Abstract
A framework has been evolving for evaluation of mode of action (MOA) of rodent toxicity and carcinogenicity findings and their relevance to humans. Folpet produces duodenal glandular tumors in mice, but is not carcinogenic in rats. A wealth of information is available regarding folpet’s mode of action, providing an excellent example of how this tumor can be evaluated using this framework. Folpet reacts with thiol groups, and is rapidly hydrolyzed at pH 7. Both reactions produce thiophosgene that reacts with thiols and other functional groups. Folpet is not genotoxic in vivo. At sufficiently high, prolonged dietary doses, folpet irritates the mouse duodenum, resulting in cytotoxicity with consequent regenerative proliferation and ultimately tumor development. Forestomach lesions secondary to cytotoxicity are also induced. Dogs have stomachs similar to humans and show no evidence of gastrointestinal toxicity or tumor formation at exposure levels at least as high as rodents. The data support a MOA in mice involving cytotoxicity and regenerative proliferation. Based on MOA analysis and assessment of human relevance, folpet, like captan, another trichloromethylthio-related fungicide with similar toxic and carcinogenic effects, is not likely to be a human carcinogen at dose levels that do not cause cytotoxicity and regenerative proliferation.
Contents
Abstract 531
1. Introduction 532
2. Summary of 2-year rodent bioassays 533
2.1. Mouse 533
2.2. Rat 535
2.3. Summary 536
3. Mode of action analysis: Mouse duodernum tumors 536
3.1. Overview 536
3.2. Key events 537
3.3. Temporal relationships 539
3.4. Dose-response relationships 539
3.5. Biological plausibility 539
3.6. Data cohesiveness 539
3.7. Possible data gaps 539
3.8. Alternative possibilities 539
3.9. Evaluation of effect of life stages 540
3.10. Evaluation of possible susceptible populations 540
4. Forestomach lesions 540
5. Analysis of human relevance 541
5.1. Evaluation of qualitative differences 541
5.2. Evaluation of quantitative differences 543
6. Summary and conclusions 543
Acknowledgments 544
Declaration of interest 544
References 544
1. Introduction
Mode of action (MOA) is increasingly being considered in the risk assessment of pesticides. During the past decade, the International Life Sciences Institute (ILSI) and the International Programme on Chemical Safety (IPCS) of the World Health Organization (WHO) have been evolving a framework for the analysis of mode of action for rodent toxicity and carcinogenicity findings along with assessment of their human relevance (CitationSonich-Mullin et al., 2001; CitationMeek et al., 2003; CitationSeed et al., 2005; CitationBoobis et al., 2006, Citation2008). Numerous case studies have been published illustrating the applicability of the framework for genotoxic and nongenotoxic cancer modes of action and for cancer and noncancer endpoints. Mode of action analysis has been incorporated into the risk assessment guidelines of various regulatory agencies, including the US Environmental Protection Agency (CitationUS EPA, 2005).
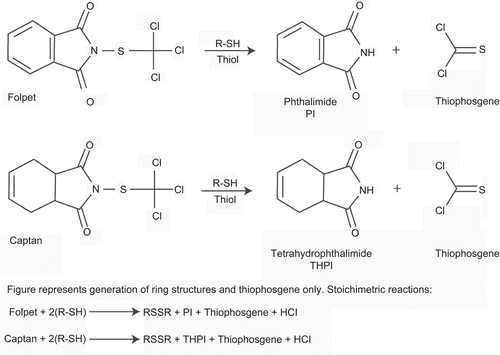
Folpet and captan are used for their fungicidal properties in both industrial and agricultural products. Their structures are shown in along with their reaction with thiols. Both compounds have Reregistration Eligibility Decisions (REDs) issued (CitationUS EPA 1999a, Citation1999b) as well as subsequent reviews (CitationUS EPA 2003, Citation2004a, Citation2004b) that included the reclassification of captan from “B2” (probable human carcinogen) to “not likely” at dietary exposures expected from agricultural use (CitationUS EPA, 2004a; CitationGordon, 2007).
Figure 1. Chemical structures of folpet and captan and their reaction products following interaction with thiols.
The major tumor finding from captan bioassays was gastrointestinal adenomas and adenocarcinomas in mice, primarily in the duodenum. By contrast, there was no carcinogenic effect of captan in rats. The 2004 cancer reclassification was based on the 1999 proposed Carcinogenic Risk Assessment guidelines (CitationUS EPA, 1999c) that were finalized in 2005 (CitationUS EPA, 2005).
Folpet, chemically and biologically similar to captan, has also been evaluated in rodent carcinogenicity bioassays and has a similar pattern of tumor development, that is, gastrointestinal tumors in mice and the absence of treatment-related tumors in rats. Studies evaluating the early stages of effects in the gastrointestinal tract support analysis of the mode of action. Folpet provides an example of how the application of the ILSI/IPCS mode of action and human relevance framework can be applied to tumors in assessing possible carcinogenic risk to humans. Folpet previously was considered by EPA a genotoxic carcinogen, like captan, and was considered a carcinogen in mice and rats (CitationQuest et al., 1993). Given the information available concerning mode of action, assessment of human relevance and the precedent setting case of captan, folpet today would likely be classified as a nongenotoxic, threshold-based carcinogen, with carcinogenicity only in mice. We describe below the basis for concluding that the rat bioassays are negative; this has also been the conclusion of Joint Food and Agriculture Organization of the United Nations (FAO/WHO) Meeting on Pesticide Residues (JMPR) (CitationFAO/WHO, 1996) and European Food Safety Authority Citation(EFSA) (2009a, Citation2009b).
In this paper, we review the folpet carcinogenicity bioassays in rodents and critically evaluate the results, taking into account data from a variety of short- and long-term studies in vivo and mechanistic information gathered from various types of investigations. We cite relevant studies with captan as these two compounds have been shown to have a common mode of toxicity (CitationBernard and Gordon, 2000). The mode of action of folpet for induction of the mouse tumors and their human relevance is then evaluated using the framework developed over the past decade by the WHO IPCS and the International Life Sciences Institute (ILSI), Risk Science Institute (RSI). The evidence supports the conclusion that folpet, like captan, is a non–DNA-reactive carcinogen in mice with a threshold, nonlinear dose-response (CitationUS EPA, 2004a; CitationGordon, 2007, Citation2010). We also suggest that humans are likely to be considerably less susceptible to its precursor cytotoxic effects in the upper gastrointestinal tract than are mice.
