Abstract
This study investigated (1) sex differences in hormonal responses to psychosocial stress; (2) the relation between variability in pre-test hormone concentrations and stress-induced hormonal changes; and (3) some possible sources of within-sex variation in pre-test hormone concentrations and in hormonal responses to the test in a large human subject population. To this end, changes in salivary concentrations of testosterone and cortisol in response to a mild psychosocial stressor (a set of computerized economic decision-making tests) were measured in a sample of over 500 MBA students. Males had higher concentrations of testosterone and cortisol than females both before and after the test. After taking effects of time of testing on hormone concentrations into account, testosterone showed a post-test decrease in males but not in females. Cortisol level increased in both sexes but the post-test increase was larger in females than in males. At the individual level, the pre-test concentrations of testosterone and cortisol predicted both the direction and the magnitude of the post-test hormone change, so that low pre-test hormone concentrations showed large post-test increases whereas high pre-test concentrations showed large post-test decreases. Within-sex variation in hormone concentrations was not accounted for by variation in 2D:4D digit length ratio, a marker of prenatal androgen exposure, but by social variables. Single males without a stable romantic partner had higher testosterone level than males with stable partners, and both males and females without a partner showed a greater cortisol response to the test than married individuals with or without children. Studies conducted with large sample sizes such as this one can help understand normative patterns of hormonal responses to psychosocial stimuli as well as identify the sources of interindividual variation in endocrine function.
Introduction
In recent years, there has been growing interest in investigating how hormones may influence many aspects of human behavior and cognition including the tendency to be in different types of social relationships (Ellison and Gray Citation2009), the propensity to take risks and to choose particular career paths (White et al. Citation2006; Sapienza et al. Citation2009), and the motivation to participate in competitive contests and the performance in these contests (Booth et al. Citation1989; Bateup et al. Citation2002; Schulteiss et al. Citation2005). Since psychosocial stress is an inevitable component of our daily lives and can affect both our decisions and our performance in a variety of situations, a complete and accurate knowledge of hormonal responses to psychosocial stress is a prerequisite for understanding the relation between hormones and behavior or cognition.
A great deal of research has investigated the effects of psychosocial stress on testosterone and cortisol levels, two hormones that are particularly sensitive to psychological and social influences. Testosterone can potentially influence many aspects of an individual's response to environmental challenges including tendency to take risks, psychomotor function and coordination, and cognitive performance (Kimura Citation2000). Cortisol is primarily a metabolic hormone and plays an important role in the mobilization of physiological resources necessary to deal with challenges (Sapolsky Citation2004).
Research on psychosocial stress often involves measuring salivary hormone concentrations before and after mildly stressful experiences, such as competitive challenges, cognitive tasks, public speaking/verbal interaction tasks, emotion induction procedures (e.g. watching a film with disturbing scenes), or exposure to disturbing noise (Dickerson and Kemeny Citation2004). Typically, the stressful experience results in an elevation of cortisol and a reduction in testosterone level, although there have been studies reporting different changes in these hormones, or no changes at all (Booth et al. Citation1989; Dickerson and Kemeny Citation2004; Schulteiss et al. Citation2005; Wirth and Schulteiss Citation2006; Wirth et al. Citation2006). One reason for the discrepancies between the results of different studies is that there is always substantial interindividual variability in hormonal responses to psychosocial stress, and because of this variability, studies with small sample sizes may report different results from those conducted with larger sample sizes. Interindividual variability in responsiveness does not simply represent noise in the system but it is a crucial element for understanding the relation between hormones and psychosocial stress; yet the determinants of this variability are still poorly understood.
Although there is some evidence that men and women differ in their hormonal responses to stress (e.g. women exhibit greater cortisol increases following stress; Dickerson and Kemeny Citation2004), this evidence is far from being unequivocal. The variability with which psychosocial stress can affect testosterone and cortisol secretion in individuals of the same sex is also poorly understood. A common explanation for this variability is that hormonal responses to psychosocial stimuli depend in large part on the subjectivity with which these stimuli are interpreted, and that this subjective interpretation can vary dramatically in relation to individuals' personalities and context (Schulteiss et al. Citation1999, Citation2004, Citation2005; Dickerson and Kemeny Citation2004; Salvador Citation2005). Other explanations have to do with variation in pre-test hormone concentrations. Studies in which testosterone and cortisol are measured before a psychosocial stress test often report marked individual differences in pre-test hormone concentrations. These differences may reflect variability in baseline hormone concentrations (which, in part, is accounted for by biological and social–experiential factors) or variability in psychological anticipation of the test (which, in part, may be accounted for by personality characteristics), or both. Since pre-test hormone concentrations can affect the magnitude and the direction of the hormonal change after exposure to stress, taking variation in pre-test hormone concentrations into account and understanding the origins of this variation can be important to study and understand variation in responsiveness to stress.
This study investigated between- and within-sex variation in salivary concentrations of testosterone and cortisol in response to a mild psychosocial stressor in a population of over 500 MBA students. The stressor was a 90-min computerized test, in which students had to make financial decisions and play competitive games with one another. The test was mandatory for the students and they were told that their performance on it could potentially affect their future career placement.
Our study had three main goals: (1) to investigate sex differences in hormonal responses to psychosocial stress; (2) to investigate the relation between variability in pre-test hormone concentrations and stress-induced hormonal changes; and (3) to investigate some possible sources of within-sex variation in pre-test hormone concentrations and in hormonal responses to the test.
With regard to the first goal, we hypothesized that both male and female participants would experience a stress-related increase in cortisol and a reduction in testosterone secretion, and that the stress-induced cortisol increase may be greater in women than in men. With regard to the second goal, we hypothesized that both between- and within-sex variation in pre-test hormone concentrations would affect the magnitude of hormone change following the test, so that females with the lowest cortisol concentrations should experience the largest increases in this hormone, while men with the highest testosterone concentrations should experience the largest decreases in secretion of this hormone.
