Abstract
The aim of the present study was to evaluate whether the anxiety-increasing effects of chronic psychosocial stress generalize to non-social (i.e. heterotypic) stressful situations. To investigate this issue, we repeatedly exposed rats to predictable or unpredictable psychosocial stress for 5 or 12 days and examined their anxiety in two markedly different contexts: the elevated plus maze and social interaction tests. Psychosocial stress and the social interaction test were administered under highly similar conditions, i.e. the two situations were homotypic. Psychosocial stress did not affect anxiety in the elevated plus-maze under any condition, but markedly increased anxiety in the social interaction test. In contrast, repeated restraint–a non-social stressor heterotypic to both the elevated plus maze and social interaction tests–increased plus-maze anxiety, demonstrating that anxiety in this test was sensitive to repeated restraint, and the effects were manifested in heterotypic situations. Thus, the anxiety-related effects of chronic psychosocial stress–unlike those of the chronic non-social stressor–were context-dependent. This is reminiscent of phobic anxiety, which manifests in specific situations only. In addition, behavior in the social interaction test showed changes that went beyond simple anxiogenesis. Socially stressed rats spent nearly 40% of total time in aggressive interactions. Based on recent data showing that social phobics are prone to violence under social pressure, and also based on the situation-dependent effects of the social stressor, we suggest that chronic psychosocial stress leads to a behavioral profile akin to social phobia.
Introduction
The physiological response to aversive stimuli–the stress response–becomes a pathological risk factor when it is long-lasting, is frequently repeated or is excessively strong. Continuous stress exposure or excessively strong stressors are relatively scarce, and occur in specific situations only. In contrast, repeated exposures to stressors are part of everyday life, the effects of which can accumulate and lead to various psychopathologies including anxiety (Reavley Citation1974; Blazer et al. Citation1987; Allen et al. Citation2008). Not surprisingly, various aspects of repeated stress exposure are intensively studied, including the relationship between the effects of subsequent stressors.
Repeated exposure to stressors of the same kind (i.e. to homotypic stressors) often leads to habituation (Armario et al. Citation2004; Vining et al. Citation2007). Various stressors are rather different in this respect. Certain stressors (e.g. cold or novelty stress) habituate rapidly while others (e.g. immobilization stress) habituate more slowly (Sabban and Serova Citation2007; Gagliano et al. Citation2008; Kvetnansky et al. Citation2009). Importantly for the present paper, social stressors (e.g. chronic social defeat) appear to be the least prone to habituation (Keeney et al. Citation2006; Barnum et al. Citation2007). Even when habituation occurs, however, the consequences of repeated homotypic stressors do not vanish altogether. On their background, a stressor of a different kind (i.e. heterotypic stressors) leads to exacerbated acute stress responses (Ma et al. Citation1999; Sabban and Serova Citation2007; Belda et al. Citation2008; Kvetnansky et al. Citation2009; Spiga et al. Citation2009). Similar to habituation, the response to heterotypic stress is also stressor-specific, e.g. chronic restraint does not affect the response to lipopolysaccharide injection, but increases the response to acute noise (Spiga et al. Citation2009).
Given the strong association between stress responses and anxiety, one can hypothesize that the complexities of the former are reflected by a similarly complex stress-anxiety relationship; i.e. the similarity between the stressful situation and the testing environment should have an impact on stress-induced anxiety. Disparate findings support this notion. It was shown for instance that the stressing and testing context has an impact on depression-like behavior in laboratory rodents (Haller et al. Citation2002; Keeney et al. Citation2006). Somewhat similar findings were obtained in the case of anxiety (Baranyi et al. Citation2005; Razzoli et al. Citation2007). There also are a series of studies where in contrast to expectations, chronic stress exposure did not affect anxiety as assessed in particular tests (D'Aquila et al. Citation1994; Zelena et al. Citation1999; Gregus et al. Citation2005). Importantly for the present paper, there was a marked dissimilarity between the stressful and testing environments in these studies. One can hypothesize that anxiety does not generalize to dissimilar situations but it would be apparent if tested in the same environment where stress was experienced. Testing this hypothesis is often difficult, because the conditions employed in stress paradigms are usually quite different from the conditions under which anxiety is tested. This may explain why this line of research has received little attention so far. Social behavior is an exception in this respect, as encounters between two animals are used both to induce chronic stress states and to study anxiety.