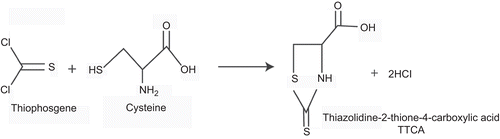
Folpet has been extensively evaluated in genotoxicity assays, and like captan, it is generally positive in vitro with or without enzymatic activation systems, but is not genotoxic in vivo. The findings in vitro are related to the strong reactivity of folpet with cellular thiols, either directly or following folpet’s initial reaction with thiols. A typical reaction in vivo is noted in , showing the reaction of thiophosgene with cysteine to produce thiazolidine-2-thione-4-carboxylic acid (TTCA), a urinary metabolite. Because of the role thiols play, the addition of S9 with its own abundant thiols serves to degrade the parent, resulting in lower concentrations and consequently lower mutagenic activity. A detailed analysis of the genotoxicity of folpet is reported in the accompanying paper (Arce et al., 2010). The overall conclusion of the mutagenicity and carcinogenicity studies is that folpet induces tumors in mice by a non–DNA-reactive mode of action that involves chemical reactivity with cellular constituents leading to irritation, cytotoxicity, and regenerative proliferation; this regenerative proliferation is then the basis for tumor development (CitationGordon, 2010; EFSA, Citation2009b).
Figure 2. Reactions of folpet and thiophosgene with cysteine, a representative thiol, producing the urinary metabolite TTCA.
Abridged study data from unpublished reports are available online at the Informa Web site (www.informahealthcare.com/txc). Full study reports of this unpublished material that have MRID numbers are available from US EPA via FOI requests.
2. Summary of 2-year bioassays
Folpet has been evaluated in several 2-year rodent bioassays (CitationQuest et al., 1993; CitationBernard and Gordon, 2000), including three mouse studies (CitationEast 1994; CitationRubin and Nyska 1985; CitationWong, 1985) and three rat studies (CitationCox et al., 1985: CitationCrown et al., 1985, Citation1989). In these dietary studies, folpet doses ranged in mice from 150 to 12,000 ppm (7.5–600 mg/kg/day); and in rats, due to increased toxicity, the doses ranged from 200 ppm to a maximum of 5000 ppm (10–250 mg/kg/day). Despite its reactivity with thiol groups, folpet has been shown to be stable in the diet administered to the rats and mice (96–100% up to 3 weeks at 5000 ppm (CitationMilburn, 1997); 96–115% of nominal levels for 12 weeks at 1000, 3000, or 10,000 ppm, but 59–67% at 300 ppm (CitationReno et al., 1981). All of the standard bioassays included high doses that met the criteria of maximum tolerated dose (MTD) and resulted in dose-related pathology. One study (CitationEast, 1994) was designed to establish a no observed adverse effect level (NOAEL) and included a high dose below the MTD but sufficient to cause tumors.
The collective data for folpet and captan show consistency within species across studies; that is, both compounds induce gastrointestinal tumors in mice and both compounds do not induce treatment-related tumors in rats.
A distinction can be made, however, between the two fungicides in that folpet produces a low incidence of squamous cell tumors of the forestomach of mice whereas captan does not. This may be related to the respective hydrolysis rates of the two compounds at low pH.
2.1. Mouse
In CD-1 and B6C3F1 mice, the consistent finding is induction of glandular tumors (adenomas and adenocarcinomas) of the duodenum ( and ; ) (CitationNyska et al., 1990; CitationQuest et al., 1993; CitationBernard and Gordon, 2000; EFSA, Citation2009a, Citation2009b; CitationGordon, 2010). The duodenum is the proximal portion of the small intestine. It transitions to the jejunum at the ligament of Treitz. The distal half of the small intestine is referred to as the ileum. In several of the short- and long-term studies involving folpet and related chemicals, some investigators have arbitrarily divided the small intestine into thirds. Regardless of whether the lesions occurred in what was stated as the duodenum or the jejunum, they occurred in the proximal portion of the small intestine, generally near the ampulla of Vater where bile and pancreatic secretions enter the intestine, with high concentrations of bicarbonate raising the pH of the intestinal luminal contents. There has also been an analysis of squamous cell tumors of the nonglandular, forestomach in mice (). It is suggested that one possibility is that the large duodenal tumors act to inhibit the passage of stomach contents to the intestine, thus increasing the resident time of reactive species in the stomach and enhancing tumor formation (CitationNyska et al., 1990). The incidences of forestomach tumors in mice were marginally statistically significant but did not show a consistent dose-response. Across these studies the no observed effect level (NOEL) is 450 ppm (approximately 50 mg/kg/day) (CitationEast, 1994).
Table 1. Tumor incidences of selective tissues in a study in CD-1 mice administered folpet in the diet (CitationWong, 1985).
Table 2. Tumor incidences of selective tissues in a study in B6C3F1 mice administered folpet in the diet (CitationRubin and Nyska, 1985).
Figure 3. Opened gastric and duodenal lumen of high-dose group mouse. A large mass (squamous cell carcinoma) growing in the nonglandular portion of the stomach is indicated by a large asterisk. The duodenal lumen is obstructed by a large nodule (small asterisk). (Reproduced by permission from A. Nyska et al., Induction of gastrointestinal tumors in mice fed the fungicide folpet: possible mechanisms. 1990. Jpn J Cancer Res 81:545–549.)

Figure 4. Opened duodenal lumen of a mouse treated with the high dose of folpet. Note the large nodule growing on the mucosa (arrow). (Reproduced by permission from A. Nyska et al., Induction of gastrointestinal tumors in mice fed the fungicide folpet: possible mechanisms. 1990. Jpn J Cancer Res 81:545–549.)
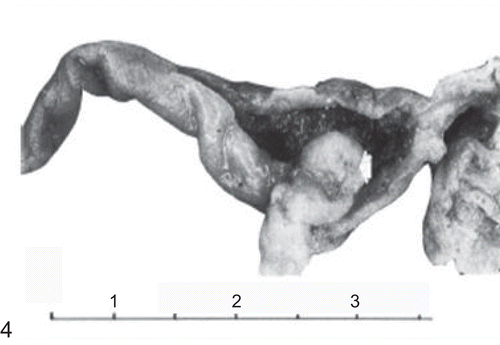
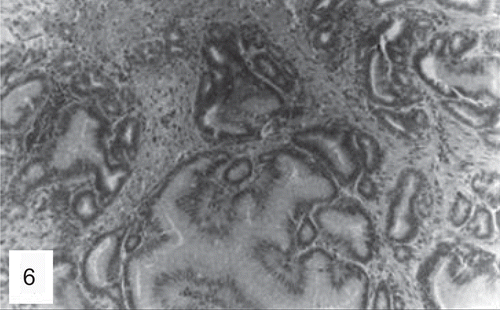
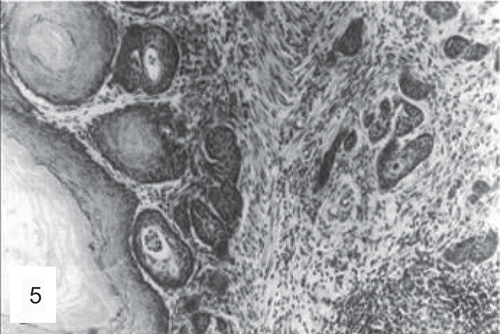
Figure 5. Histological section of the forestomach of a mouse treated with the high dose of folpet. Note squamous cell carcinoma infiltrating the wall (H&E, ×200). (Reproduced by permission from A. Nyska et al., Induction of gastrointestinal tumors in mice fed the fungicide folpet: possible mechanisms. 1990. Jpn J Cancer Res 81:545–549.)
