; Bernard et al., Citation2015; Watamura et al., Citation2003). The present study focuses on infant cortisol concentrations in center care throughout the first year of life, and investigates whether inter-individual differences can be explained by infant temperament, the quality of center care, and the quality of maternal behavior.
The way infants respond to stress may not be the same for all children, but may be moderated by temperament. For example, young children with a more fearful temperament or higher anger proneness react with higher cortisol reactivity to potentially stressful and fear-evocative laboratory tasks (Talge et al., Citation2008; Van Bakel & Riksen-Walraven, Citation2004), as well as to school entry (Groeneveld et al., Citation2013). Children with a more difficult temperament may also encounter more challenges in center care than children with a less difficult temperament (Crockenberg, Citation2003; Pluess & Belsky, Citation2009, Citation2010). Indeed, social fearfulness and negative emotionality are associated with increased cortisol in center care in older children (Dettling et al., Citation2000; Watamura et al., Citation2003).
The quality of care provided in the center may also make a difference for how an infant responds to child care. There is evidence that the challenges of infants in center care may be buffered by high-quality center care, while low-quality care can add to the challenges (Badanes et al., Citation2012; Geoffroy et al., Citation2006). Studies on 1.5- to 6-year-olds found lower quality center care associated to larger increases in cortisol across the center care day (Badanes et al., Citation2012; Legendre, Citation2003; Sims et al., Citation2006; Watamura et al., Citation2009). Whether the same applies for younger infants remains to be determined.
Finally, infants’ cortisol levels in center care may also depend on the quality of care the infants receive from their mothers at home. On one hand, high quality of maternal caregiving behavior helps infants to regulate stress in challenging situations and, in the long run, to develop self-regulatory capacities (Loman & Gunnar, Citation2010; Schore, Citation2001). On the other hand, young infants accustomed to receiving high-quality care at home may be less prepared to deal with challenges without such maternal aid, especially if the quality of the care provided by the caregivers in the center is relatively low. And indeed, infants of well-functioning dyads have been found to show high cortisol levels after entering center care (humans; Ahnert et al., Citation2004), or being separated from their mothers (macaques; Gunnar et al., Citation1980).
This longitudinal study is the first to investigate infant cortisol levels in center care throughout the first year of life, taking important covariates such as infant feeding and napping into account. Salivary samples were taken on multiple days at 10.00 h and 16.00 h. This enabled us to investigate infants’ cortisol levels in the child care centers in the morning and afternoon, as well as the cortisol decline during the day. Cortisol decline is an often-used marker of the cortisol circadian rhythm, which is normally characterized by higher levels in the morning and lower levels in the afternoon (de Weerth et al., Citation2003). Infants acquire the cortisol circadian rhythm during their first year of life (Custodio et al., Citation2007; de Weerth & van Geert, Citation2002; de Weerth et al., Citation2003). Relatively high cortisol levels and small declines have been related to (psycho) pathology in children and adults (Shirtcliff & Essex, Citation2008). In this study, we also examined whether differences between infants in their cortisol levels in center care can be explained by infant temperament, the quality of center care, and the quality of maternal behavior at home. We expected higher cortisol concentrations in center care and smaller declines in infants having a more difficult temperament and infants receiving lower quality center care. The relationship between infant cortisol and quality of maternal behavior was examined exploratively.
Methods
Participants
The sample consisted of 65 infants that were recruited from waiting lists of child care centers. Recruitment occurred in several stages. A total of 77 child care centers in the cities of Nijmegen and Arnhem and the surrounding areas in the Netherlands were randomly chosen using telephone books and the internet. The child care centers were invited by letter to participate in the study. Sixty-six child care centers (or 86%) agreed to participate. Non-participation was mainly due to having no new enrollments of infants, or organizational circumstances (moving or merging).
In the next step, the child care centers were asked to send a letter to all parents who had signed up for center care, meeting the following criteria. The infants had to be 3 or 4 months of age at the time of their entry in center care and they had to attend center care for at least two days a week. A total of 113 parents were contacted by post and 65 (or 57%) agreed to participate. Those who did not want to participate indicated that they were “too busy”, mostly with caring for the newborn baby.
As only one family dropped out, the final sample included 34 boys and 30 girls with a mean age of 14.6 weeks (SD = 2.8) upon entry in child care. The infants were born at full term, had normal 5-min Apgar scores (M = 9.57, SD = 0.65), and a mean birth weight of 3587 g (SD = 591.21). Approximately half (53%) of the sample were firstborn. All but one of the infants’ parents was married or cohabiting with a partner, with the mother being the primary caretaker. None of the mothers had only elementary education, 34 mothers (53.1%) had completed middle or higher professional education, and 29 mothers (45.3%) had at least one university degree. This indicates an overrepresentation of higher educated parents. This is in line with the general overrepresentation of children from high SES families in Dutch child care centers (Bennet & Tayler, Citation2006; Merens et al., Citation2012). The 64 infants in the sample attended 53 different care groups distributed across 38 child care centers. The infants attended center care for a mean of 2.8 d/week (range: 2–4 d).