As for the third goal, we focused on 2D:4D digit length ratios and social variables such as marital or relationship status, as potential predictors of within-sex variation in endocrine function. The ratio between the length of the second and the fourth finger (2D:4D), is a putative marker of prenatal exposure to androgens, is typically lower in males than in females, and in some studies, but not others, has been shown to account for interindividual variation in testosterone levels (Honekopp et al. Citation2007). Thus, we hypothesized that in both men and women, individuals with lower 2D:4D digit ratio would have higher salivary testosterone concentrations. Testosterone and cortisol secretion can also be affected by relationship and marital status, as studies have shown that both baseline testosterone levels and cortisol responses to stress may be different in relation to whether individuals are single and without a stable partner, are single but in a long-term romantic relationship, or are married with or without children (van Anders and Watson Citation2006). Based on these studies, we hypothesized that individuals (especially males) who were married or in stable romantic relationships would have both lower testosterone and cortisol levels initially, and lower testosterone and cortisol responses to stress than unmarried or unattached individuals.
Materials and methods
Participants
Study participants were 557 Master's students (386 males and 171 females) in the Booth Business School at the University of Chicago. Participation in the study was one of the requirements for a course and therefore it was mandatory for the entire 2008 cohort of MBA students. Students, however, were paid $20 or more for their participation. The use of human subjects was approved by the Social Science IRB of the University of Chicago, and all students were asked for informed written consent for their participation in the study. Data for 56 participants could not be used for hormonal analyses because of lack of consent or technical problems with sample collection or hormonal assays. Therefore, these individuals were excluded from this study. Of the remaining 501 participants, 348 were males (age range: 24–36 years; mean ± SE = 28.73 ± 0.13) and 153 females (age range: 23–38 years; mean ± SE = 27.42 ± 0.20).
Procedures
Study participants were asked to take a 90-min computerized test in which they played games that assessed their economic decision-making tendencies in seven different domains: irrational exuberance (an asset market game that allows players to buy and sell shares of stocks among themselves), trust, competition, cooperation, risk aversion, loss aversion, and hyperbolic discounting. For example, to assess tendencies for financial risk aversion (Sapienza et al. Citation2009), students played a game in which they were asked to choose 15 times between a guaranteed dollar amount (ranging from $50 in the first choice to $120 in the fifteenth choice) and a lottery that paid either $200 or zero with equal probability. At the end of the game, one of the 15 choices was randomly chosen and subjects were paid according to their decision (and the lottery drawn) in that choice. An extremely risk-averse individual was expected to always choose the guaranteed dollar amount, whereas a very risk-tolerant individual was expected to always choose the lottery. In between, as the guaranteed amount increases, a subject should cross over from the lottery to the guaranteed amount as a function of his/her risk aversion. Taking the test was psychologically stressful because the students were told that it was a course requirement and that its results could potentially affect their future career placement. In addition, students had the opportunity to win or lose real money depending on their decisions. Finally, competition-related stress was elicited as well because in some of the games the students had to play against each other.
All students were tested on two days (October 3 and October 5, 2006). Tests were conducted in the afternoon, between 13:30 and 17:00 h. There were two separate testing sessions each day, held in different rooms: the early session began at 13:30 h (n = 333; Day 1 = 167; Day 2 = 166), while the late session began at 14:50 h (n = 224; day 1 = 111; day 2 = 114). All sessions used an identical protocol. The students tested in the same session and in the same room took the tests simultaneously, using different laptop computers, and were asked not to communicate with each other during the test.
Two saliva samples were collected from each study participant, one at the beginning of the test session (13:30 or 14:50 h) and the other two hours later, after the participants completed their tests (15:30 or 16:50 h). Approximately, 2–3 ml of saliva was collected by passive drool into plastic vials. In some cases, saliva production was stimulated by brief (5 s) chewing of sugarless gum. Study participants did not consume food, drink, or smoke at least 1 h prior to the procedure. Previous studies have shown that although salivary concentrations of testosterone and cortisol are lower in the afternoon than in the morning, afternoon hormone levels are more stable and therefore better suited for psychoneuroendocrine studies (Gray et al. Citation2004a; Takahashi et al. Citation2005; Wirth et al. Citation2006). All samples were immediately placed into dry ice and transported to Dr Robert Chatterton's Endocrinology Laboratory at Northwestern University, where they were frozen at − 80°C until assayed. Before assay, samples were thawed and centrifuged to reduce viscosity. Salivary concentrations of testosterone and cortisol were measured by radioimmunoassay, using antisera prepared within the laboratory (Chatterton et al. Citation1997). Cross-reactivity of the cortisol antiserum with cortisone was nonexistent; cross-reactivities of the testosterone antiserum with dihydrotestosterone and androstenedione were 13 and 0.2%; those for androsterone, etiocholanone, estradiol, and dehydroepiandrosterone were all less than 0.1%. The lower sensitivity of the assays was 0.07 ng/ml for cortisol and 7.5 pg/ml for testosterone. Intra-assay coefficients of variation (CVs) were all ≤ 10% and inter-assay CVs were ≤ 15%, consistent with published data for other assays from this laboratory (Chatterton et al. Citation1997). All samples were assayed in duplicate, and the average of duplicates was used in all analyses. Cortisol data were highly skewed for both men and women, while testosterone data were less skewed, especially for men (Mehta and Josephs Citation2006; Wirth et al. Citation2006). For consistency, we log transformed all hormonal data to approximate normal distributions.