2.2. Rat
In Sprague-Dawley and F344 rats, tumors of the duodenum and the remainder of the small intestine have not been observed. There have been changes consistent with toxicity (hyperkeratosis, hyperplasia [acanthosis], edema, and inflammation) to the forestomach, but no incidence of tumors has been induced in the forestomach of rats or in the glandular stomach or esophagus. These irritation-related changes in rats are qualitatively similar to those seen in mice, but do not result in tumor development. The NOEL in rats is 200 ppm for non-neoplastic forestomach mucosal changes (CitationCox et al., 1985).
The collective data show folpet is not a rat carcinogen. Although incidences of some tumors in rats were increased compared to concurrent controls at some doses (CitationQuest et al., 1993), none are considered treatment related or biologically relevant to human risk, as noted below and as has been concluded by EFSA (Citation2009b) and FAO/WHO JMPR (CitationFAO/WHO, 1996).
In one study in Sprague-Dawley rats, there were no significantly increased incidences of tumors at any site (). The incidences of Leydig cell tumors was increased compared to controls but there was no dose-response, statistical evaluation for trend was not significant, and the incidence in the controls was considerably lower then usually observed in this strain (Sprague-Dawley) of rats.
Table 3. Tumor incidences of selective tissues in a study in Sprague-Dawley rats administered folpet in the diet (60 rats per group) (CitationCox et al., 1985).
In a second study, there was a suggested increased incidence of benign mammary gland tumors in female F344 rats, but they were judged to be not treatment related (). Based on the description of the lesions, they would now be classified as fibrodenomas.
Table 4. Tumor incidences of selective tissues in a study in F344 rats administered folpet in the diet (20 rats per group) (CitationCrown et al., 1989).
In a third study in rats, there was a slight increase in incidence of these fibroadenomas of the mammary gland in female F344 rats, and also a slight increase in thyroid tumors and malignant lymphomas (). As discussed below in greater detail, these tumors are concluded not to be treatment related.
Table 5. Tumor incidences of selective tissues in a study in F344 rats administered folpet in the diet (60 rats per group) (CitationCrown et al., 1985).
Captan shares a common mode of action with folpet (CitationBernard and Gordon, 2000; CitationGordon, 2010), It also produces gastrointestinal tumors in the duodenum in mice but does not induce treatment-related tumors in rats. In captan rat bioassays there was an increased incidence of kidney or uterine tumors, but these were deemed non–treatment-related by an independent third party (CitationTERA, 2003) and subsequently confirmed by EPA (US EPA, 2004) and by EFSA (Citation2009a).
Thus, both captan and folpet produce a carcinogenic effect in the gastrointestinal tract in mice, primarily in the duodenum, but are not carcinogenic to rats (US EPA, 2004; CitationBernard and Gordon, 2000; CitationGordon 2010).
The incidences of mammary gland, thyroid, and lymphoid tumors in rats did not follow a distinct dose-response, and were not increased at statistically significant levels above the controls. Furthermore, statistical analysis for trend was not significant. The investigators in these studies stated that they did not believe these tumors were related to chemical treatment, based on statistical comparisons, and they also concluded that the tumors lacked biological relevance to humans.
Lack of statistical significance in these studies was concluded on the basis of p >.05. Lack of statistical significance is even more strongly concluded if one utilizes the recommendation by the National Toxicology Program (NTP) (CitationHaseman, 1983) that statistical comparison of common tumors in treated animals compared to controls in rodent bioassays should utilize a p value of <.025 or even p <.01 instead of the standard p <.05 recommended for uncommon tumors. This modified standard for statistical significance is now routinely used by the NTP for their bioassay assessments, and it is also used by pharmaceutical regulatory agencies worldwide, based on International Committee on Harmonisation (ICH) recommendations (CitationLin, 1998). Thus, for folpet, it would be appropriate to use p <.05 for statistical significance for the duodenum and forestomach tumors, since these are relatively uncommon, but a lower p value should be utilized for the mammary gland, thyroid, and lymphoid tumors. This is also true for the testicular Leydig cell tumors. Regardless, the incidences of these three common tumors (mammary gland, thyroid, and lymphoma) in the rats in the 2-year bioassays with folpet were not statistically significant, even at p <.05. They certainly do not meet the criteria of a p value of <.025 and none of the tumors in any of the rat studies showed statistical significance for a trend analysis, even at p <.05. Furthermore, these tumors occur spontaneously quite frequently in control rats (CitationHaseman et al., 1998). There does not appear to be biological significance for human risk assessment for any of these three tumor types in these strains of rats (Sprague-Dawley, F344).
Mammary gland fibroadenomas in female rats often occur spontaneously, especially in Sprague-Dawley rats where spontaneous mammary gland tumor incidences can exceed 75%. In addition, these fibroadenomas are benign tumors that are not predictive of malignant tumors in the rodent and they are not predictive of malignancy in humans (CitationRusso and Russo, 1987; Russo et al. 1996). Fibroadenomas are common lesions in the human breast, and are not considered precursor lesions for carcinomas (CitationRosen and Oberman, 1993; CitationRusso et al., 1990).
The thyroid tumors that were observed in one of the three bioassays in rats are not indicative of a risk of human cancer for the following reasons: (1) The increased tumor incidence was inconsistent, occurring only in one of three studies; (2) they were not increased at a statistically significant incidence; (3) the incidences even in the high dose group are within expected incidences for control groups; and (4) there is no evidence of a dose-response, and a statistical evaluation for a trend is not significant.
Like the thyroid tumors, an increased incidence of malignant lymphomas was also observed in only one of the three rat bioassays. Again, this is not statistically significant, there was no evidence of a dose-response, they occurred in only one of the three studies, and most importantly, it represents a tumor type that occurs commonly in F344 rats. It is unlikely that the observation in one of the three rat bioassays with folpet represents a treatment-related or biologically significant finding.