Procedure
About 2 weeks before entering center care (M = 16.9 d; SD = 8.9) at approximately 12 weeks of age (Mage = 12.0, SD = 2.8), the infants were visited at home by the first author to assess the quality of maternal behavior. During the home visits, the mother–infant dyads were videotaped during a complete bathing routine (undressing, bathing, and dressing the child) that lasted about 20 min. Mothers were instructed to bathe the infant as they would normally do and to ignore the observer as much as possible. Home visits started at around 10.00 h (M = 10.11 h, SD = 41 min). In addition, at 3 months of age, mothers were asked to fill out a temperament questionnaire.
Subsequently, the infants were visited three times at the child care center by the first author and two trained graduate students. The first visit took place around the infant’s first week alone in center care (Mage = 14.7 weeks, SD = 2.8). Before being left alone an entire day, Dutch babies have usually been to the center on a couple of occasions together with a parent, or alone for only a few hours. The second center care visit was conducted 12 weeks later (Mage = 26.0 weeks, SD = 3.0), and the third and the final visit was conducted another 12 weeks later (Mage = 38.5 weeks, SD = 3.3). In order to observe the quality of the infant’s everyday interactive experiences with their primary professional caregivers in center care, we videotaped the infant and his/her primary caregiver during three different caregiving episodes at the moment that they naturally occurred. The episodes were the following: (1) changing diapers; (2) putting to bed and taking out of bed, and (3) individual feeding (i.e., bottle-feeding), lasting about 25 min in total. The professional caregivers were instructed to go about these everyday routines with the infants as they would normally do, and to ignore the observer as much as possible. Center care visits started when the infants arrived at the child care center at around 08.00 h and concluded at about 13.00 h. All procedures were carried out with the adequate understanding and written consent of the participants: i.e., parents and professional caregivers.
The infants’ cortisol concentrations were assessed on nine regular center care days, and on three regular days at home, between 12 and 52 weeks of age (i.e., during the infants’ first 9 months in child care). Saliva samples were taken twice on each assessment day (at approximately 10.00 h and 16.00 h). As cortisol in saliva represents the situation of 20–40 min before, approximately (Goldberg et al., Citation2003), cortisol concentrations measured at 10.00 h in the center reflect physiological stress in the infant related to center care (rather than, for example, stress related to transportation or change of location).
During the first 4 weeks in center care, which we considered an adaptation period, the children were sampled weekly because we expected cortisol concentrations to change relatively rapidly in this period. After the first 4 weeks, saliva samples were taken less frequently. The infants were sampled in center care in weeks 1 (Mage = 14.7 weeks, SD = 2.8), 2, 3, 4, 8, 12, 16, 24, and 36 after entry (Mage = 52.1, SD = 1.0). At home, the infants were sampled in weeks 1, 24, and 36 after entry.
The caregivers were asked to take the morning sample between 10.00 h and 10.30 h, and afternoon sample between 16.00 h and 16.30 h without disrupting normal routines. They were also instructed to sample either before naptime or at least 45 min after naptime if the sampling time conflicted with the infant’s naptime, and to sample before feeding. Moreover, the caregivers were asked to keep a diary on the sampling days. In these diaries, the infants’ nap and meal times, use of medication and saliva sampling times were recorded. Diaries were monitored to ensure that the sampling guidelines were followed.
Salivary cortisol sampling
Saliva samples were obtained using Salivette sampling devices (Sarstedt, Etten-Leur, The Netherlands). Caregivers were instructed by the first author how to obtain the infant’s saliva. The procedure was as follows: a sterile absorbent cotton dental roll was placed in the infant’s mouth, and saliva was absorbed by sucking/chewing on this cotton roll. When the cotton rolls were sufficiently moist, which took about 2–3 min, the cotton rolls were returned to the Salivette sampling devices. No oral stimulants were used to stimulate saliva flow because it has been suggested that these stimulants may compromise the validity of assay values (Schwartz et al., Citation1998). The samples were stored in the refrigerator for one day and mailed to the first author the next day. Mailing does not affect the cortisol concentrations (Clements & Parker, Citation1998). After arrival, the Salivettes were centrifuged and the supernatants were placed at −25 °C until defrosted for analysis.