Earlier we showed that chronic social instability (daily alternation of individual and crowded housing for 14 days) in female rats increases anxiety in the social interaction test but not in the elevated plus-maze (Baranyi et al. Citation2005). In that study, however, the stressing and testing environments were rather different, although both were social in nature. We have also shown that in male rats, social defeat for 30 min daily for 4 days induced subtle changes in plus-maze behavior without increasing anxiety in this test. Here we extended these observations by directly comparing the anxiety-related effects of chronic psychosocial stress in tests that are similar to, and entirely different from the stressing environment (i.e. in homotypic and heterotypic tests). Based on the findings briefly reviewed above, we hypothesized that chronic psychosocial stress-induced anxiety is context-specific, i.e. it does not generalize to heterotypic situations.
Materials and methods
Animals
The subjects were male Wistar rats (Charles River Laboratories, Budapest, Hungary) weighing 300–400 g. Before experimentation, rats were housed in groups of six per cage (35 × 50 × 25 cm) in a reversed 12:12 h day–night cycle (lights off at 10.00 h). Habituation to the schedule lasted at least 2 weeks. Room temperature and humidity were 22 ± 2°C and 60%, respectively. Tap water and standard laboratory food were available ad libitum. Each rat was used only once. Experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were reviewed and approved by the Animal Welfare Committee of the Institute of Experimental Medicine (Budapest, Hungary).
The residents for the psychosocial stress paradigm were six month-old male Wistar rats (Charles River Laboratories) weighing 450–500 g. Maintenance conditions were similar to those described above except that residents were individually housed for 2 weeks in cages measuring 60 × 40 × 50 cm. Their aggressiveness was checked 1 week prior to experimentation.
Experimental design
Experiment 1 aimed at monitoring the effects of a 1-week long psychosocial stress on plus-maze behavior. Three groups were studied. Stressed rats were exposed to the residents for 4 h on 4 consecutive days as shown below. Between encounters, rats remained in olfactory and visual but not physical contact with their residents (i.e. they were allowed to establish sensory contacts only). As the stressed group was neither socially isolated nor living in a social group, it was compared with two control groups. Group housed rats were maintained in groups of six throughout, while socially isolated rats were transferred to individual cages on day 1 and maintained as such until behavioral testing. Rats were tested on the elevated plus-maze on the 5th day. Sample size was nine rats per group.
Experiment 2 was carried out because psychosocial stress did not affect anxiety in Experiment 1. The experimental design was similar except for the duration of psychosocial stress. The subjects of this study were in sensory contact with the residents for 12 days, during which they were exposed to 4 h-long aggressive encounters daily at the same clock hour (10.30–14.30 h) except for the weekend and the last experimental day, when plus-maze testing was performed. Despite omitting aggressive encounters on certain days, psychosocial stress was continuous as subordinates remained in sensory contact with their dominants. Sample size was nine rats per group.
The stress load was further increased in Experiment 3. The procedure was similar, but the timing, duration and the daily number of stress exposures was varied, i.e. aggressive encounters were made unpredictable. Aggressive encounters were performed between 11.30 and 18.00 h (day 1), 13.00 and 14.30 h (day 2), 09.00 and 11.30 h, and 14.00 and 15.00 h (both on day 3), and between 11.30 and 16.00 h (day 4). The active (dark) period started at 10.00 h and lasted 12 h. Thus, aggressive encounters were started at variable time-points between 09.00 and 14.00 h, while the duration of direct contacts varied from 1 to 7.5 h. In addition, the aggressive episodes were repeated on one of the days. Subjects remained in sensory contact with their dominants throughout. Sample size was 9–12 rats per group.
Experiment 4 studied the effects of unpredictable social stress in the social interaction test. The protocol was similar to that employed in Experiment 3, but the elevated plus-maze test was replaced with the social interaction test. Sample size was 12 rats per group.
