Abstract
Yarrowia lipolytica has been developed as a production host for a large variety of biotechnological applications. Efficacy and safety studies have demonstrated the safe use of Yarrowia-derived products containing significant proportions of Yarrowia biomass (as for DuPont’s eicosapentaenoic acid–rich oil) or with the yeast itself as the final product (as for British Petroleum’s single-cell protein product). The natural occurrence of the species in food, particularly cheese, other dairy products and meat, is a further argument supporting its safety. The species causes rare opportunistic infections in severely immunocompromised or otherwise seriously ill people with other underlying diseases or conditions. The infections can be treated effectively by the use of regular antifungal drugs, and in some cases even disappeared spontaneously. Based on our assessment, we conclude that Y. lipolytica is a “safe-to-use” organism.
Introduction
Yarrowia lipolytica is an oleaginous yeast that grows on a variety of hydrophobic substrates and can accumulate lipids intracellularly to ≥40% of its cell dry weight (Beopoulos et al., Citation2011). Yarrowia lipolytica has been in use, still is deployed in, or is considered for multiple industrial applications, for example (i) as a high-quality protein source for livestock feeding (Bamberg, Citation2000), (ii) as a biotechnological production host for organic acids (e.g. citric acid) or hydrophobic substances such as polyunsaturated fatty acids (PUFAs) or carotenoids (Bailey et al., Citation2006; Picataggio et al., Citation2007; Rywinska et al., Citation2012; Thevenieau et al., Citation2009), (iii) as a heterologous production host for pharmaceutical and industrial proteins and enzymes (De Pourcq et al., Citation2012a, Citationb; Madzak et al., Citation2004), (iv) for the mass production of biofuels (Beopoulos et al., Citation2009), as well as (v) for bioremediation purposes (Bankar et al., Citation2009).
Given the potentially broad use of Yarrowia, the ambition of this article is to summarize and assess the pertinent information on the safety of Y. lipolytica. As indicators of its safety, the history of safe use in feed/food and other industries, the safety studies performed in the past on Y. lipolytica itself or on Yarrowia-derived products, the reported cases of Y. lipolytica acting as an (opportunistic) pathogen, and an analysis of its genome sequence will be carefully reviewed.
Taxonomy, biodiversity, physiology and identification of Y. lipolytica and related species
Yarrowia lipolytica (Wickerham, Kurtzman & Herman) van der Walt & von Arx is the only known species in the teleomorph (i.e. sexual) genus Yarrowia van der Walt & von Arx and has its anamorph (i.e. asexual state) classified in the genus Candida Berkhout as Candida lipolytica (F.C. Harrison) Diddens & Lodder (Kurtzman & Fell, Citation1998; van der Walt & von Arx, Citation1980). This species can be found readily in nature, has significant industrial value and is important to the food and medical fields (Kurtzman et al., Citation2011).
The teleomorph was discovered by Wickerham et al. (Citation1970) and published as Endomycopsis lipolytica Wickerham, Kurtzman & Herman. A year later van der Walt & Scott (Citation1971) as well as von Arx (Citation1972) pointed out that the generic name Endomycopsis Dekker was invalid and was indeed an obligate synonym of Saccharomycopsis Schiönning. Most of the Endomycopsis species were transferred by van der Walt & Scott (Citation1971) to various other genera; however, Endomycopsis lipolytica was one of the species that was not transferred. Yarrow (Citation1972) investigated this species further, transferred E. lipolytica to the genus Saccharomycopsis, and reclassified this teleomorph as Saccharomycopsis lipolytica (Wickerham, Kurtzman & Herman) Yarrow.
Van der Walt & von Arx (Citation1980) made the observation that S. lipolytica appeared unique relative to other species of Saccharomycopsis. The shape and size of its ascospores differ, as mature ascospores are usually hemi-ellipsoidal, navicular when looked at from the side, with apical, cap-like appendages, and do not contain ledges. Other differences observed by them were the presence of coenzyme Q-9 (Yamada et al., Citation1976) whereas most of the members of the families Endomycetaceae and Saccharomycetaceae show the coenzyme Q-6, Q-7 or Q-8 systems; and the whole-cellular carbohydrate composition of S. lipolytica differs from the other species mentioned due to the presence of galactose. Due to these differences van der Walt & von Arx (Citation1980) decided to introduce a new genus, Yarrowia (van der Walt & von Arx), and reassigned S. lipolytica to this new genus as Yarrowia lipolytica that is also the type species of this genus. Van der Walt & von Arx (Citation1980) concluded that direct relatives of Y. lipolytica are not known. Therefore, this unique species had an isolated position in the Endomycetales at that time.
Based on phenotypic features, the species Candida deformans (Zach) Langeron & Guerra, introduced by Langeron & Guerra (Citation1938), was considered as a variety of Candida lipolytica by van Uden & Buckley (Citation1970), namely as C. lipolytica var. deformans. It could be distinguished from C. lipolytica var. lipolytica by its ability to assimilate β-glucosides. It was later found that β-glucoside-negative and -positive strains can mate readily and can be considered as conspecific; thus, strains of both varieties were included in C. deformans (Yarrow, Citation1972). On the basis of morphology and physiology, Yarrow (Citation1972) concluded that C. deformans was the imperfect, asexual state of Y. lipolytica, which was at that time called S. lipolytica. This was also confirmed by Meyer et al. (Citation1984) who found that the type strain of C. deformans formed ascospores in mixtures with fresh isolates of Y. lipolytica, concluding that C. deformans might be considered a synonym of Y. lipolytica. Therefore, in a later revision of the genus Candida, Kurtzman & Fell (Citation1998) listed C. deformans as a synonym of Y. lipolytica.
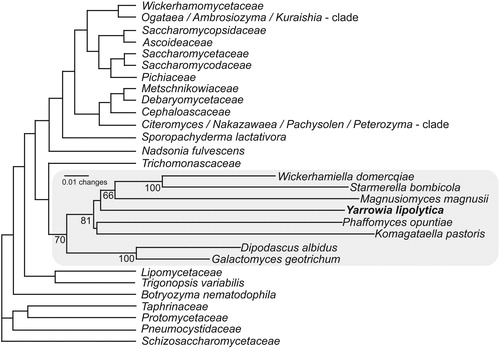
From the beginning of the 1990s, major efforts were undertaken to re-classify yeast species using phylogenetic approaches (Barns et al., Citation1991; Bigey et al., Citation2003; Knutsen et al., Citation2007; Kurtzman & Robnett, Citation1994, Citation1995, Citation1998; Suzuki et al., Citation1999). These studies provided significant insight in the phylogenetic placement of Y. lipolytica and the anamorphic species linked to this genus, as well as species boundaries within this group. Through comparisons of SSU rRNA (Barns et al., Citation1991; Suzuki et al., Citation1999) and partial 26S rRNA gene sequences (Kurtzman & Robnett, Citation1994, Citation1995, Citation1998), Y. lipolytica was found to be phylogenetically distantly related to most members of Candida and other known ascomycetous yeast genera (), with its nearest neighbor being Wickerhamiella domercqiae. It was at that time taxonomically assigned to the Hemiascomycetes, a class that contains very divergent groups of yeasts. It was shown that species within this group differ by several properties such as the relatively high G+C content of the nuclear DNA (Kurtzman & Fell, Citation1998) and the unique genomic organization of the rRNA genes (Fournier et al., Citation1986). Less than 90% sequence identity was found in the coding regions of the lipase genes YILIP2 and CdLIP1 of Y. lipolytica and C. deformans, respectively, the latter indicated by Yarrow (Citation1972) to be the asexual state of Y. lipolytica. The similarity drops to less than 70% in the untranslated regions, suggesting that C. deformans can be seen as a taxon separate from Y. lipolytica (Bigey et al., Citation2003). This was also confirmed by Knutsen et al. (Citation2007) who found significant sequence differences in the internal transcribed spacer (ITS) regions (ITS 1, ITS 2 and the intervening 5S rRNA gene) and the D1/D2 domain of the large-subunit rRNA gene of these two species. In the most recent authoritative taxonomic treatment, C. deformans is included as a species distinct from Y. lipolytica (Lachance et al., Citation2011).
Figure 1. Phylogenetic relationships among ascomycetous yeast genera and families based on neighbor-joining analysis of a concatenated dataset of gene sequences from LSU rRNA, SSU rRNA and translation elongation factor-1α showing the position of Yarrowia lipolytica. Adapted from Kurtzman et al. (Citation2011).
Knutsen et al. (Citation2007) conducted an elaborate study on Yarrowia and closely related Candida species using a vast number of techniques such as sequence analyses of the D1/D2 domain and the ITS region, PCR-mediated fingerprint analyses, DNA–DNA reassociation and mating experiments, physiology and morphology to study the Candida species close to Y. lipolytica and C. deformans. Intraspecific DNA–DNA reassociation values are very high (85–100%), except for Candia hispaniensis and Candida hollandica for which data were not available, while interspecific values (including Y. lipolytica) drop sharply (Knutsen et al., Citation2007), supporting clear delimitation of the species. Two species described earlier, Candida galli Péter, Dlauchy, Vasdinyei, Tornai-Lehoczki & Deák and Candida yakushimensis nom. inval. were treated as separate species. They also described three new species, Candida alimentaria Knutsen, V. Robert & M.Th. Smith, C. hollandica Knutsen, V. Robert & M.Th. Smith and Candida oslonensis Knutsen, V. Robert & M.Th. Smith in this group. Interspecific mating was detected among some of the isolates of Y. lipolytica, C. deformans and C. galli, but complementary conspecific mating types were not detected for any of the Candida species in this group, suggesting that separate taxa are present. Candida phangngensis Limtong, Yongmanitchai, Kawasaki & Seki was described a year later by Limtong et al. (Citation2008) based mainly on the variation found in the D1/D2 and SSU sequences of strains of this species and those of Y. lipolytica, C. deformans and C. galli.
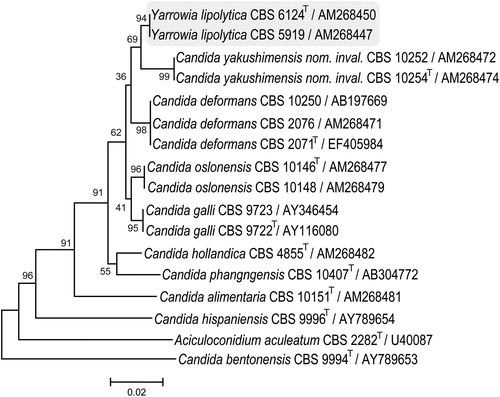
According to Kurtzman et al. (Citation2011), several Candida species are linked to the Yarrowia clade (; Kurtzman et al., Citation2011): C. deformans, C. galli, C. hollandica, C. oslonensis, C. alimentaria, C. phangngensis, C. yakushimensis (nom. inval.) and a species that is placed in a basal position in this Yarrowia clade, namely C. hispaniensis Kurtzman. Knutsen et al. (Citation2007) showed that all of the above-mentioned species (except C. phangngensis which was not included in the study) can be separated from one another based on the variation found in their D1/D2 and ITS sequences. Sequence variants of ITS within a given strain of Y. lipolytica were detected in four strains, as expected from the existence of dispersed rDNA clusters in this species (Knutsen et al., Citation2007). Fortunately, the phylogenetic signal from the different orthologues was found to be the same. The D1/D2 and ITS sequences of C. phangngensis are also unique for this species (GenBank, CBS database). In conclusion, the D1/D2 and ITS regions seem to be sufficient to separate the Yarrowia-related species from one another, taking into account the ITS orthologues that might be present.
Figure 2. Phylogenetic placement of Yarrowia lipolytica and related species determined from D1/D2 LSU rRNA gene sequences. Culture collection accession numbers and GenBank sequence numbers follow species names. T represents type strains.
The Amsterdam declaration on fungal nomenclature proposed to change the naming system of pleomorphic fungi as molecular data are now routinely available (Hawksworth et al., Citation2011). This has also been accepted in the International Code of Nomenclature for algae, fungi and plants. As from 2013, characterization of fungi will be mainly done according to their phylogenetic grouping. This will have a huge impact on fungal nomenclature and will also have an effect on the Yarrowia clade, where many of the Candida species will probably be given the genus name Yarrowia.
Multigene analyses of a limited number of yeast species placed Y. lipolytica at the base of the Saccharomycetales (Fitzpatrick et al., Citation2006; James et al., Citation2006), but a multigene analysis that included a greater number of species showed the Lipomycetaceae and C. caseinolytica to be basal to Y. lipolytica (Kurtzman et al., Citation2011). The latest phylogenetic placement of Yarrowia is in the class Saccharomycetes and the order Saccharomycetales (Kurtzman et al., Citation2011). Here Yarrowia is one of the more basal genera of the order and seems to be a sister group to Wickerhamiella van der Walt, Starmerella Rosa & Lachance and Magnusiomyces Zender (; Kurtzman et al., Citation2011). At present, the genus is not linked to a family and even the link to the Saccharomycetales is uncertain (Kurtzman et al., Citation2011) and needs to be investigated in further detail.
Traditionally yeasts have been identified using physiological growth characteristics, namely their ability to utilize a panel of carbon and nitrogen compounds, growth at different temperatures, etc. In the 5th edition of The Yeasts: a Taxonomic Study (Kurtzman et al., Citation2011), an identification key based on such characteristics is still included and, importantly, Y. lipolytica can be identified using such growth characteristics. In some cases, however, a distinction from the closely related C. deformans is not possible (Kurtzman et al., Citation2011). Sequence analyses of the ITS regions and the D1/D2 domains are reliable means for the identification of species of the Yarrowia clade (Knutsen et al., Citation2007). Recently, the ITS region was selected as the default fungal barcode (Schoch et al., Citation2012), but for yeasts, the use of both rDNA regions is recommended (Kurtzman et al., Citation2011).
A recent and promising development in microbial diagnostics is based on the analysis of the proteome by Matrix-Assisted Laser Desorption Ionization-Time Of Flight Mass Spectroscopy (MALDI-TOF MS) (Croxatto et al., Citation2012; Welker, Citation2011; Wieser et al., Citation2012). MALDI-TOF-assisted protein identification has revolutionized microbial diagnostics including that of fungal pathogens (Croxatto et al., Citation2012; Steensels et al., Citation2011). So far, the method has been used mainly for the diagnostics of human and animal pathogenic yeasts and turned out to be highly useful for the identification of clinically important yeast isolates (Dhiman et al., Citation2011; Marklein et al., Citation2009; Pinto et al., Citation2011; Van Herendael et al., Citation2012; van Veen et al., Citation2010; Welker, Citation2011). Yarrowia is not yet included in the commercially available databases, but the addition of spectra of Yarrowia can be easily realized. The Platelia Candida mannan antigen test used to diagnose invasive Candida infections did not yield a positive reaction and, therefore, cannot be used to detect Y. lipolytica in the serum of patients (Rimek et al., Citation2004).
From the data available on the CBS yeast database (www.cbs.knaw.nl/collections) and Kurtzman et al. (Citation2011), it is clear that Y. lipolytica has a broad substrate range and can be found in different geographical regions. The CBS Fungal Biodiversity Centre (www.cbs.knaw.nl) holds presently (April 15, 2012) 37 isolates of Y. lipolytica that are confirmed by bar coding of the ITS (Schoch et al., Citation2012) and D1/D2 ribosomal DNA regions. The geographical data of 22 of these strains are available and include countries from Europe [The Netherlands (n = 4), Italy (n = 3), Germany (n = 3), Norway (n = 3), UK (n = 1) and France (n = 1)], Russia (n = 2), USA (n = 3) and Argentina (n = 2). The majority of these isolates have been isolated from nonclinical substrates, such as soil (n = 7), rancid margarine or mayonnaise (n = 4), hydrocarbons (i.e. n-paraffin, kerosene or contaminated gas-oil ferment; n = 4), olives (n = 2), maize-processing plant (n = 2), apple juice (n = 1) and a probable dairy product (n = 1).
Physiological data are patchy and mainly limited to the growth tests carried out for strain identification, and often done under different conditions. Data are not available for C. yakushimensis (nom. inval.) which lacks a Latin description. Yarrowia lipolytica is generally considered being unable to grow above 37 °C and uses a limited number of sugars and polyols (i.e. glucose, mannitol, glucitol, glycerol and N-acetyl-glucosamine) as carbon source, in addition to lipids and alkanes (Barth & Gaillardin, Citation1997; Poncet & Arpin, Citation1965; CBS yeast database, www.cbs.knaw.nl/collections). However, there is some phenotypic variability among Y. lipolytica strains. Contrary to the type strain, some strains are able to grow at 37 °C or to use sugars such as galactose, sorbose, cellobiose, ribose or ribitol (Kurtzman, Citation2011). So far its inability to synthesize thiamine is reputedly a characteristic of the species, although this has not been checked systematically.