Instruments and measures
Infant temperament
The mothers completed the Infant Behavior Questionnaire – Revised (IBQ-R; Gartstein & Rothbart, Citation2003) to assess infant temperament. The IBQ-R is a 191-item questionnaire that asks about the relative frequency of specific behaviors in the past week (or past 2 weeks) in concrete situations such as “Fussing and protesting when placed on his/her back” or “Showing pleasure when playing quietly with his/her toys”. Mothers rated all 191 items on a 7-point scale ranging from 1 (never) to 7 (always). The IBQ-R consists of 14 subscales. All scales demonstrated satisfactory internal consistency (Cronbach’s alpha’s 0.63–0.91). Factor analysis on the subscales (PCA with oblique rotation via oblimin) yielded a three-factor solution that was very similar to the three-factor structure Gartstein and Rothbart (Citation2003) reported for their US sample. For the present study we used the factor Negative Emotionality that had an eigenvalue of 2.27, explained 16.2% of the variance, and was defined by high loadings of Activity level (0.60), Distress to Limitations (0.74), Fear (0.49), Sadness (0.76) and, loading negatively, Falling Reactivity (−0.62). Factor scores were used to indicate the infant’s Negative Emotionality.
Quality of maternal behavior
The caregiving behavior of the mothers was rated from the videotapes using two 9-point scales for sensitivity and cooperation versus interference developed by Ainsworth (cf. Ainsworth et al., Citation1978). Ratings were based on the observations of the whole bathing routine (undressing, bathing, and dressing again). Sensitivity represents the degree to which caregiving behavior reflects awareness of infant signals and communications, and the ability to respond appropriately and promptly to infant cues and signals. Cooperation versus interference represents the extent to which caregiving interventions and initiations of interactions break into, interrupt, or cut across the infant’s ongoing activity. Higher scores on these scales reflect more sensitive and cooperative behavior. The 9-point sensitivity rating scale has been used worldwide in the past decades. Moreover, as judged by a wealth of studies on sensitivity (de Wolff & van IJzendoorn, Citation1997), the construct has proven to be related to a secure mother–infant relationship. A cooperative, non-intrusive interaction style is another key feature of adequate caregiving in infancy; maternal intrusiveness during interactions with their infants has been shown to predict child maladaptation in the early school years, beyond maternal insensitivity (Egeland et al., Citation1993).
The videotapes were rated by two trained coders. Inter-rater reliabilities (Cohen’s kappa), calculated on 20% of the sample, were 0.77 and 0.76 for sensitivity and cooperation versus interference, respectively. Because of the high positive correlation between the two scales (r = 0.82, p < 0.001), an overall maternal caregiving behavior score was computed by averaging the standardized scores for both scales.
Quality of center care
The caregiving behavior of the professional caregivers, which is generally considered the most important aspect of child care quality for younger children (Vandell & Wolfe, Citation2002) was rated from videotapes using the same 9-point scales for sensitivity and cooperation versus interference developed by Ainsworth (cf. Ainsworth et al., Citation1978). The videotapes of the caregiving episodes in week 1, week 12, and week 24 after entry were coded by different groups of trained raters who were blind to the other scores. At each assessment time, caregiver behavior was rated once on each of the two scales, based on the observation of all videotaped caregiving episodes, i.e., the three different caregiving routines. Cohen’s kappa’s were calculated on 20% of the sample and ranged from 0.74 to 0.93 (M = 0.83) for caregiver sensitivity and from 0.80 to 1.0 (M = 0.88) for caregiver cooperation at the three ages. Within each assessment moment, the two scales were significantly correlated (rweek1 = 0.84, rweek12 = 0.88, and rweek24 = 0.85, all p < 0.001). Given the high positive correlations, overall scores for the quality of caregiver behavior were computed by averaging the standardized scores for caregiver sensitivity and cooperation at each of the three assessment moments. The three overall scores were also significantly correlated, reflecting significant stability in the quality of professional caregiving over time (rweek1-24 = 0.39, p < 0.01). To obtain a robust measure of the quality of caregiving across the first months after entering child care, the mean of the three overall scores was calculated and used as the variable representing the overall quality of child care. Higher scores on this variable reflect higher quality of care provided in the center.
Salivary cortisol analysis
Salivary cortisol was measured by a commercial Luminescense Enzyme Immunoassay (IBL, Hamburg, Germany). Briefly, 20 µl saliva were pipetted into antibody-coated microtiter plate wells, followed by 100 µl of enzyme conjugate. After 3 h incubation at room temperature, the plate was washed and luminescence reagent was added to each well, with subsequent reading of the signal in a luminometer. At concentrations of 3.3 and 27.3 nmol/l, within-assay coefficients of variation (CV) were 8.7 and 3.6%, respectively. Between assay CVs were 12.3 and 7.7%. Because the cortisol values were not normally distributed, the cortisol data were log 10-transformed prior to the analyses.
Confounders
The following possible confounders were examined: infant sex, infant age at center care entry, time since the last feeding with respect to cortisol sampling, time awake with respect to cortisol sampling, maternal age, and maternal educational level. Also, to control for maternal postpartum depression, mothers completed the Edinburgh Postnatal Depression Scale (EPDS, Cox et al., Citation1987) at 3 months postpartum. This 10-item questionnaire, scored on a 4-point scale, is widely used to measure depression.
