Abstract
CTX-M enzymes, the plasmid-mediated cefotaximases, constitute a rapidly growing family of extended-spectrum β-lactamases (ESBLs) with significant clinical impact. CTX-Ms are found in at least 26 bacterial species, particularly in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis. At least 109 members in CTX-M family are identified and can be divided into seven clusters based on their phylogeny. CTX-M-15 and CTX-M-14 are the most dominant variants. Chromosome-encoded intrinsic cefotaximases in Kluyvera spp. are proposed to be the progenitors of CTX-Ms, while ISEcp1, ISCR1 and plasmid are closely associated with their mobilization and dissemination.
Introduction
Extended-spectrum β-lactamases (ESBLs) are the most influential mechanism for cephalosporin resistance in Enterobacteriaceae, particularly in Escherichia coli and Klebsiella pneumoniae. ESBLs confer resistance to penicillins, broad-spectrum cephalosporins with an oxyimino side chain (cefotaxime, ceftriaxone and ceftazidime) and the oxyimino-monobactam aztreonam, but can be inhibited by serine-type β-lactamase inhibitors as sulbactam, clavulanate and tazobactam (Philippon et al., Citation1989; Bradford, Citation2001). SHV-2 is the first ESBL, identified in a clinical isolate of Klebsiella ozaenae in Germany (Kliebe et al., Citation1985). To date, over 10 families have been documented to be associated with ESBLs, including CTX-M, SHV, TEM, PER, VEB, BES, GES, TLA, SFO and OXA (Paterson and Bonomo, Citation2005).
CTX-M enzymes, the plasmid-mediated acquired cefotaximases from a distinct phylogenetic lineage, constitute a rapidly growing family of ESBLs with significant clinical impact (Bonnet, Citation2004; Cantón and Coque, Citation2006; Livermore et al., Citation2007; Naseer and Sundsfjord, Citation2011). Chromosome-encoded genes of intrinsic cefotaximases in Kluyvera spp. are proposed to be the progenitors of CTX-M family (Humeniuk et al., Citation2002; Olson et al., Citation2005; Decousser et al., 2011). Most of CTX-Ms exhibit powerful activity against cefotaxime and ceftriaxone but not ceftazidime. However, some CTX-Ms, such as CTX-M-15 (Poirel et al., Citation2002a), CTX-M-16 (Bonnet et al., Citation2001) and CTX-M-19 (Poirel et al., Citation2001), exhibit enhanced catalytic efficiencies against ceftazidime.
This article summarizes the epidemiology of CTX-M-producing Gram-negative bacteria and the genetics of CTX-M ESBLs, with a focus on the phylogeny, origin and genetic platforms including ISEcp1, ISCR1 and plasmid.
Epidemiology of CTX-M ESBLs
Occurrence and bacterial hosts
A plasmid-mediated cefotaximase was identified from a clinical isolate of E. coli in Munich, Germany, and designated CTX-M in reference to its hydrolytic activity and the region where it was found (Bauernfeind et al., Citation1990). To date, the numbers of CTX-M variants and the recognized organisms harboring the genes have dramatically increased. At least 109 CTX-M variants, CTX-M-1 to CTX-M-124, have been identified () and assigned in the Lahey database (Jacoby and Bush, Citation2012). The amino-acid sequences of CTX-M-14 and CTX-M-18 and of CTX-M-55 and CTX-M-57 are identical, and CTX-M-118 has been withdrawn. There is no detailed information available for the assigned members CTX-M-70, -73, -100, -103, -115, -119, -120 and -124 so far. In addition, CTX-M-76, -77, -78 and -95 are chromosome-encoded intrinsic cefotaximases in Kluyvera spp., and therefore, they are not counted into the CTX-M family. CTX-M-2, -3 and -37 are plasmid-mediated enzymes but also found on chromosomes in Kluyvera spp. To clarify the differences, the term c-CTX-M is used for such chromosome-encoded CTX-Ms in this article. Of the studied CTX-Ms, at least 19 variants display the enhanced catalytic efficiencies against ceftazidime ().
Table 1. CTX-M ESBLs and their bacterial hosts.
CTX-Ms have been detected in at least 26 bacterial species, including Acinetobacter baumannii, Aeromonas caviae, A. hydrophila, Citrobacter amalonaticus, C. freundii, C. koseri, E. coli, Enterobacter cloacae, E. aerogenes, E. gergoviae, E. hormaechei, K. pneumoniae, K. oxytoca, Morganella morganii, Proteus mirabilis, Pantoea agglomerans, Providencia rettgeri, P. stuartii, Pseudomonas aeruginosa, Salmonella enterica, Shigella flexneri, S. sonnei, Serratia marcescens, S. liquefaciens, Stenotrophomonas maltophilia and Vibrio cholera ().
CTX-M enzymes as the most prevalent ESBLs in E. coli, K. pneumoniae and P. mirabilis
The high prevalence of CTX-M ESBL genes in Enterobacteriaceae, particularly in E. coli, K. pneumoniae and P. mirabilis, has been documented worldwide (Bonnet, Citation2004; Cantón and Coque, Citation2006), while the CTX-Ms are not prominent in P. aeruginosa and A. baumannii (Zhao and Hu, Citation2010, 2012).
A study on the resistance of Enterobacteriaceae to third-generation cephalosporin was undertaken in 16 British hospitals over a 12-week period (Potz et al., Citation2006). Of 19,252 clinical isolates, CTX-M-producing strains accounted for 1.7%, higher than other ESBLs-producing strains (0.6%) and high-level AmpC-producing strains (0.4%). Particularly, of the resistance isolates of E. coli (n = 574) and Klebsiella spp. (n = 243), the CTX-M-producing strains accounted for 50.9% and 81.9%, respectively, by contrast with other ESBLs-producing strains (15.3% and 11.1%), high-level AmpC-producing strains (7.1% and 0.8%) and non-β-lactamase-producing strains (26.7% and 3.3%).
A rapid occurrence of CTX-M-producing strains in Enterobacteriaceae was documented by several longitudinal surveillances. Of 20,258 E. coli isolates studied in Italy, the prevalence of ESBL-producing strains increased from 0.2% in 1999 to 1.6% in 2003, of which CTX-M-positive strains increased from 12.5% to 38.2% (Brigante et al., Citation2005). Of 1574 P. mirabilis clinical isolates collected in a Taiwanese hospital during 1999–2005, 44 CTX-M-producing strains were detected at a rate of 0.7% in 1999 and approximately 6% after 2002 (Wu et al., Citation2008). Of 11,407 E. coli isolates from urine samples of outpatients in the USA, 107 CTX-M-producing strains were detected at a rate of 0.07% in 2003 and 1.66% in 2008 (Qi et al., Citation2010).
CTX-M-producing strains widespread not only in human but also in animals and in environments. Of 240 E. coli isolates from health and sick pets during 2007–2008 in China, 97 strains (40.4%) harbored ESBL-encoding genes, of which 96 strains were confirmed to be carriers of blaCTX-M genes (Sun et al., Citation2010). Of 16 multi-drug resistant E. coli isolates from river water during 2000–2001 in South Korea, 10 strains harbored CTX-M-14 gene (Kim et al., Citation2008). Of 79 food samples of animal origin in Tunisia, blaCTX-M-1-positive E. coli strains were isolated from 10 samples (Ben Slama et al., Citation2010).
A Japanese group surveyed the spread status of CTX-M genes in nosocomial Gram-negative bacteria collected from 132 geographically distant medical facilities during 2001–2003. Of the 1456 isolates resistant to oxyimino-cephalosporins, 21.8% were found to harbor blaCTX-M genes. The prevalent rates of CTX-Ms in ESBL-producing E. coli, K. pneumoniae and P. mirabilis were 77% (168/218), 56% (50/90) and 99% (71/72), respectively, while the rates of CTX-Ms in ESBL-producing A. baumannii and S. marcescens were 4.5% (4/89) and 7% (10/149), respectively (Shibata et al., Citation2006).
CTX-M-15 and CTX-M-14 as the most dominant variants in CTX-M family
Although the dominant variants of CTX-Ms are geographically different, CTX-M-15 and CTX-M-14 are the most common variants detected worldwide in clinically important pathogens, followed by CTX-M-2, CTX-M-3 and CTX-M-1 (). Conjugative plasmid-mediated horizontal transfer and clonal spread contributed to the increased prevalence.
Of 171 CTX-M-producing E. coli isolates from 11 Canadian medical centers in 2007, the positive rates for CTX-M-15, CTX-M-14, CTX-M-3 and CTX-M-27 were 86.5%, 9.9%, 2.9% and 0.6%, respectively (Peirano et al., Citation2010). Of 202 CTX-M-producing K. pneumoniae isolates from 41 medical centers in Hungary in 2005, 97% were CTX-M-15 producers derived from three genetically distinct clones (Damjanova et al., Citation2008). Of the CTX-M-producers (288 E. coli and 142 K. pneumoniae isolates) collected from 6 provinces in China during 1998–2002, CTX-M-14 was predominantly detected in 77.4% and 52.8% of the isolates, respectively, followed by CTX-M-3 (18.4% and 29.6%), CTX-M-24 (5.6% and 14.1%) and CTX-M-15 (0.7% and 1.4%) (Yu et al., Citation2007). An outbreak of CTX-M-producing S. enterica infection occurred in a university hospital in Algeria during 2008–2009, and all of 200 isolates from 138 patients were CTX-M-15 producers, identified to be a single clone (Naas et al., Citation2011).
Of 44 clinical isolates of CTX-M-producing P. mirabilis from a Taiwanese hospital, CTX-M-14 and CTX-M-3 positive strains accounted for 50% and 40.9%, respectively (Wu et al., Citation2008). Of 71 CTX-M-producing P. mirabilis isolates collected from 132 geographically distant hospitals in Japan, however, 100% of the strains carried the blaCTX-M-2-like genes (Shibata et al., Citation2006). CTX-M-2 was also predominant in C. koseri, accounting for 76.7% of ESBL-producing strains (n = 60) collected from 10 areas throughout Japan in a 5-month period between 2009 and 2010 (Kanamori et al., Citation2011).
Phylogeny, origin and evolution of CTX-M enzymes
Amino-acid identity and phylogeny
The deduced amino-acid sequences of CTX-Ms comprise 291 residues, with the exceptions of CTX-M-11 (282), CTX-M-107 and -108 (288), CTX-M-45 and -109 (289), CTX-M-40, -63 and -106 (290) and CTX-M-110 (292). Based on the phylogenetic tree of amino-acid sequences, CTX-M enzymes may be divided into seven clusters ().