From a biotechnological point of view, different Y. lipolytica strains are known to display differences in their ability to grow on n-alkanes or in producing lipases or proteases (Barth & Gaillardin, Citation1997). Intra- and interspecific variability was also evidenced for citric acid production from glycerol in Y. lipolytica and C. hispaniensis (Levinson et al., Citation2007). A comprehensive update on the physiology of strains of the Yarrowia clade, including their ability to catabolize and/or store hydrophobic compounds, will be published soon (S. Michely, in preparation). According to the available literature, strains of the different species differ in some aspects from Y. lipolytica, but the extent of intraspecific variability is unknown. Major phenotypic differences reported concern the following: (i) hyphae formation is not detected in the basal species C. hispaniensis, C. alimentaria and C. oslonensis, (ii) C. alimentaria is the only species that is unable to grow at 30 °C, with 21 °C as its optimal growth temperature, (iii) assimilation of galactose is usually weak or absent in Y. lipolytica, but present in C. oslonensis and C. hollandica, while C. hispaniensis is the only species able to grow on trehalose, (iv) C. galli, C. phangngensis and C. hispaniensis are unable to use N-acetyl-glucosamine as a carbon source (v) C. hispaniensis is the only species able to grow neither on erythritol nor on citrate, (vi) C. galli is able to grow on vitamin-free medium according to Peter et al. (Citation2004), while other species do not, with Y. lipolytica requiring thiamine only, (vii) C. hollandica, C. oslonensis, C. galli and C. deformans are able to grow in the presence of 10% NaCl (Knutsen et al., Citation2007; Kurtzman, Citation2005; Limtong et al., Citation2008; Peter et al., Citation2004).
Genome sequence of Y. lipolytica and of other species in the Yarrowia clade
Wild-type isolates of Y. lipolytica are poorly interfertile suggesting a mostly clonal propagation and thus a possible high degree of genetic polymorphism (Barth & Gaillardin, Citation1997). However, there are actually not much data available in favor of or against this assumption. A few reports on the intraspecific genetic diversity of the species Y. lipolytica have been published. A high level of chromosomal polymorphism was detected among natural strains and segregants of fertile diploids (Casaregola et al., Citation1997; Naumova et al., Citation1993). Chromosome size variations may however mostly reflect differences in the number and size of the subtelomeric rDNA clusters, ranging from 1 to 6 (Casaregola et al., Citation1997; Clare et al., Citation1986), largely due to recombination between and within the subtelomeric rDNA repeats (Casaregola et al., Citation1997; Naumova et al., Citation1993). Linkage groups detected by hybridizing gene probes to chromosomes separated by pulsed field gel electrophoresis tend to be largely conserved among different isolates (Casaregola et al., Citation1997). This suggests that poor interfertility of separate lineages (Barth & Gaillardin, Citation1997; Ogrydziak, Citation1988) may not only result from chromosomal polymorphisms but also from nucleotide divergence, similar to observations made in the Saccharomyces paradoxus complex (Liti et al., Citation2009). Little data are currently available on the differences between geographically distinct populations. A clear difference between American and European isolates has been observed for the distribution of the Tyl6 gypsy retrotransposon, which seems to be of American origin (Kovalchuk et al., Citation2005). Insertion sites of the line retrotransposon Ylli are conserved in sympatric populations (S. Casaregola, unpublished data), also supporting the existence of a geographic organization of wild populations. A small number of nucleotide polymorphisms was observed within the D1/D2 and ITS sequences of rDNA of 39 strains of Y. lipolytica, and 12 representatives showed DNA–DNA reassociation values of 92–100% (Knutsen et al., Citation2007), thus indicating low overall sequence variation. When the XPR2 gene sequences of three different strains were compared, 20 differences occurred over 177 nt in the terminator region, but only 1 in the 1161 nt upstream intergenic region and none in the coding region (compare YALI0F:3951321.3948508 from the Génolevures database to GenBank entries M17741, M23353). A recently initiated whole-genome sequencing effort of six different Y. lipolytica isolates, including an isolate from human lung (CBS 5589), revealed a limited number of single nucleotide polymorphisms (SNPs) and insertions or deletions (indels). Preliminary analysis revealed an overall divergence to the reference strain E150 of about 0.25%. Further analysis will shed light on positions of SNPs and indels, gene content, copy numbers and transposable element polymorphisms as well as on population structure (C. Neuvéglise, to be published).
Genomic data at partial or full coverage have also been obtained recently for six of the species in the Yarrowia clade: C. hispaniensis, C. alimentaria, C. phangngensis, C. yakushimensis, C. galli and C. deformans (C. Neuvéglise, to be published). All species are haploid and carry a single copy of one idiomorph of the mating type locus identified in Y. lipolytica, suggesting that sexual states may exist in all species of the clade. They all share with Y. lipolytica some original features such as atypical intron structure (Neuveglise et al., Citation2011), dispersed 5S RNA (Fournier et al., Citation1986) or existence of tRNA-5S fusions (Acker et al., Citation2008). Compared to the Y. lipolytica genome (Dujon et al., Citation2004), major differences concern the following: (i) haploid genome size which varies between 10.6 Mb in C. hispaniensis and 22.8 Mb in C. galli (final assembly size), (ii) GC content is around 49–50% for all species except C. phangngensis (43%) and C. hispaniensis (41%), (iii) transposable elements, which are highly diversified in Y. lipolytica and closely related species, are scarce in C. hispaniensis with a single degenerate family of gypsy retrotransposons, and are restricted to class I elements in C. phangngensis, and (iv) gene contents vary between ∼5300 (C. hispaniensis) and ∼6450 (Y. lipolytica). The average identity/similarity with Y. lipolytica at the protein level varies between 75 and 83% for C. alimentaria and between 82 and 87% for C. deformans. As expected, synteny is better conserved between closely related species (e.g. C. galli and Y. lipolytica) although internal inversions within blocks (mesosynteny) are frequent. There are large differences in the sizes of gene families, such as alkaline proteases, lipases, etc., often reflecting recent contraction and/or expansion events in the different lineages. Most families underwent significant size reduction in C. phangngensis and C. hispaniensis which, together with a more compact organization, accounts for the overall size and gene number reduction in these species. Taken together, these data suggest strong genome dynamics within the clade.
As far as we know, no exhaustive search has been done in the Y. lipolytica genome sequence for toxin genes or genes involved in virulence. Killer toxins such as those encoded by the DNA killer plasmid pGKL1 from Kluyveromyces lactis have never been found in Y. lipolytica, whereas they have been found in other species (Pichia acacia, Wingea robertsiae, Debaryomyces hansenii). Similarly, to our knowledge, there are no genes known to encode antibiotic activities nor polyketide synthases in Y. lipolytica. Gene families encoding adhesins and agglutinin-like sequences, which play a key role in adhesion of fungal pathogens to human cells, were also not found in Y. lipolytica.
Natural occurrence of Yarrowia lipolytica in food
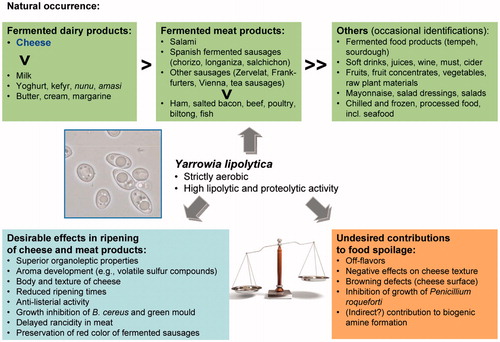
Yarrowia lipolytica is known for its pronounced lipolytic and proteolytic activities (Corbo et al., Citation2001; Deak & Beuchat, Citation1996; Gardini et al., Citation2006; Ismail et al., Citation2000; Patrignani et al., Citation2011a). In line with these properties, Y. lipolytica is found primarily in foods with high proportions of fat and/or protein, particularly in (fermented) dairy products and meat (for a schematic representation, see ).
Figure 3. Schematic representation of impact of metabolic properties of Yarrowia lipolytica on its natural occurrence, ripening and spoilage of foods. As indicated by the balance, it will be crucial for a potential commercial application in the ripening of cheese or fermented meat products to favor the intended beneficial effects while suppressing the potential objectionable side effects of Yarrowia lipolytica. For further details, see the text. “>” (in horizontal or vertical direction) means higher prevalence and/or higher rate of detection.
More than 60 scientific publications report on the occurrence of Y. lipolytica in a variety of different cheeses (mould-ripened, smear-ripened, blue-veined and fresh cheeses; see Supplementary Table 1). Yarrowia lipolytica has been identified in cheese produced and/or sold in ∼20 countries spread over all the five continents, although the majority of the studies were done in Europe. When comparing cheese produced from raw versus pasteurized milk, no obvious difference in prevalence of Y. lipolytica was seen. However, among the different milk sources, cow milk seems to be over-represented, which is not surprising when taking into account the much larger annual production volumes of cow versus ewe, goat and buffalo milk and cheese (Supplementary Table 2). Much to the contrary, when normalizing to the annual production volumes, the data in Supplementary Table 2 seem to suggest a higher prevalence of Y. lipolytica in ewe, goat and buffalo cheese as compared to cow cheese, possibly due to the differences in fat and crude protein content in the milk.
In commercial cheese production, Y. lipolytica so far has not been included deliberately in ripening cultures. Therefore, its occurrence in cheese must be due to either its presence in milk or through contamination of equipment, bodily surfaces of operators, or aprons in the cheese-making environment (Larpin-Laborde et al., Citation2011; Welthagen & Viljoen, Citation1998). Despite the fact that Y. lipolytica is not added deliberately, it has been reported frequently to be among the top-three most prevalent yeast species in cheese (e.g. Larpin et al., Citation2006; Larpin-Laborde et al., Citation2011; Monnet et al., Citation2010; Roostita & Fleet, Citation1996; Welthagen & Viljoen, Citation1998), and even to outcompete other yeast species (Lanciotti et al., Citation2005). Because Y. lipolytica is reported to be strictly aerobic (Barth & Gaillardin, Citation1997; Fickers et al., Citation2005), it has mostly been identified in the surface microflora, or in the interior of blue-veined and cottage cheese where oxygen is also available (see Supplementary Table 1). Occasionally, Y. lipolytica was also found in the interior of other cheeses with a supposedly hostile environment for growth of Yarrowia.
Due to its lipolytic and proteolytic activities, Y. lipolytica has been implicated in the ripening of cheese or conversely, if going too far, its spoilage. Yarrowia lipolytica was reported to contribute to superior organoleptic characteristics, in terms of aroma, body and/or texture of the cheese (Cantor et al., Citation2004; Fröhlich-Wyder, Citation2003; Guerzoni et al., Citation1998; Lanciotti et al., Citation2005; Wyder et al. Citation1999). Aroma development seems to be due, to a large part, to the production of volatile sulfur compounds, such as methanethiol, dimethylsulfide or dimethyldisulfide (Lopez Del Castillo-Lozano et al., Citation2007; Martin et al., Citation2001; Spinnler et al., Citation2001). In cheese-making trials, sensory panels assigned the highest organoleptic scores to cheeses inoculated with Y. lipolytica (Guerzoni et al., Citation1998; Lanciotti et al., Citation2005; Wyder et al., Citation1999). Additional benefits of Y. lipolytica include a reduction in ripening times, with associated economic benefits, and possibly also an extended shelf life of the cheese (Ferreira & Viljoen, Citation2003; Fröhlich-Wyder, Citation2003). Moreover, Y. lipolytica was suggested to have anti-listerial activity, and to inhibit the growth of Bacillus cereus and green mould (Addis et al., Citation2001; Goerges et al., Citation2006; Lanciotti et al., Citation2005; Monnet et al., Citation2010).
As far as undesirable effects in cheese ripening are concerned, Y. lipolytica was reported to produce off-flavors (Alvarez-Martín et al., Citation2008; Bintsis & Robinson, Citation2004; Gaborit et al., Citation2001), negatively affect cheese texture (Westall & Filtenborg, Citation1998), stimulate the formation of biogenic amines (Gardini et al., Citation2006; for a review, see Linares et al., Citation2012; Wyder et al., Citation1999) and to inhibit the growth of Penicillium roqueforti (Cantor et al., Citation2004; van den Tempel & Jakobsen, Citation2000). Strain-specific differences in these properties were observed (van den Tempel & Jakobsen, Citation2000), and the concentrations of biogenic amines (up to 120 mg/kg) were stated to give no reason for health concerns (Wyder et al., Citation1999). Eventually, the high proteolytic and lipolytic activities of Y. lipolytica create a precursor pool for biogenic amine production by Y. lipolytica itself and/or by other organisms in the cheese microflora. As a matter of fact, (candidate) decarboxylase genes possibly involved in biogenic amine formation are present in the Y. lipolytica genome (e.g. YALI0B11330g, YALI0A00330g and YALI0E20361g; Jiménez-Bremont et al., Citation2001).
Yarrowia lipolytica was also implicated in surface browning defects of cheese (Ross et al., Citation2000; van den Tempel & Jakobsen, Citation2000). Although this defect does not affect the nutritive quality or taste of the cheese, the appearance is sufficiently unpleasant for consumers to reject the product, with concomitant financial loss. The pyomelanin pigment responsible for this discoloration defect is thought to be formed by the extracellular auto-oxidation of the tyrosine catabolite, homogentisic acid (Carreira et al., Citation2001; Williams & Withers, Citation2007). However, some conflicting observations were also made: (i) in studies on Camembert cheese, when used in combination with Penicillium candidum, Y. lipolytica either had no effect or even reduced browning (Carreira et al., Citation2002), (ii) Candida famata, rather than Y. lipolytica, was reported to be responsible for the production of a brownish pigment by van den Tempel & Jakobsen (Citation1998), and (iii) although counts of Y. lipolytica in cottage cheese increased toward the end of the shelf life, this yeast was not identified in the visible spoilage area of the cheese (Brocklehurst & Lund, Citation1985). Thus, if Y. lipolytica indeed contributes to discoloration defects, it is safe to conclude that this trait displays pronounced strain-specific differences (Ross et al., Citation2000; van den Tempel & Jakobsen, Citation2000; Williams & Withers, Citation2007).
Whether and/or to what extent Y. lipolytica contributes to cheese spoilage appears to be the subject of some controversy: whereas some authors consider Y. lipolytica as a (prominent) cheese spoilage microorganism (Daryaei et al., Citation2010; Fleet, Citation1990; Fröhlich-Wyder, Citation2003; Ramos et al., Citation2012; Westall & Filtenborg, Citation1998), others do not even mention Y. lipolytica in the context of cheese spoilage (Pitt & Hocking, Citation1985). Also growth curves do not provide a clear-cut picture: while in some studies, counts of Y. lipolytica increased toward the end of cheese ripening (which might indicate a risk for overgrowth; Brocklehurst & Lund, Citation1985; Larpin-Laborde et al., Citation2011; Welthagen & Viljoen, Citation1998), both total yeast counts and Y. lipolytica counts stayed constant or even decreased in other studies (Ferreira & Viljoen, Citation2003; van den Tempel & Jakobsen, Citation1998).
Based on the potential benefits outlined above, a number of research groups advocated inclusion of Y. lipolytica in ripening cultures for cheese (Addis et al., Citation2001; Freitas et al., Citation1999; Lanciotti et al., Citation2005; Monnet et al., Citation2010; van den Tempel & Jakobsen, Citation2000; Zarowska et al., Citation2004). Yarrowia lipolytica was stated to be “essential for balancing the Livarot cheese ecosystem” (Larpin et al., Citation2006) or to possess “some of the essential properties for use as a cheese starter” such as proteolytic activity, compatibility with the lactic acid starter culture or the ability to compete with other naturally occurring yeasts such as Debaryomyces hansenii and Saccharomyces cerevisiae (Guerzoni et al., Citation1998; Jakobsen et al., Citation2002). It has also been postulated that “in order to improve flavor, Y. lipolytica should never be absent” (Wyder et al., Citation1999). Other authors were somewhat more cautious, stating that despite its potential as an adjunct culture, Y. lipolytica must be controlled very carefully because of its strong enzymatic activity, its inhibitory effect toward P. roqueforti and its potential involvement in discoloration defects (Cantor et al., Citation2004). In some of the studies, the synergistic effects of Y. lipolytica with other microorganisms and, in particular, with D. hansenii have been stressed (De Wit et al., Citation2005; Freitas et al., Citation1999; Wyder et al., Citation1999). Finally, Y. lipolytica is repeatedly included in complex, experimental cheese communities containing multiple bacterial, fungal and yeast species (Deetae et al., Citation2009; Delbes-Paus et al., Citation2012; Irlinger et al., Citation2012; Martin et al., Citation2001).
