Abstract
Brain-derived neurotrophic factor (BDNF) is crucial for the survival and differentiation of the central and peripheral nervous systems. Recently, BDNF has been reported to exert broader biological activity on non-neural cells. A previous study examined the effect of immobilization stress on BDNF and its receptor tyrosine receptor kinase B in male rat submandibular glands. In the present study, we found that the rat submandibular gland is the major source of plasma BDNF during acute immobilization stress. Biting modulates the mRNA and protein levels of BDNF in the rat hippocampus, so we also investigated whether the plasma BDNF concentration is influenced by biting. Two hours of acute immobilization stress significantly increased the amount of BDNF mRNA within the rat submandibular glands. Moreover, allowing biting behavior for the second half of the 2-h stress exposure significantly increased the amount of salivary gland BDNF mRNA relative to stress alone. Similar results were found with plasma BDNF concentrations under the same conditions. We confirmed that biting during stress attenuates the increases in plasma adrenocorticotropic hormone and corticosterone concentrations, but this was not dependent on the submandibular glands. Increased BDNF, mRNA and protein expressions were observed in salivary duct cells as a result of immobilization stress and biting behavior, as demonstrated by real-time polymerase chain reaction, immunohistochemistry, western blotting, and enzyme-linked immunosorbent assay. Taken together, the findings indicate that the submandibular glands evidently contribute to the increase in plasma BDNF upon biting.
| Abbreviations | ||
| ACTH | = | adrenocorticotropic hormone |
| BDNF | = | brain-derived neurotrophic factor |
| CRH | = | corticotropin releasing hormone |
| DAB | = | 3,3′-diaminobenzidine-tetrahydrochloride |
| DEPC | = | diethyl pyrocarbonate |
| EB | = | ethidium bromide |
| EGF | = | epidermal growth factor |
| ELISA | = | enzyme-linked immunosorbent assay |
| GAPDH | = | glyceraldehydes-3-phosphate dehydrogenase |
| HGF | = | hepatocyte growth factor |
| HPA | = | hypothalamic-pituitary-adrenal |
| HRP | = | horseradish peroxidase |
| NFDM | = | non-fat dry milk |
| NGF | = | nerve growth factor |
| nNOS | = | neuronal nitric oxide synthase |
| NO | = | nitric oxide |
| NT-3 | = | neurotrophin-3 |
| NT-4 | = | neurotrophin-4 |
| PCR | = | polymerase chain reaction |
| PVN | = | paraventricular nucleus |
| RT | = | reverse transcription |
| SA | = | sialoadenectomy |
| SD | = | Sprague–Dawley |
| SDS-PAGE | = | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TBS | = | Tris-buffered saline |
| TrkB | = | tyrosine receptor kinase B |
Introduction
Brain-derived neurotrophic factor (BDNF) is a crucial peptide in the central and peripheral nervous systems. It is also present in non-neural tissues, such as the heart, lungs (Timmusk et al. Citation1993), platelets (Radka et al. Citation1996), lymphocytes (Sobue et al. Citation1998), and lacrimal glands (Ghinelli et al. Citation2003). In our previous study, the rat submandibular gland in particular was identified as an organ that expresses BDNF when under acute immobilization stress (Tsukinoki et al. Citation2006). BDNF is produced by the ductal epithelium of glandular components. However, no BDNF-receptor tyrosine receptor kinase B (TrkB) mRNA was detected in submandibular gland tissues or oral and esophageal mucosa by reverse transcription (RT)-polymerase chain reaction (PCR) in either non-stress or stress conditions (Tsukinoki et al. Citation2006). Interestingly, plasma BDNF concentrations significantly increased in the stress model, which may be attributed to its secretion from the salivary gland into circulation (Tsukinoki et al. Citation2007). Reduced plasma BDNF concentrations have been found in humans with neurodegenerative disorders, major depression, and bipolar disorder (Buckley et al. Citation2007). As these disorders are believed to be stress-related, preventing the suppression of BDNF decrease under stress conditions seen in these disorders may prevent their manifestation (Piccinni et al. Citation2008). Although the role of plasma BDNF is not well understood, it has been hypothesized that the effects of plasma BDNF secreted from salivary glands may protect the human body, including the nervous system, from stress.
It has also been reported that biting modulates a hormonal stress response (Hori et al. Citation2004, Citation2005; Sasaguri et al. Citation2005; Lee et al. Citation2008). The expression of corticotropin releasing hormone (CRH) is significantly increased in the paraventricular nucleus (PVN) neurons of the hypothalamus by acute immobilization stress, and this increase has been found to be suppressed by biting (Hori et al. Citation2004). Nitric oxide (NO) modulates the activity of the endocrine system during behavioral responses to stress, and an increase in neuronal nitric oxide synthase (nNOS) mRNA expression under acute immobilization stress has been observed in the PVN of the hypothalamus, while biting a wooden stick during immobilization decreases nNOS mRNA expression in the hypothalamus (Hori et al. Citation2005). Fos protein, the expression of which is induced by acute immobilization stress, is generally used as a marker for neuronal activity in the PVN, and biting behavior during stress reduces the expression of Fos protein (Sasaguri et al. Citation2005). Adrenocorticotropic hormone (ACTH) and corticosterone circulating concentrations have been shown to be markedly elevated in stressed animals, but this elevation is suppressed in the biting group (Lee et al. Citation2008). Additionally, we have recently reported that the decreased BDNF mRNA expression in rat hippocampus induced by acute immobilization stress is recovered by biting (Lee et al. Citation2008). As biting may attenuate systemic stress responses, the changes in plasma BDNF concentrations under biting conditions may be of interest.