Finally, the responsiveness of plus-maze behavior to chronic non-social stress was investigated in Experiment 5. In this experiment, socially isolated rats were either left undisturbed in their home-cage or were restrained for 1 h per day for 4 days. Sample size was seven rats per group.
In each experiment, body weights were measured before the first stress episode and after behavioral testing. At the latter time-point, subjects were killed by conscious decapitation and their adrenals were removed and weighed.
Stress procedures
Psychosocial stress was administered in the home-cage of residents as described earlier (Zelena et al. Citation1999). On the first day of the study, rats belonging to the stressed group were introduced into the cage of the residents in the early hours of the dark phase under dim red light, and were allowed to freely interact. Attacks delivered by the residents were counted during the first 30 min of the encounter. The intruders were separated from residents by a transparent, perforated Plexiglas partition after the encounter. This allowed sensory but not physical contacts between opponents. On each of the subsequent days, subjects were moved to the home-cage of a different resident, and the partition was removed. Cage switch was employed because earlier experience showed that the aggressiveness of the resident rapidly declines to almost nil when the same rats encounter each other repeatedly. In the case of Experiments 1 and 2, the partition was always removed for 4 h between 10.00 and 14.00 h. Thus, the stressed group interacted daily with residents for 4 h, and remained in sensory contact with the resident for 5 or 12 days (in Experiments 1 and 2, respectively). As shown above, the timing and duration of the aggressive encounter was varied in Experiments 4 and 5. Psychosocial contacts were maintained for 5 days.
Restraint stress was delivered every day at the same time for 1 h between 09:00 and 12:00 h for 4 days as described previously (Zelena et al. Citation2003). Briefly, the rats were placed into transparent plastic tubes (5–6 cm inner diameter) having a 4 cm long conical head part ending with a large breathing-hole (2 cm inner diameter). Behind the body, the rear end of the tube was loosely packed with paper towels. This procedure minimized the space around the animal, prevented turning, and provided strong stress without being harmful. Rats were tested on the elevated plus-maze on the 5th day, i.e. 1 day after the last restraint session.
Behavioral testing
All behavioral experiments were performed under red light during the early hours of the dark phase. Anxiety was assessed by means of the elevated plus-maze and social interaction tests.
The elevated plus-maze test was performed as described in Pellow et al. (Citation1985). A dark grey painted wooden plus-maze was used (arm length: 50 cm, arm width: 20 cm, wall height: 30 cm, platform height: 80 cm). Closed arms faced each other. Rats were placed in the central arena, facing a closed arm. Testing lasted 5 min. Behavioral analysis was done by means of EthoVision (Noldus, The Netherlands) software, which automatically scored entries and time spent in different compartments. Furthermore, risk assessment was recorded by direct observation. Risk assessment activities considered were the frequencies of stretch-attend posture (investigating the open arm from a protected compartment in a stretched posture) and head dipping (investigating the area beneath the platform).
The social interaction test was performed as described in File and Hyde (Citation1978), slightly modified according to Guy and Gardner (Citation1985). Conditions were similar to those employed earlier (Haller et al. Citation2001). The test arenas (60 × 40 × 50 cm) were made from opaque plastic except for the front walls, which were transparent. The size and shape of the resident's home-cage and the social interaction arenas were similar, but were differently colored (the home-cages were dark grey, whereas the social interaction arenas were red). Social encounters lasted 10 min and were evaluated in pairs of unfamiliar animals that had similar stress backgrounds. The following behaviors were recorded: resting (lying or standing, infrequent movements of the head or postural changes); exploration (sniffing directed toward the environment); grooming (self-care with forepaws and scratching with hind legs); social investigation (sniffing directed towards the opponent's flank, nasal, or anogenital region); offence (aggressive grooming, lateral threat, offensive upright posture, mounting and chasing taken together); defence (defensive upright, defensive kick, fleeing and freezing taken together); dominant posture (keeping down the opponent while he lay on his back); submissive posture (laying on back while kept down by the opponent); attack (skin pulling, soft and hard bites taken together). Values were expressed as percent of time, except for attacks which were expressed as frequency. Behavior was scored by an experimenter blind to the treatments, by means of a computer-based event-recorder (H77, Budapest, Hungary).