Marital status (married or unmarried, with or without children) and relationship status of study participants at the time of testing (whether or not they had been involved in a romantic relationship for at least 6 months up to the time of testing) were assessed with a questionnaire completed after the study. This questionnaire was also used to collect information on background variables such as the participants' age, height, weight, general health, smoking and drug use, handedness, sexual orientation, stress levels, and use of contraceptives. Baseline stress levels were assessed by asking participants to rate, on a scale from 1 (minimum) to 7 (maximum), how stressful the current academic year had been so far. Women were asked whether they used hormonal contraceptives at the time of testing, the first and the last day of their most recent menstrual flow period, the average length of their menstrual cycles, and whether cycles were regular or irregular. None of the above background variables had any significant effects on the hormonal variables of interest in this study.
For a subset of study participants (155 males and 64 females), we scanned their right and left hand, measured the length of their second and fourth finger, and calculated their ratio (2D:4D ratio). Hands were scanned using Canon flatbed scanners and digit lengths were digitally measured in Adobe Photoshop CS2 using the “measurement tool”, which measures the distance between two points on the image (from the basal crease to the tip of the digit). Measurements were made in triplicate and we averaged the three readings of the fingers’ length before calculating the ratio. All data analyses were done using the average ratio of the left- and right-hand measures. The results were similar if we used the left-hand or the right-hand measurements separately.
Statistical analyses
All statistical analyses used log-transformed hormonal data, unless otherwise specified, to approximate normal distributions and allow for the use of parametric tests. Non-transformed data are reported in the figures and text to facilitate interpretation and comparisons with previously published data. All correlations between variables were assessed with Pearson's coefficient. A one-way factorial ANOVA was used to compare hormone measures in groups of individuals with different relationship or marital status. A 2 × 2 ANOVA was used to investigate sex differences and the effects of time of testing on hormone concentrations. Bonferroni–Dunn tests were used for post hoc comparisons. Repeated measures ANOVAs were used to compare pre- and post-test concentrations of testosterone and cortisol in males and females as well as to compare 2D:4D ratios for the left and the right hand in males and females. Student's t tests for paired samples were used for separate comparisons of pre- and post-test hormonal data in males and females. Student's t tests for unpaired samples were used to compare hormone measures and age between paired and unpaired individuals. The chi square test was used to compare the participation of male and female students in early and late tests as well as to compare the proportion of male and female students who were paired vs. unpaired. All tests were two tailed and probabilities < 0.05 were considered statistically significant.
Results
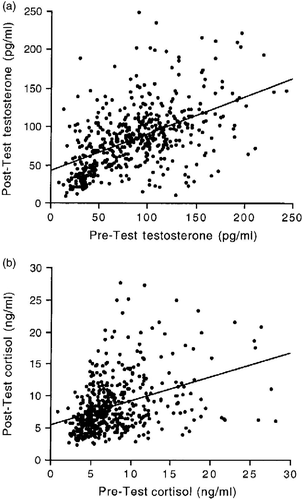
Correlations between pre- and post-test hormone concentrations, and between hormones
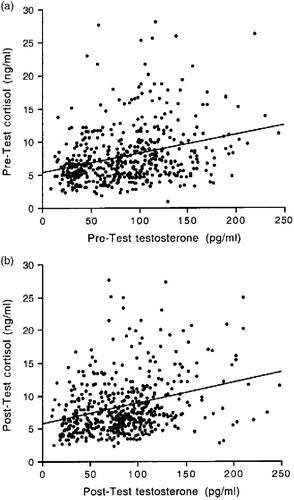
depicts the pre-test and post-test concentrations of testosterone and cortisol across all male and female participants. Pre-test concentrations of testosterone were positively correlated with post-test testosterone concentrations across all participants, and the same was found for cortisol (testosterone: r = 0.57, n = 501, p < 0.0001; cortisol: r = 0.42, n = 501, p < 0.0001; ). These correlations were also significant for males and females analyzed separately (male testosterone: r = 0.26, n = 348, p < 0.0001; male cortisol: r = 0.31, n = 348, p < 0.0001; female testosterone: r = 0.41, n = 153, p < 0.0001; female cortisol: r = 0.67, n = 153, p < 0.0001), indicating that individual differences in hormone concentrations were consistent before and after the test, regardless of gender. depicts the relationship between testosterone and cortisol concentrations in the pre-test () and the post-test () conditions across all male and female participants. The correlation between pre-test testosterone and pre-test cortisol concentrations and that between post-test testosterone and post-test cortisol concentrations was also positive and significant (pre-test: r = 0.29, n = 501, p < 0.0001; post-test: r = 0.30, n = 501, p < 0.0001; ). With one exception (the female pre-test hormone concentrations), this was the case also for males and females analyzed separately (male pre-test: r = 0.24, n = 348, p < 0.0001; male post-test: r = 0.29, n = 348, p < 0.0001; female pre-test; r = 0.12, n = 153, p = 0.11; female post-test: r = 0.31, n = 153, p < 0.0001). Therefore, individuals who differed from one another in their testosterone concentrations generally showed similar differences also in their cortisol concentrations.
Sex differences and temporal variation in testosterone and cortisol concentrations in males and females
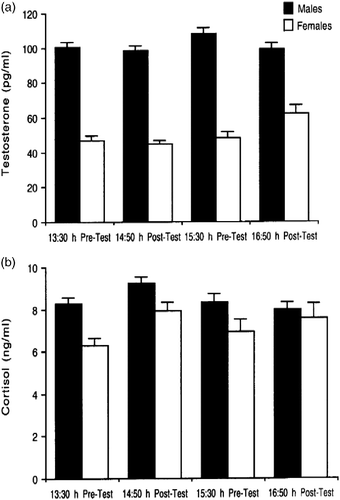
To investigate sex differences and the effect of time of testing on hormone concentrations, pre-test and post-test data for cortisol and testosterone were analyzed separately. The analyses revealed that males had significantly higher testosterone and higher cortisol concentrations than females in both the pre-test (testosterone: F1,497 = 304.63, p < 0.0001; cortisol: F1,497 = 20.01, p < 0.0001) and the post-test conditions (testosterone: F1,497 = 220.92, p < 0.0001; cortisol: F1,497 = 4.72, p = 0.03; ). There was also a main effect of time of day on testosterone and cortisol concentrations, but only in the post-test condition (testosterone: F1,497 = 10.96, p = 0.001; cortisol: F1,497 = 9.68, p = 0.002). Testosterone concentration was higher in late than in early samples (), whereas cortisol concentration was higher in early than in late samples (). A similar effect of time of day in the pre-test condition was not statistically significant (testosterone: F1,497 = 2.86, p = 0.09; cortisol: F1,497 = 0.15, p = 0.70). There were no significant interactions between gender and time of day for either hormone in the pre-test or the post-test conditions. To summarize, males had higher concentrations of testosterone and cortisol than females, the two hormones showed different effects of time of day, and these temporal effects on the two hormones were generally similar in males and females.