In the present study, we investigated whether biting affects (a) BDNF mRNA expression induced by immobilization stress within the rat submandibular gland, (b) plasma BDNF concentration induced by immobilization stress, and (c) plasma BDNF concentrations induced by immobilization stress in sialoadenectomized rats. The concentrations of BDNF mRNA and protein extracted from submandibular gland tissue under these conditions were examined.
Materials and methods
Animals
Sprague–Dawley (SD) male rats (n = 72; age, 7 weeks; weight, 220–240 g; Japan SLC, Shizuoka, Japan) were used in this study. They were housed in groups of four rats per cage in a room maintained under controlled standardized conditions of a 12-h light/12-h dark cycle (lights on at 07:00 h) and ambient temperature (22 ± 3°C) with humidity of 55 ± 5%. Rats had ad libitum access to standard food pellets and tap water.
Immobilization, biting, and sialoadenectomy (SA) procedures
Rats were divided into 3 groups of 24 rats each: control, stress, and stress+biting groups. These groups were further divided into three sub-groups of eight rats each: intact (intact), sham operation (sham), and SA groups. Control rats were not exposed to immobilization. Non-control rats were immobilized to produce acute stress according to a well-established protocol (Hori et al. Citation2004, Citation2005). Briefly, they were fixed with a leather belt onto a wooden board (18 × 25 cm) in a supine position, and all legs were fixed at an angle of 45° to the body midline with adhesive tape. Immobilization stress was continued for 2 h. Rats in the stress group were exposed to this condition only, while those in the stress+biting group were allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min) (Lee et al. Citation2008). The wooden stick was manipulated toward the rat's mouth, allowing the rat to bite it without any head or body movements. Every rat in the stress+biting group responded to the wooden stick by biting on it with a rapid and repetitive sequence of jaw opening and closing movements for at least two-thirds of the 60 min period.
In the six operated groups, SA of the bilateral major salivary glands or a sham operation was performed under sodium pentobarbital anesthesia (65 mg/kg, i.p.) 2 weeks prior to the stress and stress+biting group immobilization stress experiments (age, 5 weeks; Saruta et al. Citation2009). A 2-cm skin incision in the neck was made, the major salivary glands were isolated from the surrounding subcutaneous fat tissue, a ligature was placed around the supplying vessels, and the salivary glands were then removed. At the end of the experiments, all rats were deeply anesthetized with sodium pentobarbital (65 mg/kg, i.p.) for blood and tissue collection. Rats in both the stress and stress+biting groups were killed immediately after immobilization stress. To avoid diurnal variations in BDNF expression, all rats were killed between 07:00 and 11:00 h. The experimental protocol used in this study was reviewed and approved by the Ethics Committee on Animal Experiments of Kanagawa Dental College, and was carried out with adherence to the Guidelines for Animal Experimentation of Kanagawa Dental College.
Blood sampling
After deep anesthesia of all rats at the end of the immobilization stress experiments, blood samples were collected between 07:00 and 11:00 h by cardiac puncture into Venoject® tubes containing EDTA (Terumo, Tokyo, Japan). The tubes were immediately placed on ice and then centrifuged (500g, 15 min, 4°C). Plasma was stored at − 20°C prior to radioimmunoassay.
RNA extraction and cDNA synthesis
After blood sampling, the anaesthetized intact and sham operated rats were killed by decapitation and the submandibular glands were removed. Total RNA isolation from the submandibular glands was performed using the ISOGEN reagent (Nippon Gene, Toyama, Japan) in accordance with the manufacturer's instructions. The RNA product was resuspended in 20 μl diethyl pyrocarbonate (DEPC)-treated water. The quality of RNA was judged from the pattern of ribosomal RNA after electrophoresis through a 1.5% agarose gel containing ethidium bromide (EB) and visualization by UV illumination. RNA concentrations were determined by absorbance at 260 nm with a SmartSpec Plus spectrophotometer (Bio-Rad, Tokyo, Japan). RNA was stored at − 80°C until use. Total RNA was reverse transcribed at 50°C for 30 min, 99°C for 5 min, and 5°C for 5 min using a single-strand cDNA synthesis kit (Roche Diagnostics, Ltd, Lewes, UK) according to the manufacturer's instructions (Tsukinoki et al. Citation2006). Following the RT reaction, cDNA products were stored at − 20°C until use.