Figure 1. Phylogenetic tree of CTX-M family based on amino-acid sequences. DNASIS Pro v2.10 (Hitachi Software Engineering Co., Tokyo, Japan) was used to align the amino-acid sequences and construct the phylogenetic tree. The amino-acid sequences were downloaded from GenBank under the accession numbers cited in . The branch lengths are drawn to scale and are proportional to the number of different amino-acid residues. The scale bars of 0.05 and 0.005 represent 5% and 0.5% amino-acid difference, respectively.
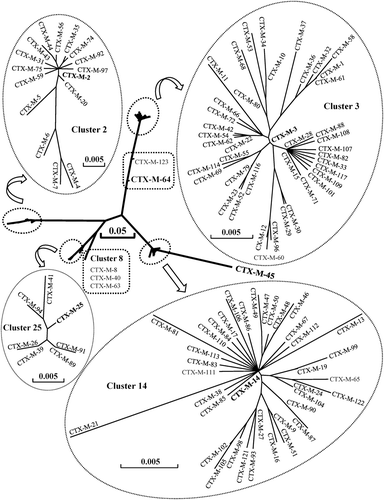
CTX-M-3 cluster includes 42 members, sharing 97.6–99.7% identity in amino-acid sequences. The other clusters are as follows: CTX-M-14 cluster, 38 members, 97.3–99.7% identity; CTX-M-2 cluster, 16 members, 95.2–99.7% identity; CTX-M-25 cluster, 7 members, 98.6–99.7% identity; CTX-M-8 cluster, 3 members, 97.9–99.7% identity; CTX-M-64 cluster, 2 members, 95.9% identity. There is only one member in CTX-M-45 cluster. Among CTX-M variants, CTX-M-4 and CTX-M-45 are most divergent with 91 amino-acid substitutions.
Variations of amino-acid sequences
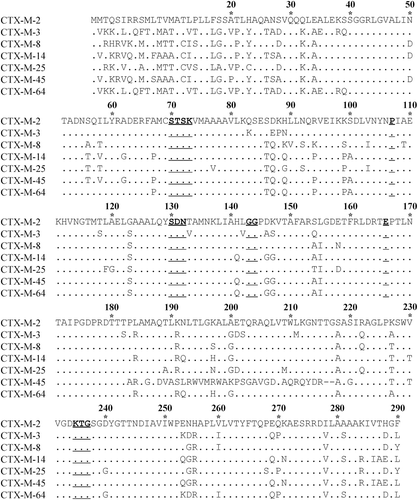
Based on the central positions in phylogenetic tree (), CTX-M-2, -3, -8, -14, -25, -45 and -64 are chosen as the representative enzymes in each cluster. The amino-acid sequences of the seven enzymes are aligned, and numbered according to the standard numbering scheme for the class A serine β-lactamases, giving the active site serine residue the Ambler number 70 (Ambler et al., Citation1991) (). The sequences of CTX-M variants are then compared with their representative in each cluster (). In the CTX-M-3 cluster, for example, a single amino-acid is substituted between CTX-M-3 and CTX-M-15, -22, -42, -54, -62, -66, -72 or -80, while 5 amino-acids are substituted between CTX-M-3 and CTX-M-58.
Table 2. Amino acid substitutions of CTX-M variants compared to their representative enzymes.
Figure 2. Comparison of amino-acid sequences of seven representative enzymes in the CTX-M family. Amino-acids are numbered according to the standard numbering scheme for the class A serine β-lactamases, giving the active site serine residue the Ambler number 70. Dots indicate identical amino-acids compared to CTX-M-2. Deletion mutations are expressed with short lines. The underlined amino-acids, 70SXXK73, 107P, 130SDN132, 143GG144, 166E and 234KXG236, represent the conserved residues in typical class A serine β-lactamases.
Origin of CTX-M family
In the family Enterobacteriaceae, the genus Kluyvera is a relatively new member, which has been isolated from various clinical specimens and regarded as a potentially virulent pathogen (Sarria et al., Citation2001). Some Kluyvera spp. harbor chromosome-encoded intrinsic genes of cefotaximases which are closely associated with CTX-Ms (Decousser, et al., 2001; Humeniuk et al., Citation2002; Rodríguez et al., Citation2004). Generally, Kluyvera spp. are susceptible to cefotaxime in despite of the presence of naturally occurring cefotaximases. However, the recombinant clones of E. coli with Kluyvera-derived cefotaximase genes exhibited a significant increase in resistance to cefotaxime (Decousser et al., Citation2001; Humeniuk et al., Citation2002; Rodríguez et al., Citation2004), suggesting that a proper genetic platform is necessary for the gene expression. The chromosome-encoded cefotaximases identified in Kluyvera spp. include KLUA, KLUG, KLUY, KLUC, c-CTX-M-2, c-CTX-M-3, c-CTX-M-37, c-CTX-M-76, c-CTX-M-77, c-CTX-M-78 and c-CTX-M-95. All of them comprise 291 amino-acid residues. An aspartate aminotransferase-encoding gene is found commonly upstream of these chromosomal bla genes, which is replaced by ISEcp1 or ISCR1 in the plasmid-harbored blaCTX-M genes (see the details under next section).
KLUA-1 to -5 and -8 to -12 (GenBank accession no. AJ272538, AJ251722, AJ427461, AJ427462, AJ427463, AJ427465, AJ427466, AJ427467, AJ427468, AJ427469) are a group of chromosomal cefotaximases identified in K. ascorbata, with minor variations (<5%) in their amino-acid sequences (Humeniuk et al., Citation2002). KLUA-2 shares 100% identity with plasmid-mediated CTX-M-5. CTX-M-2 and CTX-M-3 originally identified on plasmids were also found on the chromosomes of K. ascorbata (Rodríguez et al., Citation2004; Lartigue et al., Citation2006). The immediate upstream- and downstream-sequences of blaKLUA-1 and plasmid-mediated bla genes in CTX-M-2 cluster (blaCTX-M-2, -4, -5, -6, -7,-44) share 85 to 100% identities (Di Conza et al., Citation2002; Humeniuk et al., Citation2002). The architectures of the flanking regions corresponding to c-CTX-M-3 and plasmid-mediated CTX-M-3 are identical, including a 128 bp immediate upstream region and the first 373 bp of the downstream region of the bla gene (Rodríguez et al., Citation2004). The c-CTX-M-76, -77 and -95 (AM982520, AM982521, FN813245) identified in K. ascorbata also share high identities with the enzymes in CTX-M-2 cluster.
KLUY-1 to -4 (AY623932, AY623935, AY623934, AY623933) are a group of chromosomal cefotaximases identified in K. Georgiana (Olson et al., Citation2005). They share high homology with the enzymes in CTX-M-14 cluster. Typically, KLUY-1 exhibits 100% amino-acid identity with CTX-M-14. The upstream- and downstream-sequences of blaKLUY and blaCTX-M-9, -13, -14 also share consistent identity. A 42 bp upstream region of blaCTX-M-14 is identical to the corresponding region of blaKLUY genes. A 347 bp downstream region of blaCTX-M-9 and blaCTX-M-13 shares 95.7–98.6% identities with the corresponding region of blaKLUY genes (Olson et al., Citation2005).
KLUG-1 (AF501233) and c-CTX-M-78 (AM982522) are the chromosomal cefotaximases identified in K. Georgiana. KLUG-1 shares 99% amino-acid identity with the plasmid-mediated CTX-M-8 (Poirel et al., Citation2002b). The c-CTX-M-78 possesses high homology with the known members of CTX-M-25 cluster, sharing 95.2–96.2% identities (Rodríguez et al., Citation2010).
CTX-M-37 was also found on the chromosome of K. cryocrescens (FN813246), suggesting the c-CTX-M-37 as an origin of CTX-M-3 cluster. KLUC-1 (AY026417) and KLUC-2 (EF057432), with a single amino-acid substitution, are two chromosome-encoded cefotaximases identified in K. cryocrescens (Decousser et al., Citation2001). KLUC-1 and -2 are diverse from the known CTX-Ms, sharing only 87.6% identity with CTX-M-3. Notably, KLUC-2 was also identified on a plasmid carried by a clinical isolate of E. cloacae, indicating the transfer of blaKLUC from chromosome to the plasmid (Petrella et al., Citation2008). We would like to suggest the plasmid-mediated KLUC-2 as a novel cluster or member of CTX-M family.
CTX-M-64 shows a chimeric sequence of both CTX-M-14 (central portion) and CTX-M-15 (N- and C-terminal moieties), suggesting an origination owing to homologous recombination between the blaCTX-M-14 and -15 genes (Nagano et al., Citation2009).
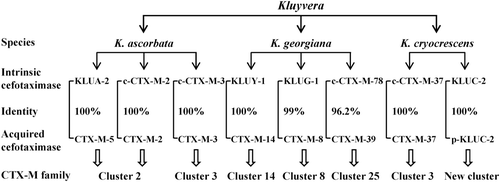
Taken together, the origins of the acquired CTX-Ms in various clusters can be traced back to the intrinsic cefotaximase genes harbored by Kluyvera spp., of which the CTX-M-2 cluster appears to be derived from K. ascorbata, the CTX-M-14, CTX-M-8 and CTX-M-25 clusters from K. georgiana, while the CTX-M-3 cluster from both K. ascorbata and K. cryocrescens ().
Figure 3. Identification of intrinsic cefotaximase genes in Kluyvera spp. as the original sources of acquired CTX-Ms based on their amino-acid identities and the homologies of neighboring sequences of the associated genes. c-CTX-M, CTX-M identified on chromosome of Kluyvera spp.; p-KLUC-2, KLUC-2 identified on plasmid in a clinical isolate of Enterobacter cloacae.
Genetic platforms of CTX-M enzymes
ISEcp1
Insertion sequences (ISs) are the smallest transposable elements (<2.5 kb) capable of independent transposition in an organism, thereby causing insertion mutations and genome rearrangements (Mahillon and Chandler, Citation1998). ISs play three basic roles in bacteria: encoding a transposase which makes a genetic element mobile; providing promoters to activate silent genes or enhance expression of downstream determinants; moving IS-mobilized genes among integrons, transposons, plasmids and chromosomes, thereby greatly increasing the opportunity a resistance determinant becomes transferable.