Statistical evaluation
For statistical analysis, Statistica software was used (StatSoft Inc., Tulsa, OK, USA). Data are shown throughout as mean ± standard error of the mean (SEM). Physiological parameters were compared by one-way ANOVA. Behavior was compared by Kruskal–Wallis ANOVA, followed by Mann–Whitney post hoc comparisons. The level of significance was set at p < 0.05.
Results
The intensity of psychosocial stress
During the first 30 min of the first aggressive encounter, the number of biting attacks received by the subjects was 8.5 ± 0.9, which gradually declined over time. On the day before behavioral testing, the number of biting attacks was 2.4 ± 0.5. Defeats as well as the continuous psychosocial stress led to a significant decrease in body weight gain when the duration of stress was 5 days (). In Experiment 2 where psychosocial stress lasted 12 days, the decrease in body weight gain was not significant, indicating a degree of habituation to the procedure. Adrenal weights were increased by chronic psychosocial stress in all experiments including Experiment 2 in which weight gain normalized (). Repeated restraint did not increase adrenal weight but significantly decreased body weight gain ().
Table I. Weight gain (g) in controls and stressed rats.
Table II. Relative adrenal weights in controls and stressed rats.
Behavioral findings
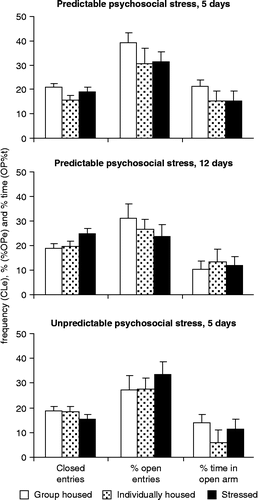
Classical plus-maze variables were not affected by psychosocial stress under any condition (). In the case of closed arm entries, H values were 2.62 (p < 0.3), 4.16 (p < 0.15), and 1.5 (p < 0.5) after 5 and 12 days of predictable, as well as after 5 days of unpredictable social stress, respectively. The similar values for percent open entries were 2.41 (p < 0.3), 0.54 (p < 0.8), and 1.93 (p < 0.2), respectively. The duration of open arm visits showed non-significant changes as well (H values were 2.87 (p < 0.3), 0.11 (p < 0.9), and 4.01 (p < 0.2) after 5 and 12 days of predictable, as well as after 5 days of unpredictable social stress, respectively). Risk assessment was not affected significantly either (data not shown).
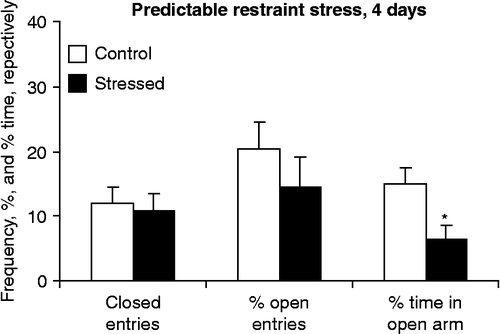
Figure 1. The effects of psychosocial stress on elevated plus-maze behavioral variables. No agonistic encounters were performed on the last (testing) day, but subjects remained in sensory contact with dominants throughout. CLe, closed entries; %OPe, percent open arm entries; and OP%t, % time in open arms. Values are group mean+SEM. No significant differences were seen under any of the conditions. Sample size was 9–12 rats per group.
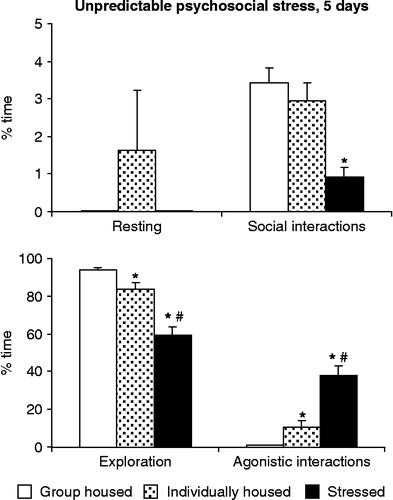
In contrast, 5 days of unpredictable social stress significantly decreased social interactions (H(2,36) = 16.94; p < 0.001) without affecting resting (H(2,36) = 4.11; p < 0.1) (). Exploration was significantly decreased by psychosocial stress (H(2,36) = 22.7; p < 0.001), a change which occurred at the expense of a dramatic increase in agonistic behaviors (H(2,36) = 27.79; p < 0.001). Agonistic behavior but not social interactions were increased by social isolation as well.