Statistical analyses
First, longitudinal regression analyses using mixed-model (multilevel) designs in SPSS 22.0 (SPSS Inc., Chicago, IL) were conducted to compare infant center care concentrations in weeks 1, 24, and 36 after entry in center care, to their home concentrations sampled in the corresponding weeks. A build-up strategy was used, adding predictors into the model one by one and examining their deviance on the −2loglikelihood ratio scale after generalized least square estimation. Linear time, quadratic time, location (center care versus home), and the interaction between time and location were entered one by one into the model. Linear time was considered a random factor.
Second, to test whether infant temperament, the quality of center care, and the quality of maternal behavior uniquely predicted infants’ cortisol concentrations at center care in weeks 1, 2, 3, 4, 8, 12, 16, 24, and 36 after entry, longitudinal regression analyses using mixed-model (multilevel) designs in SPSS 22.0 (SPSS Inc., Chicago, IL) were conducted. A major advantage of multilevel modeling is the potential to include infants with missing data. With this technique, all valid data points can be included in the model. Missing data were, therefore, not imputed. The infant cortisol concentrations in the morning (10.00 h), the afternoon (16.00 h), or the cortisol slope (10.00 h minus16.00 h) were introduced at level 1 and nested within the infants at level 2. First, the intraclass correlation was calculated using a null model, to examine whether the nested structure is appropriate. The intraclass correlation was 0.078 for the infants’ cortisol concentrations at 10.00 h, so 7.8% of the variability in infants’ cortisol concentrations at center care in the morning was associated with differences between infants, and multilevel analyses were appropriate. For infants’ cortisol concentrations in the afternoon (16.00 h), the intraclass correlation was 0.126. For the cortisol slope, the intraclass correlation was 0.053. These intraclass correlations also indicate that multilevel analyses were appropriate.
Third, a build-up strategy was used, adding predictors into the model one by one and examining their deviance on the −2loglikelihood ratio scale after generalized least square estimation. Linear time, quadratic time, and cubic time were first entered into the model. Linear time was considered a random factor. The time model which improved the model fit the best was retained, and thereafter, the confounders were entered one by one. Subsequently, the predictors depicted for explaining individual differences in cortisol concentrations in center care (infant negative emotionality, quality of center care, and quality of maternal behavior) were added. The last models also included the interactions between the predictors, and the interactions between the predictors and the time variables. The best fitting models are presented in the results.
Results
Preliminary analyses
The means and standard deviations of the variables used as predictors or confounders in explaining individual differences in cortisol concentrations in center care are depicted in . Temperament (IBQ-R) was missing for nine infants. The remainder of the dataset was complete for both the predictors and the confounding variables (see also ).
Table 1. Means and standard deviations for the (variables constituting the composite) predictors of inter-individual differences in infant cortisol concentrations in center care.
In addition, less than 10% of the cortisol values was missing (98 out of 1152), due to samples not containing enough saliva for reliable analysis or to outliers (values greater or smaller than 3 SD’s of the mean). The raw means and standard deviations of the cortisol concentrations can be seen in , while shows how they change over time. As can be seen from the figure, the infants’ morning cortisol concentrations increased sharply during their first month in child care, followed by a gradual decline in the following 8 months. The morning cortisol concentrations were higher than the afternoon cortisol concentrations, as can be expected from the circadian rhythm. The correlations among the predictors are shown in .
Figure 1. Average morning and afternoon cortisol concentrations (nmol/L) in center care across the first 9 months after entering center care at 3 months of age.
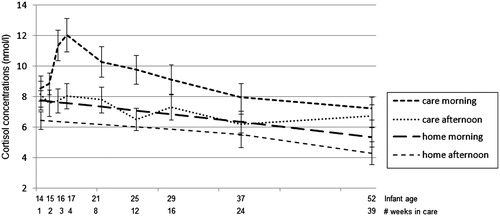
Table 2. Raw Means and Standard Deviations for the Cortisol Levels (nmol/L) in Center Care and at Home.
Table 3. Correlations among the variables predicting infants’ cortisol concentrations in center care.
Longitudinal regression analyses: infant center care cortisol versus home cortisol
The final longitudinal regression models are presented in . For the morning cortisol concentrations (10.00 h), there was a significant effect of linear time indicating that morning cortisol concentrations decreased over the first year of life. A significant effect of location can also be seen, indicating that morning cortisol concentrations were higher in center care. For the afternoon cortisol concentrations (16.00 h), there was a significant effect of linear time, indicating that afternoon cortisol concentrations decreased over the first year of life. In addition, a significant interaction between time and location was found. As can be seen in , morning and afternoon cortisol concentrations were the highest in center care; and the difference between center care and home concentrations became larger over time. For the cortisol slope, no significant results were found.
Table 4. Results of the longitudinal regression analyses (multilevel) comparing infants’ cortisol concentrations, and cortisol slope, in center care and at home in weeks 1, 24, and 36 after entry in center care.