2.3. Summary
Thus, in summary, the only reproducible, statistically and biologically significant, treatment-related tumorigenic finding was the duodenal glandular tumors in mice. There was also a statistically marginal increase in squamous cell lesions of the forestomach in mice but the dose-response was inconsistent between studies and sexes. The duodenal tumors occur subsequent to irritation and toxic changes with consequent regenerative proliferation. Cytotoxicity and regeneration is also likely the basis for the squamous cell tumors of the forestomach. There is no carcinogenic effect in the rat that is statistically significant, reproducible, or biologically relevant to human risk. Based on this summary of the findings in the 2-year bioassays, an analysis of the mode of action and human relevance is only appropriate for the mouse duodenal (small intestine) tumors. We present a detailed analysis of the mode of action and human relevance of the mouse duodenal tumors. We also discuss the forestomach changes, emphasizing their lack of relevance to humans, since humans lack a forestomach or comparable organ.
3. Mode of action analysis: Mouse duodenum tumors
3.1. Overview
For the mode of action analysis and assessment of human relevance, we utilize the framework that has evolved from the initial mode of action document published by International Programme on Chemical Safety (IPCS) and the US EPA, and subsequent publications on the evaluation of the human relevance for cancer and noncancer endpoints that have been developed through the International Life Sciences Institute/Risk Science Institute (ILSI/RSI) sponsored by EPA and Health Canada and the continued efforts by the IPCS (CitationSonich-Mullin, 2001; CitationMeek et al., 2003; CitationSeed et al., 2005; CitationBoobis et al., 2006, Citation2008). The mode of action framework is based on the Bradford Hill criteria originally developed for an evaluation of etiologic significance for human epidemiologic investigations (CitationHill 1965). We rely primarily on the framework presented by CitationMeek et al. (2003) in Critical Reviews in Toxicology, with some additional incorporation of recent suggestions regarding life stages and default assumptions (CitationBoobis et al., 2006, Citation2008; CitationSeed et al., 2005).
3.2. Key events
For the duodenal tumors induced in mice by folpet, the mode of action involves irritation-related cytotoxicity with consequent cellular regeneration ultimately leading to the development of tumors. The critical key event involves consumption of sufficiently high levels of folpet that yield a cytotoxic concentration of folpet and its degradation product, thiophosgene. Combined, these chemicals can cause cytotoxicity in the duodenum (). In the stomach and duodenum there is cytotoxicity from the direct reaction of folpet with cellular components containing thiol groups as well as from the highly reactive thiophosgene, a hydrolysis product of folpet, which reacts with thiols as well as other cellular constituents (see and ). Folpet itself, like captan (CitationWilkinson et al., 2004), is reactive with thiol groups, which generates thiophosgene (). However, thiophosgene is also reactive with thiol groups (), with a half-life of less than 1 s (0.6 s in human blood). Thiophosgene, thus, is generated either by the reaction of folpet with thiol groups or following hydrolysis of folpet. Direct interaction of folpet with thiol groups is the primary source of the thiophosgene. The hydrolysis of folpet to thiophosgene is highly pH dependent. The reaction occurs readily at neutral to alkaline pH but significantly less at low pH. Because of their marked reactivity, folpet and thiophosgene will react rapidly with thiol groups and not reach DNA or DNA-related targets (e.g., histones) (Couch and Siegel, Citation1977; Couch et al., Citation1977; CitationLiu and Fishbein, 1967; CitationLukens, 1966; CitationLukens et al., 1965; CitationSiegel, 1971a, Citation1971b; CitationBernard and Gordon, 2000). This is highlighted by a pair of key in vivo studies that show folpet and captan do not induce genotoxicity, as evidenced by the lack of increased nuclear aberrations in the tumor target site, the duodenum (CitationChidiac and Goldberg, 1987; CitationGudi and Krsmanovic, 2001). Thus, DNA damage is highly unlikely and not detected in studies designed to assess this effect at the target tissue in vivo (Arce et al., 2010).
Table 6. Concordance table for analysis of potential human relevance of cytotoxicity mode of action of folpet induction of duodenal tumors in mice.
The reaction of folpet and thiophosgene with thiol groups in glutathione and proteins (Couch and Siegel, Citation1972, Citation1977; CitationJernstrom et al, 1993; CitationLiu and Fishbein, 1967; CitationLukens, 1966; CitationLukens et al., 1965; CitationMoriya et al., 1978; CitationSiegel, 1971a, Citation1971b; Couch et al., Citation1977) leads to cytotoxicity and an inflammatory reaction, with a consequent regenerative increase in cell proliferation and ultimately the development of tumors (). The sequence of events involving cytotoxicity and regenerative proliferation is a common mode of action for tumorigenesis by non–DNA-reactive chemicals (CitationMeek et al., 2003). Cytotoxicity and regeneration is the same mode of action that has been demonstrated for the duodenal tumors induced in mice by captan (CitationBernard and Gordon, 2000; CitationGordon, 2007; Citation2010; US EPA, 2004a).
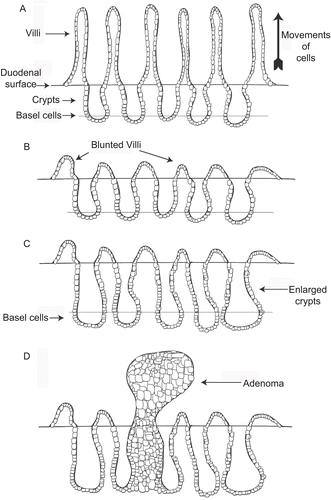
Figure 7. Diagrams illustrating the sequence of events in folpet-induced duodenal tumors. (A) Simplified diagram of normal duodenum showing normal crypt and villous structures. (B) Folpet-induced damage of duodenum with blunting of the villi and widening of the crypts. (C) Enlargement of crypts in response to duodenal damage, associated with increased proliferation and number of crypt cells. (D) Proliferation of crypt cells leading to development of adenoma, the precursor lesion for adenocarinoma.
The mode of action for folpet, including generation of thiophosgene, is similar to that seen with chloroform. Chloroform is metabolically activated to phosgene, which leads to liver and kidney cytotoxicity, regeneration, and ultimately tumors. Like folpet, this is a threshold phenomenon (CitationAndersen et al., 2000; CitationMeek et al., 2003). Thiophosgene is not available systemically based on the chemical reactivity of folpet in the gastrointestinal tract and thiophosgene’s rapid degradation in blood, in contrast to the systemic distribution of chloroform and the enzymatic generation of phosgene. In contrast to chloroform, folpet is considerably less toxic, probably related to the rapid extracellular hydrolysis leading to thiophosgene, which must then react with cells, compared to the intracellular generation of phosgene by metabolism of chloroform. Exogenous exposure to phosgene by inhalation, a highly toxic event, is unrelated to the process of the intracellular generation of phosgene from chloroform.