Of the genetic platforms associated with CTX-Ms, ISEcp1 is one of the most important elements (). ISEcp1 was first identified on the plasmid pST01 in E. coli strain 79 (AJ242809), hence its name (Stapleton, Citation1999). ISEcp1 is composed of an orf encoding a transposase with 420 amino-acids and two imperfect and inverted repeats. ISEcp1 can mobilize the downstream-located blaCTX-M gene and provide a promoter for its expression (Karim et al., Citation2001; Cao et al., Citation2002; Poirel et al., Citation2003, 2005; Dhanji et al., Citation2011b).
Table 3. Genetic platforms of CTX-M enzymes.
Co-existence of ISEcp1 and blaCTX-M at a high rate in CTX-M-producing E. coli isolates is well documented. ISEcp1 was identified upstream of blaCTX-M genes in 86.9% of the isolates (93/107) recovered from health and sick pets in China, and no major clonal relatedness was observed (Sun et al., Citation2010). Similarly, ISEcp1 was identified upstream of blaCTX-M-14 in 91.4% of the clinical isolates (32/35) in Korea (Kim et al., Citation2011), and upstream of blaCTX-M-1 in 69.2% of the isolates (9/13) from food samples in Tunisia (Ben Slama et al., Citation2010). In addition, variations of ISEcp1 were also observed. ISEcp1B, originally identified upstream of a blaCTX-M-19 gene cassette (AF458080), differs from ISEcp1 by three nucleotide substitutions (Poirel, et al., 2003). Of the 174 ISEcp1-like and blaCTX-M-15 complex from E. coli isolates, the intact ISEcp1, truncated ISEcp1 with various lengths and a 24 bp remnant of ISEcp1 accounted for 62%, 33.3% and 4.6%, respectively (Dhanji et al., Citation2011b). Notably, ISEcp1 was also detected upstream of chromosomal blaCTX-M-2 genes in 4 P. mirabilis isolates in Japan (Harada et al., Citation2012), highlighting the ISEcp1-mediated movement of blaCTX-M genes between plasmids and chromosomes.
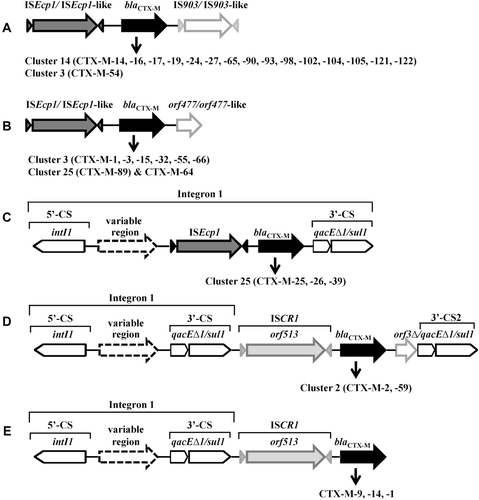
ISEcp1-blaCTX-M-IS903 () and ISEcp1-blaCTX-M-orf477 () are two major genetic platforms. In some cases, ISEcp1-mobilized blaCTX-M is inserted in a class 1 integron (). IS903 (V00359) encodes a transposase with 307 amino-acids and was originally found on a kanamycin resistance transposon Tn903 (Oka et al., Citation1981). IS903 and IS903-like elements, such as IS903C and IS903D, are located downstream of blaCTX-M genes (), including blaCTX-M-14-like genes (blaCTX-M-14, -16, -17, -19, -24, -27, -65, -90, -93, -98, -102, -104, -105, -121, -122) and blaCTX-M-3-like gene (blaCTX-M-54). orf477 encodes a protein of 158 amino-acids with unknown function and the orf477 and orf477-like elements were found downstream of plasmid-harbored blaCTX-M-3-like genes (blaCTX-M-1, -3, -15, -22, -32, -53, -55, -66), blaCTX-M-89 and blaCTX-M-64 (). The orf477 was also identified downstream of the chromosomal blaCTX-M-3 in K. ascorbata, of the chromosomal blaKLUY-1, -2, -3, -4 in K. georgiana, and of the chromosomal blaCTX-M-37 (FN813246) in K. cryocrescens (Rodriguez et al., 2004; Olson et al., Citation2005), footnoting the ISEcp1-mediated transfer of blaCTX-M genes together with the orf477 from the chromosomes of Kluyvera spp. to plasmids.
Figure 4. Typical genetic platforms of CTX-M enzymes. A & B: the blaCTX-M gene cassettes bracketed upstream by ISEcp1/ISEcp1-like and downstream by IS903/IS903-like (A) or orf477/orf477-like (B); C: blaCTX-M genes associated with class 1 integron-ISEcp1; D & E: blaCTX-M genes associated with class 1 integron-ISCR1 complex. CS, conserved segment; intI, integrase gene; qacE▵1, quaternary ammonium resistance gene; sul1, sulphonamide resistance gene; 3′-CS2, the second copy of 3′-conserved segment.
Class 1 integron-ISCR1 complex
Integrons are defined as mobile DNA elements that can capture genes by site-specific recombination (Stokes and Hall, Citation1989). A typical class 1 integron consists of a 5′ conserved segment (5′-CS), a variable region and a 3′ conserved segment (3′-CS). The 5′-CS consists of the gene encoding integrase (intI1), the site adjacent to intI1 for the insertion of captured genes (attI), and a promoter region (Pc). The 3′-CS often consists of a partially deleted qac gene (qacEΔ1) fused to a sul1 gene, and confers resistance to antiseptics and sulfonamide, respectively. Class 1 integrons play a critical role in acquiring and spreading metallo-β-lactamases (Mazel, Citation2006; Zhao and Hu, Citation2011a,b). The role of integrons in CTX-M gene acquisition and dissemination, however, is still unclear. The physical link of some blaCTX-M genes with class 1 integron-ISEcp1 complex () and class 1 integron-ISCR1 complex (, ) indicates a possible association among the three genetic elements.
ISCR1 is another important element in the genetic platforms associated with the mobilization and dissemination of CTX-M genes (Rodriguez-Martinez et al. Citation2006; Toleman et al., Citation2006). Common region 1 (CR1) was first found as element associated with but distinct from class 1 integrons (Stokes et al., Citation1993). The CR1 element was renamed ISCR1 because it possesses the key motifs of IS91-like element and accommodates orf513 gene which codes a putative transposase of 513 amino-acids (Toleman et al., Citation2006). ISCR1 is particularly important for CTX-M-2 and CTX-M-9 genes (). In most instance, the ISCR1-blaCTX-M-2 is located between a typical class 1 integron and a fuse type of orf3Δ and qacEΔ1/sul1 (, ). Notably, the genes harbored by class 1 integrons in their variable regions, such as blaOXA-2, aacA4, cmlA and dfr, are also associated with bacterial resistance to β-lactam, aminoglycoside, chloramphenicol and trimethoprim, respectively.
Molecular epidemiological study performed in Argentine during 1993–2000 showed that class 1 integron-ISCR1 complex was adjacent to blaCTX-M-2 in all the CTX-M-2 producers (n = 35), including Acinetobacter spp., E. cloacae, E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. enterica and S. marcescens, while only 1.5% of the blaCTX-M-2-negative isolates (n = 65) harbored ISCR1 (Arduino et al., Citation2003). These data strongly implicate the association of ISCR1 with the emergence and dissemination of blaCTX-M-2 gene. In addition, ISCR1 is also related to blaCTX-M-59, -74, -75 (members of CTX-M-2 cluster) and blaCTX-M-1, -9, -14 ().
Other IS and phage-related sequences
Besides ISEcp1, IS903 and ISCR1 described above, IS1, IS5, IS10, IS26, IS50A, IS1294, IS1326, IS3000, IS4321 and IS6100 were also found to be adjacent to blaCTX-M genes (). In some cases, several IS elements co-existed in a gene complex, for example, intI1-dfrA12-orfF-aadA2-qacEΔ1-sul1-ISCR1-IS6100-ISCR1-ISEcp1Δ-blaCTX-M-14-IS903D (Bae et al., Citation2008). Such heterogeneity may be explained by a continuously recombinatorial exchange of gene cassettes, denoting the sophisticated genetic rearrangement strategies that organisms acquire and dispense resistance genes.
A 12.2-kb DNA fragment containing blaCTX-M-10 gene in plasmid pRYCE21 was cloned from K. pneumoniae, and further detected in other bacterial species including E. coli, E. cloacae and E. gergoviae. Analysis of the sequence showed a phage-related 3.5-kb element immediately upstream of the blaCTX-M-10 gene cassettes. This phage-related fragment corresponds to four orfs, of which orf2, orf3 and orf4 display homology to the genes of conserved phage tail proteins (Oliver et al., Citation2005). Although there is a limited report on phage-related CTX-M genes, this finding indicates that phages may also function as a tool for blaCTX-M-associated genetic elements to become transferable.
Plasmids
The movement of IS-mobilized genes between chromosomes and plasmids greatly increase the opportunity a resistance determinant becomes transferable. Particularly, conjugative plasmid is one of the most important mechanisms for intra-species, inter-species and inter-genus gene transfers.
Plasmids are usually classified on their incompatibility (Inc), defined as the inability of two plasmids to be propagated stably in the same bacterial strain; thus, only compatible plasmids can be rescued in transconjugants (Novick et al., Citation1976). At least 29 Inc groups have been recognized among plasmids of enteric bacteria, including IncFI, IncFII, IncFIII, IncFIV, IncFV, IncFVI, IncI1, IncI2, IncIy, IncHI1, IncHI2, IncHI3, IncA/C, IncB, IncD, IncJ, IncK, IncL/M, IncN, IncO, IncP, IncS, IncT, IncU, IncV, IncW, IncX, IncY and com9 (Novick et al., Citation1976; Couturier et al., Citation1988). The IncFII, IncA/C, IncL/M, and IncI1 plasmids show the highest occurrence among the typed resistance plasmids (Carattoli, Citation2009).
Molecular epidemiological studies have revealed a close and significant linkage of blaCTX-M genes to plasmids, mainly belonged to IncF, IncI, IncN, IncHI2, IncL/M and IncK groups (). The IncF group (FIA, FIB and FII) is the most prevalent in transmitting blaCTX-M-15 genes, while IncF, IncK and IncI1 are closely related to the widespread of blaCTX-M-14 genes. In addition, the blaCTX-M-1 gene is dominantly harbored by IncN and IncI1, blaCTX-M-3 gene by IncL/M and IncI1, and blaCTX-M-9 gene by IncHI2.