As outlined above, there seems to be a rather thin line between the desirable, beneficial effects of Y. lipolytica in cheese ripening and its unintentional, occasional contribution to spoilage. In addition, taste and flavor preferences can be quite different between subjects; therefore, what may be desirable for some may be considered as spoilage by others (Bourdichon et al., Citation2012). For commercial cheese production, the eventual fear of Y. lipolytica overgrowth, appearance of off-odors and inhibition of growth of P. roqueforti (Devoyod, Citation1990; Romano et al., Citation2006) may be overcome by (i) using balanced microbial communities rather than single strains as ripening cultures, (ii) using isolated enzymes or extracts from Y. lipolytica rather than live cultures (Cantor et al., Citation2004; M. Wojtatowicz, personal communication), or (iii) adding cheese flavor produced with the aid of Y. lipolytica (Jakobsen et al., Citation2002).
Yarrowia lipolytica has been identified, at lower frequencies, in a number of other dairy products: in milk of cow, ewe and water buffalo (Baroiller & Schmidt, Citation1990; Chen et al., Citation2010; Corbo et al., Citation2001; Suárez & Iñigo, Citation1982), in (traditionally) fermented milk products such as yoghurt, kefir, nunu and amasi (Akabanda et al., Citation2010; Bai et al., Citation2010; Fröhlich-Wyder, Citation2003; Gadaga et al., Citation2000; Lourens-Hattingh & Viljoen, Citation2002; Rohm et al., Citation1992; Viljoen et al., Citation2003), as well as in butter, cream and margarine (Bours & Mossel, Citation1973; Lanciotti et al., Citation1992; Lopandic et al., Citation2006). In yoghurt, the use of Y. lipolytica in starter cultures, to support the growth of probiotic bacteria, has been considered (Lourens-Hattingh & Viljoen, Citation2002).
Similar to the situation in milk and milk-derived products, Y. lipolytica was frequently observed in meat and fish (Deak & Beuchat, Citation1996). Yarrowia lipolytica has been detected in salami (Abunyewa et al., Citation2000; Gardini et al., Citation2001; Gianni et al., Citation2008; Nielsen et al., Citation2008), Spanish fermented sausages such as chorizo, longaniza and salchichon (Encinas et al., Citation2000; Martinez et al., Citation2004), German (dry fermented) sausages such as Zervelat and Frankfurters (Entel, Citation1961; Samelis & Sofos, Citation2003), as well as in Vienna, tea and other sausages (Leistner & Bem, Citation1970; Viljoen et al., Citation1993; Zivanovic & Ristic, Citation1974). The prevalence of Y. lipolytica was higher at the surface than in the interior of the products (Leistner & Bem, Citation1970). Yarrowia lipolytica has also been reported to occur in ham (Fung & Liang, Citation1990; Leistner & Bem, Citation1970), salted bacon (Nielsen et al., Citation2008), beef (Aboukheir & Kilbertus, Citation1974; Fung & Liang, Citation1990), poultry (Ismail et al., Citation2000; Sinigaglia et al., Citation1994; Viljoen et al., Citation1998), biltong (Wolter et al., Citation2000), other meat samples (Abunyewa et al., Citation2000; Fleet, Citation1992; Martinez et al., Citation2004) and – occasionally – also in fish (Oliveira Leme et al., Citation2011; Ross & Morris, Citation1965). As a caveat, Y. lipolytica and Candida alimentaria, a recently identified member of the Yarrowia clade (Knutsen et al., Citation2007), were found to co-exist in processed meat products (Nielsen et al., Citation2008); thus, it may be hypothesized that some of the earlier identifications of Y. lipolytica in meat might actually relate to C. alimentaria.
As described above for cheese, Y. lipolytica has been implicated in the ripening and/or spoilage of sausages and other meat products, with eventually a narrow border in between the two phenomena. The use of Y. lipolytica in starter cultures for dry fermented sausages has been advocated, based on evidence suggesting that this yeast contributes to superior sensory properties and overall quality of the sausages, and that it may help to reduce ripening time (Gardini et al., Citation2001; Patrignani et al., Citation2007, Citation2011a, Citationb; Romano et al., Citation2006). In addition, the yeast microflora is supposed to delay rancidity and to protect nitrosomyoglobin from oxidative breakdown, thereby preserving the appealing red color of fermented sausages (Romano et al., Citation2006). On the other hand, Y. lipolytica seemed to cause production of off-flavors (ten Cate, Citation1960) and an increase in biogenic amines, and its use in sausage ripening was not well appreciated by sensory panelists (Iucci et al., Citation2007). No significant effect on sausage ripening, neither positive nor negative, was seen by still another research group (Selgas et al., Citation2003).
The evidence around a potential contribution of Y. lipolytica to meat spoilage is ambiguous. Some authors infer a prominent role of Y. lipolytica in meat spoilage (Deak & Beuchat, Citation1996; Ismail et al., Citation2000; Kniewallner & Haas, Citation1966; Samelis & Sofos, Citation2003; Viljoen et al., Citation1998). According to Viljoen et al. (Citation1998), “it would appear that yeasts are substantially represented in the total microbial ecology of spoiled poultry carcasses, although yeasts are rarely the direct cause of spoilage”. On the other hand, in salami, Y. lipolytica counts were shown to sharply decrease toward the end of ripening (Gianni et al., Citation2008), and to be undetectable at the end of shelf life (Nielsen et al., Citation2008). Furthermore, Y. lipolytica was concluded not to contribute to fermentative gas production in meat spoilage (Martinez et al., Citation2004), and to be associated primarily with fresh meat (Fleet, Citation1992; Viljoen et al., Citation1993).
More anecdotal reports exist on the occurrence of Y. lipolytica in a broad range of food products other than those mentioned so far (for overviews, see Deak & Beuchat, Citation1987, Citation1996; Fleet, Citation1992; Heard & Fleet, Citation2000; Sinigaglia et al., Citation1994). For instance, Y. lipolytica has been detected in other fermented food products, such as tempeh and sourdough (Romano et al., Citation2006; Samson et al., Citation1987), in soft drinks, juices, wine, must and cider (Stratford, Citation2006), in fruits, fruit concentrates, vegetables and raw plant materials (Sancho et al., Citation2000), in mayonnaise, salad dressings and salads (Baleiras Couto et al., Citation1996), as well as in chilled and frozen, processed food, including seafood. All these examples suggest that Y. lipolytica may be a regular, although probably minor, component of food microbiota. Despite the fact that, next to the examples for cheese and meat already mentioned, there are also reports suggesting that Y. lipolytica might be involved in spoilage of butter (Lin & Fung, Citation1987; Rodrigues Paula et al., Citation1988), margarine (Castañon-Vélez & Leal, Citation1973), yoghurt (Liu & Tsao, Citation2009; Rodrigues & Pais, Citation2000), mayonnaise-based salad (Buick & Damoglou, Citation1989), as well as fish and seafood (for overviews, see Fleet, Citation1992; Deak & Beuchat, Citation1996; Oh et al., Citation1998), it is important to stress that in all these cases, spoilage was exclusively related to taste or appearance. In no single case was there any evidence of food poisoning or of another health hazard related to Y. lipolytica (Lin & Fung, Citation1987; Peppler, Citation1977).
In line with its regular occurrence in food, Y. lipolytica has been detected in feces of cattle and probably fish (Mehnert, Citation1957; Ross & Morris, Citation1965), as well as in the oral cavity of human beings (Bremenkamp et al., Citation2011). The frequency of occurrence of Y. lipolytica in saliva seemed to be higher in diabetic patients than in the control group. To the best of our knowledge, none of the human gastrointestinal microbiome studies reported so far identified Y. lipolytica as a “normal” inhabitant of the human intestinal microflora. When Y. lipolytica was administered to mice, viable cells were found in the feces only until discontinuation of the feeding (Mehnert, Citation1957). Thus, based on the very limited evidence available so far, Y. lipolytica does not seem to colonize the intestinal tract, but to be subject to transient passage. Finally, Y. lipolytica has been proposed as a probiotic (Liu & Tsao, Citation2009), and its cell-free extract as a prebiotic (Kumura et al., Citation2009). Effectiveness as a probiotic will depend on further clarification of whether Y. lipolytica is capable of surviving in or, even better, colonizing the intestinal tract.
Opportunistic infections by Yarrowia lipolytica
Only four isolates in the CBS yeast collection originated from human sources, namely a corneal lesion (CBS 2070), lung (CBS 5570, CBS 5589) and the infected skin of an offset printer (CBS 7133). A fifth isolate (CBS 2787) was probably also obtained from a human. Note that the two isolates from lung (CBS 5570 and CBS 5589) were deposited by the same person, R.E. Halbinger from Argentina under numbers 2752 and 2753 with mating type B and A, respectively. Unfortunately, no publication could be found referring to these isolates, but it seems probable that both were obtained from the same patient. This presumption is based on (i) the fact that infections by Y. lipolytica are very rare and those from lung have not been further reported and (ii) the two isolates have sequential collector’s numbers, and belong to two mating types that could be a result from a single ascogenous and sporulating culture. In the recent taxonomic study by Knutsen et al. (Citation2007), no more isolates from clinical origin were reported. Some CBS strains that previously were classified as Y. lipolytica, but that are now assigned to other species, originated from human-related sources, namely CBS 2076 and CBS 2071 (=C. deformans), isolated from an unspecified part of the body and fingernail, respectively.
In recent years, a number of reports have been published on the occurrence of Y. lipolytica in clinical samples. In almost all cases, the patients were immunocompromised and received parenteral nutrition or antibiotic treatment via a catheter. Nitzulescu & Niculescu (Citation1976) referred to an early paper published by Nannizzi (1928) [cited in Lodder & Kreger-van Rij (Citation1952)] in which a corneal infection due to Monilia cornealis was described. The original strain from Nannizzi is preserved as CBS 2070. Using sequence analysis of the D1/D2 domains and the ITS 1 + 2 regions of the rDNA as well as DNA–DNA reassociation experiments, this strain turned out to represent Y. lipolytica (Knutsen et al., Citation2007).
Nitzulescu & Niculescu (Citation1976) described three cases of ocular mycosis due to C. lipolytica var. lipolytica. According to these authors, the infections were a consequence of eye trauma caused by wood chips, wire or metal inclusions. The strains were only identified using limited growth characteristics and the ability to hydrolyze fat. Unfortunately, these authors did not provide any further growth characteristics, nor were the strains preserved. The presence of distinct fusiform blastoconidia along the pseudohyphae does not seem to resemble those formed by Y. lipolytica (see, e.g. Kurtzman, Citation2011; Lodder & Kreger van Rij, Citation1952). Therefore, the identity of these isolates as Y. lipolytica cannot be confirmed and is doubtful at best.
Since 1985, 55 cases of human infections due to Y. lipolytica have been described (Agarwal et al., Citation2008; Belet et al., Citation2006; Blanco et al., Citation2009; Chang et al., Citation2001; D’Antonio et al., Citation2002; Franck et al., Citation2010; Kang et al., Citation2008; Ninin et al., Citation1997; Özdemir et al., Citation2011; Shin et al., Citation2000; Walsh et al., Citation1989; Wehrspann & Füllbrandt, Citation1985; Yoon, Citation2009). Ninin et al. (Citation1997) reported nine cases in their hospital that were isolated within only two years, which may suggest that infections by Y. lipolytica occur more frequently than presently anticipated. Except for two, all infections were related to the use of intravenous catheters, in patients with serious underlying diseases (Supplementary Table 3). In several cases, a co-infection with bacteria and other yeast species was reported. Wehrspann & Füllbrandt (Citation1985) described an infection by Y. lipolytica in an immunocompromised 57-year-old female patient. Candida agglutination tests revealed high titers of 1:2560 (June 25), 1:1280 (June 27) and 1:320 (July 19). This patient was infected with C. albicans that was isolated from urine (June 20). However, approximately one month later (July 25), Y. lipolytica was cultivated from a blood culture and one day later from the intravenous catheter. The authors assumed that Y. lipolytica entered the body via the catheter. Walsh et al. (Citation1989) described several cases, including a catheter-related infection of blood in a 54-year-old male with a history of alcohol abuse who suffered from catheter-associated thrombophlebitis, cholangitis, cholelithiasis and cholecystitis, and who underwent cholecystectomy. One other case was related to a localized infection of the sinuses with Staphylococcus aureus and Pseudomonas aeruginosa as co-occurring microorganisms. These authors described an additional four people who were colonized by the yeast, which was isolated from stool, oropharyngeal swab and sputum (Walsh et al., Citation1989). Six patients infected with Y. lipolytica suffered from diverse forms of leukemia (Chang et al., Citation2001; D’Antonio et al., Citation2002; Ninin et al., Citation1997; Shin et al., Citation2000; Ye et al., Citation2011). Other cases where Y. lipolytica infections were diagnosed included a neuroblastoma patient who received high-dose chemotherapy and was highly neutropenic (Özdemir et al., Citation2011), a 5-year-old boy who suffered from aplastic anemia and was immunocompromised because of globulin and cyclosporine therapies (Shin et al., Citation2000), a newborn treated with broad-spectrum antibiotics who was also co-infected with C. albicans (Belet et al., Citation2006), a 3-year-old male with tubercular meningitis who also received broad spectrum antibiotics (Agarwal et al., Citation2008), a 2-month-old boy with streptococcal meningitis (Shin et al., Citation2000) and a 4-month-old malnourished girl who received parenteral nutrition, was treated with vancomycin and later received broad spectrum antibiotics, and was also infected with C. albicans isolated from blood and the catheter. One case occurred in an 86-year-old female who suffered from many disorders, such as arterial hypertension, diabetes mellitus type 2, vesical neoformation with peritoneal fibrosis, bilateral hydronephrosis and recurrent urinary tract infections by many bacterial and yeast species other than Y. lipolytica (Blanco et al., Citation2009). Two cases were reported to be related to the intake of nutraceuticals and food. A 13-year-old boy who suffered from acute lymphoblastic leukemia was found to consume yeast extract preserved in capsules. Culturing from these capsules yielded Y. lipolytica (Ye et al., Citation2011), suggesting that the infection originated from these yeast capsules. In the other case, the patient was reported to have a history of raw meat consumption (Kang et al., Citation2008). However, it remains to be seen whether a causal relationship exists between the Y. lipolytica infection of the blood and the food habits of the patient. In another study on 24 patients, Y. lipolytica was grown from the lung mass of 17 patients with a suspicion of malignancy, and other isolates came from stool, duodenal mass, a mesenteric mass, two cutaneous nodules and one bronchoalveolar lavage (Franck et al., Citation2010). It is important to note that these authors concluded that the species is part of the normal human mycobiota, and especially of the adult respiratory tract (Franck et al., Citation2010). Related to this commensal nature of the yeast on the human body seems the presence of Y. lipolytica on rat-inflicted foot wounds of a 73-year-old man who was in shock, and suffered from respiratory failure, a cerebrovascular incident and renal failure (Levy et al., Citation2003).
Yarrowia lipolytica appears to be of low pathogenicity. Walsh et al. (Citation1989) inoculated two mice with a clinical strain and the mice survived. One kidney was found to be infected, but no infections were observed in the brain, liver, spleen and the other kidney. Similarly, the autopsy of a patient with a previous blood stream infection by Y. lipolytica who died due to dyspnea and cytomegalovirus interstitial pneumonia did not reveal any deep visceral infections nor could the yeast be isolated from autopsy material (D’Antonio et al., Citation2002). Some large-scale studies confirm that Y. lipolytica causes infections rarely. Only 4 (0.07%) isolates of Y. lipolytica were present among 6082 yeast isolates collected from blood stream infections in 250 medical centers distributed over 32 countries between 1992 and 2001 (Pfaller & Diekema, Citation2004). In another study, 2 Y. lipolytica isolates were observed among 337 isolates during six years in an intensive care unit in Medellín (Colombia) and one of them was found to be resistant to fluconazole (Zuluaga Rodríguez et al., Citation2010).