Thiol groups are present in many proteins (e.g., those containing the amino acid cysteine). When folpet enters the duodenal lumen, it first encounters villi, since they project into the lumen. Cells of the crypts are not readily accessible due to their location well below the surface of the duodenum and the presence of a surface mucus layer (CitationGreaves, 2007). Disruption of the integrity of duodenal proteins in the villi due to the interaction of folpet with thiols leads to cytotoxicity and inflammation (CitationMilburn, 1997).
Studies with folpet and captan have demonstrated that the early changes after administration at high doses are related to the presence of blunting of the intestinal villi and an increase in the mononuclear inflammatory infiltrate in the lamina propria. The terminology of blunting of the villi and villous atrophy are commonly used in human pathology, whereas in studies in rodents, including those with folpet, this is frequently referred to as hypertrophy of the villi and crypts. This is the common pattern of reaction with any type of toxicity in the small intestine, whether chemical, infectious, inherited or immunologic reactions whether in rodents (CitationGreaves, 2007) or in humans (CitationNoffsinger and Waxman, 2007). More commonly, the reaction in the small intestinal mucosa is that of a mononuclear inflammatory cell infiltrate without preceding acute inflammation. The time course of changes following folpet administration have been extensively evaluated (CitationMilburn, 1997). In addition, these changes appear to be reversible upon cessation of folpet consumption.
The localization of the lesions following dietary folpet administration corresponds to the potential for reaction with thiol groups and generation of thiophosgene (CitationBernard and Gordon, 2000; CitationMilburn, 1997). In the stomach, reactivity may be inhibited by the increased hydrolytic stability of folpet in acid media. No cytotoxicity is seen in the glandular stomach with a luminal pH of approximately 1–2 (CitationProctor et al., 2007; CitationMcConnell et al., 2009). Some reactivity of folpet occurs in the forestomach, as there is an inflammatory and consequent regenerative process seen in the squamous epithelium. The luminal pH of the forestomach is approximately 5–6 (CitationProctor et al., 2007; CitationMcConnell et al., 2009). Once the folpet enters the duodenum, the pH rises abruptly and significantly (approximately 5–6 in mouse, 6–7 in rat) because of the presence of the intestinal secretions and the presence of pancreatic secretions, rich in bicarbonate, entering the duodenum through the ampulla of Vater (CitationMcConnell et al., 2009). Thus, it is consistent with the chemical and physical properties of folpet that increased hydrolytic instability occurs with increased pH. Based on the pH of the forestomach, glandular stomach, and duodenum, the tumors occur, therefore, predominately in the duodenum. At this higher pH, nearly all of the dietary folpet reacts with thiol groups or is hydrolyzed before it can proceed beyond the duodenum. The folpet and the generated thiophosgene react with the duodenum mucosa. Little folpet or thiophosgene will be left by the time they reach the proximal jejunum, where occasional lesions were also observed. However, most of the changes are observed in the proximal 1.5 cm of the small intestine, which is where one would anticipate the rapid hydrolysis of the folpet and reactivity with the mucosa to occur. The reactivity of solubilized folpet and that of thiophosgene at neutral pH occurs in a matter of a few seconds. Systemic effects are unlikely because the folpet, if absorbed, would be rapidly degraded (half life of folpet: 4.9 s; half life of thiophosgene: 0.6 s). The lesions in the forestomach and in the duodenum reflect contact toxicity that results in degradation of the parent substance.
The lack of effect on the glandular stomach mucosa is consistent with numerous other highly reactive substances administered directly into the gastric lumen by gavage, which also do not produce toxicity in the glandular stomach (see below). The forestomach squamous mucosa appears to be much more susceptible to chemical reactivity whereas the glandular stomach mucosa is highly resistant. This may be due to the presence of a thick mucus layer on the glandular stomach as well as its normal, physiologic resistance to the extremely low pH (pH of 1.0–2.0) to which it is naturally exposed. Furthermore, folpet’s hydrolytic stability increases at low pH, resulting in limited evolution of thiophosgene. In the forestomach, the pH of the contents approximates pH of 5 (Procter et al., 2007; CitationMcConnell et al., 2009); at this pH hydrolysis would increase with the concurrent generation of thiophosgene.
As long as the folpet is present in the diet at sufficiently high doses, cytotoxicity of the duodenum will result. For folpet, the cytotoxicity is clearly a threshold phenomenon and is reversible. The necessity for continued exposure to folpet to produce cytotoxicity and the continued increased proliferation was noted in a reversibility study (CitationWaterson, 1995). Following 28 days of exposure, an additional 28 days without folpet administration resulted in complete recovery of the duodenal lesions. This is quite typical for chemicals having a cytotoxic mode of action. It is also typical for duodenal disorders involving blunting (hypertrophy) of the villi and chronic inflammation, in rodents or in humans (CitationNoffsinger and Waxman, 2007). Once the offending stimulus is removed, the villi are able to return to normal, usually within a matter of days (CitationPotten and Loeffler, 1990; CitationPotten et al., 1990).
With this cytotoxicity in mice, there is consequent regenerative proliferation (). This is seen in the duodenal mucosa as measured both by an increase in bromodeoxyuridine (BrdU) or proliferating cell nuclear antigen (PCNA) labeling index and widening of the length of the stem cell portion of the small intestinal crypts, indicating an expansion of the target cell population that can evolve into tumors (CitationMilburn, 1997; CitationWaterson, 1995). It is only the stem cell population of the small intestine that can actually evolve into a malignant tumor (CitationPotten and Loeffler, 1990; CitationPotten et al., 1990). Expansion of this stem cell population in the crypts represents an increase in the number of potential cells that can develop the spontaneous genetic errors that are necessary for the development of malignancy. This combination of an expanded number of cells with a higher proliferative rate than normal provides an ample background for which a tumorigenic response can evolve (Cohen and Ellwein, Citation1990; CitationGreenfield et al., 1984; CitationKnudson, 1971; CitationMoolgavkar and Knudson, 1981). A similar process appears to be occurring in the mouse forestomach (see below). It is also noted that there is a low incidence of spontaneously transformed cells resident in the crypt compartment, based on the incidence of duodenal tumors found in control mice. These cells would also be subject to proliferative pressure following damage to the villi by folpet or captan. This pressure may well act to promote these cells to tumors.