Figure 3 (a) Pre- and post-test salivary testosterone concentrations (mean ± SEM) in males and females in early and late tests. Times of test are indicated on the x-axis. Males had significantly higher testosterone level than females in both the pre-test and the post-test conditions (both p < 0.0001). Testosterone concentration was significantly higher in late than in early samples, irrespective of gender, but only in the post-test condition (p = 0.001). (b) Pre- and post-test salivary cortisol concentrations (mean ± SEM) in males and females in early and late tests. Males had significantly higher cortisol concentration than females in both the pre-test (p < 0.0001) and the post-test conditions (p = 0.03). Cortisol concentration was significantly higher in early than in late samples, irrespective of gender, but only in the post-test condition (p = 0.002). See Results text for detailed statistical results. Sample sizes are: males, early test, n = 201; late test, n = 147; females, early test, n = 101, late test, n = 52. Sample sizes are the same for pre- and post-test data, and for testosterone and cortisol data.
Testosterone and cortisol responses to the psychosocial test in males and females
Since males and females were equally likely to participate in early and late tests (male early = 201; male late = 147; female early = 101; female late = 52; χ2 = 3.02; p = 0.1), and showed similar temporal variation in hormone concentrations, data from early and late testing sessions were combined for this analysis.
For testosterone, there was no main significant effect of test (F1,499 = 0.18; p = 0.67) but a significant interaction between test and gender (F1,499 = 3.75; p = 0.05), such that testosterone concentration tended to decrease in males (mean ± SE; pre-test: 102.8 ± 2.20 pg/ml; post-test: 97.89 ± 2.14 pg/ml) but not in females (pre-test: 48.2 ± 2.35 pg/ml; post-test: 51.0 ± 2.38 pg/ml). When data were analyzed separately for males and females, the post-test decrease in testosterone in males was statistically significant (t = 2.05, df = 347; p = 0.04), whereas there was no significant effect of the test on testosterone concentration in females (t = − 1.28; df = 152; p = 0.20).
For salivary cortisol concentration, there were both a main effect of test (F1,499 = 13.97; p = 0.0002), with cortisol being significantly higher in the post-test (8.35 ± 0.19 ng/ml) than in the pre-test (7.79 ± 0.19 ng/ml), and a significant interaction between test and gender (F1,499 = 6.94; p = 0.008), with the post-test increase in cortisol concentration being larger for females (mean ± SE, pre-test: 6.52 ± 0.28 ng/ml; post-test: 7.82 ± 0.33 ng/ml) than for males (pre-test: 8.35 ± 0.25 ng/ml; post-test: 8.58 ± 0.24 ng/ml). When the data were analyzed separately by gender, the post-test increase in cortisol was significant for females (t = − 5.70; df = 152; p < 0.0001) but non-significant for males (t = − 1.52; df = 347; p = 0.13).
Taken together, these results indicate that taking the test significantly reduced salivary testosterone concentration in males and significantly increased salivary cortisol concentration in all participants, especially in females.
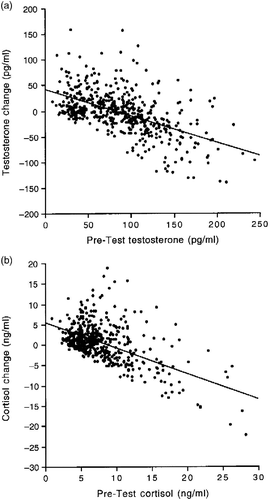
Between-subjects analyses revealed significant negative correlations between the pre-test concentrations of testosterone and cortisol and the difference between the post-test and the pre-test concentrations (testosterone: r = − 0.55, n = 501, p < 0.0001; cortisol: r = − 0.56, n = 501, p < 0.0001; ). Thus, the individuals with the lowest pre-test hormone concentrations generally exhibited large increments (positive difference scores) after the test, whereas the individuals with the highest pre-test concentrations generally exhibited large reductions (negative difference scores). This was apparent in both males and females (data not shown). For subsequent analyses, hormone changes from the pre-test to the post-test were calculated using the regressor variable method (Allison Citation1990), namely as the unstandardized residuals of a regression analysis with pre-test hormone as the predictor and post-test hormone as the dependent variable. This method ensures that the measure of hormone change is independent of the pre-test scores (Schulteiss et al. Citation2005; Mehta and Josephs Citation2006; Wirth et al. Citation2006).
Figure 4 (a) Correlation between salivary pre-test testosterone concentrations and the difference between post-test and pre-test testosterone concentrations (r = 0.55; n = 501; p < 0.0001). (b) Correlation between pre-test salivary cortisol concentrations and the difference between post-test and pre-test cortisol concentrations (r = 0.56; n = 501; p < 0.0001). Data are for all subjects.
Predictors of within-sex variation in hormone concentrations
To investigate whether 2D:4D digit ratios and marital/relationship status accounted for interindividual variation in hormonal variables, two hormone measures were used: the average of the testosterone and cortisol concentrations calculated from the nontransformed pre- and post-test values, and the unstandardized residuals of the regression between pre- and post-test hormone concentrations.