Real-time PCR analysis
Real-time PCR was performed using a LightCycler (Roche) according to the manufacturer's instructions (Lee et al. Citation2008). Reactions were performed in a 20 μl volume [BDNF: 0.3 μM of each primer and 4 mM MgCl2; neurotrophin-3 (NT-3): 0.5 μM of each primer and 3 mM MgCl2]. Reactions with Taq DNA polymerase, nucleotides, and buffer for BDNF were performed with LightCycler-DNA Master SYBR Green I mix (Roche Diagnostics). Oligonucleotide primers designed to amplify rat BDNF were specific for the coding region of exon 5. BDNF-specific primers were 5′-CAGGGGCATAGACAAAAG-3′ (forward) and 5′-CTTCCCCTTTTAATGGTC-3′ (reverse) (BDNF PCR product: 167 bp; Tsukinoki et al. Citation2006, Citation2007; Lee et al. Citation2008) as designed and synthesized by Nippon Gene Laboratory. Real-time PCR for amplification of the rat β-actin housekeeping gene was performed using a LightCycler Primer/Probe set, 5′-CCTGTATGCCTCTGGTCGTA-3′ (forward) and 5′-CCATCTCTTGCTCGAAGTCT-3′ (reverse) (β-actin PCR product: 260 bp), following the manufacturer's instructions (Nihon Gene Research Labs, Inc., Sendai, Japan). Denaturation was performed at 95°C for 10 min, after which segment 1 (95°C for 10 s), segment 2 (60°C for 10 s), and segment 3 (72°C for 10 s) were repeated for 40 cycles. Melting analysis and agarose gel electrophoresis were performed to confirm the specificity of the PCR products obtained using each primer pair. Gene expression is given in terms of the ratio of the copy number of BDNF mRNA to β-actin mRNA for each sample.
Tissue preparation for immunohistochemistry
The rats were killed under deep anesthesia as above, between 07:00 and 11:00 h; rats in the experimental groups were killed immediately after blood sampling. Resected rat submandibular gland tissue samples were fixed in 10% buffered formaldehyde (pH 7.4) for 24 h and embedded in paraffin, and serial 3-μm sections were cut and stained with hematoxylin and eosin and processed for immunohistochemistry. Immunohistochemical analysis was performed using Simple stain MAX-PO (Nichirei, Tokyo, Japan). Slides were pre-incubated in 3% H2O2 for 5 min. Sections were then incubated with anti-human BDNF monoclonal antibody (1:100, Techne, Minneapolis, MN, USA) for 1 h at room temperature. After washing with PBS, sections were reacted with the secondary antibody, horseradish peroxidase (HRP)-labeled anti-rabbit IgG with amino acid polymer (Nichirei), for 30 min at room temperature. Color was developed using 0.02% 3,3′-diaminobenzidine-tetrahydrochloride (DAB) containing 0.0003% H2O2 in Tris-buffered saline (TBS) for 5 min, and sections were then counterstained with hematoxylin. To provide negative controls, non-immunized rabbit or mouse IgG was used instead of the primary antibody.
Measurement of plasma ACTH and corticosterone
ACTH was assayed using a radioimmunoassay kit [Lot No. 5055889, enzyme-linked immunosorbent assay (ELISA)-ACTH, CIS; Atomic Energy Laboratory of Biochemical Products, Gif sur Yvette, France), following the manufacturer's instructions. Using these kits, ACTH can be quantified in a range of 1.0–2000 pg/ml; ACTH concentrations are reported in pg/ml. A COAT-A-COUNT Rat Corticosterone kit (Lot No. 5715-7, Siemens Medical Solutions Diagnostics, New York, NY, USA) was used for measurement of plasma corticosterone (ng/ml). Using this kit, corticosterone can be quantified in a range of 0.2–99,999 ng/ml (Nabors et al. Citation1974).
Submandibular gland protein extraction
Submandibular gland tissue samples were homogenized in ice-cold lysis buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium vanadate. The tissue homogenate solutions were centrifuged at 14,000g for 5 min at 4°C. The supernatants were collected and used for quantification of total protein and neurotrophin levels. Total protein concentrations were determined by the Bradford method using absorbance at 595 nm with a SmartSpec Plus spectrophotometer (Bio-Rad).
Bdnf Elisa Analysis
BDNF concentrations were assayed in plasma using an ELISA kit (Promega, Co., Madison, WI, USA). Briefly, standard 96-well flat-bottom NUNC-ImmunoMaxisorp ELISA plates were incubated with the corresponding captured antibody, which binds the neurotrophin of interest, overnight at 4°C. The plates were blocked by incubating for 1 h at room temperature with a 1 × blocking and sample buffer. Serial dilutions of a known amount of BDNF ranging from 0 to 500 pg/ml were performed in duplicate for standard curve determination. Wells containing the standard curves and plasma were incubated at room temperature for 2 or 6 h, as specified by the protocol. They were then incubated with a second specific antibody overnight at 4°C or for 2 h at room temperature. A species-specific antibody conjugated to HRP was used for a tertiary reaction for 2 or 2.5 h at room temperature following this incubation step. TMB One Solution was used to develop color in the wells. This reaction was terminated with 1 M HCl at a specific time (10–15 min) at room temperature, and absorbance was then determined at 450 nm in a plate reader within 30 min of stopping the reaction. BDNF concentrations were determined by comparison with the regression line for each proposed BDNF standard. Using this kit, BDNF can be quantified in a range of 0–500 pg/ml. For the BDNF assay kit, cross-reactivity with other neurotrophic proteins is < 2–3%.