The sequence of proliferative lesions seen in the duodenum is that usually seen in the development of gastrointestinal glandular carcinoma occurring in response to cytotoxicity, that is, progression from reactive hyperplasia to adenoma and then to adenocarcinoma.
In summary, there is strong evidence for the sequence of key events involving the direct interaction of folpet with thiol groups as well as the interaction of thiophosgene, produced both by hydrolysis of folpet as well as folpet’s initial reaction with thiols. Thiophosgene reacts with numerous cellular components in addition to thiols. These reactions produce cytotoxicity, inflammatory processes, consequent regenerative proliferation, and ultimately, tumor development ( and ). The collective bioassay data in mice have shown that the threshold for proliferative changes and subsequent tumor development is above dietary levels of 450 ppm (approximately 50 mg/kg/day).
3.3. Temporal relationships
The temporal sequence of events has been well established in a time course experiment (CitationWaterson, 1995). With periodic sacrifices through the first 28 days of folpet administration, the study demonstrated that the initial event is blunting of the villi with a mononuclear inflammatory cell infiltrate in the lamina propria, with a consequent widening of the crypts and increase in cell proliferation. Development of hyperplastic lesions then occurred. The chronic bioassays in mice demonstrated the later development of adenomas and ultimately carcinomas.
3.4. Dose-response relationships
There is a clear dose-response for precursor events and the development of tumors ( and ). At doses of 1000 ppm and higher duodenal tumors develop. There is also a low (but not zero) incidence of duodenal lesions in the control mice, reflecting a background of transformed cells resident in the duodenum (CitationRubin and Nyska, 1985; CitationWong, 1985). The NOAEL for tumor development is above 450 ppm (approximately 50 mg/kg/day) (CitationEast, 1994).
3.5. Biological plausibility
This sequence of events is clearly biologically plausible. Cytotoxicity followed by consequent regenerative proliferation is a common mode of action for carcinogenesis, having been demonstrated in numerous tissues and in a variety of species (Cohen and Ellwein, Citation1991; CitationMeek et al., 2003). This is based on the probability that every time DNA replicates in a stem cell population there is a certain probability that a mistake critical to the development of cancer can occur (Cohen and Ellwein, Citation1990; Citation1991; CitationGreenfield et al., 1984; CitationKnudson, 1971; CitationMoolgavkar and Knudson, 1981). Although this probability is extremely low, it is not zero. With numerous DNA replications occurring over time, sufficient replications can occur so that all of the necessary errors in the DNA can occur in a single cell over time. By increasing the number of DNA replications (by both increasing the rate and the number of stem cells available), it increases the possibility of all of the mistakes occurring at a greater incidence. This sequence has been well described based on animal models (Cohen and Ellwein, Citation1990, Citation1991; CitationGreenfield et al., 1984), and based on epidemiologic evidence (CitationMoolgavkar and Knudson, 1981). The presence of the low background incidence of transformed cells contributes to the population of cells available for progression to tumors.
3.6. Data cohesiveness
There is clearly cohesiveness to the data. The duodenal tumorigenic results have been reproducible in two different studies, in both CD-1 and B6C3F1 mice, and the precursor lesions have also been observed repeatedly in this species (CitationMilburn, 1997; CitationRubin and Nyska, 1985; CitationWaterson, 1995; CitationWong, 1985). A lack of treatment-related carcinogenicity in rats is also consistent across studies.
3.7. Possible data gaps
As with all data sets, there are some gaps in our knowledge of the sequence of events following folpet administration. Clearly, it is unknown which thiol groups are targeted in specific proteins leading to the cytotoxicity that eventually evolves into a carcinogenic response. However, these gaps in the data do not prohibit an assessment of the overall mode of action. The detailed biochemical and molecular events have not been defined, but these concern the mechanism of action rather than mode of action.
3.8. Alternative possibilities
The other two possibilities for folpet would be a direct mitogenic stimulus to the duodenum (a non–DNA-reactive process) or a DNA-reactive (genotoxic) mechanism. With respect to mitogenesis, there is no evidence that folpet is directly mitogenic to the duodenal mucosa. Direct mitogenesis here refers to a direct stimulus that increases cell proliferation without the necessity of a precursor cytotoxicity stimulus. This is most commonly seen in response to hormonal stimuli or growth factors. Folpet does not have these biologic capabilities. Furthermore, the increased proliferative changes seen in the duodenum associated with folpet administration are always associated with an inflammatory, cytotoxic process in mice (CitationMilburn, 1997; CitationRubin and Nyska, 1985; CitationWaterson, 1995). Thus, the proliferative process seen with folpet administration in the duodenum involves the sequence of cytotoxicity with consequent increased proliferation rather than a direct stimulus of the increased proliferation (direct mitogenesis).
It is clear that folpet is an irritant. The irritant effect is most evident in tests evaluating mucosal irritation of the eye (CitationBullock et al., 1982). Irritation is seen to a limited extent following dermal (FAO/WHO, 1996) or inhalation exposure (CitationCracknell, 1993), but is more obvious by oral exposure (CitationCox et al., 1985; CitationCrown et al., 1985; Citation1989; CitationRubin and Nyska, 1985; CitationWaterson, 1995; CitationWong, 1985). The effects with these exposures are localized as would be expected for contact cytotoxicity with compounds that are transient and not systemic.
Of critical concern is the potential for a mode of action involving DNA reactivity. Folpet (like captan) has been evaluated in an extensive number of genotoxicity assays (summarized in CitationBernard and Gordon, 2000; CitationGordon, 2010), and discussed in greater detail in Arce et al. (2010). Frequently these assays have produced positive results in vitro, with positive results higher when S9 is absent. It is likely that S9 proteins serve not as a mechanism for metabolizing the folpet but serve, in contrast, as a source of thiol groups in the culture medium that folpet and thiophosgene may react with, thus limiting the potential for interactions with cellular protein and DNA. Support for this hypothesis is provided by the decreasing mutagenicity of folpet with increasing concentrations of cysteine present in the medium in vitro. The more cysteine present, the less the mutagenic effect as evaluated in the Escherichia coli revertant assay (CitationMoriya et al., 1978). The rapid reaction with thiol groups precludes DNA reactivity in vivo. Consistent with this explanation is the lack of any genotoxicity occurring in a variety of in vivo assays. Furthermore, no covalent binding to DNA was detected following exposure to captan (CitationProvan, 1995). Thus, although folpet produces a positive genotoxicity response in various in vitro assays, the effect is inhibited by increasing availability of thiol groups, and it does not occur in vivo. This precludes DNA reactivity as a mode of action for the carcinogenic effect of folpet in vivo (EFSA, Citation2009b), similar to the conclusion regarding captan (EFSA, Citation2009a). In addition, phthalimide, the relatively stable degradation product of folpet, has been judged to be nongenotoxic (CitationSchnaubelt, 1995; Arce et al., 2010).