A repeated measures ANOVA comparing 2D:4D ratios for the left and the right hand in males and females revealed a main effect of gender (F1,177 = 15.11, p < 0.0001), with males having lower digit ratios (mean ± SEM, right: 0.957 ± 0.02; left: 0.954 ± 0.01) than females (mean ± SEM, right: 0.975 ± 0.01; left: 0.975 ± 0.01), no significant main effect of hand (F1,177 = 1.13, p = 0.28), and no significant interaction between gender and hand (F1,177 = 0.38; p = 0.53). Therefore, the digit ratios in the right and left hand were generally similar, and a similar difference between males and females was detected in both hands. Furthermore, the digit ratios in the two hands were positively correlated (r = 0.28; n = 181; p < 0.0001) across male and female participants (Honekopp and Schuster Citation2010 showed a stronger correlation between hands). Therefore, only scores for the right hand were used in subsequent analyses.
In males, digit ratios were not significantly correlated with either average testosterone concentrations (r = 0.06; n = 115; p = 0.47) or with testosterone residuals (r = 0.05; n = 115; p = 0.58). However, they tended to be positively correlated with average cortisol concentrations (r = 0.16; n = 115; p = 0.09) and negatively with cortisol residuals (r = − 0.16; n = 115; p = 0.07). In females, digit ratios were not correlated with any measures of testosterone (means: r = 0.06; n = 64; p = 0.61; residuals: r = 0.07; n = 64; p = 0.55) or cortisol (means: r = 0.02; n = 64; p = 0.86; residuals: r = 0.04; n = 64; p = 0.73). To summarize, although there was a significant sex difference in digit ratio, within-sex variation in digit ratios appeared to be unrelated to within-sex variation in testosterone or cortisol concentrations.
All study participants for whom information on relationship status was available (n = 463) were first divided into two groups, depending on whether they were single without a partner (“unpaired”: n = 254) or had a partner (“paired”: n = 209; this group included both unmarried and married individuals). A higher proportion of males were unpaired (58.5%) than females (46.9%; χ2 = 5.41; df = 1; p = 0.02). Furthermore, unpaired individuals were, on average, 1 year younger than paired ones (mean ± SEM, unpaired = 27.88 ± 0.15 years; paired = 28.87 ± 0.18 years; t = − 4.33; p < 0.0001). Given the sex differences in hormone concentrations and in relationship status, the relation between social and hormonal variables was analyzed separately for males and females.
Unpaired males (n = 186) had significantly higher average salivary testosterone concentration than paired males (n = 132; t = 2.07; df = 316; p = 0.03; ) but were similar in testosterone residuals (t = 0.57; df = 316; p = 0.57; ). There was no significant difference in average cortisol concentrations between unpaired and paired males (t = 0.12; df = 316; p = 0.90; ). Unpaired males, however, had significantly higher cortisol residuals than paired males (t = 3.36; df = 316; p = 0.0009; ). There were no significant differences between unpaired (n = 68) and paired females (n = 77) in average testosterone concentrations (t = − 1.04; df = 143; p = 0.31), average cortisol (t = 1.33; df = 143; p = 0.18), testosterone residuals (t = 0.02; df = 143; p = 0.98), or cortisol residuals (t = 1.14; df = 143; p = 0.25; ).
Table I. Mean ± SEM testosterone (T, pg/ml) and cortisol (cort, ng/ml) concentrations and their residuals in male and female study participants with respect to relationship and marital status.
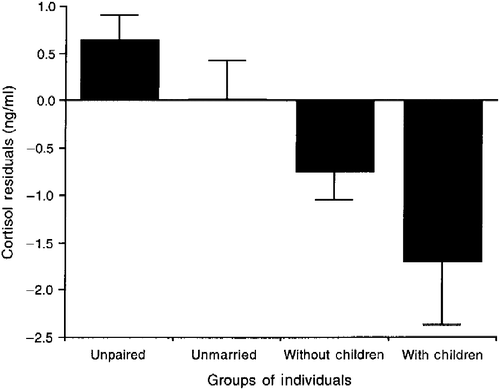
In order to investigate the relationship between marital status and hormone concentrations paired individuals were subdivided into three groups: unmarried (n = 69), married without children (n = 116), and married with children (n = 24). There were no significant group differences among males or females in average testosterone concentration (males: F2,131 = 0.17, p = 0.85; females: F2,76 = 0.84, p = 0.43), average cortisol concentration (males: F2,131 = 0.30; p = 0.75; females: F2,76 = 1.07; p = 0.35), testosterone residuals (males: F2,131 = 0.58; p = 0.56; females: F2,76 = 0.002; p = 0.99), or cortisol residuals (males: F2,131 = 0.81; p = 0.44; females: F2,76 = 2.32; p = 0.10; ). Finally, since the testosterone and cortisol residuals were independent of the pre-test concentrations, and therefore also of sex differences in the pre-test concentrations, we pooled together the data for males and females and compared the residuals in four groups of individuals: unpaired, paired but unmarried, married without children, and married with children. There was no significant difference among the four groups in testosterone residuals (F3,462 = 0.80; p = 0.49). There was, however, a significant difference in the cortisol residuals (F3,462 = 5.43, p = 0.001). Post hoc tests indicated that unpaired individuals had higher cortisol residuals than both married individuals without children (p < 0.05) and married individuals with children (p < 0.05; ), whereas there were no significant differences between the other groups. To summarize, unpaired males had higher average testosterone and higher cortisol residuals than paired males. More generally, unpaired individuals had higher cortisol residuals than married individuals with or without children, whereas paired but unmarried individuals did not differ significantly from unpaired or from married individuals in cortisol residuals.