Figure 2. The effects of psychosocial stress in the social interaction test. No agonistic encounters were performed on the last (testing) day, but subjects remained in sensory contact with dominants throughout. Values are group mean+SEM. *Significantly different from control (p < 0.05 at least); #significantly different from both control and isolated rats (p < 0.05 at least). Sample size was nine rats per group.
Repeated restraint stress increased anxiety-like behavior in the elevated plus-maze (). Closed arm entries were not affected significantly (H(1,14) = 0.21, p < 0.7). The decrease in percent open arm entries did not reach statistical significance (H(1,14) = 0.92, p < 0.3), but the duration of open arm exploration was significantly decreased by 4 days of restraint stress (H(1,14) = 4.45, p < 0.04).
Correlations
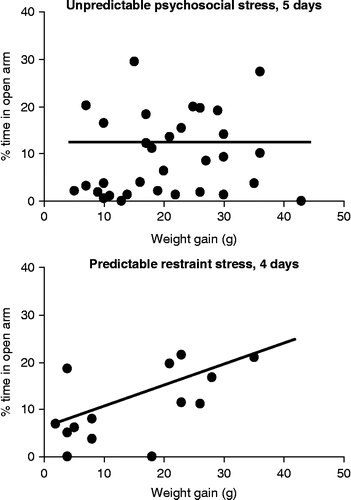
Correlations were calculated to investigate the possibility that psychosocial stress had a hidden impact on plus-maze behavior. On average, the behavior of control and psychosocially stressed rats was similar in the elevated plus-maze. Yet, the stress response was not uniform in stressed rats, which raised the possibility that the indicators of stress and the indicators of anxiety correlated significantly. This may have revealed effects that were not conspicuous when averages were compared. We evaluated the Spearman rank correlation between body weight gain and open arm exploration to further characterize the interaction between the psychosocial stress and anxiety-like behavior shown on the plus-maze. No significant correlations were found in Experiments 1–3 (R values were between − 0.064 and − 0.483; p values were between 0.74 and 0.18). illustrates the lack of correlation for 5 days of unpredictable psychosocial stress. In contrast, a significant correlation was found between these two variables when rats were exposed to restraint stress (R = 0.564; p = 0.035) ().
Figure 4. The correlation between body weight gain and the duration of open arm exploration. No correlation was seen in the case of psychosocial stress (R = − 0.064; p>0.7) (upper panel; N = 32). In contrast, the correlation was significant in the case of repeated restraint (R = 0.564; p < 0.04) (lower panel; N = 14).
Discussion
Both psychosocial stress with repeated aggressive encounters and repeated restraint robustly decreased body weight and increased adrenal weight, indicating that rats were chronically stressed in these paradigms. A degree of habituation occurred after 12 days of psychosocial stress, as weight gain tended to normalize, but adrenal hypertrophy persisted. Anxiety-like behavior was increased by psychosocial stress in the social interaction test but not in the elevated plus-maze. The decrease in social interactions was not secondary to locomotor suppression, as neither resting nor exploration was affected by chronic psychosocial stress. In addition, aggressiveness was dramatically increased in the social interaction test, which precludes a decrease in locomotion. Behavior in the elevated plus-maze was sensitive to stressful manipulations, as repeated restraint increased anxiety in this test. Taken together, these findings show that psychosocial stress robustly increases anxiety in homotypic (i.e. social) contexts, but anxiety does not generalize to heterotypic (i.e. non-social) situations.