The various clinical cases have been treated by the prescription of different antifungal drugs. In vitro susceptibility tests revealed that Y. lipolytica is susceptible to amphotericin B, ketoconazole, fluconazole, itraconazole, voriconazole and caspofungin (Belet et al., Citation2006; Blanco et al., Citation2009; D’Antonio et al., Citation2002; Özdemir et al., Citation2011; Walsh et al., Citation1989), whereas in some isolates resistance has been reported to 5-flucytosine (5FC) and itraconazole (Belet et al., Citation2006; Özdemir et al., Citation2011). A Y. lipolytica blood stream isolate showed high MIC values to amphotericin B (MIC50 1 µg/ml after 24 h in both M27-P and microdilution methods and 2 µg/ml after 48 h using microdilution) and fluconazole (MIC50 64 µg/ml using M27-P method, 32 µg/ml after 24 h with the microdilution method and 64 µg/ml after 48 h using microdilution). Despite these high in vitro MIC values the patient was treated successfully with fluconazole (Rex et al., Citation1995). Decreased susceptibilities of Y. lipolytica isolates to fluconazole, itraconazole and the triazoles posaconazole and voriconazole were also observed (Pfaller et al., Citation2003). Another study showed high MIC values for fluconazole, ketoconazole, itraconazole and flucytosine and cross resistance was observed for fluconazole and ketoconazole, and fluconazole and itraconazole (Barchiesi et al., Citation1999). Unfortunately, the isolates from the above studies were not maintained so their identity could not be confirmed with molecular tools. In an extensive study in which the antifungal susceptibility profiles were studied for approximately 1700 strains belonging to 992 yeast species, some strains of Y. lipolytica and the closely related C. hispaniensis and Candida umkomasiana showed high MICs to all eight antifungals tested, namely the azoles fluconazole, itraconazole, posaconazole and voriconazole, the echinocandin caspofungin, the polyene amphotericin B, the antimetabolite 5FC and the allylamine terbinafine. Candida bentonensis had high MICs for all antifungals tested, except for 5FC, and C. galli had high MICs for 5FC, fluconazole, voriconazole and amphotericin B (Desnos-Ollivier et al., Citation2012). These data clearly demonstrated that resistance to various antifungals may occur in species belonging to the Yarrowia clade.
Importantly, almost all infections due to Y. lipolytica were catheter related. Removal of the catheter or its replacement, in some cases combined with antifungal therapy, resulted invariably in clearance of the pathogen (Agarwal et al., Citation2008; Belet et al., Citation2006; Blanco et al., Citation2009; Chang et al., Citation2001; D’Antonio et al., Citation2002; Kang et al., Citation2008; Ninin et al., Citation1997; Shin et al., Citation2000; Walsh et al., Citation1989; Wehrspann & Füllbrandt, Citation1985; Yoon, Citation2009). In addition, treatment with single or various combinations of antifungals was used, i.e. ketoconazole (Walsh et al., Citation1989; Wehrspann & Füllbrandt, Citation1985), fluconazole (Shin et al., Citation2000; Ye et al., Citation2011), amphotericin B (Agarwal et al., Citation2008; D’Antonio et al., Citation2002; Shin et al., Citation2000), liposomal amphotericin B (Belet et al., Citation2006), amphotericin B and fluconazole (Ninin et al., Citation1997), amphotericin B and caspofungin (Belet et al., Citation2006), liposomal amphotericin B and caspofungin (Belet et al., Citation2006), fluconazole and caspofungin (Özdemir et al., Citation2011) and voriconazole (Blanco et al., Citation2009). In one case, no antifungal treatment was done and the catheter was removed only during the third episode, but the authors indicated clearly that in their opinion this was an unnecessary act (Chang et al., Citation2001). However, most of the above authors recommended that removal of the catheter, when possible, would help to eliminate the infection. In summary, the following treatments of Y. lipolytica infections have been recommended: (i) removal of the catheter with systemic antifungal therapy (D’Antonio et al., Citation2002; Walsh et al., Citation1989; Ye et al., Citation2011), (ii) removal of the catheter with amphotericin B treatment (Shin et al., Citation2000), (iii) use of caspofungin in children (Belet et al., Citation2006) and other patients (Özdemir et al., Citation2011), and (iv) no treatment (Chang et al., Citation2001).
In conclusion, opportunistic infections by Y. lipolytica occur occasionally in strongly immunocompromised patients and, fortunately, can be treated well and/or disappear even without treatment.
Industrial uses of Y. lipolytica: Past, present and future
The industrial use of Y. lipolytica was pioneered by British Petroleum (BP). In the 1950s to 1970s, a considerable number of companies around the globe explored different microbial hosts (bacteria, molds, yeasts and algae) for the large-scale production of high-quality protein (so-called “single-cell protein”), to secure adequate nutritional supply for a rapidly growing global population (Babel et al., Citation2000; Litchfield, Citation1983). The low-cost substrates for biomass growth used at that time were methanol, ethanol and different fractions of petroleum, as well as waste streams from different industries.
BP started its activities in the field in 1957, in its majority-owned French subsidiary, Société Française des Pétroles BP (Bamberg, Citation2000). Over the coming years, BP developed two microbial systems, Candida tropicalis and C. ( = Yarrowia) lipolytica, using gas oil and n-alkanes as substrates, respectively. For both organisms, pilot plants and demonstration production plants were built, for C. tropicalis in Lavera, France (annual production capacity = 17 000 tons; brand name of the product: Toprina L), and for Y. lipolytica in Grangemouth, UK (4000 tons per annum; Toprina G). In 1971, BP and the Italian ANIC entered into a Joint Venture, called Italproteine, and built a 100 kton/a production plant for Toprina G in Sarroch, Sardinia. However, despite ample evidence for the safety of the product (see below), the Italian authorities refused to grant the required production permits. This setback, as well as the sharp increase in raw material prices with the 1973 oil crisis, seriously jeopardized the economic foundation for commercial success of Toprina G, and drove BP in 1978 to step out of Italproteine and of single-cell protein (SCP) production altogether.
While most of its competitors had targeted their SCP products for human nutrition, BP deliberately chose to develop its Toprina products for livestock nutrition. Efficacy studies were performed, among others, with broilers, pigs, lambs, calves and rainbow trout, and demonstrated that Toprina can be included in the diet, in selected cases, up to levels of 35% with no negative impact on the pertinent performance indicators (Hanssen & Farstad, Citation1980; Matty & Smith, Citation1978; Shannon & McNab, Citation1972; Van der Wal, Citation1976). In addition, an unprecedented safety program was run on the Toprina products (see below), using more than 50 000 animals at the time, and following rats and Japanese quails over at least 19 and 29 consecutive generations of exposure to Toprina G, respectively (Champagnat, Citation1975; De Groot, Citation1976). Commercial sales of Toprina as milk replacer in calf feeds and as replacements for fishmeal and soybean in pig and poultry feeds started in 1971 in the United Kingdom and in 1972 in France (Bamberg, Citation2000).
Recently, commercial production of Y. lipolytica biomass, for use as fodder yeast in Europe (currently at approximately 600 tons per year) has been resumed by the Polish company Skotan SA (Rywinska et al., Citation2013; see also <http://www.skotansa.pl> and <http://www.feedmaterialsregister.eu>). In addition, Skotan SA is developing Y. lipolytica for prebiotic and probiotic use in feed and food.
Citric acid production using Y. lipolytica has been granted “Generally Regarded as Safe” (GRAS) status by the US FDA (21 CFR 173.165). Approximately 40 years ago, Pfizer Inc. developed a Yarrowia-based production process for citric acid (Fried, Citation1972; Nubel et al., Citation1979). In 1990, Pfizer sold its citric acid business to Archer Daniels Midland (Connor, Citation2008), and since then, several statements have been made in the open literature that ADM has been and/or still is using Y. lipolytica for citric acid production at its Decatur, IL, plant (Fickers et al., Citation2005). However, no trustworthy evidence is currently available on the history of commercial production, nor on the annual production volumes of citric acid derived from Y. lipolytica. The high potential of Y. lipolytica for organic acid production has been confirmed in the recent scientific literature not only for citric acid (Liu et al., Citation2013; Moeller et al., Citation2011; Rywinska et al., Citation2012), but also for isocitric, α-ketoglutaric, pyruvic, succinic and acetic acid (Chatzifragkou et al., Citation2011; Heretsch et al., Citation2008; Holz et al., Citation2011; Kamzolova et al., Citation2009, Citation2012; Otto et al., Citation2011, Citation2012; Zhou et al., Citation2012; Yu et al., Citation2012; Yuzbashev et al., Citation2011).
Yarrowia lipolytica has been chosen as production host for mannitol and erythritol (De Zeeuw & Tynan, Citation1973a, Citationb; Tomaszewska et al., Citation2012). Since 2003, Baolingbao Biology Co., Ltd., from Shandong, China, produces erythritol with Y. lipolytica, for addition to foods as a nutritive sweetener, flavor enhancer, formulation aid, humectant, stabilizer and thickener, sequestrant and texturizer. The product was distributed in China, Japan, Korea and Norway. Recently, Baolingbao Biology Co., Ltd., has also submitted a GRAS Notice for Yarrowia-derived erythritol (http://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000382.pdf).
The fact that Y. lipolytica can accumulate large amounts of lipids intracellularly, in the so-called lipid bodies, has been a trigger to also attempt overproduction of lipophilic compounds in this oleaginous yeast. Linoleic acid is the major PUFA synthesized by wild-type Y. lipolytica, but genetic engineering can be used to generate recombinant strains overproducing, e.g., eicosapentaenoic acid (EPA), docosahexaenoic acid, arachidonic acid, conjugated linoleic acid or γ-linolenic acid (Chuang et al., Citation2010; Damude et al., Citation2009a, Citationb; Picataggio et al., Citation2007; Zhang et al., Citation2012). In 2010, DuPont submitted a GRAS Notice for its EPA-enriched oil derived from Y. lipolytica to the FDA (http://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000355.pdf), and sells the product as a dietary supplement under the trade name New Harvest™ (http://www.newharvest.com).
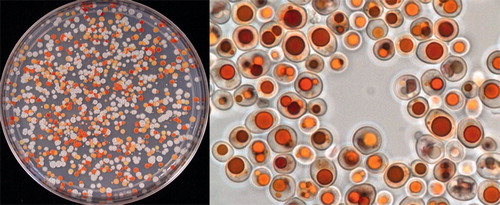
Microbia (now part of DSM) and DuPont independently developed Y. lipolytica as a production host for carotenoids, a class of natural coloring and stabilizing agents for food and feed (Bailey et al., Citation2006, Citation2008; Sharpe et al., Citation2008; see ). At Microbia, several candidate carotenoid production strains were brought to pilot scale, and a GRAS self-affirmation has been prepared for β-carotene produced with Y. lipolytica (unpublished data).
Figure 4. Carotenoid production in Yarrowia lipolytica. Left: mating of two carotenoid-overproducing Yarrowia lipolytica strains, creating a progeny with a rich genetic diversity. White colonies have lost the trait to produce colored carotenoids. Colonies with different colors produce different carotenoids, and/or different quantities of carotenoids. Right: Yarrowia lipolytica cells from a fed-batch culture, showing canthaxanthin accumulation in the lipid bodies. Pictures courtesy of María Mayorga (DSM).
Yarrowia lipolytica can also be used for the production of aroma chemicals (Bialecka-Florjanczyk et al., Citation2012; Garcia et al., Citation2009) and, in particular, of γ-decalactone, which has a characteristic peach flavor. γ-Decalactone is typically produced using methyl ricinoleate derived from castor oil as substrate (Gomes et al., Citation2012; Guo et al., Citation2012; Rabenhorst & Gatfield, Citation2002; Schrader et al., Citation2004).
Yarrowia lipolytica has been explored as a production host for therapeutic and industrial proteins and enzymes by – among others – some major industrial players (e.g. Pfizer or Novozymes; James & Strick, Citation1998; Müller et al., Citation1998). The Y. lipolytica LIP2 lipase, homologously overexpressed in Y. lipolytica, may serve as a therapeutic agent for the treatment of exocrine pancreatic insufficiency (Turki et al., Citation2010b). It is currently under clinical development by the French Mayoly Spindler group (http://www.mayoly-spindler.fr; Fickers et al., Citation2011; Leblond et al., Citation2012). In May 2011, in a joint press release with its partner, Protea Biosciences, Inc., Mayoly Spindler stated that clinical phase I/IIa trials on patients with chronic pancreatitis had been completed successfully.
Oxyrane, headquartered in the United Kingdom, is a biopharmaceutical start-up company dedicated to the development of enzyme replacement therapies (ERTs) to treat lysosomal storage diseases (http://www.oxyrane.com). Oxyrane has developed and optimized Y. lipolytica for the production of human lysosomal enzymes with high levels of bound mannose 6-phosphate, the sugar-based targeting mechanism that enables clinically effective enzyme uptake and localization (Callewaert et al., Citation2008; Geysens & Vervecken, Citation2011; Ryckaert & Lerondel, Citation2011). Oxyrane is currently at the preclinical stage with its first potential ERT treatment, targeting Pompe disease, and is expecting to start clinical trials in 2013. Yarrowia lipolytica is also deployed by Oxyrane as a platform for the expression of various epoxide hydrolases that are used for the cost-effective manufacturing of enantiomerically pure pharmaceuticals (Botes & Mitra, Citation2006; Botes et al., Citation2007). According to Thevenieau et al. (Citation2009), Y. lipolytica is applied by Oxyrane in South Africa for the industrial production of a wide range of chiral epoxides and diols, using multi-molar substrate concentrations in a simple, batch, stirred-tank reactor process. In addition to these examples, Y. lipolytica was demonstrated to be suited for the expression of a large variety of homologous and heterologous proteins and enzymes, for use in different applications (De Pourcq et al., Citation2012b; Madzak et al., Citation2004).
Yarrowia lipolytica has been assessed for many more applications. However, based on available evidence, the developments in these fields seem to be at an exploratory and/or more premature stage of commercialization. Yarrowia lipolytica itself, or enzyme preparations derived from it, have been investigated for the production of polyhydroxyalkanoates and other (biodegradable) polymers, single-cell oil and specialty lipids (Barrera-Rivera et al., Citation2012; Haddouche et al., Citation2011; Papanikolaou & Aggelis, Citation2011; Sabirova et al., Citation2011). Yarrowia lipids have also been explored as a cocoa butter substitute (Papanikolaou & Aggelis, Citation2011). Moreover, various applications of Y. lipolytica in bioremediation and detoxification have been proposed, for example (i) bioremediation of hydrocarbon-contaminated soils or aquatic environments, (ii) detoxification of 2,4,6-trinitrotoluene (TNT), methyl parathion, aflatoxins or brominated organics, (iii) treatment and upgrading of waste streams (e.g. olive mill wastewater, palm oil mill effluents), or (iv) metal detoxification (Bankar et al., Citation2009; Mann & Rehm, Citation1977; Shinde et al., Citation2012; Song et al., Citation2011; Vatsal et al., Citation2011; Wang et al., Citation2012). The Belgian company Artechno (http://www.artechno.be) has a small business using a non-GMO Yarrowia lipase product for the treatment of lipid-rich wastewater (Bordes et al., Citation2011). For more widespread deployment in bioremediation, however, it is critical whether the use of genetically modified microorganisms (GMM) will be conceivable, with the aim to tailor Y. lipolytica to given applications and thereby to secure economic viability. Again, the safety of Y. lipolytica itself should be an important factor in these considerations.
Crucial in determining Y. lipolytica’s potential for bioremediation may be its capacity to produce biosurfactants; such biosurfactants may also be applied to enhance oil recovery, and in crude oil drilling, food processing and cosmetic formulations (Amaral et al., Citation2006; Trindade et al., Citation2008). Yarrowia lipolytica seems to produce a variety of different emulsifiers, for example Liposan isolated from cultures grown on hexadecane, Yansan from cultures grown on glucose or Rufisan from cultures grown on soybean oil refinery residue (Amaral et al., Citation2006; Cirigliano & Carman, Citation1985; Fontes et al., Citation2010; Rufino et al., Citation2011).
Yarrowia lipolytica may also be used for biofuel production. Either biodiesel may be prepared from, e.g. soybean oil, by using Y. lipolytica lipase (Meng et al., Citation2011; Ribeiro et al., Citation2011), or alternatively, Y. lipolytica itself may be used as a lipid source for biodiesel production (Karatay & Donmez, Citation2010; Katre et al., Citation2012; Tsigie et al., Citation2012). The properties of Y. lipolytica for biofuel production can be further improved by genetic engineering (Stephanopoulos & Abidi, Citation2011).
Finally, Y. lipolytica has been considered for starter or ripening cultures in cheese manufacturing (Ferreira & Viljoen, Citation2003; Lanciotti et al., Citation2005; Wyder et al., Citation1999, see also above), as a probiotic (Kumura et al., Citation2004), or as a platform for basic research, e.g. as a screening host for directed protein evolution (Bordes et al., Citation2011; Duquesne et al., Citation2012; Tanaka et al., Citation2012), or for the screening of substances interacting with human hormone receptors (Cho et al., Citation2010). All examples described in this section are testimony to the great variety of potential commercial applications of Y. lipolytica on our path toward a more bio-based economy.