SDS-PAGE and western blot analysis
Control, stress, stress+biting, control-sham, stress-sham, and stress+biting-sham group salivary gland lysates were used for western blot analysis of both BDNF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total protein (40 μg) from each sample was boiled for 3 min with Laemmli sample buffer solution (Wako, Tokyo, Japan) and allowed to cool to room temperature. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% gel; 2.5 ng of rBDNF (R&D Systems, Minnneapolis, MN, USA) was run as a positive control. The movement of the proteins was monitored using Precision Plus All Blue Standards (Bio-Rad). Proteins were transferred onto a PVDF membrane (Millipore, Corp., Bedford, MA, USA) in transfer buffer (25 mM Tris, 190 mM glycine, 20% MeOH) for 1 h at 100 V and 4°C, then blocked for 1 h at room temperature in blocking buffer (PBS, 0.1% Tween-20, 1% NP-40) with 5% non-fat dry milk (NFDM; w/v). For immunodetection, PVDF membranes were incubated overnight at 4°C with primary antibodies. Antibodies against the following antigens were used: anti-BDNF rabbit polyclonal antibody (1:1000; molecular weight 14 kDa; sc-546, Santa Cruz Biochemistry, Santa Cruz, CA, USA), anti-GAPDH rabbit polyclonal antibody (1:1000; molecular weight 37 kDa; 14C10, Cell Signaling Technology, Danvers, MA, USA) in blocking buffer with 5% NFDM. After washing in PBST, membranes were incubated in anti-rabbit IgG conjugated with HRP (Dako Cytomation, Glostrup, Denmark), diluted 1:2000 in blocking buffer with 5% NFDM, for 1 h at room temperature. Membranes were washed again and an ECL Plus Chemiluminescence system (Amersham Biotech, Piscataway, NJ, USA) was used for detection. The specificity of the primary antibody was tested both by peptide neutralization and by determining cross-reactivity to 2.5 ng of recombinant nerve growth factor (NGF), NT-3, and NT-4 (R&D Systems). Negative controls using only the secondary antibody were also run. For semi-quantification, the specific signals on immunoblotted membrane detected by the antibody were computer-captured and quantified with the Multi Gauge Version 3.1 of Science Lab 2005 (Fujifilm, Tokyo, Japan). The signal intensities were adjusted relative to the GAPDH intensity, used as an internal control. There were eight individual samples used in each experimental group, and each protein sample was evaluated three times.
Statistical analysis
Statistical analyses were carried out using the SPSS (Version 17.0) statistics program and comprised two-way analysis of variance (ANOVA) followed by Tukey's post-test to assess differences among groups. All data are expressed as the group mean ± SEM. A probability level of < 0.05 was accepted as significant.
Results
Plasma concentrations of ACTH and corticosterone in control, stress, or stress+biting (intact, sham, or SA) groups
In a previous study, we demonstrated a similar result with plasma ACTH and corticosterone concentrations (Tsukinoki et al. Citation2007; Lee et al. Citation2008). Plasma ACTH concentrations were greater in stress-intact, -sham, or -SA rats than in either the control or stress+biting group, and greater in stress+biting-intact, -sham, or -SA rats than in control rats, with no intra-group differences between the intact, sham, or SA rats of either the control, stress, or stress+biting groups (F(8, 66) = 49.46; p < 0.01, ANOVA; ). Similarly, plasma corticosterone concentrations were greater in stress-intact, -sham, or -SA rats than in either the control or stress+biting group, and greater in stress+biting-intact, -sham, or -SA rats than in control rats, with no intra-group differences between the intact, sham, and SA rats of either the control, stress, or stress+biting groups (F(8, 66) = 50.89; p < 0.01, ANOVA; ).
Table I. Plasma concentrations of ACTH: control, stress, or stress+biting (intact, sham, or SA).
Table II. Plasma concentrations of corticosterone: control, stress, or stress+biting (intact, sham, or SA).
Quantitative analysis of BDNF mRNA in the submandibular glands of stress and stress+biting-intact groups
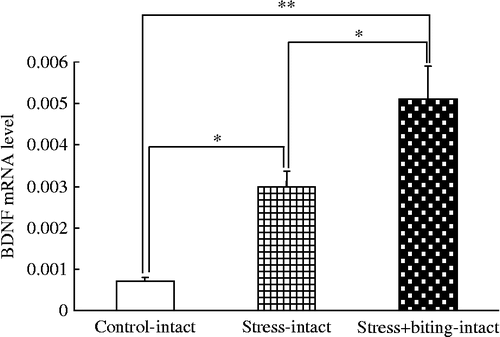
Melting curve analysis demonstrated a single fluorescent peak representing the Tm of BDNF mRNA in all samples, except for the negative control sample (data not shown). In addition, a single band was observed following agarose gel electrophoresis (data not shown). These findings confirmed that the PCR product was BDNF mRNA. The BDNF/β-actin mRNA ratio was greater in stress+biting-intact rats than in control or stress rats, and greater in stress-intact rats than in control rats (F(5, 44) = 19.69; p < 0.05, ANOVA; ).