Figure 5 Residuals for salivary cortisol concentrations in unpaired individuals (n = 254), paired but unmarried individuals (n = 69), and married individuals with (n = 166) and without children (n = 24). Data for both males and females are presented. See text for statistical results. Values are mean ± SEM. The difference among the four groups is statistically significant (p = 0.001); see Results text for detailed statistical results. Unpaired individuals had significantly higher cortisol residuals than both married individuals without children (Bonferroni–Dunn post hoc test, p < 0.05) and married individuals with children (Bonferroni–Dunn post hoc test, p < 0.05).
Discussion
The measurement of salivary concentrations of testosterone and cortisol before and after a set of economic decision-making tests in a sample of over 500 MBA students allowed us to confirm and extend previous findings concerning interindividual variation in human endocrine function and stress responsiveness, including (1) the consistency of individual differences in cortisol and testosterone concentrations and the correlations between levels of these hormones; (2) sex differences in baseline endocrine function and in hormonal responses to a psychological stressor; (3) the relationship between pre-test hormone concentrations and change in response to stress; and (4) the relationship, or lack thereof, between 2D:4D digit length ratios, marital/relationship status, and salivary hormone levels.
Salivary testosterone and cortisol concentrations showed a great deal of interindividual variability, both between and within sexes. The positive correlations between the pre-test and the post-test values suggest that individual differences in hormone concentrations were relatively stable over the 2-h testing period. Previous studies have reported stability in hormone concentrations over, not only hours, but also days or weeks (Dabbs Citation1990; Liening et al. Citation2010), indicating that individual differences in these variables are a robust phenomenon, which is relatively independent of context. In our study, individual differences in testosterone concentrations were positively correlated with differences in cortisol in both males and females. Positive correlations between basal testosterone and basal cortisol levels in both sexes have been reported previously (Gray et al. Citation1991; Popma et al. Citation2007; Mehta et al. Citation2008) and may reflect overlap in adrenal release of both hormones (Mehta et al. Citation2008). Positive correlations between testosterone and cortisol levels may also reflect reciprocal influences between these two hormones, as previous research has shown that testosterone can influence the activity of the hypothalamic–pituitary–adrenal (HPA) axis (Viau Citation2002), while increased cortisol secretion can suppress testosterone level (Rivier and Rivest Citation1991).
Males had higher concentrations of salivary testosterone and cortisol than females. Sex differences in testosterone levels are well known (Granger et al. Citation2004), whereas sex differences in cortisol secretion are still a matter of debate: most previous studies have either reported higher baseline cortisol levels in men (Zumoff et al. Citation1974; Schoneshofer and Wagner Citation1977; Vierhapper et al. Citation1998; Wirth and Schulteiss Citation2006) or no sex differences in basal cortisol (Kirschbaum et al. Citation1992). We found that males had significantly higher salivary cortisol levels than females in both the pre-test and the post-test condition, despite the finding that female cortisol level was more responsive to the test than male cortisol was (see below). Therefore, our findings are consistent with those of previous studies reporting higher basal cortisol levels in men than in women. By using a large sample size to confirm the existence of a sex difference in basal cortisol levels, our study contributes to research on biological sex differences and HPA axis function.
Sex differences were also evident in the hormonal responses to the stressor: the test was accompanied by a larger increase in salivary cortisol level in females than in males and by a significant reduction in testosterone level in males but not in females. Previous research has shown that the magnitude of the cortisol increase in response to stress can differ between the sexes in relation to the type of stressor (Stroud et al. Citation2002). Thus, it is possible that the stronger effects of the test on female cortisol level than on male cortisol level in our study were associated with differences in the perception of the test as being stressful. Similar to our findings, previous studies in both animals and humans have reported that a stress-related increase in cortisol level is accompanied by a decrease in testosterone level (Sapolsky Citation2004). Previous studies, however, rarely included a direct comparison of males and females and the demonstration that the association between increased cortisol and decreased testosterone levels occurs in males but not in females.
The sex difference in the testosterone response to the test observed in our study may suggest that female testosterone secretion is less sensitive to psychosocial stimuli and to stress-related cortisol increases than is male testosterone (but the opposite has also been argued; Bateup et al. Citation2002). Alternatively, it is possible that measures of salivary testosterone underestimate the effects of psychosocial stimuli on this hormone in females because salivary testosterone level is less strongly correlated with serum testosterone in females than in males (Shirtcliff et al. Citation2002). Contrary to this explanation, however, in the same subject population of MBA students, we reported a stronger correlation between testosterone and financial risk aversion in females than in males (Sapienza et al. Citation2009), suggesting that our measure of women's salivary testosterone is biologically meaningful. The hypothesis that testosterone secretion in men is more sensitive to stress and to glucocorticoid hormones than testosterone secretion in women needs to be further investigated and has potentially important implications for understanding stress and reproductive physiology in men and women.
Similar to what has been reported by some other studies, we found that both testosterone and cortisol levels increased following the tests in some men and women, while they decreased in others. The pre-test hormone concentrations generally predicted both the direction and the magnitude of change following the test. The individuals with lowest pre-test scores exhibited a large increase in the post-test, those with highest pre-test concentrations dropped dramatically, and those with intermediate scores exhibited relatively small hormonal changes. This pattern was evident in the negative correlation between the pre-test hormone concentrations and the difference in concentrations between the post- and the pre-test. This correlation was significant for both hormones, and in both men and women. We interpreted this pattern as the product of regression to the mean, a statistical phenomenon in which individuals with high pre-test scores tend to move down in the post-test, while individuals with low pre-test scores tend to move up (Cronbach and Furby Citation1970; Allison Citation1990). Given that many studies of hormonal responses to psychological stress have a test-retest experimental design, regression to the mean can represent a potentially significant confound in the data; yet, this phenomenon is not always taken into consideration. This can be particularly problematic for studies with small sample sizes, in which a few individuals who have extreme values of hormone concentrations in the pre-test condition can bias the overall pattern of hormonal response to stress. Our study, therefore, underscores the importance of using large sample sizes and of minimizing the influence of regression to the mean effects, for example, using experimental designs with two conditions instead of one and using residuals instead of change scores.