Safety studies on Yarrowia and Yarrowia-derived products
Yarrowia lipolytica is generally regarded as a biosafety class 1 microorganism. This biosafety class encompasses microorganisms which are not known to cause disease in healthy adult humans (Lelieveld et al., Citation1996). The only exception we are aware of is in Switzerland, where Y. lipolytica – due to the reported cases of opportunistic infection (see above) – is classified as biosafety level 2. In many countries, Y. lipolytica is also on the positive list of microorganisms suited for the construction of GMM which can be used in large-scale production facilities under conditions not exceeding the Good Industrial Large-Scale Practice (GILSP) level of physical containment. One of the basic elements for such listings is the fact that the microorganism is biosafety class 1 and not known to produce toxins. Criteria for GMM complying with the GILSP level of physical containment have been developed by OECD (Organisation for Economic Co-operation and Development, Citation1992).
Yarrowia lipolytica is considered to be nonpathogenic, although it has been isolated in association with disease (see above). In three separate studies (Holzschu et al., Citation1979; Walsh et al., Citation1989; Yoshida & Hashimoto, Citation1986), Y. lipolytica was injected intravenously at high cell concentrations (107–108 CFU) into mice, with little or no indication that this organism could invade, colonize and produce disease. Although the relevance of these studies for occupational health is limited, they do provide potency information relative to other pathogenic and nonpathogenic yeasts. Holzschu et al. (Citation1979), e.g., concluded that C. albicans was clearly pathogenic, while S. cerevisiae was clearly nonpathogenic. Yarrowia lipolytica acted similarly to S. cerevisiae in the same test.
With respect to toxigenicity, there are no reports on the production of substances by Y. lipolytica that are toxic in humans or animals, apart from its potential contribution to biogenic amine formation in cheese and meat (see above). Pariza & Johnson (Citation2001) developed guidelines that can be used to evaluate the safety of microbial enzyme preparations for use in food processing, including the safety of metabolites of the production strain. Based on these guidelines, the primary consideration in an evaluation of safety of a production strain is its toxigenic potential. Pathogenic potential typically is not a concern, as food enzyme preparations rarely contain viable production organisms. Additionally, Pariza & Johnson (Citation2001) noted that it is important to distinguish between pathogenicity and opportunistic infection. When given access to tissue normally protected by the host, many microorganisms will produce opportunistic infections. True pathogens, however, are able to cross uncompromised host barriers and produce infections in individuals that are generally regarded as healthy.
The decision tree as developed by Pariza & Johnson (Citation2001) for enzyme preparations for use in food processing can equally be used and be helpful for other products derived from Y. lipolytica, such as citric acid, oils or carotenoids. However, in the context of this review which is focused on the safety of Y. lipolytica as a production microorganism, it does not seem relevant to apply the decision tree.
The most extensive tests on the safety of Y. lipolytica were done when BP developed its SCP products by fermentation of Y. lipolytica on alkanes derived from crude oil. The resulting products Toprina G (Y. lipolytica) and Toprina L (C. tropicalis) were extensively tested for safety in the 1960s by the Centraal Instituut voor Voedingsonderzoek (CIVO) in Zeist, The Netherlands (De Groot, Citation1976; De Groot et al., Citation1970a, Citationb, Citation1971, Citation1975; Engel, Citation1972). CIVO is part of TNO and a well-known, independent research institute. In these tests, acute (3–6 weeks), subchronic (90 d) and chronic (1.5–2 years) toxicity was assessed, using mainly rats and mice, although guppies, chickens and quail were used from time to time. Dried biomass of Y. lipolytica grown on pure n-paraffins (and, in parallel studies, C. tropicalis) was fed at dietary levels of 10%, 20% or 30% to groups of 30 male and 30 female rats in a two-year study and to groups of 10 male and 20 female rats in a reproduction study over three generations. Each study included two control groups, one on feed similar to the yeast diets but with soybean meal substituted for the yeast, and the other on the Institute’s stock diet. In the two-year study, the feeding of the yeast had no adverse effect on mortality, rate of body-weight gain, hematology, urine composition or kidney function tests. The multi-generation study revealed no effects on fertility, the number of offspring or the growth and mortality of the offspring during lactation. In a 90-day study on rats from the third generation, no changes attributable to yeast feeding were apparent in any of the parameters investigated. It was concluded that the yeast did not exert a harmful effect in rats at dietary levels up to 30% for two years and over three generations. In an overview, Champagnat (Citation1975) mentioned that higher proportions in the diets would, from a nutritional point of view, affect the optimal ratios of protein, fat and carbohydrates and thus would lead to adverse effects as a consequence of nutritional imbalance. Shacklady (Citation1969, Citation1972) concluded that, although it is not entirely clear whether all data refer to the Yarrowia product, BP has carried out probably the most comprehensive series of toxicity tests and efficacy studies so far undertaken on a product of this nature. In the CIVO studies, about 50 000 test animals were used and no adverse effects were found. The efficacy studies with the Toprina products were done by ILOB, Wageningen, an independent institute for research on feed, nowadays part of Wageningen University. Also other groups have studied the effects of animal feed protein products based on Y. lipolytica dried cell mass for health effects in animals, such as pigs and beagles (Bizzi et al., Citation1980; Davies et al., Citation1977; Hanssen & Farstad, Citation1980; Jackson & Kirkpatrick, Citation1978; Newberne & Young, Citation1975; Sedgman et al., Citation1985).
What is clear from both safety and efficacy studies is that the microbial biomass can be safely used as a valuable feed source for animals up to high proportions in the diet and that when efficacy was negatively affected, this could be attributed to factors such as a high zinc content (Davies et al., Citation1977), a low calcium content (Engel, Citation1972) or suboptimal physical properties of the product which affected palatability and mouthfeel. Based on their safety and efficacy studies, BP obtained authorizations to market their products as an ingredient in the diet of animals in France, Germany, the Netherlands, Belgium, Denmark, the United Kingdom, Ireland, Italy and South Africa (Champagnat, Citation1975).
The use of n-alkanes as raw material for the production of SCP caused some concern as it was shown that they could be absorbed from the SCP product and end up in the meat of the target animals. This even caused suspension of the approvals in Italy; before the issue was finally resolved (Alimenti et al., Citation1979), the production of SCP from Y. lipolytica was stopped for economic reasons (see above). For comparison, in modern, state-of-the-art Yarrowia fermentation processes, crude oil-derived carbon sources are mostly replaced by renewable ones.
Besides BP, also the French company ELF got engaged in the development of SCP products based on Y. lipolytica but, as far as we are aware, the results of the safety studies performed on their products were not published in the open literature.
A production process for citric acid using Y. lipolytica grown on n-paraffins was developed by Pfizer in the United States, and a GRAS petition for the product was submitted to the FDA in 1973 as GRP0014 (Charles Pfizer & Co., Citation1973). In this process, the cells of the production organism are completely removed. Similar to the existing process of citric acid production using Aspergillus niger on molasses, the process included a carbon treatment step prior to the final crystallization of the product. The possibility that polycyclic aromatic hydrocarbons present in the n-paraffin substrate might carry over to the final citric acid product was the main subject of concern and was extensively investigated. To back up the safety of the production microorganism, Y. lipolytica, at that time still known under the name C. lipolytica, the studies of De Groot et al. (Citation1970b) were used. In the final approval of citric acid for human consumption by the FDA, 21 CFR 173.165, C. lipolytica is recognized as a nonpathogenic organism.
Recently, FDA also listed erythritol produced with Y. lipolytica on its inventory of GRAS notifications for which it has no questions (http://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000382.pdf). In the manufacturing process, the notifier uses a strain of Y. lipolytica traditionally improved for overproduction of erythritol. No specific safety studies were performed; rather, the notifier concluded that the strain is genetically equivalent to the strain approved for citric acid production. In addition, the product is considered as substantially equivalent to various sources of erythritol previously approved as GRAS by the FDA.
Safety studies were also published on products produced with genetically modified (GM) strains of Y. lipolytica. The most detailed studies have been done on eicosapentaenoic acid triglyceride oil (EPA oil) produced by a GM Y. lipolytica strain. In a 28-day repeat-dose oral and genetic toxicity study (Belcher et al., Citation2011), no adverse test substance-related effects were observed on body weight, nutritional or other clinical or anatomic pathology parameters. The oil was not mutagenic in an in vitro Ames test and in a mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus test. In conclusion, exposure for 28 d to EPA oil derived from yeast did not produce adverse effects at doses up to 2820 mg/kg/day and was not genotoxic. The safety profile of the EPA oil in these tests was comparable to a commercial fish oil. In addition, the safety of EPA oil produced from the GM Y. lipolytica yeast was evaluated following 90 d of exposure in a study by MacKenzie et al. (Citation2010). Groups of rats received 0 (olive oil), 98, 488 or 976 mg EPA kg−1 day−1, or GRAS fish oil or deionized water by oral gavage. Rats were evaluated for in-life, neurobehavioral, anatomic and clinical pathology parameters. The results of the study demonstrate a lack of adverse effects attributed to oral exposure at doses up to 3 ml EPA oil kg−1 day−1, equivalent to 976 mg EPA kg−1 day−1. The safety profile of EPA oil was comparable to that of GRAS fish oil. These results support the use of EPA oil produced from Y. lipolytica as a safe source for use in dietary supplements.
Yarrowia lipolytica extracellular lipase Lip2 is an enzyme under development for use as a pharmaceutical in ERT for the treatment of pancreatic insufficiency. Its safety was preliminarily tested in acute and 28-day repeated dose oral toxicity studies in rats (Turki et al., Citation2010a). The results demonstrated that there were no toxicologically severe changes in clinical signs, growth, hematology, clinical chemistry, organ weights and pathology related to oral administration of extracellular Y. lipolytica lipase in rats. The no observed adverse effect level (NOAEL) as determined in these studies markedly exceeds the maximal recommended daily intake by humans for pancreatic insufficiency treatment. Results of a follow-up 90-day toxicity study in rats are not yet available.
Finally, an authoritative list of microorganisms with documented safe use in foods was published by Mogensen et al. (Citation2002a,Citationb). The list was recently updated and published by Bourdichon et al. (Citation2012) and includes Y. lipolytica as one of the (safe) yeast species.
It is meaningful to compare the safety record of Y. lipolytica, having a long history of safe use, with that of another important food-related yeast, S. cerevisiae, widely used for millennia predominantly in food, but more recently also in feed-related applications. The clinical cases caused by Y. lipolytica showed that the yeast may occasionally cause opportunistic infections in human beings with a severely debilitated immune system. Such infections can be treated effectively with available antifungal drugs, and some patients recovered even without treatment. Saccharomyces cerevisiae, with an even broader use in the food and fermentation industries, is emerging as an opportunistic pathogen, especially in immunocompromised or severely ill patients, but one case actually concerned an infection in an apparently immunocompetent person (Enache-Angoulvant & Hennequin, Citation2005, and references therein). When comparing the safety of Y. lipolytica with that of S. cerevisiae we consider that the former will stand the comparison, because (i) fewer clinical cases have been documented, (ii) the patients involved suffered from either an immune deficiency or an otherwise underlying disease or condition, (iii) the infections are largely related to the presence of invasive catheters, and (iv) the infections could be readily treated, and in some cases disappeared spontaneously.
Conclusion
The evidence reviewed in this paper supports the safety of Y. lipolytica when used in feed or food, or when deployed as a production host for a large variety of biotechnological applications. In efficacy and safety studies performed on farm and laboratory animals, respectively, Yarrowia-derived products were shown to be without mentionable side effects not only when highly purified, but also when significant portions of Yarrowia biomass remained in the final product (as for DuPont’s EPA-rich oil), or when Y. lipolytica itself was the final product (as for BP’s SCP product). The natural occurrence of Y. lipolytica in food, particularly in cheese, other dairy products, and meat, is an additional argument in favor of its safety. The occasional occurrence of opportunistic infections of Y. lipolytica in immunocompromised and catheterized patients does not differ from other microorganisms with a history of safe use, such as S. cerevisiae. Our assessment, based on currently available knowledge, that Y. lipolytica is a “safe-to-use” organism, is in full agreement with conclusions drawn by others, namely (i) Y. lipolytica is considered a class 1 organism in most countries and (ii) Y. lipolytica is included in the 2012 update of the “authoritative list of microorganisms with a documented use in food”, originally established in a joint project between the International Dairy Federation (IDF) and the European Food and Feed Cultures Association (EFFCA; Bourdichon et al., Citation2012).
Declaration of interest
Piet van Dijck and Markus Wyss are employees of DSM, which develops Yarrowia lipolytica as a production host for carotenoids. All other authors declare to have no conflicts of interest.
| Abbreviations | ||
| BP, | = | British Petroleum |
| CBS, | = | Centraalbureau voor Schimmelcultures |
| EPA, | = | eicosapentaenoic acid |
| ITS, | = | internal transcribed spacer |
| LSU, | = | large ribosomal subunit |
| MIC, | = | minimal inhibitory concentration |
| SCP, | = | single-cell protein |
| SSU, | = | small ribosomal subunit |
Supplementary Material
Download PDF (232.7 KB)Acknowledgements
Marie-Therese Fröhlich-Wyder (Research Station Agroscope, Bern, Switzerland) is gratefully acknowledged for stimulating discussions, Maria Wojtatowicz (Wroclaw University of Environmental and Life Sciences, Poland) for sharing unpublished information, Joshua Trueheart, Dan Grenfell-Lee and James Edwards (DSM) for valuable inputs and critical reading of the manuscript, Bart Theelen (CBS) for preparing and , Ann-Katrin Glatthar and Renata Corkovic (DSM) for preparing , and María Mayorga (DSM) for .
Notes
*Yarrowia lipolytica is listed as BSL 1 (De Hoog, G.S., Guarro, J., Gené, J. and Figueras, M.J. (2000) Atlas of Clinical Fungi, 2nd ed., CBS, Utrecht, The Netherlands and update in 2010), Biological agent class 1 [COGEM 24.10.2011 Classificatie apathogene schimmels (CGM/111024-02) http://www.cogem.net/index.cfm/nl/publicaties/publicatie/classificatie-apathogene-schimmels; Boekhout, T. (2011) Classificatie humaan- en dierpathogene fungi, Report COGEM, Bilthoven, The Netherlands]. The species is not listed in the European list [Directive 2000/54/EC], the Dutch list [Nederlandse Vereniging voor Microbiologie (2000) Veilig werken met micro-organismen, parasieten en cellen in laboratoria en andere werkruimten, Bilthoven, The Netherlands, pp. 1–79], the List of Biological Agents of the Dutch Ministry of Social Affairs and Employment [AI-9], the German list [Classification of Biological Agents: Fungi, BG Chemie, Brochure B 007e 9/2003 (2003)], the review by de Hoog, G.S. [Risk assessment of fungi reported from humans and animals. Mycoses 39, 407–417, 1996], or the Risk group classification for infectious agents [American Biological Safety Association].
†The Swiss list [Guidelines – Classification of organisms – Fungi (http://www.uab.cat/Document/468/768/fongs_2004.pdf) lists the species as class 2; note that this list and the COGEM list (2011) have two different classifications each for S. cerevisiae, namely class 1 (non-pathogenic strains) and class 2 (pathogenic strains)].