Longitudinal regression analyses: predicting individual differences in center care cortisol
The final longitudinal regression models are presented in . For the morning cortisol concentrations (10.00 h), significant linear time, quadratic time, and cubic time effects can be seen. The mean course of morning cortisol concentrations over the first year of life can be found in . Next to the time effects, a significant effect for quality of maternal behavior was found. This effect indicates that infants receiving higher quality of maternal behavior display higher concentrations of morning cortisol in center care throughout the first year of life. In addition, infant negative emotionality tended to be related to higher morning cortisol concentrations in center care. The following predictors and confounders were not retained in the model: infant sex and age, time since the last feeding (morning), maternal age and educational level, maternal depression, and quality of center care.
Table 5. Results of the longitudinal regression analyses (multilevel) predicting infants’ morning and afternoon cortisol concentrations, and cortisol slope, in center care in weeks 1, 2, 3, 4, 8, 12, 16, 24, and 36 after entry.
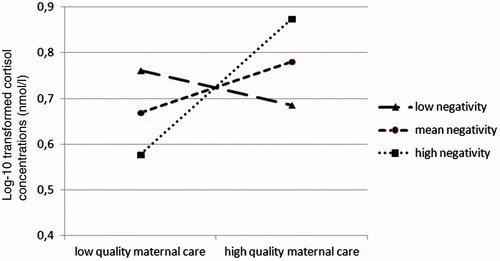
For the afternoon cortisol concentrations (16.00 h), there was a significant effect of linear time indicating that afternoon cortisol concentrations in center care decrease over the first year of life. Moreover, the analyses showed a significant interaction effect between the quality of maternal behavior and infant negative emotionality. This interaction effect is depicted in , and shows that higher quality of maternal behavior is related to higher concentrations of afternoon cortisol in center care, but only for infants high in negative emotionality. The following predictors and confounders were not retained in the model: infant sex and age, time since the last feeding (afternoon), maternal age and educational level, maternal depression, and quality of center care.
Figure 2. Interaction effect between infant negative emotionality and maternal quality of care predicting infant afternoon cortisol concentrations in center care.
For the cortisol slope (10.00 h–16.00 h), there were no significant time effects. In addition, the analyses showed one marginally significant effect indicating that the quality of maternal behavior tended to predict a steeper cortisol slope. The following predictors and confounders were not retained in the model: infant sex and age, time since the last feeding (afternoon), maternal age and educational level, maternal depression, and quality of center care.
Discussion
The aim of this study was to investigate infant cortisol concentrations in center care throughout the first year of life, and to examine whether inter-individual differences can be explained by infant temperament, the quality of center care, and the quality of maternal behavior. First, we found that infant morning and afternoon cortisol concentrations were higher on center care days, compared with home days. Second, we found that infants receiving higher quality of maternal behavior displayed higher morning cortisol in center care, compared to infants receiving lower quality of maternal behavior. In addition, higher quality of maternal behavior was also related to higher concentrations of afternoon cortisol in center care, but this was only true for infants high in negative emotionality, i.e. with a more difficult temperament.
Studies mostly in older children have repeatedly reported higher cortisol in center care as compared with home days (for a review, see Sumner et al., Citation2010; Vermeer & van IJzendoorn, Citation2006; Watamura et al., Citation2010). This study found that morning and afternoon cortisol concentrations are also higher on center care days in young infants. Because infants are brought to center care early in the morning, the 10.00 h and 16.00 h measures reflect the physiological stress experienced by the infant in the center care setting. Earlier studies compared cortisol patterns at home and at center care at one point in time. The present study additionally showed that morning cortisol concentrations remain high, while afternoon concentrations even become higher, during the first nine months after entry in care. These findings resemble the recent findings from Bernard et al. (Citation2015) who also found afternoon cortisol levels to remain elevated throughout a 10-week transition to a new childcare setting, possibly indicating that childcare continues to serve as a cortisol-eliciting context, even months into the transition.
Our results show that mothers who behave more sensitively and less intrusively during home interactions with their infants, have infants with higher concentrations of morning cortisol in center care. This effect of quality of maternal behavior is not moderated by time, indicating that this effect remains stable throughout the first year of life. This finding points in the direction of one of our alternative hypotheses, namely that young infants with sensitive mothers are used to the mother helping them regulate their arousal in stressful situations. For these infants, the unavailability of the mother for an extended period of time might be more stressful than for infants not used to high levels of maternal sensitivity. The possibility should be considered that this finding is specific to the Dutch center care context. As noticed earlier, the parents in our sample were highly educated, which is usual for parents choosing center care for their children in the Netherlands. Moreover, higher educated parents have been shown to be more sensitive in interactions with their infants (e.g. Deynoot-Schaub Gevers & Riksen-Walraven, Citation2008; van Bakel & Riksen-Walraven, Citation2002), which is also visible in their relatively high sensitivity scores (see ). As the general quality of Dutch center care at the time of assessment was on average low (Vermeer et al., Citation2008), the contrast between the quality of the care provided at home and in center care was probably rather high, especially for infants of highly sensitive mothers. In addition, the stability of the quality of care provided in the centers over time was only moderate (r = 0.39), while there is evidence that the quality of maternal behavior is more consistent across time (Albers et al., Citation2010) and also across situations (Maas et al., Citation2012). Infants of more sensitive mothers may also be more sensitive to discrepancies in care and hence stressed by the higher variability and unpredictability of care at center care.