Table 4. Plasmids associated with the spread of CTX-M genes.
Unlike the plasmids with broad host range, such as IncP, IncA/C and IncQ, IncF plasmids are limited by host range to the genera of Enterobacteriaceae (Toukdarian, Citation2004), footnoting the high prevalence and widespread of CTX-M genes in Enterobacteriaceae, but not in Acinetobacter and Pseudomonas.
Various resistance genes frequently co-exist on a plasmid, facilitating the dissemination of resistance determinants and the survival of bacteria under the pressure of various antibiotics. For example, plasmid pEK499 (a fusion of type FII and FIA replicons) identified in a UK variant of the internationally prevalent E. coli O25:H4-ST131 lineage is confirmed to harbor 10 resistance genes, conferring resistance to seven antibiotic classes, β-lactams (blaCTX-M-15, blaOXA-1, blaTEM-1), aminoglycoside (aac6′-Ib-cr, aadA5), macrolides (mph(A)), chloramphenicol (catB4), tetracycline (tet(A)), trimethoprim (dfrA7) and sulfonamide (sul1) (Woodford et al., Citation2009).
Secondary chromosomal integration
Most of the blaCTX-M genes are harbored by plasmids and the secondary chromosomal insertions of blaCTX-M genes are also confirmed, particularly in P. mirabilis. Of 25 clinical isolates of CTX-M-producing P. mirabilis collected in Korea, 21 strains harbored blaCTX-Ms on their chromosomes (Song et al., Citation2011). The genes of blaCTX-M-25 and-41 were also found on the chromosomes of P. mirabilis in Israel (Navon-Venezia et al., Citation2008).
In addition, chromosomal integration of blaCTX-M-15 gene was reported in E. coli, K. pneumoniae and S. enterica (Coque et al., Citation2008; Coelho et al., 2010; Fabre et al., Citation2009). Chromosomal blaCTX-M-9 was observed in one strain of 30 E. coli isolates collected in Barcelona during 1996–1999 (García et al., Citation2005).
Conclusion
Plasmid-mediated CTX-M enzymes are the most prevalent ESBLs, particularly in E. coli, K. pneumoniae and P. mirobilis. At least 109 members in CTX-M family are identified and can be divided into seven clusters based on their phylogeny. CTX-M-15 and CTX-M-14 are the most dominant variants in the family, followed by CTX-M-2, CTX-M-3 and CTX-M-1.
The CTX-M genes can be traced back to the chromosome-encoded cefotaximas genes in Kluyvera spp., strongly indicating that the plasmid-mediated CTX-M enzymes are originally from Kluyvera. Multiple genetic elements, especially ISEcp1 and ISCR1, are involved in the mobilization of blaCTX-M genes from the chromosomes to plasmids. Conjugative plasmids are responsible for the transfer of the blaCTX-M genes to new hosts, while the properties of plasmid incompatibility and host range are closely associated with the high prevalence and widespread of the CTX-M genes in Enterobacteriaceae, but not in Acinetobacter and Pseudomonas.
Declaration of interest
This work was supported by a grant (No. 24591489) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by a grant from Showa University Medical Foundation, Tokyo, Japan.
References
- Abdalhamid B, Pitout JD, Moland ES, Hanson ND. (2004). Community-onset disease caused by Citrobacter freundii producing a novel CTX-M beta-lactamase, CTX-M-30, in Canada. Antimicrob Agents Chemother, 48, 4435–4437.
- Acikgoz ZC, Gulay Z, Bicmen M, Gocer S, Gamberzade S. (2003). CTX-M-3 extended-spectrum beta-lactamase in a Shigella sonnei clinical isolate: first report from Turkey. Scand J Infect Dis, 35, 503–505.
- Aibinu I, Pfeifer Y, Peters F, Ogunsola F, Adenipekun E, Odugbemi T, Koenig W. (2012). Emergence of blaCTX-M-15, qnrB1 and aac(6′)-Ib-cr resistance genes in Pantoea agglomerans and Enterobacter cloacae from Nigeria (sub-Saharan Africa). J Med Microbiol, 61, 165–167.
- Alobwede I, M’Zali FH, Livermore DM, Heritage J, Todd N, Hawkey PM. (2003). CTX-M extended-spectrum beta-lactamase arrives in the UK. J Antimicrob Chemother, 51, 470–471.
- Ambler RP, Coulson AF, Frère JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. (1991). A standard numbering scheme for the class A beta-lactamases. Biochem J, 276 (Pt 1), 269–270.
- Arduino SM, Catalano M, Orman BE, Roy PH, Centrón D. (2003). Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob Agents Chemother, 47, 3945–3949.
- Arduino SM, Roy PH, Jacoby GA, Orman BE, Pineiro SA, Centron D. (2002). blaCTX-M-2 is located in an unusual class 1 integron (In35) which includes Orf513. Antimicrob Agents Chemother, 46, 2303–2306.
- Bae IK, Lee BH, Hwang HY, Jeong SH, Hong SG, Chang CL, Kwak HS, Kim HJ, Youn H. (2006a). A novel ceftazidime-hydrolysing extended-spectrum beta-lactamase, CTX-M-54, with a single amino acid substitution at position 167 in the omega loop. J Antimicrob Chemother, 58, 315–319.
- Bae IK, Lee YH, Jeong HJ, Hong SG, Lee SH, Jeong SH. (2008). A novel blaCTX-M-14 gene-harboring complex class 1 integron with an In4-like backbone structure from a clinical isolate of Escherichia coli. Diagn Microbiol Infect Dis, 62, 340–342.
- Bae IK, Lee YN, Hwang HY, Jeong SH, Lee SJ, Kwak HS, Song W, Kim HJ, Youn H. (2006b). Emergence of CTX-M-12 extended-spectrum beta-lactamase-producing Escherichia coli in Korea. J Antimicrob Chemother, 58, 1257–1259.
- Bae IK, Lee YN, Lee WG, Lee SH, Jeong SH. (2007). Novel complex class 1 integron bearing an ISCR1 element in an Escherichia coli isolate carrying the blaCTX-M-14 gene. Antimicrob Agents Chemother, 51, 3017–3019.
- Baraniak A, Fiett J, Hryniewicz W, Nordmann P, Gniadkowski M. (2002a). Ceftazidime-hydrolysing CTX-M-15 extended-spectrum beta-lactamase (ESBL) in Poland. J Antimicrob Chemother, 50, 393–396.
- Baraniak A, Fiett J, Sulikowska A, Hryniewicz W, Gniadkowski M. (2002b). Countrywide spread of CTX-M-3 extended-spectrum beta-lactamase-producing microorganisms of the family Enterobacteriaceae in Poland. Antimicrob Agents Chemother, 46, 151–159.
- Bauernfeind A, Grimm H, Schweighart S. (1990). A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection, 18, 294–298.
- Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas JM. (1996). Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrob Agents Chemother, 40, 509–513.
- Ben Slama K, Jouini A, Ben Sallem R, Somalo S, Sáenz Y, Estepa V, Boudabous A, Torres C. (2010). Prevalence of broad-spectrum cephalosporin-resistant Escherichia coli isolates in food samples in Tunisia, and characterization of integrons and antimicrobial resistance mechanisms implicated. Int J Food Microbiol, 137, 281–286.
- Billard-Pomares T, Tenaillon O, Le Nagard H, Rouy Z, Cruveiller S, Médigue C, Arlet G, Denamur E, Branger C. (2011). Complete nucleotide sequence of plasmid pTN48, encoding the CTX-M-14 extended-spectrum ß-lactamase from an Escherichia coli O102-ST405 strain. Antimicrob Agents Chemother, 55, 1270–1273.
- Bonnet R, Dutour C, Sampaio JL, Chanal C, Sirot D, Labia R, De Champs C, Sirot J. (2001). Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240–>Gly. Antimicrob Agents Chemother, 45, 2269–2275.
- Bonnet R, Recule C, Baraduc R, Chanal C, Sirot D, De Champs C, Sirot J. (2003). Effect of D240G substitution in a novel ESBL CTX-M-27. J Antimicrob Chemother, 52, 29–35.
- Bonnet R, Sampaio JL, Labia R, De Champs C, Sirot D, Chanal C, Sirot J. (2000). A novel CTX-M beta-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob Agents Chemother, 44, 1936–1942.
- Bonnet R. (2004). Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother, 48, 1–14.
- Bouallègue-Godet O, Ben Salem Y, Fabre L, Demartin M, Grimont PA, Mzoughi R, Weill FX. (2005). Nosocomial outbreak caused by Salmonella enterica serotype Livingstone producing CTX-M-27 extended-spectrum beta-lactamase in a neonatal unit in Sousse, Tunisia. J Clin Microbiol, 43, 1037–1044.
- Bradford PA, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. (1998). CTX-M-5, a novel cefotaxime-hydrolyzing beta-lactamase from an outbreak of Salmonella Typhimurium in Latvia. Antimicrob Agents Chemother, 42, 1980–1984.
- Bradford PA. (2001). Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev, 14, 933–51, table of contents.
- Brasme L, Nordmann P, Fidel F, Lartigue MF, Bajolet O, Poirel L, Forte D, Vernet-Garnier V, Madoux J, Reveil JC, Alba-Sauviat C, Baudinat I, Bineau P, Bouquigny-Saison C, Eloy C, Lafaurie C, Siméon D, Verquin JP, Noël F, Strady C, De Champs C. (2007). Incidence of class A extended-spectrum beta-lactamases in Champagne-Ardenne (France): a 1 year prospective study. J Antimicrob Chemother, 60, 956–964.
- Brenwald NP, Jevons G, Andrews JM, Xiong JH, Hawkey PM, Wise R. (2003). An outbreak of a CTX-M-type beta-lactamase-producing Klebsiella pneumoniae: the importance of using cefpodoxime to detect extended-spectrum beta-lactamases. J Antimicrob Chemother, 51, 195–196.
- Brigante G, Luzzaro F, Perilli M, Lombardi G, Colì A, Rossolini GM, Amicosante G, Toniolo A. (2005). Evolution of CTX-M-type beta-lactamases in isolates of Escherichia coli infecting hospital and community patients. Int J Antimicrob Agents, 25, 157–162.