References
- Aboukheir S, Kilbertus G. (1974). Fréquence des levures dans les denrées alimentaires à base de viande (in French). Ann Nutr Aliment 28:539–47
- Abunyewa AAO, Laing E, Hugo A, Viljoen BC. (2000). The population change of yeasts in commercial salami. Food Microbiol 17:429–38
- Acker J, Ozanne C, Kachouri-Lafond R, et al. (2008). Dicistronic tRNA-5S rRNA genes in Yarrowia lipolytica: an alternative TFIIIA-independent way for expression of 5S rRNA genes. Nucleic Acids Res 36:5832–44
- Addis E, Fleet GH, Cox JM, et al. (2001). The growth, properties and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses. Int J Food Microbiol 69:25–36
- Agarwal S, Thakur K, Kanga A, et al. (2008). Catheter-related candidemia caused by Candida lipolytica in a child with tubercular meningitis. Indian J Pathol Microbiol 51:298–300
- Akabanda F, Owusu-Kwarteng J, Glover RLK, Tano-Debrah K. (2010). Microbiological characteristics of Ghanaian traditional fermented milk product, nunu. Nature Sci 8:178–87
- Alimenti R, Antonucci G, Bernardini MP, et al. (1979). Nutritional research on Toprina (yeasts cultivated on n-paraffins) (in Italian). Ann Ist Super Sanita 15:649–89
- Alvarez-Martín P, Flórez AB, Hernández-Barranco A, Mayo B. (2008). Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control 19:62–70
- Amaral PF, Lehocky M, Barros-Timmons AM, et al. (2006). Cell surface characterization of Yarrowia lipolytica IMUFRJ 50682. Yeast 23:867–77
- Babel W, Pöhland H-D, Soyez K. (2000). Single cell proteins. In: Ullmann’s encyclopedia of industrial chemistry. Wiley Online Library, 1–21
- Bai M, Qing M, Guo Z, et al. (2010). Occurrence and dominance of yeast species in naturally fermented milk from the Tibetan Plateau of China. Can J Microbiol 56:707–14
- Bailey R, Madden KT, Trueheart J. (2006). Production of carotenoids in oleaginous yeast and fungi. International Patent Application WO 2006/102342 A2
- Bailey RB, Madden KT, Trueheart J, et al. (2008). Production of carotenoids in oleaginous yeast and fungi. International Patent Application WO 2008/042338 A2
- Baleiras Couto MM, Hartog BJ, Huis in’t Veld JHJ, et al. (1996). Identification of spoilage yeasts in a food-production chain by microsatellite polymerase chain reaction fingerprinting. Food Microbiol 13:59–67
- Bamberg J. (2000). Nutrition. In: British petroleum and global oil 1950–1975. Cambridge, UK: Cambridge University Press, 424–44
- Bankar AV, Kumar AR, Zinjarde SS. (2009). Environmental and industrial applications of Yarrowia lipolytica. Appl Microbiol Biotechnol 84:847–65
- Barchiesi F, Tortorano AM, Di Francesco LF, et al. (1999). In-vitro activity of five antifungal agents against uncommon clinical isolates of Candida spp. J Antimicrob Chemother 43:295–9
- Barns SM, Lane DJ, Sogin ML, et al. (1991). Evolutionary relationships among pathogenic Candida species and relatives. J Bacteriol 173:2250–5
- Baroiller C, Schmidt JL. (1990). Study on the origins of yeasts from Camembert cheese (in French). Lait 70:67–84
- Barrera-Rivera KA, Flores-Carreon A, Martinez-Richa A. (2012). Synthesis of biodegradable polymers using biocatalysis with Yarrowia lipolytica lipase. Methods Mol Biol 861:485–93
- Barth G, Gaillardin C. (1997). Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19:219–37
- Belcher LA, MacKenzie SA, Donner M, et al. (2011). Safety assessment of EPA-rich triglyceride oil produced from yeast: genotoxicity and 28-day oral toxicity in rats. Regul Toxicol Pharmacol 59:53–63
- Belet N, Ciftci E, Ince E, et al. (2006). Caspofungin treatment in two infants with persistent fungaemia due to Candida lipolytica. Scand J Infect Dis 38:559–62
- Beopoulos A, Cescut J, Haddouche R, et al. (2009). Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–87
- Beopoulos A, Nicaud J-M, Gaillardin C. (2011). An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90:1193–206
- Bialecka-Florjanczyk E, Krzyczkowska J, Stolarzewicz I, Kapturowska A. (2012). Synthesis of 2-phenylethyl acetate in the presence of Yarrowia lipolytica KKP 379 biomass. J Mol Catal B Enzym 74:241–5
- Bigey F, Tuery K, Bougard D, et al. (2003). Identification of a triacylglycerol lipase gene family in Candida deformans: molecular cloning and functional expression. Yeast 20:233–48
- Bintsis T, Robinson RK. (2004). A study of the effects of adjunct cultures on the aroma compounds of Feta-type cheese. Food Chem 88:435–41
- Bizzi A, Veneroni E, Tacconi MT, et al. (1980). Accumulation and metabolism of uneven fatty acids present in single cell protein. Toxicol Lett 5:227–40
- Blanco MT, Garcia-Martos P, Garcia-Tapia A, et al. (2009). Fungemia caused by Candida lipolytica: reporting on two cases (in Spanish). Rev Iberoam Micol 26:211–2
- Bordes F, Tarquis L, Nicaud JM, Marty A. (2011). Isolation of a thermostable variant of Lip2 lipase from Yarrowia lipolytica by directed evolution and deeper insight into the denaturation mechanisms involved. J Biotechnol 156:117–24
- Botes AL, Labuscagne M, Roth R, et al. (2007). Recombinant yeasts for synthesizing epoxide hydrolases. International Patent Application WO 2007/010403 A2
- Botes AL, Mitra RK. (2006). Epoxide hydrolases: a biocatalytic technology platform for the production of chiral pharmaceutical intermediates. Innov Pharmaceut Technol 21:86–9
- Bourdichon F, Casaregola S, Farrokh C, et al. (2012). Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154:87–97
- Bours J, Mossel DAA. (1973). A comparison of methods for the determination of lipolytic properties of yeasts mainly isolated from margarine, moulds, and bacteria. Arch Lebensmittelhyg 24:197–203
- Bremenkamp RM, Caris AR, Jorge AO, et al. (2011). Prevalence and antifungal resistance profile of Candida spp. oral isolates from patients with type 1 and 2 diabetes mellitus. Arch Oral Biol 56:549–55
- Brocklehurst TF, Lund BM. (1985). Microbiological changes in cottage cheese varieties during storage at +7 °C. Food Microbiol 2:207–33
- Buick RK, Damoglou AP. (1989). Effect of modified atmosphere packaging on the microbial development and visible shelf life of a mayonnaise-based vegetable salad. J Sci Food Agric 46:339–47
- Callewaert NLM, Vervecken W, Marcel de Pourcq KJ, et al. (2008). Glycosylation of molecules. International Patent Application WO 2008/120107 A2
- Cantor MD, van den Tempel T, Hansen TK, Ardö Y. (2004). Blue cheese. In: Fox PF, McSweeney PLH, Cogan TM, Guinee TP, eds. Cheese: chemistry, physics and microbiology, Vol 2: major cheese groups. London, UK: Elsevier Academic Press, 175–98
- Carreira A, Dillinger K, Eliskases-Lechner F, et al. (2002). Influence of selected factors on browning of Camembert cheese. J Dairy Res 69:281–92
- Carreira A, Ferreira LM, Loureiro V. (2001). Brown pigments produced by Yarrowia lipolytica result from extracellular accumulation of homogentisic acid. Appl Environ Microbiol 67:3463–8
- Casaregola S, Feynerol C, Diez M, et al. (1997). Genomic organization of the yeast Yarrowia lipolytica. Chromosoma 106:380–90
- Castañon-Vélez M, Leal BI. (1973). Microorganisms in margarine deterioration. LWT Report 6:70–2
- Champagnat A. (1975). Les protéines de fermentation des hydrocarbures (in French). Z Ernährungswiss 14:125–32
- Chang CL, Park TH, Lee EY, et al. (2001). Recurrent self-limited fungemia caused by Yarrowia lipolytica in a patient with acute myelogenous leukemia. J Clin Microbiol 39:1200–01
- Charles Pfizer & Co. (1973). GRAS affirmation of citric acid from n-paraffin fermentation. GRAS Petition GRP 0014 (document can be obtained upon request from the FDA)
- Chatzifragkou A, Makri A, Belka A, et al. (2011). Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36:1097–108
- Chen L-S, Ma Y, Maubois J-L, et al. (2010). Identification of yeasts from raw milk and selection for some specific antioxidant properties. Int J Dairy Technol 63:47–54
- Cho EM, Lee HS, Eom CY, Ohta A. (2010). Construction of high sensitive detection system for endocrine disruptors with yeast n-alkane-assimilating Yarrowia lipolytica. J Microbiol Biotechnol 20:1563–70
- Chuang LT, Chen DC, Nicaud JM, et al. (2010). Co-expression of heterologous desaturase genes in Yarrowia lipolytica. N Biotechnol 27:277–82
- Cirigliano MC, Carman GM. (1985). Purification and characterization of liposan, a bioemulsifier from Candida lipolytica. Appl Environ Microbiol 50:846–50
- Clare JJ, Davidow LS, Gardner DC, Oliver SG. (1986). Cloning and characterisation of the ribosomal RNA genes of the dimorphic yeast, Yarrowia lipolytica. Curr Genet 10:449–52
- Connor JM. (2008). Global price fixing. 2nd ed. Berlin, Heidelberg, Germany: Springer-Verlag
- Corbo MR, Lanciotti R, Albenzio M, Sinigaglia M. (2001). Occurrence and characterization of yeasts isolated from milks and dairy products of Apulia region. Int J Food Microbiol 69:147–52
- Croxatto A, Prod'hom G, Greub G. (2012). Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev 36:380–407
- D’Antonio D, Romano F, Pontieri E, et al. (2002). Catheter-related candidemia caused by Candida lipolytica in a patient receiving allogeneic bone marrow transplantation. J Clin Microbiol 40:1381–6
- Damude HG, Gillies PJ, Macool DJ, et al. (2009a). High arachidonic acid producing strains of Yarrowia lipolytica. United States Patent 7,588,931
- Damude HG, Macool DJ, Picataggio SK, et al. (2009b). Docosahexaenoic acid producing strains of Yarrowia lipolytica. United States Patent 7,550,286 B2
- Daryaei H, Coventry J, Versteeg C, Sherkat F. (2010). Combined pH and high hydrostatic pressure effects on Lactococcus starter cultures and Candida spoilage yeasts in a fermented milk test system during cold storage. Food Microbiol 27:1051–6
- Davies NT, Soliman HS, Corrigall W, Flett A. (1977). The susceptibility of suckling lambs to zinc toxicity. Br J Nutr 38:153–6
- De Groot AP. (1976). Safety evaluation studies with SCP. PAG Bull 6:41–4
- De Groot AP, Til HP, Feron VJ. (1970a). Safety evaluation of yeast grown on hydrocarbons. I. One-year feeding study in rats with yeast grown on gas oil. Food Cosmet Toxicol 8:267–76
- De Groot AP, Til HP, Feron VJ. (1970b). Safety evaluation of yeast grown on hydrocarbons. II. One-year feeding study in rats with yeast grown on pure n-paraffins. Food Cosmet Toxicol 8:499–507
- De Groot AP, Til HP, Feron VJ. (1971). Safety evaluation of yeast grown on hydrocarbons. III. Two-year feeding and multigeneration study in rats with yeast grown on gas oil. Food Cosmet Toxicol 9:787–800
- De Groot AP, van der Meulen CD, Til HP, Feron VJ. (1975). Safety evaluation of yeast grown on hydrocarbons. IV. Two-year feeding and multigeneration study in rats with yeast grown on pure n-paraffins. Food Cosmet Toxicol 13:619–27
- De Pourcq K, Tiels P, Van Hecke A, et al. (2012a). Engineering Yarrowia lipolytica to produce glycoproteins homogeneously modified with the universal Man3GlcNAc2 N-glycan core. PLoS One 7:e39976
- De Pourcq K, Vervecken W, Dewerte I, et al. (2012b). Engineering the yeast Yarrowia lipolytica for the production of therapeutic proteins homogeneously glycosylated with Man8GlcNAc2 and Man5GlcNAc2. Microb Cell Fact 11:53
- De Wit M, Osthoff G, Viljoen BC, Hugo A. (2005). A comparative study of lipolysis and proteolysis in Cheddar cheese and yeast-inoculated Cheddar cheeses during ripening. Enzyme Microb Technol 37:606–16
- De Zeeuw JR, Tynan EJ. (1973a). Fermentation process for the production of D-mannitol. United States Patent 3,736,229
- De Zeeuw JR, Tynan EJ. (1973b). Fermentation process for the production of erythritol. United States Patent 3,756,917
- Deak T, Beuchat LR. (1987). Identification of foodborne yeasts. J Food Prot 50:243–64
- Deak T, Beuchat LR. (1996). Handbook of food spoilage yeasts. Boca Raton, FL: CRC Press
- Deetae P, Mounier J, Bonnarme P, et al. (2009). Effects of Proteus vulgaris growth on the establishment of a cheese microbial community and on the production of volatile aroma compounds in a model cheese. J Appl Microbiol 107:1404–13
- Delbes-Paus C, Pochet S, Helinck S, et al. (2012). Impact of Gram-negative bacteria in interaction with a complex microbial consortium on biogenic amine content and sensory characteristics of an uncooked pressed cheese. Food Microbiol 30:74–82
- Desnos-Ollivier M, Robert V, Raoux-Barbot D, et al. (2012). Antifungal susceptibility profiles of 1698 yeast reference strains revealing potential emerging human pathogens. PLoS One 7:e32278
- Devoyod J-J. (1990). Yeasts in cheese-making. In: Spencer JFT, Spencer DM, eds. Yeast technology. New York: Springer-Verlag, 228–40
- Dhiman N, Hall L, Wohlfiel SL, et al. (2011). Performance and cost analysis of matrix-assisted laser desorption ionization-time of flight mass spectrometry for routine identification of yeast. J Clin Microbiol 49:1614–6
- Dujon B, Sherman D, Fischer G, et al. (2004). Genome evolution in yeasts. Nature 430:35–44
- Duquesne S, Bordes F, Fudalej F, et al. (2012). The yeast Yarrowia lipolytica as a generic tool for molecular evolution of enzymes. Methods Mol Biol 861:301–12
- Enache-Angoulvant A, Hennequin C. (2005). Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis 41:1559–68
- Encinas JP, Lopez-Diaz TM, Garcia-Lopez ML, et al. (2000). Yeast populations on Spanish fermented sausages. Meat Sci 54:203–8
- Engel C. (1972). Safety evaluation of yeast grown on hydrocarbons. In: Gounelle de Pontanel H, ed. Proteins from hydrocarbons. London: Academic Press, 53–81
- Entel HJ. (1961). Die in Rohwürsten vorkommenden Hefen (in German). Fleischwirtschaft, 387–390
- Ferreira AD, Viljoen BC. (2003). Yeasts as adjunct starters in matured Cheddar cheese. Int J Food Microbiol 86:131–40
- Fickers P, Benetti PH, Wache Y, et al. (2005). Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–43
- Fickers P, Marty A, Nicaud JM. (2011). The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol Adv 29:632–44
- Fitzpatrick DA, Logue ME, Stajich JE, Butler G. (2006). A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol 6:99
- Fleet G. (1992). Spoilage yeasts. Crit Rev Biotechnol 12:1–44
- Fleet GH. (1990). Yeasts in dairy products. J Appl Bacteriol 68:199–211
- Fontes GC, Amaral PF, Nele M, Coelho MA. (2010). Factorial design to optimize biosurfactant production by Yarrowia lipolytica. J Biomed Biotechnol 2010:821306
- Fournier P, Gaillardin C, Persuy MA, et al. (1986). Heterogeneity in the ribosomal family of the yeast Yarrowia lipolytica: genomic organization and segregation studies. Gene 42:273–82
- Franck R, Baluch A, Sandin R, Green J. (2010). Yarrowia lipolytica as normal human flora: a case series of 24 patients with positive cultures and no attributable disease. Poster 656, 48th Annual Meeting, Infectious Diseases Society of America, Vancouver, Canada
- Freitas AC, Pintado AE, Pintado ME, Malcata FX. (1999). Role of dominant microflora of Picante cheese on proteolysis and lipolysis. Int Dairy J 9:593–603
- Fried JH. (1972). Method of producing citric acid by fermentation. United States Patent 3,632,476
- Fröhlich-Wyder M-T. (2003). Yeasts in dairy products. In: Boekhout T, Robert V, eds. Yeasts in food: beneficial and detrimental aspects. Hamburg, Germany: Behr’s Verlag, 209–37
- Fung DY, Liang C. (1990). Critical review of isolation, detection, and identification of yeasts from meat products. Crit Rev Food Sci Nutr 29:341–79
- Gaborit P, Menard A, Morgan F. (2001). Impact of ripening strains on the typical flavour of goat cheeses. Int Dairy J 11:315–25
- Gadaga TH, Mutukumira AN, Narvhus JA. (2000). Enumeration and identification of yeasts isolated from Zimbabwean traditional fermented milk. Int Dairy J 10:459–66