In addition, higher quality of maternal behavior also predicted higher afternoon cortisol concentrations in center care across the first year of life, but only for infants with a more difficult temperament, i.e., infants scoring higher on negative emotionality. This finding is in line with the “biological sensitivity to context theory” (Boyce & Ellis, Citation2005), and adds to the rising body of research acknowledging that children are differentially affected by early environmental conditions depending on early-appearing individual differences (Phillips et al., Citation2010). It is, however, less clear why higher quality of maternal behavior is related to heightened morning cortisol concentrations in all children, and to heightened afternoon cortisol concentrations only in children with a more difficult temperament. It has been suggested that children with a more difficult temperament might encounter more challenges in center care compared with children with a less difficult temperament (Crockenberg, Citation2003; Phillips et al., Citation2010). These on-going challenges throughout the center care day, including social interactions and exposure to often unpredictable and intense stimulation, might build up stress and prevent these infants from recovering. In contrast, the challenges throughout the day might be more manageable for infants with a more easy-going temperament, who would be able to use their better regulatory capacities (i.e., a product of the high quality of maternal behavior they received) to recover from the initial physiological stress response in the morning. In this line, in an earlier study on 3-month-olds we found that the quality of maternal behavior was important for cortisol recovery, and not reactivity, from everyday stressful situations (Albers et al., Citation2008).
In contrast to our expectation, the quality of the care provided in the centers did not predict infant cortisol concentrations at center care days. This is in contrast with what has been found in other studies, although it should be noted that these studies focused on older children attending higher quality centers than the centers included in the present study (Badanes et al., Citation2012; Legendre, Citation2003; Sims et al., Citation2006; Watamura et al., Citation2009). This makes the comparability of our results with those of the previous studies difficult, and our discussion of a tentative nature. Given the considerable variation in center care quality (see ), it is not probable that the lack of association is due to restricted variation. It may be, however, that we should have extended our observations of the caregiver–child interaction beyond the present three caregiving situations (diapering, feeding, putting to bed/waking up). Although most of the everyday caregiver–child interactions occur during these caregiving routines, caregivers’ (non)response to infants’ signals and needs during the rest of the day may also be of importance in explaining variation in infant cortisol concentrations in center care. In addition, it is possible that for infants under a year of age other indicators of center care quality are of equivalent or greater importance. Candidates are poor sleeping arrangements, over-exposure to noise and/or visual stimuli, and poor interactions with (older) peers.
An interesting finding of the present study is that the infants’ morning cortisol in child care steeply increased in the first weeks after entry (see ). One possible explanation for this “delayed” cortisol response might be that it has taken some time for these young infants to recognize the novel environment and associate it with the prolonged separation from the mother. Another possibility might be that the professional caregivers were more attentive and sensitive to the infants during the first weeks after entry, than in later weeks, hence initially buffering the infants’ cortisol responses to separation from the mother and other challenges in center care. Although reveals no differences in the quality of the center care caregivers over time, as mentioned above, the caregivers’ (non)response to infants’ signals and needs during other situations might also be important.
Also interesting is that this peak in morning cortisol concentrations only disappears very gradually, taking several months to reach the morning levels of the first week again. During the first year of life, the infant is rapidly developing regulatory capacities and gaining more control over emotions and behavior (Schore, Citation2001). At the same time, each motor and mental developmental step will also bring about more social and environmental challenges to cope with, probably sustaining the challenging or even stressful nature of center care for young infants.
It is important to note that the biological significance of the heightened cortisol upon entering center care remains to be determined. These heightened cortisol concentrations may be part of the normal physiological reactions needed to cope with the challenges of center care, and may thus very well be adaptive. On the other hand, the heightened cortisol concentrations may also be indicating biologically significant chronic stress in the infants, or at least in a subgroup. As can be seen from the standard deviations in , there was a large variation in infant cortisol concentrations, indicating that there may be a subgroup of infants consistently showing much higher concentrations of cortisol with possible accompanied negative effects on their stress system and subsequent development (Loman & Gunnar, Citation2010).