Although some variation in baseline hormone measurements and in hormonal responses to psychosocial stress is likely to reflect sampling artifacts, part of it is likely to result from real differences in the characteristics of individuals. The subject population for this study was relatively homogeneous in terms of age, socioeconomic status, and cultural background and interests. In women, sex hormone fluctuations across the menstrual cycle and the use of contraceptives can affect salivary measurements of testosterone and cortisol. For example, testosterone secretion is greater around the time of ovulation than in other phases of the menstrual cycle (van Anders and Watson Citation2006), and women on hormonal contraceptives have generally lower testosterone values than others (Wirth and Schulteiss Citation2006). The effects of menstrual cycle variation and of the use of oral contraceptives on female testosterone and cortisol, however, are relatively small in comparison to circadian fluctuations in these hormones or to the effects of psychosocial stress (Dabbs and de La Rue Citation1991; Kirschbaum et al. Citation1999). Indeed, these variables had no significant effects on hormone concentrations in our study.
There is conflicting evidence as to whether the 2D:4D digit length ratio, a marker of prenatal exposure to androgens (Putz et al. Citation2004), is a significant predictor of adult hormone levels, particularly for testosterone in males. A recent meta-analysis and review of the literature concluded that such a relationship is weak or nonexistent (Honekopp et al. Citation2007). In our study, we confirmed the occurrence of a robust sex difference in 2D:4D digit length ratios, the ratio being lower in males than in females, but failed to detect any significant relationship between digit ratios and hormone measures in males or females.
In this study, we made no attempt to assess whether personality characteristics accounted for interindividual variation in hormonal variables. We did, however, investigate the role of social variables by comparing hormone levels, first between individuals who were not in a stable romantic relationship and those who were at the time of testing, and then among the latter, in relation to whether they were married or unmarried, and with or without children. We found significant relationships between hormone levels and marital/relationship status, but these relationships were different for the two hormones: for testosterone, the critical variable was the average concentrations in the pre-test and post-test conditions (which can be considered an approximation of baseline levels), whereas for cortisol the critical variable was the residuals, which is a measure of change in cortisol level in response to stress. Consistent with reports from a growing number of studies, we found that men who are not in stable relationships have higher testosterone levels than men who have a partner, regardless of marital status (Booth and Dabbs Citation1993; Burnham et al. Citation2003; Gray et al. Citation2004a,Citationb; van Anders and Watson Citation2007; van Anders et al. Citation2007). Whether this difference reflects social relationship orientation, i.e. men with high testosterone levels are less likely to engage in committed relationships, or a causal effect of social or sexual interactions on testosterone levels remains unclear (Gray et al. Citation2004a; van Anders and Watson Citation2006, Citation2007). The observed relationship between change in cortisol level and marital/relationship status in both males and females represents more of a novel contribution to the socio-endocrinology research literature. We found that unpaired individuals of both sexes had higher cortisol residuals than married individuals with or without children, whereas paired but unmarried individuals did not differ significantly from unpaired or from married individuals in cortisol residuals. These results suggest that single and unpaired individuals are more responsive to psychological stress than married individuals, a finding consistent with a growing body of evidence showing that marriage and social support can buffer against stress and result in lower physiological activation in response to challenges (Waite and Gallagher Citation2000; Robles and Kiecolt-Glaser Citation2003; Coan et al. Citation2006). Unlike the association between relationship status and testosterone, in which the relevant social variable appears to be involvement in a stable relationship, with regard to responsiveness to stress marital status had a stronger association with cortisol level than social relationship status. Although the results of our study do not speak to the physiological mechanisms underlying this association, other research has suggested that oxytocin may be involved (Robles and Kiecolt-Glaser Citation2003; Coan et al. Citation2006). The observed relationships between marital/relationship status and testosterone and cortisol levels are remarkable because our population of MBA students was rather homogeneous in age, educational background, and presumably also in lifestyle when compared to a random sample of a human population. Our results indicate that being single vs. in a stable relationship vs. being married can be associated with significant variation in baseline cortisol and testosterone levels or in their responses to stress even in young individuals, who have similar lifestyles and professional interests, and who have had similar social and life experiences.
The use of a large sample of MBA students at an elite academic institution may limit the generalizability of our results to other human populations. However, our results are relevant to contemporary research on the effects of psychosocial stimuli on cortisol and testosterone levels, much of which is conducted with college students and with similar procedures. Moreover, although our study investigated hormonal responses to economic decision-making tests, our results are generally consistent with those of stress research and therefore are likely to generalize also to studies using different psychosocial stress paradigms.
Acknowledgements
This study is part of a larger project funded by the Templeton Foundation and the Zell Center; without their support none of this would have been possible. In addition, Luigi Zingales thanks the CRSP center and the Global Financial Market Initiative at the University of Chicago for financial support. We thank Ernesto Reuben for his excellent research assistantship during the project and Moshe Hoffman and Paul Rogerson for their help in hand scanning. We also thank Sari Van Anders for her helpful comments on the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allison PD. 1990. Change scores as dependent variables in regression analysis. Sociol Method. 20:93–114.
- Bateup HS, Booth A, Shirtcliff EA, Granger DA. 2002. Testosterone, cortisol, and women's competition. Evol Hum Behav. 23:181–192.
- Booth A, Dabbs JMJr. 1993. Testosterone and men's marriages. Soc Forces. 72:463–477.
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. 1989. Testosterone, and winning and losing in human competition. Horm Behav. 23:556–571.
- Burnham TC, Flynn Chapman J, Gray PB, McIntyre MH, Lipson SF, Ellison PT. 2003. Men in committed, romantic relationships have lower testosterone. Horm Behav. 44:119–122.
- Chatterton RT, Vogelsong KM, Lu YC, Hudgens GA. 1997. Hormonal responses to psychological stress in men preparing for skydiving. J Clin Endocrin Metab. 82:2503–2509.