Figure 1. BDNF mRNA quantification in rat submandibular glands in stress and stress+biting-intact groups. Data are BDNF/β-actin mRNA ratios. Control: no stress group; stress: 2 h acute immobilization stress group; stress+biting: 2 h acute immobilization stress group allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min); intact: no surgery. Values are means ± SEM; n = 8 rats in each group. *p < 0.05, **p < 0.01, ANOVA/Tukey's.
Plasma BDNF concentrations in the submandibular glands of stress and stress+biting-intact groups
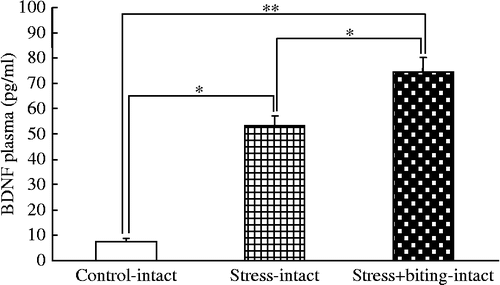
Plasma BDNF concentrations were greater in stress+biting-intact rats than in control or stress rats, and greater in stress-intact rats than in control rats (F(5, 44) = 46.62; p < 0.05, ANOVA; ).
Figure 2. BDNF plasma concentrations in stress and stress+biting-intact groups. Data are plasma BDNF concentrations in terminal cardiac puncture blood samples. Control: no stress group; stress: 2 h acute immobilization stress group; stress+biting: 2 h acute immobilization stress group allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min); Intact: no surgery. Values are means ± SEM; n = 8 rats in each group. *p < 0.05, **p < 0.01, ANOVA/Tukey's.
BDNF immunohistochemistry in submandibular gland-intact rats
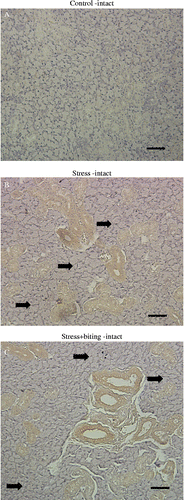
Rat brain sections were used as a positive control for BDNF immunohistochemistry, and showed high levels of BDNF expression in neural cells (data not shown). Non-stressed submandibular gland tissue from unstressed rats revealed little to no expression of BDNF in duct-type cells (). Granular duct cells weakly expressed BDNF protein with the exception of duct-type cells. Intense BDNF expression was observed in duct-type cells of the stress-intact () and stress+biting-intact groups (); however, BDNF expression was not consistently observed in acinar cells in the stress-intact () and stress+biting-intact groups ().
Figure 3. BDNF immunohistochemistry in submandibular glands of control, stress, and stress+biting-intact groups. Photomicrographs show the immunohistochemical localization of BDNF protein, identified with an anti-BDNF monoclonal antibody in paraffin-embedded sections of submandibular gland from: (A) control, non-stressed rat: only faint staining was observed; (B) stressed rat after 2 h acute immobilization stress: BDNF protein was observed in duct cells, but there was no obvious BDNF expression in acinar cells (arrows); and (C) stress+biting rat, after 2 h acute immobilization stress allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min); BDNF protein was observed in duct cells but there was no obvious BDNF expression in acinar cells (arrows). Intact: no surgery. Scale bar = 50 μm (A–C).
Effects of stress and stress+biting on BDNF expression in the rat submandibular gland
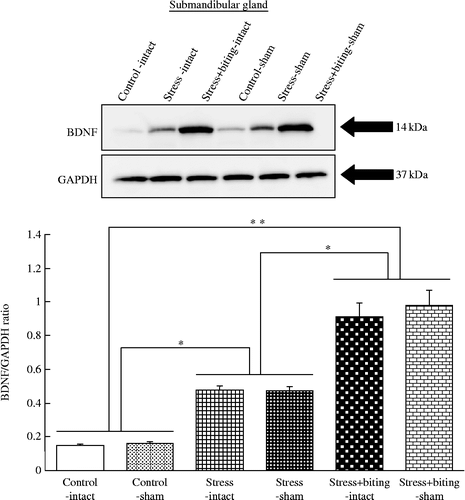
Western blot analysis of equal amounts of protein in submandibular glands from control, stress, stress+biting, control-sham, stress-sham, and stress+biting-sham rats revealed that the BDNF concentration was higher in both stress and stress+biting groups than in control rats. The BDNF concentration was also higher in both stress-sham and stress+biting-sham groups than control-sham rats (). GAPDH expression did not change in control, stress, stress+biting, control-sham, stress-sham, or stress+biting-sham rats (). The BDNF–GAPDH ratio was greater in stress+biting-intact or -sham rats than in control or stress rats, and greater in stress-intact or -sham rats than in control rats, with no intra-group differences between intact and sham rats (F(5, 30) = 26.35; p < 0.05, ANOVA; ).