- Garcia EE, Aguedo M, Gomes N, et al. (2009). Production of 3-hydroxy-γ-decalactone, the precursor of two decenolides with flavouring properties, by the yeast Yarrowia lipolytica. J Mol Catal B 57:22–6
- Gardini F, Suzzi G, Lombardi A, et al. (2001). A survey of yeasts in traditional sausages of southern Italy. FEMS Yeast Res 1:161–7
- Gardini F, Tofalo R, Belletti N, et al. (2006). Characterization of yeasts involved in the ripening of Pecorino Crotonese cheese. Food Microbiol 23:641–8
- Geysens SCJ, Vervecken W. (2011). Yeast strains producing mammalian-like complex N-glycans. International Patent Application WO 2011/061629 A2
- Gianni C, Lopez C, Medina LM, et al. (2008). Dynamics and characterization of a yeast population from an Italian fermented sausage. Ital J Food Sci 20:239–44
- Goerges S, Aigner U, Silakowski B, Scherer S. (2006). Inhibition of Listeria monocytogenes by food-borne yeasts. Appl Environ Microbiol 72:313–8
- Gomes N, Teixeira JA, Belo I. (2012). Fed-batch versus batch cultures of Yarrowia lipolytica for γ-decalactone production from methyl ricinoleate. Biotechnol Lett 34:649–54
- Guerzoni ME, Gobbetti M, Lanciotti R, et al. (1998). Yarrowia lipolytica as potential ripening agent in milk products. In: Jakobsen M, Narvhus J, Viljoen BC, eds. Yeasts in dairy industry: positive and negative aspects. IDF Symposium, Copenhagen, Denmark, 1996. International Dairy Federation, Brussels, Belgium, 23–33
- Guo Y, Song H, Wang Z, Ding Y. (2012). Expression of POX2 gene and disruption of POX3 genes in the industrial Yarrowia lipolytica on the γ-decalactone production. Microbiol Res 167:246–52
- Haddouche R, Poirier Y, Delessert S, et al. (2011). Engineering polyhydroxyalkanoate content and monomer composition in the oleaginous yeast Yarrowia lipolytica by modifying the β-oxidation multifunctional protein. Appl Microbiol Biotechnol 91:1327–40
- Hadjipanayiotou M. (1995). Composition of ewe, goat and cow milk and of colostrum of ewes and goats. Small Ruminant Res 18:255–62
- Hanssen JT, Farstad L. (1980). Effects of feeding large amounts of “Pruteen” and “Toprina” on some biological parameters in growing finishing pigs. Acta Agric Scand 30:74–80
- Hawksworth DL, Crous PW, Redhead SA, et al. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–12
- Heard GM, Fleet GH. (2000). Yarrowia (Candida) lipolytica. In: Robinson RK, Batt CA, Patel P, eds. Encyclopedia of food microbiology. New York: Academic Press, 360–5
- Heretsch P, Thomas F, Aurich A, et al. (2008). Syntheses with a chiral building block from the citric acid cycle: (2R,3S)-isocitric acid by fermentation of sunflower oil. Angew Chem Int Ed Engl 47:1958–60
- Holz M, Otto C, Kretzschmar A, et al. (2011). Overexpression of alpha-ketoglutarate dehydrogenase in Yarrowia lipolytica and its effect on production of organic acids. Appl Microbiol Biotechnol 89:1519–26
- Holzschu DL, Chandler FW, Ajello L, Ahearn DG. (1979). Evaluation of industrial yeasts for pathogenicity. Sabouraudia 17:71–8
- Irlinger F, Yung SA, Sarthou AS, et al. (2012). Ecological and aromatic impact of two Gram-negative bacteria (Psychrobacter celer and Hafnia alvei) inoculated as part of the whole microbial community of an experimental smear soft cheese. Int J Food Microbiol 153:332–8
- Ismail SA, Deak T, El-Rahman HA, et al. (2000). Presence and changes in populations of yeasts on raw and processed poultry products stored at refrigeration temperature. Int J Food Microbiol 62:113–21
- Iucci L, Patrignani F, Belletti N, et al. (2007). Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 2: evaluation of their effects on sensory quality and biogenic amine content. Meat Sci 75:669–75
- Jackson N, Kirkpatrick GM. (1978). A study of the nutritive value of Toprina G in the diet of two hybrid strains of caged laying hens. J Sci Food Agric 29:1030–6
- Jakobsen M, Larsen MD, Jespersen L. (2002). Production of bread, cheese and meat. In: Osiewacz HD, ed. The Mycota, Vol X: industrial applications. Berlin, Germany: Springer-Verlag, 3–22
- James LC, Strick CA (1998) Multiple integrative vectors and Yarrowia lipolytica transformants. United States Patent 5,786,212
- James TY, Kauff F, Schoch CL, et al. (2006). Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–22
- Jiménez-Bremont JF, Ruiz-Herrera J, Dominguez A. (2001). Disruption of gene YlODC reveals absolute requirement of polyamines for mycelial development in Yarrowia lipolytica. FEMS Yeast Res 1:195–204
- Kamzolova SV, Chiglintseva MN, Lunina JN, Morgunov IG. (2012). α-Ketoglutaric acid production by Yarrowia lipolytica and its regulation. Appl Microbiol Biotechnol 96:783–91
- Kamzolova SV, Yusupova AI, Vinokurova NG, et al. (2009). Chemically assisted microbial production of succinic acid by the yeast Yarrowia lipolytica grown on ethanol. Appl Microbiol Biotechnol 83:1027–34
- Kang K-W, Yoon HJ, Jung SH, et al. (2008). A case of Yarrowia lipolytica fungemia after raw beef ingestion (in Korean). Korean J Med 74:566–9
- Karatay SE, Donmez G. (2010). Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour Technol 101:7988–90
- Katre GK, Joshi CJ, Khot MK, et al. (2012). Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2:36
- Kniewallner K, Haas J. (1966). Fehlfabrikate bei Rohwürsten durch Hefen (in German). Wiener Tierärztl Monatsschr 53:466–8
- Knutsen AK, Robert V, Poot GA, et al. (2007). Polyphasic re-examination of Yarrowia lipolytica strains and the description of three novel Candida species: Candida oslonensis sp. nov., Candida alimentaria sp. nov. and Candida hollandica sp. nov. Int J Syst Evol Microbiol 57:2426–35
- Kovalchuk A, Senam S, Mauersberger S, Barth G. (2005). Tyl6, a novel Ty3/gypsy-like retrotransposon in the genome of the dimorphic fungus Yarrowia lipolytica. Yeast 22:979–91
- Kumura H, Ikezu Y, Tajima E, Shimazaki K-I. (2009). Growth and viability effects of cell-free extracts from dairy origin yeast on bifidobacteria. Milchwissenschaft 64:256–9
- Kumura H, Tanoue Y, Tsukahara M, et al. (2004). Screening of dairy yeast strains for probiotic applications. J Dairy Sci 87:4050–6
- Kurtzman CP. (2005). New species and a new combination in the Hyphopichia and Yarrowia yeast clades. Antonie Van Leeuwenhoek 88:121–30
- Kurtzman CP. (2011). Yarrowia van der Walt & von Arx (1980). In: Kurtzman CP, Fell JW, Boekhout T, eds. The yeasts, a taxonomic study, vol 2. Amsterdam, The Netherlands: Elsevier, 927–9
- Kurtzman CP, Fell JW, eds. (1998). The yeasts: a taxonomic study. Amsterdam: Elsevier
- Kurtzman CP, Fell JW, Boekhout T, eds. (2011). The yeasts: a taxonomic study. Amsterdam, The Netherlands: Elsevier
- Kurtzman CP, Robnett CJ. (1994). Orders and families of ascosporogenous yeasts and yeast-like taxa compared from ribosomal RNA sequence similarities. In: Hawksworth DL, ed. Ascomycete systematics: problems and perspectives in the nineties. New York: Plenum Press, 249–58
- Kurtzman CP, Robnett CJ. (1995). Molecular relationships among hyphal ascomycetous yeasts and yeastlike taxa. Can J Bot 73:S824–30
- Kurtzman CP, Robnett CJ. (1998). Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73:331–71
- Lachance MA, Boekhout T, Scorzetti G, et al. (2011). Candida Berkhout (1923). In: Kurtzman CP, Fell JW, Boekhout T, eds. The yeasts, a taxonomic study. Amsterdam, The Netherlands: Elsevier, 987–1278
- Lanciotti R, Massa S, Guerzoni ME, Di Fabio G. (1992). Light butter: natural microbial population and potential growth of Listeria monocytogenes and Yersinia enterocolitica. Lett Appl Microbiol 15:256–8
- Lanciotti R, Vannini L, Chaves Lopez C, et al. (2005). Evaluation of the ability of Yarrowia lipolytica to impart strain-dependent characteristics to cheese when used as a ripening adjunct. Int J Dairy Technol 58:89–99
- Langeron M, Guerra P. (1938). Nouvelles recherches de zymologie médicale 2me Partie. Etudes monographiques sur les Mycotoruloidées. Le genre Candida Berkhout 1923 (in French). Ann Parasitol Hum Comp 16:481–525
- Larpin-Laborde S, Imran M, Bonaiti C, et al. (2011). Surface microbial consortia from Livarot, a French smear-ripened cheese. Can J Microbiol 57:651–60
- Larpin S, Mondoloni C, Goerges S, et al. (2006). Geotrichum candidum dominates in yeast population dynamics in Livarot, a French red-smear cheese. FEMS Yeast Res 6:1243–53
- Leblond Y, Mouz N, Marty A, Uribelarrea J-L. (2012). Method for producing lipase, transformed Yarrowia lipolytica cell capable of producing said lipase and their uses. United States Patent Application US 2012/0003716 A1
- Leistner L, Bem Z. (1970). Vorkommen und Bedeutung von Hefen bei Pökelfleischwaren (in German). Fleischwirtschaft 50:492–3
- Lelieveld HL, Boon B, Bennett A, et al. (1996). Safe biotechnology. 7. Classification of microorganisms on the basis of hazard. Working party “Safety in Biotechnology” of the european federation biotechnology. Appl Microbiol Biotechnol 45:723–9
- Levinson WE, Kurtzman CP, Kuo TM. (2007). Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb Technol 41:292–5
- Levy JM, Levin RS, Clancy JT. (2003). Lower-extremity wounds inflicted by rats. J Am Podiatr Med Assoc 93:58–61
- Limtong S, Youngmanitchai W, Kawasaki H, Seki T. (2008). Candida phangngensis sp. nov., an anamorphic yeast species in the Yarrowia clade, isolated from water in mangrove forests in Phang-Nga Province, Thailand. Int J Syst Evol Microbiol 58:515–9
- Lin CCS, Fung DYC. (1987). Comparative biochemical reactions and identification of food yeasts by the conventional method, Fung’s mini-method, Minitek, and the automicrobic system. Crit Rev Biotechnol 7:1–16
- Linares DM, Del Rio B, Ladero V, et al. (2012). Factors influencing biogenic amines accumulation in dairy products. Front Microbiol 3:180
- Litchfield JH. (1983). Single-cell proteins. Science 219:740–6
- Liti G, Carter DM, Moses AM, et al. (2009). Population genomics of domestic and wild yeasts. Nature 458:337–41
- Liu SQ, Tsao M. (2009). Enhancement of survival of probiotic and non-probiotic lactic acid bacteria by yeasts in fermented milk under non-refrigerated conditions. Int J Food Microbiol 135:34–8
- Liu X-Y, Chi Z, Liu G-L, et al. (2013). Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar Biotechnol (NY) 15:26–36
- Lodder J, Kreger van Rij NJW. (1952). The yeasts, a taxonomic study. Amsterdam, The Netherlands: North Holland Publ. Co.
- Lopandic K, Zelger S, Banszky LK, et al. (2006). Identification of yeasts associated with milk products using traditional and molecular techniques. Food Microbiol 23:341–50
- Lopez Del Castillo-Lozano M, Delile A, et al. (2007). Comparison of volatile sulphur compound production by cheese-ripening yeasts from methionine and methionine-cysteine mixtures. Appl Microbiol Biotechnol 75:1447–54
- Lourens-Hattingh A, Viljoen BC. (2002). Survival of dairy-associated yeasts in yoghurt and yoghurt-related products. Food Microbiol 19:597–604
- MacKenzie SA, Belcher LA, Sykes GP, et al. (2010). Safety assessment of EPA-rich oil produced from yeast: results of a 90-day subchronic toxicity study. Regul Toxicol Pharmacol 58:490–500
- Madzak C, Gaillardin C, Beckerich JM. (2004). Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: a review. J Biotechnol 109:63–81
- Mann R, Rehm HJ. (1977). Degradation of aflatoxin B1 by various microorganisms. Z Lebensm Unters-Forsch 163:39–43
- Marklein G, Josten M, Klanke U, et al. (2009). Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J Clin Microbiol 47:2912–7
- Martin N, Berger C, Le Du C, Spinnler HE. (2001). Aroma compound production in cheese curd by coculturing with selected yeast and bacteria. J Dairy Sci 84:2125–35
- Martinez J, Wrent P, Quiros M, et al. (2004). Alteración de embutidos por levaduras productoras de gas (in Spanish). Alim Equipos Tecnol 193:72–8
- Matty AJ, Smith P. (1978). Evaluation of a yeast, a bacterium and an alga as a protein source for rainbow trout. I. Effect of protein level on growth, gross conversion efficiency and protein conversion efficiency. Aquaculture 14:235–46
- Mehnert B. (1957). Über das vorkommen und die biologische bedeutung von hefen im kot von menschen und tieren (in German). Zentralbl Bakteriol Parasitenk Infektionskrankh Hyg 110:50–81
- Meng Y, Wang G, Yang N, et al. (2011). Two-step synthesis of fatty acid ethyl ester from soybean oil catalyzed by Yarrowia lipolytica lipase. Biotechnol Biofuels 4:6
- Meyer SA, Ahearn DG, Yarrow D. (1984). Candida Berkhout. In: Kreger-van Rij NJW, ed. The yeasts, a taxonomic study. Amsterdam, The Netherlands: Elsevier, 585–844
- Moeller L, Grunberg M, Zehnsdorf A, et al. (2011). Repeated fed-batch fermentation using biosensor online control for citric acid production by Yarrowia lipolytica. J Biotechnol 153:133–7
- Mogensen G, Salminen S, O’Brien J, et al. (2002a). Food microorganisms: health benefits, safety evaluation and strains with documented history of use in foods. Bull IDF 377:4–9
- Mogensen G, Salminen S, O’Brien J, et al. (2002b). Inventory of microorganisms with a documented history of use in food. Bull IDF 377:10–19
- Monnet C, Bleicher A, Neuhaus K, et al. (2010). Assessment of the anti-listerial activity of microfloras from the surface of smear-ripened cheeses. Food Microbiol 27:302–10
- Müller S, Sandal T, Kamp-Hansen P, Dalboge H. (1998). Comparison of expression systems in the yeasts Saccharomyces cerevisiae, Hansenula polymorpha, Kluyveromyces lactis, Schizosaccharomyces pombe and Yarrowia lipolytica. Cloning of two novel promoters from Yarrowia lipolytica. Yeast 14:1267–83
- Naumova E, Naumov G, Fournier P, et al. (1993). Chromosomal polymorphism of the yeast Yarrowia lipolytica and related species: electrophoretic karyotyping and hybridization with cloned genes. Curr Genet 23:450–4
- Neuveglise C, Marck C, Gaillardin C. (2011). The intronome of budding yeasts. C R Biol 334:662–70
- Newberne PM, Young VR. (1975). An acute toxicity study with three yeast protein sources in beagle dogs. Folia Vet Lat 5:515–26
- Nielsen DS, Jacobsen T, Jespersen L, et al. (2008). Occurrence and growth of yeasts in processed meat products: implications for potential spoilage. Meat Sci 80:919–26
- Ninin E, Morin O, Le Tortorec S, et al. (1997). Invasive Candida lipolytica infection after allogenic bone marrow transplantation (in French). J Mycol Med 7:212–4
- Nitzulescu V, Niculescu M. (1976). Considérations sur trois cas de candidose oculaire à Candida lipolytica (in French). Arch Roum Path Exp Microbiol 35:269–72
- Nubel R, Fitts R, Findlay G. (1979). Fermentation process for the production of citric acid. United States Patent 4,155,811
- OECD. (1992). Safety considerations for biotechnology. Available from: http://www.oecd.org/science/biotechnologypolicies/2375496.pdf [last accessed on 1 Aug 2012]
- Ogrydziak DM. (1988). Development of genetic maps of non-conventional yeasts. J Basic Microbiol 28:185–96
- Oh E-G, Park M-Y, Chang D-S. (1998). Proteolytic yeasts isolated from mackerel (Scomber japonicus). J Korean Fish Soc 31:471–6
- Oliveira Leme FC, de Barros Negreiros MM, Koga FA, et al. (2011). Evaluation of pathogenic fungi occurrence in traumatogenic structures of freshwater fish. Rev Soc Brasil Med Trop 44:182–5
- Otto C, Yovkova V, Aurich A, et al. (2012). Variation of the by-product spectrum during α-ketoglutaric acid production from raw glycerol by overexpression of fumarase and pyruvate carboxylase genes in Yarrowia lipolytica. Appl Microbiol Biotechnol 95:905–17
- Otto C, Yovkova V, Barth G. (2011). Overproduction and secretion of α-ketoglutaric acid by microorganisms. Appl Microbiol Biotechnol 92:689–95