This study is the first comprehensive one-year longitudinal design with infant cortisol concentrations determined on multiple days, together with the repeated lengthy behavioral observations of both maternal and professional caregiver quality of care. However, limitations should also be noted. First, the comprehensive design set limits to the sample size, which was relatively small. Further studies with larger samples are recommended that focus on the periods of interesting changes in cortisol concentrations that were detected in the present study, including the first months after entry. Also, almost all mothers lived together with their partner and were highly educated. Although this is in line with the general overrepresentation of children from high SES families in Dutch child care centers (Bennet & Tayler, Citation2006; Merens et al., Citation2012), this limits the generalizability of the study results. It would be interesting to investigate infants’ cortisol in center care in relation to quality of care in a low SES sample, as there are indications that HPA-axis functioning might be compromised in low SES samples (Clearfield et al., Citation2014), but also that high-quality center care can benefit children’s socio-emotional development, especially among children from low-income families (Phillips & Lowenstein, Citation2011). Another limitation is the number of saliva samples collected during the day. Salivary samples were taken at 10.00 h and 16.00 h in center care (in post-entry weeks 1, 2, 3, 4, 8, 12, 16, 24, and 36) and at home (in post-entry weeks 1, 24, and 36). However, as cortisol concentrations at the end of the morning show high intra-individual differences (Tollenaar et al., Citation2010), and there are indications in older infants that cortisol concentrations on center care days decreases to bedtime concentrations comparable with non-center care days concentrations (Sumner et al., Citation2010), future studies should collect more cortisol samples throughout the day for two or more consecutive days.
In addition, as we coded maternal and professional caregiver quality of care only during specified types of care, we do not know how sensitive the mother or caregiver are during other types of activities. Observing the infants’ behavior and social interactions at home and in the child care center beyond the routine caregiving episodes may provide a more complete picture of the quality of care received by the infants in both settings. Another direction for future research would be to study developmental trajectories of individual infants in center care and compare, for example, children that show important cortisol increases upon entry and then quickly recover versus children that gradually increase their cortisol levels over time (i.e., where chronically elevated cortisol steadily develops), and to examine how these different trajectories of cortisol in center care are related to children’s later development and health.
Acknowledgements
The authors wish to thank the mothers and infants who kindly participated in this study, and R. van den Berg from the Department of Laboratory Medicine, Radboud University Nijmegen Medical Centre, for cortisol measurements.
Declaration of interest
The authors report that they have no conflicts of interest. This study was supported by a grant from the Netherlands Organization for Scientific Research (NWO, Grant 411-02-561) to the third author.
References
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. (2004). Transition to child care: associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Dev 75:639–50
- Ainsworth MD, Blehar MC, Waters E, Wall S. (1978). Patterns of attachment: a psychological study of the strange situation. Hillsdale, NJ: Erlbaum
- Albers EM, Riksen-Walraven JMA, de Weerth C. (2010). Developmental stimulation in child care centers contributes to young infants' cognitive development. Infant Behav Dev 33:401–8
- Albers EM, Riksen-Walraven JMA, Sweep FCGJ, de Weerth C. (2008). Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry 49:97–103
- Badanes LS, Dmitrieva J, Watamura SE. (2012). Understanding cortisol reactivity across the day at child care: the potential buffering role of secure attachments to caregivers. Early Child Res Q 1:156–65
- Beijers R, Riksen-Walraven JMA, Putnam S, de Jong M, de Weerth C. (2013). Early non-parental care and toddler behaviour problems: links with temperamental negative affectivity and inhibitory control. Early Child Res Q 28:714–22
- Bennet J, Tayler CP. (2006). Starting strong II: early childhood education and care. Paris: OECD
- Bernard K, Peloso E, Laurenceau JP, Zhang ZY, Dozier M. (2015). Examining change in cortisol patterns during the 10-week transition to a new child-care setting. Child Dev 86:456–71
- Boyce WT, Ellis BJ. (2005). Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 17:271–301
- Clearfield MW, Carter-Rodrguez A, Merali A, Shober R. (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behav Dev 37:298–304
- Clements AD, Parker CR. (1998). The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology 23:613–16
- Cox JL, Holden JM, Sagovsky R. (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150:782–6
- Crockenberg SC. (2003). Rescuing the baby from the bathwater: how gender and temperament (may) influence how child care affects child development? Child Dev 74:1034–8
- Custodio RJ, Martinelli CE, Milani SLS, Simoes AL, de Castro M, Moreira AC. (2007). The emergence of the cortisol circadian rhythm in monozygotic and dizygotic twin infants: the twin-pair synchrony. Clin. Endocrinol. (Oxf) 66:192–7