Figure 4. Effects of stress and stress-biting on salivary gland BDNF expression. Upper panel: Western blot analysis of BDNF expression with equal amounts of protein from submandibular glands of control, stress, stress+biting, control-sham, stress-sham, and stress+biting-sham rats. Control: no stress group; stress: 2 h acute immobilization stress group; stress+biting: 2 h acute immobilization stress group allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min); intact: no surgery; sham: sham SA. Blots for BDNF show that level of this protein is increased in the submandibular glands of rats subjected to stress and is more strongly increased in the submandibular glands of rats subjected to stress+biting. BDNF protein level is also increased in the submandibular glands of stress-sham rats and more strongly increased in the submandibular glands of stress+biting-sham rats. GAPDH expression is similar in control, stress, stress+biting, control-sham, stress-sham, and stress+biting-sham rats. Lower panel: Semi-quantitative analysis of western blots by densitometry. The intensities of BDNF bands were normalized with respect to the intensities of GAPDH bands detected on the same blots. Values are means ± SEM; n = 8 rats in each group. *p < 0.05, **p < 0.001, ANOVA/Tukey's.
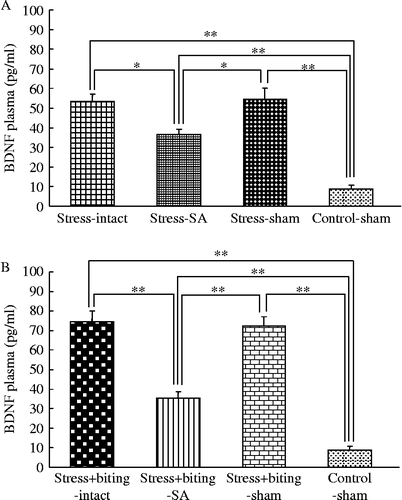
Plasma BDNF concentrations in stress or stress+biting and SA rats
There were significant differences in BDNF plasma concentrations between the intact and SA groups, and between SA and sham rats in the stress group. Plasma BDNF concentrations were greater in intact or sham rats than in SA rats in the stress group and greater in stress-intact, -SA, or -sham rats than in control-sham rats (F(2, 21) = 5.90; p < 0.05, ANOVA; ). There were also significant differences in BDNF plasma concentrations between the intact and SA rats, and between SA and sham rats in the stress+biting group. Plasma BDNF concentrations were greater in intact or sham rats than in SA rats in the stress+biting group and greater in stress+biting-intact, -SA, or -sham rats than in control-sham rats (F(2, 22) = 22.40; p < 0.001, ANOVA; ).
Figure 5. Plasma BDNF concentrations in stress or stress+biting and SA groups. Data are terminal plasma BDNF concentrations in cardiac puncture blood samples. Control: no stress group; stress: 2 h acute immobilization stress group; stress+biting: 2 h acute immobilization stress group allowed to bite a wooden stick (diameter, 0.5 cm) during the latter half of the immobilization period (60 min); intact: no surgery; SA: removal of submandibular glands before acute immobilization stress or stress and biting; sham: sham SA before stress or stress and biting. Values are means ± SEM; n = 8 rats in each group. *p < 0.05, **p < 0.001, ANOVA/Tukey's.
Discussion
In the present study, a stress-biting model allowing 1 h of biting during the latter half of a 2-h immobilization stress exposure was used to investigate changes in BDNF concentration under stress and biting conditions. The biting condition of this model is characterized by an attenuated stress reaction, because biting significantly decreases both ACTH and corticosterone concentrations relative to the 2-h stressed rats without biting (Lee et al. Citation2008). However, biting prevents a decrease in BDNF expression in rat hippocampus in this model (Lee et al. Citation2008), and the model seems to be useful for investigating the relationship between the reduction in hypothalamic-pituitary-adrenal (HPA) responses and BDNF expression. We previously reported positive correlations between the acute stress condition and the expression of salivary BDNF protein and mRNA levels (Tsukinoki et al. Citation2006). However, in the current study, biting significantly further increased BDNF mRNA expression in rat submandibular glands of 2-h stressed rats. Corticosterone secretion caused by chronic stress decreases the expression of BDNF in the hippocampus (Zheng et al. Citation2006) and this may underlie neurodegenerative disorders such as schizophrenia and post-traumatic stress disorder (Terpstra et al. Citation2003; Roceri et al. Citation2004; Zoladz et al. Citation2008). Corticosterone is one of the suppression factors for BDNF (Schaaf et al. Citation2005). However, neither ACTH nor corticosterone is likely to regulate the expression of BDNF in salivary gland tissues (Tsukinoki et al. Citation2007; Lee et al. Citation2008). Conversely, as the reduced ACTH and corticosterone responses to immobilization stress when rats were allowed to bite were not modified by prior removal of the submandibular salivary glands, it seems that circulating BDNF is not responsible for the reduced HPA axis responses seen during stress with biting (Lee et al. Citation2008).