- Coan JA, Schaefer HS, Davidson RJ. 2006. Lending a hand: Social regulation of neural response to threat. Psychol Sci. 17:1032–1039.
- Cronbach LJ, Furby L. 1970. How should we measure “change”: Or should we?. Psychol Bull. 74:68–80.
- Dabbs JMJr. 1990. Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiol Behav. 48:83–86.
- Dabbs JMJr, de La Rue D. 1991. Salivary testosterone measurements among women: Relative magnitude of circadian and menstrual cycles. Horm Res. 35:182–184.
- Dickerson SS, Kemeny ME. 2004. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 130:355–391.
- Ellison PT, Gray PB, editors. 2009. Endocrinology of social relationships. Cambridge, MA: Harvard University Press.
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. 2004. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 29:1229–1240.
- Gray A, Jackson D, McKinlay J. 1991. The relation between dominance, anger, and hormones in normally aging men: Results from the Massachusetts male aging study. Psychosom Med. 53:375–385.
- Gray PB, Campbell BC, Marlowe FW, Lipson SF, Ellison PT. Social variables predict between-subject but not day-to-day variation in the testosterone of US men. Psychoneuroendocrinology. 2004a; 29:1153–1162.
- Gray PB, Flynn Chapman J, Burnham TC, McIntyre MH, Lipson SF, Ellison PT. Human male pair bonding and testosterone. Hum Nat. 2004b; 15:119–131.
- Honekopp J, Schuster M. 2010. A meta-analysis on 2D:4D and athletic prowess: Substantial relationships but neither hand out-predicts the other. Pers Individ Dif. 48:4–10.
- Honekopp J, Bartholdt L, Beier L, Liebert A. 2007. Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: New data and a meta-analytic review. Psychoneuroendocrinology. 32:313–321.
- Kimura D. 2000. Sex and cognition. Cambridge, MA: MIT Press.
- Kirschbaum C, Wust S, Hellhammer D. 1992. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 54:648–657.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 61:154–162.
- Liening SH, Stanton SJ, Saini EK, Schulteiss OC. 2010. Salivary testosterone, cortisol and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol Behav. 99:8–16.
- Mehta PH, Josephs RA. 2006. Testosterone change after losing predicts the decision to compete again. Horm Behav. 50:684–692.
- Mehta PH, Jones AC, Josephs RA. 2008. The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. J Pers Soc Psychol. 94:1078–1093.
- Popma A, Vermeiren R, Geluk CAML, Rinne T, van den Brink W, Knol DK, Jansen LMC, van Engeland H, Doreleijers TAH. 2007. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biol Psychiatry. 61:405–411.
- Putz DA, Gaulin SJC, Sporter RJ, McBurney DH. 2004. Sex hormones and finger length. What does 2D:4D indicate?. Evol Hum Behav. 25:182–199.
- Rivier C, Rivest S. 1991. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biol Reprod. 45:523–532.
- Robles TF, Kiecolt-Glaser JK. 2003. The physiology of marriage: Pathways to health. Physiol Behav. 79:409–416.
- Salvador A. 2005. Coping with competitive situations in humans. Neurosci Biobehav Rev. 29:195–205.
- Sapienza P, Zingales L, Maestripieri D. 2009. Gender differences in financial risk aversion and career choices are affected by testosterone. Proc Natl Acad Sci USA. 106:15268–15273.
- Sapolsky RM. 2004. Why zebras don't get ulcers. 4th ed., New York: Holt.
- Schoneshofer M, Wagner GG. 1977. Sex differences in corticosteroids in man. J Clin Endocrinol Metab. 45:814–817.
- Schulteiss OC, Campbell KL, McClelland DC. 1999. Implicit power motivation moderates men's testosterone responses to imagined and real dominance success. Horm Behav. 36:234–241.
- Schulteiss OC, Wirth MM, Stanton SJ. 2004. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Horm Behav. 46:592–599.
- Schulteiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. 2005. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. J Pers Soc Psychol. 88:174–188.
- Shirtcliff EA, Granger DA, Likos A. 2002. Gender differences in the validity of testosterone measured in saliva by immunoassay. Horm Behav. 42:62–69.
- Stroud LR, Salovey P, Epel ES. 2002. Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiatry. 52:318–327.
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, Kameda T. 2005. Interpersonal trust and social stress-induced cortisol elevation. Neuroreport. 16:197–199.
- van Anders SM, Dawn Hamilton L, Watson NV. 2007. Multiple partners are associated with higher testosterone in North American men and women. Horm Behav. 51:454–459.
- van Anders SM, Watson NV. 2006. Social neuroendocrinology: Effects of social contexts and behaviors on sex steroids in humans. Hum Nat. 17:212–237.
- van Anders SM, Watson NV. 2007. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Horm Behav. 51:286–291.
- Viau V. 2002. Functional cross-talk between the hypothalamic-pituitary-gonadal and adrenal axes. J Neuroendocrinol. 14:506–513.
- Vierhapper H, Nowotny P, Waldhausl W. 1998. Sex-specific differences in cortisol production rates in humans. Metabolism. 47:974–976.
- Waite LJ, Gallagher M. 2000. The case for marriage: Why married people are happier, healthier, and better off financially. New York: Doubleday.
- White RE, Thornhill S, Hampson E. 2006. Entrepreneurs and evolutionary biology: The relationship between testosterone and new venture creation. Organ Behav Hum Decis Process. 100:21–34.
- Wirth MM, Schulteiss OC. 2006. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm Behav. 50:786–795.
- Wirth MM, Welsh KM, Schulteiss OC. 2006. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Horm Behav. 49:346–352.
- Zumoff B, Fukushima DK, Weitzman ED, Kream J, Hellman L. 1974. The sex difference in plasma cortisol concentration in man. J Clin Endocrinol Metab. 39:805–808.