These results were not entirely unexpected, as chronic social instability in female rats (the daily alternation of individual and crowded housing) decreased anxiety in the social interaction test but not in the plus-maze test (Baranyi et al. Citation2005). We have also shown that repeated brief episodes of defeat fail to increase anxiety in the elevated plus-maze test (Zelena et al. Citation1999). In line with these findings, psychosocial stress administered to adolescents did not affect, or even decreased anxiety in the elevated plus-maze, albeit anxiety did develop in later stages of life (McCormick et al. Citation2008). Taken together, the present and earlier findings suggest that chronic social stress induces a situation-specific anxiety that does not generalize to non-social situations. This inference is in line with the hypothesis of Berton et al. (Citation1997) who submitted six inbred rat strains to multivariate analysis and concluded that the behavioral reactivity to chronic social stress is a specific feature, dissociable from emotionality shown in nonsocial settings. These earlier studies, however, did not directly compare homotypic and heterotypic situations. In addition to demonstrating the stressor-specificity of psychosocial stress-induced anxiety, the present findings indicate that the anxiety-related effects of repeated restraint do generalize because restraint and plus-maze testing are not homotypic.
Interestingly, acute social stress readily decreases anxiety in the elevated plus-maze; moreover, this effect persists for 1–2 weeks if rats are prevented from establishing further social contacts (Rodgers and Cole Citation1993; Berton et al. Citation1999; Haller and Bakos Citation2002; Nakayasu and Ishii Citation2008). The lack of effect of chronic, but the robust effect of acute social stress is difficult to interpret. One can tentatively hypothesize that the emotional background of psychosocially stressed rats gradually shifts from anxiety to a state that is akin to social phobia. It is important in this respect that the aggressiveness of psychosocially stressed rats increased dramatically. These rats spent nearly 40% of total time in agonistic interactions. Although aggressive and social interactions tend to change in opposite directions in the social interaction test, the duration of agonistic interactions is usually a small fraction of that seen in the present experiment (Guy and Gardner Citation1985; Baranyi et al. Citation2005; Gregus et al. Citation2005). The unusually high aggressiveness of psychosocially stressed rats suggests that their behavior was altered beyond a simple increase in anxiety. Our hypothesis on the development of social phobia is difficult to demonstrate, as no laboratory counterpart of this disorder is available, i.e. we have no terms of comparison at present. Yet, it has to be noted that (i) social phobia in people is often viewed as a form of, or a state deriving from, subordination (Hermans and van Honk Citation2006); (ii) mildly negative social situations are interpreted by social phobics in a ‘catastrophic’ fashion (Stopa and Clark Citation2000), and (iii) social phobics are prone to excessive violence under social pressure, including threatening with weapon, assault and murder (Alden and Taylor Citation2004; Casiano et al. Citation2008). In addition, phobic anxiety is defined as an anxiety response triggered by specific situations, i.e. which does not generalize. The behavior of psychosocially stressed rats appears to fulfill this basic condition.
In conclusion, chronic psychosocial stress in male rats did not affect anxiety as assessed by behavior on the elevated plus-maze, but profoundly changed behavior in the social interaction test. Based on the present and earlier findings we hypothesize that rats habituate to the anxiogenic effects of psychosocial stress but develop a social phobia-like state.
Acknowledgements
This research was supported by OTKA grant No. T 046785 EMB to HJ.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alden LE, Taylor CT. 2004. Interpersonal processes in social phobia. Clin. Psychol. Rev.. 24:857–882.
- Allen JL, Rapee RM, Sandberg S. 2008. Severe life events and chronic adversities as antecedents to anxiety in children: A matched control study. J. Abnorm. Child Psychol.. 36:1047–1056.
- Armario A, Valles A, Dal-Zotto S, Marquez C, Belda X. 2004. A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress. 7:157–172.
- Baranyi J, Bakos N, Haller J. 2005. Social instability in female rats: The relationship between stress-related and anxiety-like consequences. Physiol. Behav.. 84:511–518.
- Barnum CJ, Blandino PJr, Deak T. 2007. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J. Neuroendocrinol.. 19:632–642.
- Belda X, Rotllant D, Fuentes S, Delgado R, Nadal R, Armario A. 2008. Exposure to severe stressors causes long-lasting dysregulation of resting and stress-induced activation of the hypothalamic-pituitary-adrenal axis. Ann. N.Y. Acad. Sci.. 1148:165–173.