- Cantón R, Coque TM. (2006). The CTX-M beta-lactamase pandemic. Curr Opin Microbiol, 9, 466–475.
- Cantón R, Oliver A, Coque TM, Varela Mdel C, Pérez-Díaz JC, Baquero F. (2002). Epidemiology of extended-spectrum beta-lactamase-producing Enterobacter isolates in a Spanish hospital during a 12-year period. J Clin Microbiol, 40, 1237–1243.
- Cao V, Lambert T, Courvalin P. (2002). ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum beta-lactamase CTX-M-17. Antimicrob Agents Chemother, 46, 1212–1217.
- Cao X, Cavaco LM, Lv Y, Li Y, Zheng B, Wang P, Hasman H, Liu Y, Aarestrup FM. (2011). Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J Clin Microbiol, 49, 2496–2501.
- Carattoli A. (2009). Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother, 53, 2227–2238.
- Cartelle M, Canle D, Llarena FJ, Molina F, Villanueva R, Bou G. (2006). Characterisation of the first CTX-M-10-producing isolate of Salmonella enterica serotype Virchow. Clin Microbiol Infect, 12, 285–287.
- Cartelle M, del Mar Tomas M, Molina F, Moure R, Villanueva R, Bou G. (2004). High-level resistance to ceftazidime conferred by a novel enzyme, CTX-M-32, derived from CTX-M-1 through a single Asp240-Gly substitution. Antimicrob Agents Chemother, 48, 2308–2313.
- Celenza G, Pellegrini C, Caccamo M, Segatore B, Amicosante G, Perilli M. (2006). Spread of blaCTX-M-type and blaPER-2 beta-lactamase genes in clinical isolates from Bolivian hospitals. J Antimicrob Chemother, 57, 975–978.
- Chanawong A, M’Zali FH, Heritage J, Xiong JH, Hawkey PM. (2002). Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People’s Republic of China. Antimicrob Agents Chemother, 46, 630–637.
- Cheng J, Gao W, Yin J, Sun Z, Ye Y, Gao YF, Li X, Li JB. (2010). Phenotypic and molecular characterization of two novel CTX-M enzymes carried by Klebsiella pneumoniae. Mol Biol Rep, 37, 1261–1267.
- Cheng J, Wang Q, Chen Y, Ye Y, Li H, Li X, Li JB. (2009). Phenotypic and molecular characterization of a novel beta-lactamase carried by Klebsiella pneumoniae, CTX-M-72, derived from CTX-M-3. J Gen Appl Microbiol, 55, 207–216.
- Cheng J, Ye Y, Wang YY, Li H, Li X, Li JB. (2008). Phenotypic and molecular characterization of 5 novel CTX-M enzymes carried by Klebsiella pneumoniae and Escherichia coli. Acta Pharmacol Sin, 29, 217–225.
- Chmelnitsky I, Carmeli Y, Leavitt A, Schwaber MJ, Navon-Venezia S. (2005). CTX-M-2 and a new CTX-M-39 enzyme are the major extended-spectrum beta-lactamases in multiple Escherichia coli clones isolated in Tel Aviv, Israel. Antimicrob Agents Chemother, 49, 4745–4750.
- Choi SH, Lee JE, Park SJ, Kim MN, Choo EJ, Kwak YG, Jeong JY, Woo JH, Kim NJ, Kim YS. (2007). Prevalence, microbiology, and clinical characteristics of extended-spectrum beta-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea. Eur J Clin Microbiol Infect Dis, 26, 557–561.
- Cloeckaert A, Praud K, Lefevre M, Doublet B, Pardos M, Granier SA, Brisabois A, Weill FX. (2010). IncI1 plasmid carrying extended-spectrum-beta-lactamase gene blaCTX-M-1 in Salmonella enterica isolates from poultry and humans in France, 2003 to 2008. Antimicrob Agents Chemother, 54, 4484–4486.
- Coelho A, González-López JJ, Miró E, Alonso-Tarrés C, Mirelis B, Larrosa MN, Bartolomé RM, Andreu A, Navarro F, Johnson JR, Prats G. (2010). Characterisation of the CTX-M-15-encoding gene in Klebsiella pneumoniae strains from the Barcelona metropolitan area: plasmid diversity and chromosomal integration. Int J Antimicrob Agents, 36, 73–78.
- Coque TM, Novais A, Carattoli A, Poirel L, Pitout J, Peixe L, Baquero F, Cantón R, Nordmann P. (2008). Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerging Infect Dis, 14, 195–200.
- Coque TM, Oliver A, Pérez-Díaz JC, Baquero F, Cantón R. (2002). Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum beta-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob Agents Chemother, 46, 500–510.
- Couturier M, Bex F, Bergquist PL, Maas WK. (1988). Identification and classification of bacterial plasmids. Microbiol Rev, 52, 375–395.
- Cui S, Li J, Sun Z, Hu C, Jin S, Li F, Guo Y, Ran L, Ma Y. (2009). Characterization of Salmonella enterica isolates from infants and toddlers in Wuhan, China. J Antimicrob Chemother, 63, 87–94.
- Cullik A, Pfeifer Y, Prager R, von Baum H, Witte W. (2010). A novel IS26 structure surrounds blaCTX-M genes in different plasmids from German clinical Escherichia coli isolates. J Med Microbiol, 59, 580–587.
- Damjanova I, Tóth A, Pászti J, Hajbel-Vékony G, Jakab M, Berta J, Milch H, Füzi M. (2008). Expansion and countrywide dissemination of ST11, ST15 and ST147 ciprofloxacin-resistant CTX-M-15-type beta-lactamase-producing Klebsiella pneumoniae epidemic clones in Hungary in 2005–the new ‘MRSAs’? J Antimicrob Chemother, 62, 978–985.
- De Champs C, Sirot D, Chanal C, Bonnet R, Sirot J. (2000). A 1998 survey of extended-spectrum beta-lactamases in Enterobacteriaceae in France. The French Study Group. Antimicrob Agents Chemother, 44, 3177–3179.
- Decousser JW, Poirel L, Nordmann P. (2001). Characterization of a chromosomally encoded extended-spectrum class A beta-lactamase from Kluyvera cryocrescens. Antimicrob Agents Chemother, 45, 3595–3598.
- Dhanji H, Doumith M, Rooney PJ, O’Leary MC, Loughrey AC, Hope R, Woodford N, Livermore DM. (2011a). Molecular epidemiology of fluoroquinolone-resistant ST131 Escherichia coli producing CTX-M extended-spectrum beta-lactamases in nursing homes in Belfast, UK. J Antimicrob Chemother, 66, 297–303.
- Dhanji H, Patel R, Wall R, Doumith M, Patel B, Hope R, Livermore DM, Woodford N. (2011b). Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J Antimicrob Chemother, 66, 1005–1012.
- Di Conza J, Ayala JA, Power P, Mollerach M, Gutkind G. (2002). Novel class 1 integron (InS21) carrying blaCTX-M-2 in Salmonella enterica serovar Infantis. Antimicrob Agents Chemother, 46, 2257–2261.
- Diestra K, Juan C, Curiao T, Moyá B, Miró E, Oteo J, Coque TM, Pérez-Vázquez M, Campos J, Cantón R, Oliver A, Navarro F; Red Española de Investigación en Patología Infecciosa (REIPI), Spain. (2009). Characterization of plasmids encoding blaESBL and surrounding genes in Spanish clinical isolates of Escherichia coli and Klebsiella pneumoniae. J Antimicrob Chemother, 63, 60–66.
- Djamdjian L, Naas T, Tandé D, Cuzon G, Hanrotel-Saliou C, Nordmann P. (2011). CTX-M-93, a CTX-M variant lacking penicillin hydrolytic activity. Antimicrob Agents Chemother, 55, 1861–1866.
- Doi Y, Adams-Haduch JM, Paterson DL. (2008). Escherichia coli isolate coproducing 16S rRNA Methylase and CTX-M-type extended-spectrum beta-lactamase isolated from an outpatient in the United States. Antimicrob Agents Chemother, 52, 1204–1205.
- Dolejska M, Jurcickova Z, Literak I, Pokludova L, Bures J, Hera A, Kohoutova L, Smola J, Cizek A. (2011). IncN plasmids carrying blaCTX-M-1 in Escherichia coli isolates on a dairy farm. Vet Microbiol, 149, 513–516.
- Doublet B, Granier SA, Robin F, Bonnet R, Fabre L, Brisabois A, Cloeckaert A, Weill FX. (2009). Novel plasmid-encoded ceftazidime-hydrolyzing CTX-M-53 extended-spectrum beta-lactamase from Salmonella enterica serotypes Westhampton and Senftenberg. Antimicrob Agents Chemother, 53, 1944–1951.
- Eckert C, Gautier V, Arlet G. (2006). DNA sequence analysis of the genetic environment of various blaCTX-M genes. J Antimicrob Chemother, 57, 14–23.
- Fabre L, Delauné A, Espié E, Nygard K, Pardos M, Polomack L, Guesnier F, Galimand M, Lassen J, Weill FX. (2009). Chromosomal integration of the extended-spectrum beta-lactamase gene blaCTX-M-15 in Salmonella enterica serotype Concord isolates from internationally adopted children. Antimicrob Agents Chemother, 53, 1808–1816.
- Fernández A, Gil E, Cartelle M, Pérez A, Beceiro A, Mallo S, Tomás MM, Pérez-Llarena FJ, Villanueva R, Bou G. (2007). Interspecies spread of CTX-M-32 extended-spectrum beta-lactamase and the role of the insertion sequence IS1 in down-regulating blaCTX-M gene expression. J Antimicrob Chemother, 59, 841–847.
- Galani I, Souli M, Chryssouli Z, Giamarellou H. (2007). Detection of CTX-M-15 and CTX-M-33, a novel variant of CTX-M-15, in clinical Escherichia coli isolates in Greece. Int J Antimicrob Agents, 29, 598–600.
- Galimand M, Sabtcheva S, Courvalin P, Lambert T. (2005). Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob Agents Chemother, 49, 2949–2953.
- García A, Navarro F, Miró E, Mirelis B, Campoy S, Coll P. (2005). Characterization of the highly variable region surrounding the blaCTX-M-9 gene in non-related Escherichia coli from Barcelona. J Antimicrob Chemother, 56, 819–826.