Using multiple techniques, we demonstrated increased expression of BDNF mRNA and protein in rat submandibular gland tissue following stress with or without a biting period. That is, when rats were exposed to stress and stress+biting, plasma BDNF concentrations were greater in stress+biting rats than in control or stress only rats, and greater in stress only rats than in control rats. It has been reported that elevated plasma BDNF level protects against neural damage from methamphetamine (Kim et al. Citation2005). However, because a decrease in the plasma BDNF level is correlated with the severity of schizophrenia accompanied by tardive dyskinesia, tardive dyskinesia may be induced by the reduction in neural cell protection provided by BDNF (Tan et al. Citation2005). In addition, as BDNF is able to pass through the blood brain barrier (Pan et al. Citation1998) and utero-placental barrier (Kodomari et al. Citation2009), free BDNF entering the plasma (endogenous BDNF) is likely to protect neural cells and maintain neural cell function. Therefore, in the early stages, an increase in plasma BDNF may contribute to the protection of neural cells against damage caused by acute stress conditions.
In recent years, many studies have reported that BDNF is produced by various organs outside the nervous system. BDNF is found in blood cells such as lymphocytes (Sobue et al. Citation1998), macrophages (Rost et al. Citation2005), and eosinophils (Raap et al. Citation2005), indicating it may be involved in the protection of neural cells in inflamed tissue. In allergic diseases, such as atopic dermatitis, BDNF is released from eosinophils, raising its plasma level (Raap et al. Citation2005). In patients with asthma, enhanced local BDNF production in the lung contributes to neural hyper-reactivity and pathological bronchoconstriction (Braun et al. Citation2004). In addition, alterations in plasma BDNF levels are also observed in acute coronary syndrome (Manni et al. Citation2005) and during the menstrual cycle (Lommatzsch et al. Citation2005). At the cellular level, vascular endothelial cells are considered to be an important source of BDNF (Nakahashi et al. Citation2000). Taken together, these reports indicate that plasma BDNF has different roles in various conditions and processes, including inflammation, allergies, and heart disease.
Oral parafunctional activity, such as tooth-clenching, nail-biting, or the biting of objects, has been suggested to provide an outlet for emotional tension or stress in humans. It has been shown in rats that aggressive biting behavior during stress exposure suppresses the stress-induced dopamine metabolism in the striatum (Gomez et al. Citation1999) and restraint-induced enhancement in turnover of catecholamines in the central nervous system (Tsuda et al. Citation1988; Tanaka et al. Citation1998). However, no study has reported the effect of parafunctional masticatory activity on stress-induced expression of BDNF in the salivary glands. Therefore, this study investigated the effects of stress and biting on the expression of BDNF in the rat salivary gland.
The increase in plasma BDNF concentration was significantly greater in intact and sham rats than in SA rats. Rat submandibular glands were therefore confirmed to be the major source of plasma BDNF under the biting condition. Biting involves movement of masticatory muscles including the masseter muscles. Movement of masseter muscles contributes to the development of major salivary glands (Jensen Kjeilen et al. Citation1987). In addition, mastication accelerates the production of saliva. The salivary glands produce various growth factors such as NGF, hepatocyte growth factor (HGF), and epidermal growth factor (EGF) (Aloe et al. Citation1986; Taylor et al. Citation1971; Tsukinoki et al. Citation2004). While exercise leads to increases in blood flow and glucose utilization (Broderick et al. Citation2005), it also induces changes in several salivary components such as immunoglobulins, hormones, lactate, proteins, and electrolytes (Chicharro et al. Citation1998). The salivary glands are likely to affect not only oral health but also systemic organs. Although the physiological mechanisms responsible for the increase in salivary BDNF under the biting condition are not understood, upregulation of salivary tissue BDNF may directly affect the salivary glands via the biting behavior. Our results show that biting influences the expression of BDNF in the salivary glands. In this respect, the response of salivary BDNF to incremental levels of exercise may be of particular interest.
In conclusion, the effect of masticatory activity on the response to acute immobilization stress in rats is important and leads to relaxation of the stress response, which may provide protective effects for general health. Future studies should investigate the mechanisms of this connection to general health.
Acknowledgements
This research was supported in part by a Grant-in-Aid for Young Scientists (Start-up, #1989023), Scientific Research (B, #20390467), Research Institute on Occlusive Medicine of Kanagawa Dental College, and Open Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aloe L, Alleva E, Böhm A, Levi-Montalcini R. 1986. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci USA. 83:6184–6187.
- Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. 2004. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol. 141:431–440.
- Broderick TL, Poirier P, Gillis M. 2005. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes Metab Res Rev. 21:44–50.
- Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. 2007. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 91:1–5.
- Chicharro JL, Lucía A, Pérez M, Vaquero AF, Ureña R. 1998. Saliva composition and exercise. Sports Med. 26:17–27.
- Ghinelli E, Johansson J, Rios JD, Chen LL, Zoukhri D, Hodges RR, Dartt DA. 2003. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest Opthalmol Vis Sci. 44:3352–3357.
- Gomez FM, Giralt MT, Sainz B, Arrue A, Prieto M, Garcia-Vallejo P. 1999. A possible attenuation of stress-induced increases in striatal dopamine methabolism by the expression of non-functional masticatory activity in the rat. Eur J Oral Sci. 107:461–467.