- Berton O, Ramos A, Chaouloff F, Mormde P. 1997. Behavioral reactivity to social and nonsocial stimulations: A multivariate analysis of six inbred rat strains. Behav. Genet.. 27:155–166.
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. 1999. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 92:327–341.
- Blazer D, Hughes D, George LK. 1987. Stressful life events and the onset of a generalized anxiety syndrome. Am. J. Psychiatry. 144:1178–1183.
- Casiano H, Belik SL, Cox BJ, Waldman JC, Sareen J. 2008. Mental disorder and threats made by noninstitutionalized people with weapons in the national comorbidity survey replication. J. Nerv. Ment. Dis.. 196:437–445.
- D'Aquila PS, Brain P, Willner P. 1994. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol. Behav.. 56:861–867.
- File SE, Hyde JRG. 1978. Can social interaction be used to measure anxiety?. Br. J. Pharmacol.. 62:19–24.
- Gagliano H, Fuentes S, Nadal R, Armario A. 2008. Previous exposure to immobilisation and repeated exposure to a novel environment demonstrate a marked dissociation between behavioral and pituitary-adrenal responses. Behav. Brain Res.. 187:239–245.
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. 2005. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res.. 156:105–114.
- Guy AP, Gardner CR. 1985. Pharmacological characterisation of a modified social interaction model of anxiety in the rat. Neuropsychobiology. 13:194–200.
- Haller J, Bakos N. 2002. Stress-induced social avoidance: A new model of stress-induced anxiety?. Physiol. Behav.. 77:327–332.
- Haller J, Leveleki C, Halasz J, Baranyi J, Makara GB. 2001. The effect of glucocorticoids on the anxiolytic efficacy of buspirone. Psychopharmacology. 157:388–394.
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. 2002. Behavioral responses to social stress in noradrenaline transporter knockout mice: Effects on social behavior and depression. Brain. Res. Bull.. 58:279–284.
- Hermans EJ, van Honk J. 2006. Toward a framework for defective emotion processing in social phobia. Cogn. Neuropsychiatry. 11:307–331.
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. 2006. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol.. 18:330–338.
- Kvetnansky R, Sabban EL, Palkovits M. 2009. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol. Rev.. 89:535–606.
- Ma XM, Lightman SL, Aguilera G. 1999. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 140:3623–3632.
- McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain. Res.. 187:228–238.
- Nakayasu T, Ishii K. 2008. Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav. Process.. 78:477–480.
- Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 14:149–167.
- Razzoli M, Carboni L, Guidi A, Gerrard P, Arban R. 2007. Social defeat-induced contextual conditioning differentially imprints behavioral and adrenal reactivity: A time-course study in the rat. Physiol. Behav.. 92:734–740.
- Reavley W. 1974. The relationship of life events to several aspects of ‘anxiety’. J. Psychosom. Res.. 18:421–424.
- Rodgers RJ, Cole JC. 1993. Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol. Behav.. 53:383–388.
- Sabban EL, Serova LI. 2007. Influence of prior experience with homotypic or heterotypic stressor on stress reactivity in catecholaminergic systems. Stress. 10:137–143.
- Spiga F, Harrison LR, MacSweeney CP, Thomson FJ, Craighead M, Lightman SL. 2009. Effect of vasopressin 1b receptor blockade on the hypothalamic-pituitary-adrenal response of chronically stressed rats to a heterotypic stressor. J. Endocrinol.. 200:285–291.
- Stopa L, Clark DM. 2000. Social phobia and interpretation of social events. Behav. Res. Ther.. 38:273–283.
- Vining C, Iyer V, Bhatnagar S. 2007. Intracerebroventricular administration of corticotrophin-releasing hormone receptor antagonists produces different effects on hypothalamic pituitary adrenal responses to novel restraint depending on the stress history of the animal. J. Neuroendocrinol.. 19:198–207.
- Zelena D, Haller J, Halasz J, Makara GB. 1999. Social stress of variable intensity: Physiological and behavioral consequences. Brain Res. Bull.. 48:297–302.
- Zelena D, Mergl Z, Foldes A, Kovacs KJ, Toth Z, Makara GB. 2003. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am. J. Physiol. Endocrinol. Metab.. 285:E1110–E1117.