- García Fernández A, Cloeckaert A, Bertini A, Praud K, Doublet B, Weill FX, Carattoli A. (2007). Comparative analysis of IncHI2 plasmids carrying blaCTX-M-2 or blaCTX-M-9 from Escherichia coli and Salmonella enterica strains isolated from poultry and humans. Antimicrob Agents Chemother, 51, 4177–4180.
- Gazouli M, Tzelepi E, Markogiannakis A, Legakis NJ, Tzouvelekis LS. (1998a). Two novel plasmid-mediated cefotaxime-hydrolyzing beta-lactamases (CTX-M-5 and CTX-M-6) from Salmonella Typhimurium. FEMS Microbiol Lett, 165, 289–293.
- Gazouli M, Tzelepi E, Sidorenko SV, Tzouvelekis LS. (1998b). Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A beta-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother, 42, 1259–1262.
- Gierczynski R, Szych J, Cieslik A, Rastawicki W, Jagielski M. (2003). The occurrence of the first two CTX-M-3 and TEM-1 producing isolates of Salmonella enterica serovar Oranienburg in Poland. Int J Antimicrob Agents, 21, 497–499.
- Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. (1998). Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing beta-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother, 42, 827–832.
- Gonullu N, Aktas Z, Kayacan CB, Salcioglu M, Carattoli A, Yong DE, Walsh TR. (2008). Dissemination of CTX-M-15 beta-lactamase genes carried on Inc FI and FII plasmids among clinical isolates of Escherichia coli in a university hospital in Istanbul, Turkey. J Clin Microbiol, 46, 1110–1112.
- Govinden U, Mocktar C, Moodley P, Sturm AW, Essack SY. (2006). CTX-M-37 in Salmonella enterica serotype Isangi from Durban, South Africa. Int J Antimicrob Agents, 28, 288–291.
- Gómez-Garcés JL, Saéz D, Almagro M, Fernández-Romero S, Merino F, Campos J, Oteo J. (2011). Osteomyelitis associated to CTX-M-15-producing Aeromonas hydrophila: first description in the literature. Diagn Microbiol Infect Dis, 70, 420–422.
- Harada S, Ishii Y, Saga T, Kouyama Y, Tateda K, Yamaguchi K. (2012). Chromosomal integration and location on IncT plasmids of the blaCTX-M-2 gene in Proteus mirabilis clinical isolates. Antimicrob Agents Chemother, 56, 1093–1096.
- Hasman H, Mevius D, Veldman K, Olesen I, Aarestrup FM. (2005). beta-Lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J Antimicrob Chemother, 56, 115–121.
- Heffernan HM, Woodhouse RE, Pope CE, Blackmore TK. (2009). Prevalence and types of extended-spectrum beta-lactamases among urinary Escherichia coli and Klebsiella spp. in New Zealand. Int J Antimicrob Agents, 34, 544–549.
- Ho PL, Ho AY, Chow KH, Wong RC, Duan RS, Ho WL, Mak GC, Tsang KW, Yam WC, Yuen KY. (2005a). Occurrence and molecular analysis of extended-spectrum beta-lactamase-producing Proteus mirabilis in Hong Kong, 1999-2002. J Antimicrob Chemother, 55, 840–845.
- Ho PL, Lo WU, Wong RC, Yeung MK, Chow KH, Que TL, Tong AH, Bao JY, Lok S, Wong SS. (2011). Complete sequencing of the FII plasmid pHK01, encoding CTX-M-14, and molecular analysis of its variants among Escherichia coli from Hong Kong. J Antimicrob Chemother, 66, 752–756.
- Ho PL, Shek RH, Chow KH, Duan RS, Mak GC, Lai EL, Yam WC, Tsang KW, Lai WM. (2005b). Detection and characterization of extended-spectrum beta-lactamases among bloodstream isolates of Enterobacter spp. in Hong Kong, 2000-2002. J Antimicrob Chemother, 55, 326–332.
- Hopkins KL, Deheer-Graham A, Threlfall EJ, Batchelor MJ, Liebana E. (2006). Novel plasmid-mediated CTX-M-8 subgroup extended-spectrum beta-lactamase (CTX-M-40) isolated in the UK. Int J Antimicrob Agents, 27, 572–575.
- Hopkins KL, Threlfall EJ, Karisik E, Wardle JK. (2008). Identification of novel plasmid-mediated extended-spectrum beta-lactamase CTX-M-57 in Salmonella enterica serovar Typhimurium. Int J Antimicrob Agents, 31, 85–86.
- Hrabák J, Empel J, Gniadkowski M, Halbhuber Z, Rébl K, Urbásková P. (2008). CTX-M-15-producing Shigella sonnei strain from a Czech patient who traveled in Asia. J Clin Microbiol, 46, 2147–2148.
- Humeniuk C, Arlet G, Gautier V, Grimont P, Labia R, Philippon A. (2002). Beta-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob Agents Chemother, 46, 3045–3049.
- Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. (1995). Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother, 39, 2269–2275.
- Izumiya H, Mori K, Higashide M, Tamura K, Takai N, Hirose K, Terajima J, Watanabe H. (2005). Identification of CTX-M-14 beta-lactamase in a Salmonella enterica serovar Enteritidis isolate from Japan. Antimicrob Agents Chemother, 49, 2568–2570.
- Jacoby G, Bush K. (2012). β-Lactamase classification and amino acid sequences for TEM, SHV and OXA extended-spectrum and inhibitor resistant enzymes. http://www.lahey.org/Studies/. Accessed on 1 March, 2012.
- Kanamori H, Yano H, Hirakata Y, Endo S, Arai K, Ogawa M, Shimojima M, Aoyagi T, Hatta M, Yamada M, Nishimaki K, Kitagawa M, Kunishima H, Kaku M. (2011). High prevalence of extended-spectrum ß-lactamases and qnr determinants in Citrobacter species from Japan: dissemination of CTX-M-2. J Antimicrob Chemother, 66, 2255–2262.
- Karim A, Poirel L, Nagarajan S, Nordmann P. (2001). Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol Lett, 201, 237–241.
- Kariuki S, Corkill JE, Revathi G, Musoke R, Hart CA. (2001). Molecular characterization of a novel plasmid-encoded cefotaximase (CTX-M-12) found in clinical Klebsiella pneumoniae isolates from Kenya. Antimicrob Agents Chemother, 45, 2141–2143.
- Kim J, Bae IK, Jeong SH, Chang CL, Lee CH, Lee K. (2011). Characterization of IncF plasmids carrying the blaCTX-M-14 gene in clinical isolates of Escherichia coli from Korea. J Antimicrob Chemother, 66, 1263–1268.
- Kim J, Kang HY, Lee Y. (2008). The identification of CTX-M-14, TEM-52, and CMY-1 enzymes in Escherichia coli isolated from the Han River in Korea. J Microbiol, 46, 478–481.
- Kim J, Lim YM, Jeong YS, Seol SY. (2005). Occurrence of CTX-M-3, CTX-M-15, CTX-M-14, and CTX-M-9 extended-spectrum beta-lactamases in Enterobacteriaceae clinical isolates in Korea. Antimicrob Agents Chemother, 49, 1572–1575.
- Kiratisin P, Apisarnthanarak A, Saifon P, Laesripa C, Kitphati R, Mundy LM. (2007). The emergence of a novel ceftazidime-resistant CTX-M extended-spectrum beta-lactamase, CTX-M-55, in both community-onset and hospital-acquired infections in Thailand. Diagn Microbiol Infect Dis, 58, 349–355.
- Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B. (1985). Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother, 28, 302–307.
- Komatsu M, Ikeda N, Aihara M, Nakamachi Y, Kinoshita S, Yamasaki K, Shimakawa K. (2001). Hospital outbreak of MEN-1-derived extended spectrum beta-lactamase-producing Klebsiella pneumoniae. J Infect Chemother, 7, 94–101.
- Lartigue MF, Poirel L, Aubert D, Nordmann P. (2006). In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring beta-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob Agents Chemother, 50, 1282–1286.
- Lartigue MF, Poirel L, Heritier C, Tolun V, Nordmann P. (2003). First description of CTX-M-15-producing Klebsiella pneumoniae in Turkey. J Antimicrob Chemother, 52, 315–316.
- Lartigue MF, Poirel L, Nordmann P. (2004). Diversity of genetic environment of blaCTX-M genes. FEMS Microbiol Lett, 234, 201–207.
- Lee MY, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. (2011). High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. Int J Antimicrob Agents, 38, 160–163.
- Liu G, Ling BD, Xie YE, Lin L, Zeng Y, Zhang X, Lei J. (2007). Characterization of CTX-M-22 and TEM-141 encoded by a single plasmid from a clinical isolate of Enterobacter cloacae in China. Jpn J Infect Dis, 60, 295–297.
- Liu W, Chen L, Li H, Duan H, Zhang Y, Liang X, Li X, Zou M, Xu L, Hawkey PM. (2009). Novel CTX-M beta-lactamase genotype distribution and spread into multiple species of Enterobacteriaceae in Changsha, Southern China. J Antimicrob Chemother, 63, 895–900.
- Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, Ayala J, Coque TM, Kern-Zdanowicz I, Luzzaro F, Poirel L, Woodford N. (2007). CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother, 59, 165–174.
- Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. (1998). Cloning and sequencing of the gene encoding Toho-2, a class A beta-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother, 42, 1181–1186.
- Mahillon J, Chandler M. (1998). Insertion sequences. Microbiol Mol Biol Rev, 62, 725–774.
- Marcadé G, Deschamps C, Boyd A, Gautier V, Picard B, Branger C, Denamur E, Arlet G. (2009). Replicon typing of plasmids in Escherichia coli producing extended-spectrum beta-lactamases. J Antimicrob Chemother, 63, 67–71.
- Mazel D. (2006). Integrons: agents of bacterial evolution. Nat Rev Microbiol, 4, 608–620.
- McGettigan SE, Hu B, Andreacchio K, Nachamkin I, Edelstein PH. (2009). Prevalence of CTX-M beta-lactamases in Philadelphia, Pennsylvania. J Clin Microbiol, 47, 2970–2974.
- Mendonça N, Ferreira E, Louro D, Caniça M; ARSIP Participants. (2009). Molecular epidemiology and antimicrobial susceptibility of extended- and broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolated in Portugal. Int J Antimicrob Agents, 34, 29–37.