- Hori N, Yuyama N, Tamura K. 2004. Biting suppresses stress-induced expression of corticotrophin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 83:124–128.
- Hori N, Lee MC, Sasaguri K, Ishii H, Kamei M, Kimoto K, Toyoda M, Sato S. 2005. Suppression of stress-induced nNOS expression in the rat hypothalamus by biting. J Dent Res. 84:624–628.
- Jensen Kjeilen JC, Brodin P, Aars H, Berg T. 1987. Parotid salivary flow in response to mechanical and gustatory stimulation in man. Acta Physiol Scand. 131:169–175.
- Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, Kim YK. 2005. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 388:112–115.
- Kodomari I, Wada E, Nakamura S, Wada K. 2009. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem Int. 54:95–98.
- Lee T, Saruta J, Sasaguri K, Sato S, Tsukinoki K. 2008. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 1195:43–49.
- Lommatzsch M, Zingler D, Schuhbaeck K, Scholetcke K, Zingler C, Schuff-Werner P, Virchow JC. 2005. The impact of age, weight, and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 26:115–123.
- Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. 2005. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 102:169–171.
- Nabors CJJr, West CD, Mahajan DK, Tyler FH. 1974. Radioimmunoassay of human plasma corticosterone: Method, measurement of episodic secretion and adrenal suppression and stimulation. Steroids. 23:363–378.
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. 2000. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 470:113–117.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. 1998. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 37:1553–1561.
- Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, Mariotti A, Roncaglia I, Palla A, Consoli G, Giovannini L, Massimetti G, Dell'osso L. 2008. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 5:279–283.
- Raap U, Goltz C, Deneka N, Bruder M, Renz H, Kapp A, Wedi B. 2005. Brain-derived neurotrophic factor is increased in atophic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 115:1268–1275.
- Radka SF, Holst PA, Fritsche M, Altar CA. 1996. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 709:122–131.
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. 2004. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 55:708–714.
- Rost B, Hanf G, Ohnemus U, Otto-Knapp R, Groneberg DA, Kunkel G, Noga O. 2005. Monocytes of allergics and non-allergics produce, store, and release the neurotrophins NGF, BDNF, and NT-3. Regul Pept. 124:19–25.
- Saruta J, Lee T, Shirasu M, Takahashi T, Sato C, Sato S, Tsukinoki K. Chronic stress affects the expression of BDNF in rat salivary glands. Stress. 2009 (in press).
- Sasaguri K, Kikuchi M, Hori N, Yuyama N, Onozuka M, Sato S. 2005. Suppression of stress immobilization-induced phosphorylation of ERK 1/2 by biting in the rat hypothalamic paraventricular nucleus. Neurosci Lett. 383:160–164.
- Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. 2005. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 48:334–341.
- Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T. 1998. Expression of mRNAs for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75NGFR, trk, trkB, and trkC) in human peripheral neuropathies. Neurochem Res. 23:821–829.
- Tan YL, Zhou DF, Zhang XY. 2005. Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: Association with dyskinetic movements. Schizoph Res. 74:263–270.
- Tanaka T, Yoshida M, Yokoo H, Tomita M, Tanaka M. 1998. Expression of aggression attenuates both stress-induced gastric ulcer formation and increases in noradrenaline release in the rat amygdale assessed by intracerebral microdialysis. Pharmacol Biochem Behav. 59:27–31.
- Taylor JM, Cohen S, Mitchell WM. 1971. Epidermal growth factor: High and low molecular weight forms. Proc Natl Acad Sci USA. 67:164–171.
- Terpstra J, Gispen-De Wied CC, Broekhoven MH, Frankhuijzen AC, Kahn RS, van Ree JM, Wiegant VM. 2003. Attenuated stress responsiveness in an animal model for neurodevelopmental psychopathological disoreders. Eur Neuropsychopharmacol. 13:249–256.
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. 1993. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 10:475–489.
- Tsuda A, Tanaka M, Ida Y, Shirao I, Gondoh Y, Oguchi M, Yoshida M. 1988. Expression of aggression attenuates stress-induced increases in rat brain noradrenaline turnover. Brain Res. 474:174–180.
- Tsukinoki K, Yasuda M, Mori Y, Asano S, Naito H, Ota Y, Osamura RY, Watanabe Y. 2004. Hepatocyte growth factor and c-Met immunoreactivity are associated with metastasis in high grade salivary gland carcinoma. Oncol Rep. 12:1017–1021.
- Tsukinoki K, Saruta J, Sasaguri K, Miyoshi Y, Jinbu Y, Kusama M, Sato S, Watanabe Y. 2006. Immobilization stress induces BDNF in rat submandibular glands. J Dent Res. 85:844–848.
- Tsukinoki K, Sarura J, Muto M, Sasaguri K, Sato S, Tan-Ishii N, Matanabe Y. 2007. Submandibular glands contribute to increases in plasma BDNF levels. J Dent Res. 86:260–264.
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. 2006. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 168:47–55.
- Zoladz PR, Conrad CD, Fleshner M, Diamond DM. 2008. Acute episodes of predator exposure in conjunction with chronic social in stability as an animal model of post-traumatic stress disorder. Stress. 11:259–281.