3.9. Evaluation of effect of life stages
An additional consideration in evaluating the mode of action and eventually extrapolating to human relevance is an evaluation of the possible influence of life stages or other susceptible populations. Since the mode of action of folpet is not DNA reactive, but involves the proliferation of the small intestine (duodenum), there should be no influence of life stage on susceptibility to the cytotoxic effect of folpet. Since the small intestine is a rapidly proliferating tissue in the adult as it is in the infant (CitationPotten and Loeffler, 1990, CitationPotten et al., 1990), it is anticipated that there will be no difference in response at these different life stages. Furthermore, there would not appear to be any reason to assume that there would be a greater or lesser susceptibility to the toxic effects of folpet. Since conversion of folpet to thiophosgene is due to chemical hydrolysis or generation from folpet’s reaction with thiol groups, enzyme-related metabolic differences in the young compared to the adult are not relevant. Thus, infants should have no differences in susceptibility compared to adults, and furthermore, the developing fetus is not at risk as there is effectively no systemic exposure due to folpet and thiophosgene’s rapid degradation in blood.
There is no information available regarding the differential hydrolysis or absorption of folpet in the proximal gastrointestinal tract between young and old animals. However, there is no reason to believe that early life stages would be dissimilar in their response to folpet compared to adults.
3.10. Evaluation of possible susceptible populations
With respect to identifying a susceptible population with increased risk, the major consideration is the extremely high dose of folpet that is required to reach cytotoxicity. As long as an individual is not exposed above that threshold amount for a sufficiently long time, there would be no toxic consequence. Are there populations that might have a somewhat lower threshold than others? There are no data that directly support this; however, data are lacking to establish that the possibility does not exist.
Since folpet is not enzymatically activated, differences in enzyme activity, enzyme induction, or enzyme polymorphisms would not influence folpet’s toxic potential. Thus, there would not be anticipated effects of life stages on susceptibility, nor populations with increased susceptibility.
4. Forestomach lesions
In both rats and mice, folpet administration in the diet produces irritation-related toxic and proliferative lesions in the forestomach (CitationCox et al., 1985; CitationCrown et al., 1985, Citation1989; CitationRubin and Nyska, 1985; CitationWong, 1985). This occasionally also involves the distal esophagus. In mice, this leads to a slight increase in forestomach tumors, squamous cell papillomas and carcinomas (see and ). The more common finding is related to cytotoxic, irritating effects, including hyperkeratosis, hyperplasia (acanthosis), and an inflammatory infiltrate. There is a commonality of early pathologic findings in rats and mice, but a low incidence of tumors are only produced in mice and only with folpet (but not captan).
The mode of action for folpet-induced forestomach tumors is the same as for the mouse duodenal tumors; reactivity of folpet and thiophosgene with tissue thiol substituents, induction of cytotoxicity (accompanied by inflammation) with consequent increased squamous cell proliferation, hyperplasia (accompanied by hyperkeratosis), and ultimately tumors. The non-neoplastic changes were observed in mice and rats, but the extent of proliferation was adequate for tumor induction only in mice. The temporal relationship of this sequence of events is similar to that seen for a variety of agents, such as ethyl acrylate (CitationGhanayem et al., 1994) and butylated hydroxy anisole (BHA) (CitationClayson et al., 1990; CitationIto and Hirose, 1989). The dose-response shows a somewhat lower dose for the toxicity, inflammatory, and hyperplastic changes compared to the doses required for tumors, also similar to the situation with other agents inducing forestomach tumors (CitationGhaneyem et al., 1994; CitationClayson et al., 1990; CitationIto and Hirose, 1989).
It is not surprising that the forestomach is affected by folpet. Squamous epithelial cells contain large amounts of cytokeratins, which notably contain a large percentage of cysteine moieties with available thiol groups. In the forestomach, the pH is generally mildly acidic, but not as acidic as in the lumen of the glandular stomach. To the extent that folpet reacts at higher pH levels (due to an increased production of thiophosgene through hydrolysis), cytotoxicity in the forestomach would be expected to be considerably greater than in the glandular stomach but somewhat less than in the proximal duodenum. The glandular stomach, in contrast to the forestomach, is markedly acidic (pH = 1.0–2.0), which would tend to stabilize folpet from hydrolysis. The half-life of folpet solubilized in blood (average pH is 7.4) is 4.9 s (CitationGordon et al., 2001). By comparison, solubilized thiophosgene has a half-life of 0.6 s (CitationArndt and Dohn, 2004). The half-life of folpet in blood is somewhat longer than captan, which has a half life of 0.9 s, but the reverse is true for hydrolytic degradation in mild acid media, such as in the forestomach. Folpet is approximately 7-fold less stable, hydrolytically, at pH 5 than captan. Thus, in the stomach, folpet would be expected to generate thiophosgene faster than captan; this may explain the increased susceptibility of mice to folpet for forestomach tumors compared to captan.
In mice, the proliferation in the forestomach induced by folpet occasionally leads to the development of forestomach tumors (see and ). Progression to tumors is not seen in the rat, although pathological inflammatory and proliferative findings are similar. The types of lesions of the forestomach and their incidences in one of the rat studies are listed in . The difference in extent of the tumorigenicity between these species is likely due in part to the exposure levels and possibly due to the degree of generation of thiophosgene occurring between the two species. Because of generalized toxicity, the doses used for the studies in rats are lower than in the mouse. These dose levels in rats, however, attained the MTD, inducing frank toxicity, and were higher than doses producing stomach tumors in mice but folpet did not induce tumors in rats. Differences between the species have been investigated. In mice with duodenal tumors, there was occasionally obstruction of the gastric outlet due to the size of the duodenal tumors. Such obstruction might have led to more folpet being retained in the stomach for longer periods of time with consequent greater irritation, cytotoxicity, and regenerative proliferation (CitationNyska et al. (1990) ( and ). Since duodenal tumors did not occur in the rats administered folpet, such an effect on the forestomach could not occur. Mice also appear to have a greater sensitivity to glutathione depletion than rats (CitationChasseaud, 1991). Although multiple differences in response to folpet administration were identified between rats and mice, the explanation for the differences in species tumorigenicity has not been resolved.