- Özdemir H, Karbuz A, Ciftci E, et al. (2011). Successful treatment of central venous catheter infection due to Candida lipolytica by caspofungin-lock therapy. Mycoses 54:e647–9
- Papanikolaou S, Aggelis G. (2011). Lipids of oleaginous yeasts. Part II: technology and potential applications. Eur J Lipid Sci Technol 113:1052–73
- Pariza MW, Johnson EA. (2001). Evaluating the safety of microbial enzyme preparations used in food processing: update for a new century. Regul Toxicol Pharmacol 33:173–86
- Patrignani F, Iucci L, Vallicelli M, et al. (2007). Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 1: evaluation of their effects on microbial evolution, lipolytic and proteolytic patterns. Meat Sci 75:676–86
- Patrignani F, Vannini L, Gardini F, et al. (2011a). Variability of the lipolytic activity and volatile molecules production by a strain of Yarrowia lipolytica in pork fat and its dependence on environmental conditions. Meat Sci 89:21–6
- Patrignani F, Vannini L, Gardini F, et al. (2011b). Variability of the lipolytic activity in Yarrowia lipolytica strains in pork fat. Meat Sci 88:689–93
- Peppler HJ. (1977). Yeast properties adversely affecting food fermentations. Food Technol 31:62–5
- Peter G, Dlauchy D, Vasdinyei R, et al. (2004). Candida galli sp. nov., a new yeast from poultry. Antonie Van Leeuwenhoek 86:105–10
- Pfaller MA, Diekema DJ. (2004). Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect 10:11–23
- Pfaller MA, Diekema DJ, Messer SA, et al. (2003). In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J Clin Microbiol 41:78–83
- Picataggio SK, Yadav NS, Zhu QQ. (2007). Production of polyunsaturated fatty acids in oleaginous yeasts. United States Patent 7,238,482
- Pinto A, Halliday C, Zahra M, et al. (2011). Matrix-assisted laser desorption ionization-time of flight mass spectrometry identification of yeasts is contingent on robust reference spectra. PLoS One 6:e25712
- Pitt JI, Hocking AD. (1985). Yeasts. In: Fungi and food spoilage. Sydney, Australia: Academic Press, 335–64
- Poncet S, Arpin M. (1965). Candida species without fermentative capacity (Cryptococcoceae). Antonie Van Leeuwenhoek 31:433–64
- Rabenhorst J, Gatfield I. (2002). Method of producing γ-decalactone using Yarrowia lipolytica strain HR 145 (DSM 12397). United States Patent 6,451,565
- Ramos O, Santos A, Leão M, et al. (2012). Antimicrobial activity of edible coatings prepared from whey protein isolate and formulated with various antimicrobial agents. Int Dairy J 25:132–41
- Rex JH, Pfaller MA, Barry AL, et al. (1995). Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother 39:40–4
- Ribeiro BD, de Castro AM, Coelho MAZ, Freire DMG. (2011). Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res 2011:Art ID 615803
- Rimek D, Redetzke K, Singh J, et al. (2004). Performance of the Candida mannan antigen detection in patients with fungemia. Mycoses 47:23–6
- Rodrigues G, Pais C. (2000). The influence of acetic and other weak carboxylic acids on growth and cellular death of the yeast Yarrowia lipolytica. Food Technol Biotechnol 38:27–32
- Rodrigues Paula C, Gambale W, Iaria ST, Reis Filho SA. (1988). Occurrence of fungi in butter available for public consumption in the city of São Paulo (in Portuguese). Rev Microbiol (Sao Paulo) 19:317–20
- Rohm H, Eliskases-Lechner F, Bräuer M. (1992). Diversity of yeasts in selected dairy products. J Appl Bacteriol 72:370–6
- Romano P, Capece A, Jespersen L. (2006). Taxonomic and ecological diversity of food and beverage yeasts. In: Querol A, Fleet GH, eds. The yeast handbook: yeasts in food and beverages. Berlin, Germany: Springer-Verlag, 13–53
- Roostita R, Fleet GH. (1996). The occurrence and growth of yeasts in Camembert and blue-veined cheeses. Int J Food Microbiol 28:393–404
- Ross HM, Harden TJ, Nichol AW, Deeth HC. (2000). Isolation and investigation of micro-organisms causing brown defects in mould-ripened cheeses. Aust J Dairy Technol 55:5–8
- Ross SS, Morris EO. (1965). An investigation of the yeast flora of marine fish from Scottish coastal waters and a fishing ground off Iceland. J Appl Bacteriol 28:224–34
- Rufino RD, Luna JM, Sarubbo LA, et al. (2011). Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf B Biointerfaces 84:1–5
- Ryckaert S, Lerondel G. (2011). Methods and compositions for displaying a polypeptide on a yeast cell surface. International Patent Application WO 2011/089527 A1
- Rywinska A, Juszczyk P, Wojtatowicz M, et al. (2013). Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 48:148–66
- Rywinska A, Musial I, Rymowicz W, et al. (2012). Effect of agitation and aeration on the citric acid production by Yarrowia lipolytica grown on glycerol. Prep Biochem Biotechnol 42:279–91
- Sabirova JS, Haddouche R, Van Bogaert IN, et al. (2011). The ‘LipoYeasts’ project: using the oleaginous yeast Yarrowia lipolytica in combination with specific bacterial genes for the bioconversion of lipids, fats and oils into high-value products. Microb Biotechnol, 4:47–54
- Samelis J, Sofos JN. (2003). Yeasts in meat and meat products. In: Boekhout T, Robert V, eds. Yeasts in Food. Boca Raton, FL: CRC Press, 239–65
- Samson RA, van Kooij JA, de Boer E. (1987). Microbiological quality of commercial tempeh in the Netherlands. J Food Protect 50:92–4
- Sancho T, Giménez-Jurado G, Malfeito-Ferreira M, Loureiro V. (2000). Zymological indicators: a new concept applied to the detection of potential spoilage yeast species associated with fruit pulps and concentrates. Food Microbiol 17:613–24
- Schoch CL, Seifert KA, Huhndorf S, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA 109:6241–6
- Schrader J, Etschmann MM, Sell D, et al. (2004). Applied biocatalysis for the synthesis of natural flavour compounds: current industrial processes and future prospects. Biotechnol Lett 26:463–72
- Sedgman CA, Roy JH, Thomas J. (1985). Digestion, absorption and utilization of single-cell protein by the preruminant calf. Abomasal outflow and its composition from calves given milk-substitute diets containing varying amounts of either bacterial or yeast protein. Br J Nutr 53:673–89
- Selgas MD, Ros J, Garcia ML. (2003). Effect of selected yeast strains on the sensory properties of dry fermented sausages. Eur Food Res Technol 217:475–80
- Shacklady CA. (1969). The production and evaluation of protein derived from organisms grown on hydrocarbon residues. Proc Nutr Soc 28:91–7
- Shacklady CA. (1972). Yeasts grown on hydrocarbons as new sources of protein. World Rev Nutr Diet 14:154–79
- Shannon DWF, McNab JM. (1972). The effect of different dietary levels of a n-paraffin-grown yeast on the growth and food intake of broiler chicks. Br Poultry Sci 13:267–72
- Sharpe PL, Ye RW, Zhu QQ. (2008). Carotenoid production in a recombinant oleaginous yeast. International Patent Application WO 2008/073367 A1
- Shin JH, Kook H, Shin DH, et al. (2000). Nosocomial cluster of Candida lipolytica fungemia in pediatric patients. Eur J Clin Microbiol Infect Dis 19:344–9
- Shinde NR, Bankar AV, Kumar AR, Zinjarde SS. (2012). Removal of Ni (II) ions from aqueous solutions by biosorption onto two strains of Yarrowia lipolytica. J Environ Manage 102:115–24
- Sinigaglia M, Lanciotti R, Guerzoni ME. (1994). Biochemical and physiological characteristics of Yarrowia lipolytica strains in relation to isolation source. Can J Microbiol 40:54–9
- Song H, Zhou L, Zhang L, et al. (2011). Construction of a whole-cell catalyst displaying a fungal lipase for effective treatment of oily wastewaters. J Mol Catal B 71:166–70
- Spinnler HE, Berger C, Lapadatescu C, Bonnarme P. (2001). Production of sulfur compounds by several yeasts of technological interest for cheese ripening. Int Dairy J 11:245–52
- Steensels D, Verhaegen J, Lagrou K. (2011). Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the identification of bacteria and yeasts in a clinical microbiological laboratory: a review. Acta Clin Belg 66:267–73
- Stephanopoulos G, Abidi SHI. (2011). Microbial engineering for the production of fatty acids and fatty acid derivatives. United States Patent Application US 2011/0223641 A1
- Stratford M. (2006). Food and beverage spoilage yeasts. In: Querol A, Fleet GH, eds. The yeast handbook: yeasts in food and beverages. Berlin, Germany: Springer-Verlag, 335–79
- Suárez JA, Iñigo B. (1982). Contribution to study of Mahon cheese. II. Yeast microflora. Chem Mikrobiol Technol Lebensm 7:173–5
- Suzuki M, Suh SO, Sugita T, Nakase T. (1999). A phylogenetic study on galactose-containing Candida species based on 18S ribosomal DNA sequences. J Gen Appl Microbiol 45:229–38
- Tanaka T, Yamada R, Ogino C, Kondo A. (2012). Recent developments in yeast cell surface display toward extended applications in biotechnology. Appl Microbiol Biotechnol 95:577–91
- ten Cate L. (1960). The flavour of dry sausage (in Dutch). Tijdschr Diergeneesk 85:859–64
- Thevenieau F, Nicaud J-M, Gaillardin C. (2009). Applications of the non-conventional yeast Yarrowia lipolytica. In: Satyanarayana T, Kunze G, eds. Yeast biotechnology: diversity and applications. Dordrecht: Springer Science + Business Media, 589–613
- Tomaszewska L, Rywinska A, Gladkowski W. (2012). Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J Ind Microbiol Biotechnol 39:1333–43
- Trindade JR, Freire MG, Amaral PFF, et al. (2008). Aging mechanisms of oil-in-water emulsions based on a bioemulsifier produced by Yarrowia lipolytica. Colloids Surf A Physicochem Eng Aspects 324:149–54
- Tsigie YA, Huynh LH, Ahmed IN, Ju YH. (2012). Maximizing biodiesel production from Yarrowia lipolytica Po1g biomass using subcritical water pretreatment. Bioresour Technol 111:201–7
- Turki S, Jabloun Z, Mrabet G, et al. (2010a). Preliminary safety assessment of Yarrowia lipolytica extracellular lipase: results of acute and 28-day repeated dose oral toxicity studies in rats. Food Chem Toxicol 48:2393–400
- Turki S, Mrabet G, Jabloun Z, et al. (2010b). A highly stable Yarrowia lipolytica lipase formulation for the treatment of pancreatic exocrine insufficiency. Biotechnol Appl Biochem 57:139–49
- van den Tempel T, Jakobsen M. (1998). Yeasts associated with Danablu. Int Dairy J 8:25–31
- van den Tempel T, Jakobsen M. (2000). The technological characteristics of Debaryomyces hansenii and Yarrowia lipolytica and their potential as starter cultures for production of Danablu. Int Dairy J 10:263–70
- Van der Wal P. (1976). Experience with SCP in animal feeding in Europe. PAG Bull 6:7–18
- van der Walt JP, Scott DB. (1971). The yeast genus Saccharomycopsis Schiönning. Mycopathol Mycol Appl 43:279–88
- van der Walt JP, von Arx JA. (1980). The yeast genus Yarrowia gen. nov. Antonie Van Leeuwenhoek 46:517–21
- Van Herendael BH, Bruynseels P, Bensaid M, et al. (2012). Validation of a modified algorithm for the identification of yeast isolates using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS). Eur J Clin Microbiol Infect Dis 31:841–8
- Van Uden N, Buckley HR. (1970). Candida Berkhout. In: Lodder J, ed. The yeasts, a taxonomic study. Amsterdam, The Netherlands: North-Holland, 893–1087
- van Veen SQ, Claas EC, Kuijper EJ. (2010). High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol 48:900–7
- Vatsal A, Zinjarde SS, Kumar AR. (2011). Growth of a tropical marine yeast Yarrowia lipolytica NCIM 3589 on bromoalkanes: relevance of cell size and cell surface properties. Yeast 28:721–32
- Viljoen BC, Dykes GA, Callis M, von Holy A. (1993). Yeasts associated with Vienna sausage packaging. Int J Food Microbiol 18:53–62
- Viljoen BC, Geornaras I, Lamprecht A, von Holy A. (1998). Yeast populations associated with processed poultry. Food Microbiol 15:113–7
- Viljoen BC, Lourens-Hattingh A, Ikalafeng B, Peter G. (2003). Temperature abuse initiating yeast growth in yoghurt. Food Res Int 36:193–7
- von Arx JA. (1972). On Endomyces, Endomycopsis and related yeast-like fungi. Antonie Van Leeuwenhoek 38:289–309
- Walsh TJ, Salkin IF, Dixon DM, Hurd NJ. (1989). Clinical, microbiological, and experimental animal studies of Candida lipolytica. J Clin Microbiol 27:927–31
- Wang XX, Chi Z, Ru SG, Chi ZM. (2012). Genetic surface-display of methyl parathion hydrolase on Yarrowia lipolytica for removal of methyl parathion in water. Biodegradation 23:763–74
- Wehrspann P, Füllbrandt U. (1985). Yarrowia lipolytica (Wickerman et al.) van der Walt & von Arx isolated from a blood culture (in German). Mykosen 28:217–22
- Welker M. (2011). Proteomics for routine identification of microorganisms. Proteomics 11:3143–53
- Welthagen JJ, Viljoen BC. (1998). Yeast profile in Gouda cheese during processing and ripening. Int J Food Microbiol 41:185–94
- Westall S, Filtenborg O. (1998). Spoilage yeasts of decorated soft cheese packed in modified atmosphere. Food Microbiol 15:243–9
- Wickerham LJ, Kurtzman CP, Herman AI. (1970). Sexuality in Candida lipolytica. In: Ahearn DG, ed. Recent trends in yeast research (Spectrum, vol. 1). Atlanta, GA: Georgia State University, 31–92
- Wieser A, Schneider L, Jung J, Schubert S. (2012). MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review). Appl Microbiol Biotechnol 93:965–74
- Williams AG, Withers SE. (2007). Tyrosine metabolism in pigment-forming Yarrowia lipolytica strains isolated from English and European specialty mould-ripened cheese exhibiting a brown discolouration defect. Int J Dairy Technol 60:165–74
- Wolter H, Laing E, Viljoen BC. (2000). Isolation and identification of yeasts associated with intermediate moisture meats. Food Technol Biotechnol 38:69–75
- Wyder M-T, Bachmann H-P, Puhan Z. (1999). Role of selected yeasts in cheese ripening: an evaluation in foil wrapped raclette cheese. Lebensm-Wiss Technol 32:333–43
- Yamada Y, Nojiri M, Matsuyama M, Kondo K. (1976). Coenzyme Q systems in the classification of the ascosporogenous yeast genera Debaryomyces, Saccharomyces, Kluyveromyces and Endomycopsis. J Gen Appl Microbiol 22:325–37
- Yarrow D. (1972). Four new combinations in yeasts. Antonie Van Leeuwenhoek 38:357–60
- Ye Q, Xu X, Li J, Cao H. (2011). Fungemia caused by Yarrowia lipolytica in a patient with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 33:e120–1
- Yoon HJ. (2009). A case of Yarrowia lipolytica fungemia after raw beef ingestion. Mycoses 52:124
- Yoshida M, Hashimoto K. (1986). Assessment of the pathogenicity of yeasts used in the production of single cell protein. Agric Biol Chem 50:2117–8
- Yu Z, Du G, Zhou J, Chen J. (2012). Enhanced α-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by an improved integrated fed-batch strategy. Bioresour Technol 114:597–602
- Yuzbashev TV, Yuzbasheva EY, Laptev IA, et al. (2011). Is it possible to produce succinic acid at a low pH? Bioeng Bugs 2:115–9
- Zarowska B, Wojtatowicz M, Polomska X, et al. (2004). Factors affecting killer activity of some yeast species occurring in Rokpol cheese. Folia Microbiol (Praha) 49:713–7
- Zhang B, Rong C, Chen H, et al. (2012). De novo synthesis of trans-10, cis-12 conjugated linoleic acid in oleaginous yeast Yarrowia lipolytica. Microb Cell Fact 11:51
- Zivanovic R, Ristic D. (1974). Finding of yeasts in tea sausages during their technological process (abstract in English). Tehnologija Mesa 15:51–2
- Zhou J, Yin X, Madzak C, et al. (2012). Enhanced α-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J Biotechnol 161:257–64
- Zuluaga Rodríguez A, de Bedout Gómez C, Agudelo Restrepo CA, et al. (2010). Susceptibility to fluconazole and voriconazole of Candida species isolated from intensive care units patients in Medellin, Colombia (2001–2007) (in Spanish). Rev Iberoam Micol 27:125–9