- Datler W, Ereky-Stevens K, Hover-Reisner N, Malmberg LE. (2012). Toddlers' transition to out-of-home day care: settling into a new care environment. Infant Behav Dev 35:439–51
- Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. (2000). Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology 25:819–36
- De Weerth C, van Geert P. (2002). A longitudinal study of basal cortisol in infants: intra-individual variability, circadian rhythm and developmental trends. Infant Behav Dev 25:375–98
- De Weerth C, Zijl RH, Buitelaar JK. (2003). Development of cortisol circadian rhythm in infancy. Early Hum Dev 73:39–52
- De Wolff MS, van IJzendoorn MH. (1997). Sensitivity and attachment: a meta-analysis on parental antecedents of infant attachment. Child Dev 4:571–91
- Deynoot-Schaub Gevers MAJJM, Riksen-Walraven JMA. (2008). Infants in group care: their interactions with professional caregivers and parents across the second year of life. Infant Behav Dev 31:181–9
- Egeland B, Pianta R, O’Brien MA. (1993). Maternal intrusiveness in infancy and child maladaptation in early school years. Dev Psychopathol 5:359–70
- Gartstein MA, Rothbart MK. (2003). Studying infant temperament via the Revised infant behavior questionnaire. Infant Behav Dev 26:64–86
- Geoffroy M, Côté SM, Parent S, Séguin JR. (2006). Daycare attendance, stress and mental health. Can J Psychiatry 51:607–15
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. (2003). Cortisol concentrations in 12-to 18-month-old infants: stability over time, location and stressor. Biol Psychiatry 54:719–26
- Groeneveld MG, Vermeer HJ, Linting M, Noppe G, Van Rossum EFC, Van IJzendoorn MH. (2013). Children's hair cortisol as a biomarker of stress at school entry. Stress 16:711–15
- Gunnar MR, Gonzalez CA, Levine S. (1980). The role of peers in modifying behavioral distress and pituitary-adrenal response to a novel environment in year-old rhesus monkeys. Physiol Behav 25:795–8
- Legendre A. (2003). Environmental features influencing toddlers’ bioemotional reactions in day care centers. Environ Behav 35:523–49
- Loman MM, Gunnar MR. (2010). Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev 34:867–76
- Maas AJBM, Vreeswijk CMJM, van Bakel HJA. (2012). Effect of situation on mother–infant interaction. Infant Behav Dev 36:42–9
- Merens A, Hartgers M, van den Brakel M. (2012). Emancipatiemonitor 2012 (Emancipation monitor 2012). The Hague, SCP/CBS, 2012
- Phillips DA, Fox NA, Gunnar MR. (2010). Same place, different experiences: bringing individual differences to research in child care. Child Dev Perspect 5:44–9
- Phillips DA, Lowenstein AE. (2011). Early care, education, and child development. Annu Rev Psychol 62:483–500
- Pluess M, Belsky J. (2009). Differential susceptibility to rearing experience: the case of childcare. J Child Psychol Psychiatry 50:396–404
- Pluess M, Belsky J. (2010). Differential susceptibility to parenting and quality child care. Dev Psychol 46:379–90
- Schore AN. (2001). Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Ment Health J 22:7–66
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. (1998). Assessing salivary cortisol in studies of child development. Child Dev 69:1503–13
- Shirtcliff EA, Essex MJ. (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol 7:690–703
- Shonkoff JP, Boyce WT, McEwen BS. (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 301:2252–9
- Sims M, Guilfoyle A, Parry TS. (2006). Children's cortisol levels and quality of child care provision. Child Care Health Dev 32:453–66
- Sumner MM, Bernard K, Dozier M. (2010). Young children's full-day patterns of cortisol production on child care days. Arch Pediatr Adolesc Med 164:567–71
- Talge NM, Donzella B, Gunnar MR. (2008). Fearful temperament and stress reactivity among preschool-aged children. Infant Child Dev 17:427–45
- Tollenaar MS, Jansen J, Beijers R, Riksen-Walraven JMA, de Weerth C. (2010). Cortisol in the first year of life: normative values and intra-individual variability. Early Hum Dev 1:13–6
- Van Bakel H, Riksen-Walraven JMA. (2004). Stress reactivity in 15-month-old infants: links with infant temperament, cognitive competence, and attachment security. Dev Psychobiol 44:157–67
- Van Bakel HJA, Riksen-Walraven JMA. (2002). Parenting and development of one-year-olds: links with parental, contextual, and child characteristics. Child Dev 73:256–73
- Vandell D, Wolfe B. (2002). Child care quality: does it matter and does it need to be improved? Madison: Institute for Research on Poverty, University of Wisconsin
- Vermeer HJ, van IJzendoorn MH. (2006). Children’s elevated cortisol levels at daycare: a review and meta-analysis. Early Child Res Q 21:390–401
- Vermeer HJ, van IJzendoorn MH, de Kruif REL, Fukkink RG, Tavecchio LWC, Riksen-Walraven JMA, van Zeijl J. (2008). Child care in the Netherlands: trends in quality over the years 1995–2005. J Genet Psychol 169:360–85
- Watamura SE, Coe CL, Laudenslager ML, Robertson SS. (2010). Child care setting affects salivary cortisol and antibody secretion in Young children. Psychoneuroendocrinology 35:1156–66
- Watamura SE, Donzella B, Alwin A, Gunnar MR. (2003). Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: age differences and behavioral correlates. Child Dev 74:1006–20
- Watamura SE, Kryzer EM, Robertson SS. (2009). Cortisol patterns at home and child care: afternoon differences and evening recovery in children attending very high quality full-day center-based child care. J Appl Dev Psychol 30:475–85