- Minarini LA, Poirel L, Trevisani NA, Darini AL, Nordmann P. (2009). Predominance of CTX-M-type extended-spectrum beta-lactamase genes among enterobacterial isolates from outpatients in Brazil. Diagn Microbiol Infect Dis, 65, 202–206.
- Miró E, Mirelis B, Navarro F, Rivera A, Mesa RJ, Roig MC, Gómez L, Coll P. (2005). Surveillance of extended-spectrum beta-lactamases from clinical samples and faecal carriers in Barcelona, Spain. J Antimicrob Chemother, 56, 1152–1155.
- Moubareck C, Daoud Z, Hakimé NI, Hamzé M, Mangeney N, Matta H, Mokhbat JE, Rohban R, Sarkis DK, Doucet-Populaire F. (2005). Countrywide spread of community- and hospital-acquired extended-spectrum beta-lactamase (CTX-M-15)-producing Enterobacteriaceae in Lebanon. J Clin Microbiol, 43, 3309–3313.
- Mshana SE, Imirzalioglu C, Hossain H, Hain T, Domann E, Chakraborty T. (2009). Conjugative IncFI plasmids carrying CTX-M-15 among Escherichia coli ESBL producing isolates at a University hospital in Germany. BMC Infect Dis, 9, 97.
- Munday CJ, Boyd DA, Brenwald N, Miller M, Andrews JM, Wise R, Mulvey MR, Hawkey PM. (2004). Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26. Antimicrob Agents Chemother, 48, 4829–4834.
- Naas T, Bentchouala C, Cuzon G, Yaou S, Lezzar A, Smati F, Nordmann P. (2011). Outbreak of Salmonella enterica serotype Infantis producing ArmA 16S RNA methylase and CTX-M-15 extended-spectrum ß-lactamase in a neonatology ward in Constantine, Algeria. Int J Antimicrob Agents, 38, 135–139.
- Nagano N, Nagano Y, Cordevant C, Shibata N, Arakawa Y. (2004). Nosocomial transmission of CTX-M-2 beta-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J Clin Microbiol, 42, 3978–3984.
- Nagano Y, Nagano N, Wachino J, Ishikawa K, Arakawa Y. (2009). Novel chimeric beta-lactamase CTX-M-64, a hybrid of CTX-M-15-like and CTX-M-14 beta-lactamases, found in a Shigella sonnei strain resistant to various oxyimino-cephalosporins, including ceftazidime. Antimicrob Agents Chemother, 53, 69–74.
- Naseer U, Sundsfjord A. (2011). The CTX-M conundrum: dissemination of plasmids and Escherichia coli clones. Microb Drug Resist, 17, 83–97.
- Navon-Venezia S, Chmelnitsky I, Leavitt A, Carmeli Y. (2008). Dissemination of the CTX-M-25 family beta-lactamases among Klebsiella pneumoniae, Escherichia coli and Enterobacter cloacae and identification of the novel enzyme CTX-M-41 in Proteus mirabilis in Israel. J Antimicrob Chemother, 62, 289–295.
- Novais A, Cantón R, Valverde A, Machado E, Galán JC, Peixe L, Carattoli A, Baquero F, Coque TM. (2006). Dissemination and persistence of blaCTX-M-9 are linked to class 1 integrons containing CR1 associated with defective transposon derivatives from Tn402 located in early antibiotic resistance plasmids of IncHI2, IncP1-alpha, and IncFI groups. Antimicrob Agents Chemother, 50, 2741–2750.
- Novick RP, Clowes RC, Cohen SN, Curtiss R 3rd, Datta N, Falkow S. (1976). Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev, 40, 168–189.
- Oka A, Sugisaki H, Takanami M. (1981). Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol, 147, 217–226.
- Oliver A, Coque TM, Alonso D, Valverde A, Baquero F, Cantón R. (2005). CTX-M-10 linked to a phage-related element is widely disseminated among Enterobacteriaceae in a Spanish hospital. Antimicrob Agents Chemother, 49, 1567–1571.
- Oliver A, Pérez-Díaz JC, Coque TM, Baquero F, Cantón R. (2001). Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-10) isolated in Spain. Antimicrob Agents Chemother, 45, 616–620.
- Olson AB, Silverman M, Boyd DA, McGeer A, Willey BM, Pong-Porter V, Daneman N, Mulvey MR. (2005). Identification of a progenitor of the CTX-M-9 group of extended-spectrum beta-lactamases from Kluyvera georgiana isolated in Guyana. Antimicrob Agents Chemother, 49, 2112–2115.
- Oteo J, Delgado-Iribarren A, Vega D, Bautista V, Rodríguez MC, Velasco M, Saavedra JM, Pérez-Vázquez M, García-Cobos S, Martínez-Martínez L, Campos J. (2008). Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int J Antimicrob Agents, 32, 534–537.
- Pai H, Choi EH, Lee HJ, Hong JY, Jacoby GA. (2001). Identification of CTX-M-14 extended-spectrum beta-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol, 39, 3747–3749.
- Pallecchi L, Bartoloni A, Fiorelli C, Mantella A, Di Maggio T, Gamboa H, Gotuzzo E, Kronvall G, Paradisi F, Rossolini GM. (2007). Rapid dissemination and diversity of CTX-M extended-spectrum beta-lactamase genes in commensal Escherichia coli isolates from healthy children from low-resource settings in Latin America. Antimicrob Agents Chemother, 51, 2720–2725.
- Paterson DL, Bonomo RA. (2005). Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev, 18, 657–686.
- Peirano G, Richardson D, Nigrin J, McGeer A, Loo V, Toye B, Alfa M, Pienaar C, Kibsey P, Pitout JD. (2010). High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-beta-lactamase-producing Escherichia coli isolates from Canada. Antimicrob Agents Chemother, 54, 1327–1330.
- Petrella S, Ziental-Gelus N, Mayer C, Renard M, Jarlier V, Sougakoff W. (2008). Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A beta-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob Agents Chemother, 52, 3725–3736.
- Philippon A, Labia R, Jacoby G. (1989). Extended-spectrum beta-lactamases. Antimicrob Agents Chemother, 33, 1131–1136.
- Poirel L, Decousser JW, Nordmann P. (2003). Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M beta-lactamase gene. Antimicrob Agents Chemother, 47, 2938–2945.
- Poirel L, Gniadkowski M, Nordmann P. (2002a). Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J Antimicrob Chemother, 50, 1031–1034.
- Poirel L, Kämpfer P, Nordmann P. (2002b). Chromosome-encoded Ambler class A beta-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum beta-lactamases. Antimicrob Agents Chemother, 46, 4038–4040.
- Poirel L, Lartigue MF, Decousser JW, Nordmann P. (2005). ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob Agents Chemother, 49, 447–450.
- Poirel L, Naas T, Le Thomas I, Karim A, Bingen E, Nordmann P. (2001). CTX-M-type extended-spectrum beta-lactamase that hydrolyzes ceftazidime through a single amino acid substitution in the omega loop. Antimicrob Agents Chemother, 45, 3355–3361.
- Pornruangwong S, Hendriksen RS, Pulsrikarn C, Bangstrakulnonth A, Mikoleit M, Davies RH, Aarestrup FM, Garcia-Migura L. (2011). Epidemiological investigation of Salmonella enterica serovar Kedougou in Thailand. Foodborne Pathog Dis, 8, 203–211.
- Potz NA, Hope R, Warner M, Johnson AP, Livermore DM; London & South East ESBL Project Group. (2006). Prevalence and mechanisms of cephalosporin resistance in Enterobacteriaceae in London and South-East England. J Antimicrob Chemother, 58, 320–326.
- Power P, Galleni M, Di Conza J, Ayala JA, Gutkind G. (2005). Description of In116, the first blaCTX-M-2-containing complex class 1 integron found in Morganella morganii isolates from Buenos Aires, Argentina. J Antimicrob Chemother, 55, 461–465.
- Qi C, Pilla V, Yu JH, Reed K. (2010). Changing prevalence of Escherichia coli with CTX-M-type extended-spectrum beta-lactamases in outpatient urinary E. coli between 2003 and 2008. Diagn Microbiol Infect Dis, 67, 87–91.
- Quinteros M, Radice M, Gardella N, Rodriguez MM, Costa N, Korbenfeld D, Couto E, Gutkind G; Microbiology Study Group. (2003). Extended-spectrum beta-lactamases in enterobacteriaceae in Buenos Aires, Argentina, public hospitals. Antimicrob Agents Chemother, 47, 2864–2867.
- Ranjbar R, Giammanco GM, Aleo A, Plano MR, Naghoni A, Owlia P, Mammina C. (2010). Characterization of the first extended-spectrum beta-lactamase-producing nontyphoidal Salmonella strains isolated in Tehran, Iran. Foodborne Pathog Dis, 7, 91–95.
- Rodriguez-Martinez JM, Poirel L, Canton R, Nordmann P. (2006). Common region CR1 for expression of antibiotic resistance genes. Antimicrob Agents Chemother, 50, 2544–2546.
- Rodríguez I, Barownick W, Helmuth R, Mendoza MC, Rodicio MR, Schroeter A, Guerra B. (2009). Extended-spectrum beta-lactamases and AmpC beta-lactamases in ceftiofur-resistant Salmonella enterica isolates from food and livestock obtained in Germany during 2003-07. J Antimicrob Chemother, 64, 301–309.
- Rodríguez MM, Power P, Radice M, Vay C, Famiglietti A, Galleni M, Ayala JA, Gutkind G. (2004). Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob Agents Chemother, 48, 4895–4897.
- Rodríguez MM, Power P, Sader H, Galleni M, Gutkind G. (2010). Novel chromosome-encoded CTX-M-78 beta-lactamase from a Kluyvera georgiana clinical isolate as a putative origin of CTX-M-25 subgroup. Antimicrob Agents Chemother, 54, 3070–3071.
- Romero L, López L, Martínez-Martínez L, Guerra B, Hernández JR, Pascual A. (2004). Characterization of the first CTX-M-14-producing Salmonella enterica serotype Enteritidis isolate. J Antimicrob Chemother, 53, 1113–1114.
- Sabaté M, Navarro F, Miró E, Campoy S, Mirelis B, Barbé J, Prats G. (2002). Novel complex sul1-type integron in Escherichia coli carrying blaCTX-M-9. Antimicrob Agents Chemother, 46, 2656–2661.
- Sabaté M, Tarragó R, Navarro F, Miró E, Vergés C, Barbé J, Prats G. (2000). Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-9) from Escherichia coli in Spain. Antimicrob Agents Chemother, 44, 1970–1973.
- Saladin M, Cao VT, Lambert T, Donay JL, Herrmann JL, Ould-Hocine Z, Verdet C, Delisle F, Philippon A, Arlet G. (2002). Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol Lett, 209, 161–168.
- Sarria JC, Vidal AM, Kimbrough RC 3rd. (2001). Infections caused by Kluyvera species in humans. Clin Infect Dis, 33, E69–E74.
- Schneider I, Queenan AM, Markovska R, Markova B, Keuleyan E, Bauernfeind A. (2009). New variant of CTX-M-type extended-spectrum beta-lactamases, CTX-M-71, with a Gly238Cys substitution in a Klebsiella pneumoniae isolate from Bulgaria. Antimicrob Agents Chemother, 53, 4518–4521.
- Seputiene V, Linkevicius M, Bogdaite A, Povilonis J, Planciuniene R, Giedraitiene A, Pavilonis A, Suziedeliene E. (2010). Molecular characterization of extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates from hospitals in Lithuania. J Med Microbiol, 59, 1263–1265.
- Shakil S, Khan AU. (2010). Detection of CTX-M-15-producing and carbapenem-resistant Acinetobacter baumannii strains from urine from an Indian hospital. J Chemother, 22, 324–327.
- Shibata N, Kurokawa H, Doi Y, Yagi T, Yamane K, Wachino J, Suzuki S, Kimura K, Ishikawa S, Kato H, Ozawa Y, Shibayama K, Kai K, Konda T, Arakawa Y. (2006). PCR classification of CTX-M-type beta-lactamase genes identified in clinically isolated gram-negative bacilli in Japan. Antimicrob Agents Chemother, 50, 791–795.
- Soler Bistué AJ, Martín FA, Petroni A, Faccone D, Galas M, Tolmasky ME, Zorreguieta A. (2006). Vibrio cholerae InV117, a class 1 integron harboring aac(6′)-Ib and blaCTX-M-2, is linked to transposition genes. Antimicrob Agents Chemother, 50, 1903–1907.
- Song W, Kim J, Bae IK, Jeong SH, Seo YH, Shin JH, Jang SJ, Uh Y, Shin JH, Lee MK, Lee K. (2011). Chromosome-encoded AmpC and CTX-M extended-spectrum ß-lactamases in clinical isolates of Proteus mirabilis from Korea. Antimicrob Agents Chemother, 55, 1414–1419.
- Stapleton PD. (1999). Novel insertion sequence, ISEcp1, mobilizes the plasmid-mediated class C β-lactamase-coding gene, blaCMY-4. In Program and Abstracts of the Thirty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 1999. Abstract 1457, p. 132. American Society for Microbiology, Washington, DC, USA.
- Stepanova MN, Pimkin M, Nikulin AA, Kozyreva VK, Agapova ED, Edelstein MV. (2008). Convergent in vivo and in vitro selection of ceftazidime resistance mutations at position 167 of CTX-M-3 beta-lactamase in hypermutable Escherichia coli strains. Antimicrob Agents Chemother, 52, 1297–1301.
- Stokes HW, Hall RM. (1989). A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol, 3, 1669–1683.
- Stokes HW, Tomaras C, Parsons Y, Hall RM. (1993). The partial 3′-conserved segment duplications in the integrons In6 from pSa and In7 from pDGO100 have a common origin. Plasmid, 30, 39–50.
- Stürenburg E, Kühn A, Mack D, Laufs R. (2004). A novel extended-spectrum beta-lactamase CTX-M-23 with a P167T substitution in the active-site omega loop associated with ceftazidime resistance. J Antimicrob Chemother, 54, 406–409.
- Su Z, Dai X, Chen J, Kong F, Wang H, Li Y, Peng S, Wang S, Shao Q, Lv L, Xu H. (2008). The blaCTX-M-1 gene located in a novel complex class I integron bearing an ISCR1 element in Escherichia coli isolates from Zhenjiang, China. J Antimicrob Chemother, 62, 1150–1151.
- Sun Y, Zeng Z, Chen S, Ma J, He L, Liu Y, Deng Y, Lei T, Zhao J, Liu JH. (2010). High prevalence of blaCTX-M extended-spectrum ß-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin Microbiol Infect, 16, 1475–1481.
- Tian GB, Adams-Haduch JM, Qureshi ZA, Wang HN, Doi Y. (2010). CTX-M-35 extended-spectrum beta-lactamase conferring ceftazidime resistance in Citrobacter koseri. Int J Antimicrob Agents, 35, 412–413.
- Tian SF, Chen BY, Chu YZ, Wang S. (2008). Prevalence of rectal carriage of extended-spectrum beta-lactamase-producing Escherichia coli among elderly people in community settings in China. Can J Microbiol, 54, 781–785.
- Toleman MA, Bennett PM, Walsh TR. (2006). ISCR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev, 70, 296–316.
- Toukdarian A. (2004). Plasmid strategies for broad-host-range replication in Gram-negative bacteria.. In: Funnell BPhillips G, ed. Plasmid Biology. Washington DC: ASM Press, 259–270.
- Valverde A, Cantón R, Galán JC, Nordmann P, Baquero F, Coque TM. (2006). In117, an unusual In0-like class 1 integron containing CR1 and blaCTX-M-2 and associated with a Tn21-like element. Antimicrob Agents Chemother, 50, 799–802.
- Valverde A, Cantón R, Garcillán-Barcia MP, Novais A, Galán JC, Alvarado A, de la Cruz F, Baquero F, Coque TM. (2009). Spread of blaCTX-M-14 is driven mainly by IncK plasmids disseminated among Escherichia coli phylogroups A, B1, and D in Spain. Antimicrob Agents Chemother, 53, 5204–5212.
- Valverde A, Coque TM, Sánchez-Moreno MP, Rollán A, Baquero F, Cantón R. (2004). Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol, 42, 4769–4775.
- Weill FX, Perrier-Gros-Claude JD, Demartin M, Coignard S, Grimont PA. (2004). Characterization of extended-spectrum-beta-lactamase (CTX-M-15)-producing strains of Salmonella enterica isolated in France and Senegal. FEMS Microbiol Lett, 238, 353–358.
- Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, Livermore DM. (2009). Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother, 53, 4472–4482.
- Wu JJ, Chen HM, Ko WC, Wu HM, Tsai SH, Yan JJ. (2008). Prevalence of extended-spectrum beta-lactamases in Proteus mirabilis in a Taiwanese university hospital, 1999 to 2005: identification of a novel CTX-M enzyme (CTX-M-66). Diagn Microbiol Infect Dis, 60, 169–175.
- Yan JJ, Ko WC, Tsai SH, Wu HM, Jin YT, Wu JJ. (2000). Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J Clin Microbiol, 38, 4320–4325.
- Ye Y, Xu XH, Li JB. (2010). Emergence of CTX-M-3, TEM-1 and a new plasmid-mediated MOX-4 AmpC in a multiresistant Aeromonas caviae isolate from a patient with pneumonia. J Med Microbiol, 59, 843–847.
- Yin J, Cheng J, Sun Z, Ye Y, Gao YF, Li JB, Zhang XJ. (2009). Characterization of two plasmid-encoded cefotaximases found in clinical Escherichia coli isolates: CTX-M-65 and a novel enzyme, CTX-M-87. J Med Microbiol, 58, 811–815.
- Yu Y, Ji S, Chen Y, Zhou W, Wei Z, Li L, Ma Y. (2007). Resistance of strains producing extended-spectrum beta-lactamases and genotype distribution in China. J Infect, 54, 53–57.
- Zhang W, Luo Y, Li J, Lin L, Ma Y, Hu C, Jin S, Ran L, Cui S. (2011). Wide dissemination of multidrug-resistant Shigella isolates in China. J Antimicrob Chemother, 66, 2527–2535.
- Zhang Y, Zhou H, Shen XQ, Shen P, Yu YS, Li LJ. (2008). Plasmid-borne armA methylase gene, together with blaCTX-M-15 and blaTEM-1, in a Klebsiella oxytoca isolate from China. J Med Microbiol, 57, 1273–1276.
- Zhao WH, Hu ZQ. (2010). Beta-lactamases identified in clinical isolates of Pseudomonas aeruginosa. Crit Rev Microbiol, 36, 245–258.
- Zhao WH, Hu ZQ. (2011a). IMP-type metallo-ß-lactamases in Gram-negative bacilli: distribution, phylogeny, and association with integrons. Crit Rev Microbiol, 37, 214–226.
- Zhao WH, Hu ZQ. (2011b). Epidemiology and genetics of VIM-type metallo-ß-lactamases in Gram-negative bacilli. Future Microbiol, 6, 317–333.
- Zhao WH, Hu ZQ. (2012). Acinetobacter: a potential reservoir and dispenser for ß-lactamases. Crit Rev Microbiol, 38, 30–51.
- Zong Z, Partridge SR, Iredell JR. (2010). ISEcp1-mediated transposition and homologous recombination can explain the context of blaCTX-M-62 linked to qnrB2. Antimicrob Agents Chemother, 54, 3039–3042.
- Zong Z, Partridge SR, Thomas L, Iredell JR. (2008). Dominance of blaCTX-M within an Australian extended-spectrum beta-lactamase gene pool. Antimicrob Agents Chemother, 52, 4198–4202.
- al Naiemi N, Bart A, de Jong MD, Vandenbroucke-Grauls CM, Rietra PJ, Debets-Ossenkopp YJ, Wever PC, Spanjaard L, Bos AJ, Duim B. (2006). Widely distributed and predominant CTX-M extended-spectrum beta-lactamases in Amsterdam, The Netherlands. J Clin Microbiol, 44, 3012–3014.
- de Oliveira Garcia D, Doi Y, Szabo D, Adams-Haduch JM, Vaz TM, Leite D, Padoveze MC, Freire MP, Silveira FP, Paterson DL. (2008). Multiclonal outbreak of Klebsiella pneumoniae producing extended-spectrum beta-lactamase CTX-M-2 and novel variant CTX-M-59 in a neonatal intensive care unit in Brazil. Antimicrob Agents Chemother, 52, 1790–1793.