Table 7. Histopathologic changes in the forestomach of Sprague-Dawley rats administered folpet in the diet (CitationCox et al., 1985).
The sequence of events seen with folpet in the forestomach are those commonly seen with non–DNA-reactive, irritative chemicals that produce tumors in the forestomach of rodents (CitationAdams et al., 2008; CitationClayson et al., 1990; CitationGhanayem et al., 1994; CitationGrice, 1988; CitationNTP, 2000; CitationWester and Kroes, 1988; CitationProctor et al., 2007). An irritation process occurs with cytotoxicity, and focal erosion, and occasionally even ulceration occurs. In studies with other chemicals than folpet involving gavage administration rather than dietary or drinking water administration, there is the added toxicity of the instrumentation itself. The toxicity from the chemical and/or the gavage instrument leads to an inflammatory reaction with associated regenerative hyperplasia. As in all of these instances, if the inciting stimulus is removed, the proliferative and inflammatory reaction subsides and the forestomach returns to normal. Eventually, however, once tumors are formed the process becomes irreversible.
Considerations of alternative modes of action for folpet-induced forestomach tumors are the same as for the duodenum. Thus, a DNA-reactive mechanism is unlikely for the reasons described above, and there is no evidence of a direct mitogenic effect of folpet or thiophosgene on cells.
5. Analysis of human relevance
5.1. Evaluation of qualitative differences
In addressing the issue of human relevance, the first consideration is whether the sequence of key events can occur in humans if they were exposed to doses that were sufficiently high as the levels in the test species. In the IPCS Human Relevance Framework this is posed as a question to address (CitationBoobis et al., 2006): Can human relevance of the MOA be reasonably excluded on the basis of fundamental qualitative differences in key events between experimental animals and humans?
The sequence of events for mouse duodenal tumors is tabulated in . These events involve ingestion of cytotoxic doses, generation of thiophosgene, reaction with cellular thiol groups by folpet and thiophosgene (with thiophosgene also reacting with many other cellular constituents), cytotoxicity, regenerative proliferation, and tumor formation. A similar sequence of events occurs in the mouse forestomach, although at a low incidence. This sequence does not progress to the stage of tumor formation in the rat forestomach (see above).
Could each of these key events occur in humans? The chemistry of folpet would be expected to be the same whether in rodents or humans. Thus, it is anticipated that if ingested, folpet would enter the stomach and then transit directly to the duodenum. Little if any will be hydrolyzed in the stomach because of the gastric acidity, except for the conditions of lower acid output as described above. Because of the acidity, folpet would not be very labile in the human stomach. Thiophosgene and folpet would be expected to chemically react in humans similar to rodents. Folpet itself is also expected to react with thiol groups similarly in humans as in mice. Thus, if it reached the duodenum at sufficiently high concentrations for prolonged times, it would be anticipated that it could cause cytotoxicity, with a consequent inflammatory reaction and regenerative proliferation. There is evidence in humans that once the stage of increased proliferation with blunting (hypertrophy) of the villi and increased inflammation in the lamina propria is reached, if sustained, an individual is at increased risk of developing tumors, albeit at a very low level of susceptibility given the rarity of small intestinal tumors in general (CitationNoffsinger and Waxman, 2007; CitationRiddell et al., 2003). Thus, patients with diseases that have a similar histologic appearance as seen following folpet toxicity in rodents, such as celiac sprue, are associated with a slight increased risk of intestinal carcinoma.
The entire sequence of events, whether in mice or in humans, is dependent on a high dietary exposure sufficient to generate a cytotoxic level of folpet and thiophosgene. The sequence of events leading to tumors is thus qualitatively possible in humans, but only at exposure levels sufficiently high and prolonged to produce cytotoxicity and the cascade of subsequent events.
Is the human stomach or esophagus also susceptible? Given the lack of reactivity in the glandular stomach of rats and mice to the toxicity of folpet, it is highly unlikely that the human glandular stomach would be susceptible to folpet toxicity. The human does not have a forestomach, but some have tried to relate the distal esophagus of the human to the forestomach. In reality, the distal esophagus is not the same as the rodent forestomach for a number of reasons: (1) the lumen of the forestomach has a slightly acidic pH whereas the distal esophagus does not have an acid pH; (2) the distal esophagus is acutely sensitive to the irritant effects of acid pH, as anyone with reflux esophagitis is aware, whereas the rodent forestomach tolerates acidic pH; and (3) the rodent forestomach usually has a thin layer of keratin on the surface whereas the human distal esophagus is a nonkeratinized epithelium. In rats and mice, there is some evidence that the distal esophagus can also undergo an irritant effect in response to folpet dietary administration (CitationCox et al., 1985; CitationCrown, et al., 1989; CitationRubin and Nyska, 1985; CitationWong, 1985) with consequent hyperkeratosis and hyperplasia (acanthosis). Thus, there is the possibility that a similar effect could occur in the distal esophagus of the human, but given the anatomy of the human esophagus and stomach compared to the rodent, it is unlikely. However, the possibility of cytotoxicity and regeneration in the esophagus in response to high-level exposure of folpet cannot be excluded.
Although there is the possibility of cytotoxicity of the esophagus in response to high levels of folpet exposure in humans, there is virtually no risk to the esophagus or stomach of a carcinogenic effect, even at high doses, based on predictions derived from rodent forestomach findings (CitationProctor et al., 2007). Numerous agents have been identified as inducing neoplasms of the forestomach in rodents, including mice, rats, and hamsters. For genotoxic carcinogens, it appears to be a combination of the DNA reactivity plus cytotoxicity and regenerative proliferation that are involved as a combined mode of action. In contrast, non–DNA-reactive chemicals, such as folpet in vivo, appear to induce forestomach lesions by the sequence of events including cytotoxicity, regenerative proliferation, and formation of squamous cell papillomas and ultimately squamous cell carcinomas. Preneoplastic lesions are always reversible if exposure to the inciting agent is discontinued before their conversion to neoplasia. The cytotoxicity and proliferation are clearly threshold phenomena. However, the forestomach is not considered to be analogous to any structure in humans, and carcinogenicity involving forestomach neoplasms in rodents are generally not considered relevant to human risk (CitationProctor et al., 2007). Thus, the antioxidant food additive, butylated hydroxyanisole (BHA), like many antioxidants, produces squamous cell tumors in rodents, including rats, mice, and hamsters. Nevertheless, because of the considerations described above, these neoplasms were not considered relevant to human risk (CitationClayson et al., 1990, CitationGrice, 1988); and the substance continues to be used widely in foods.
Another example is ethyl acrylate. Similar to BHA, at high exposure levels it produces forestomach tumors in rodent, following cytotoxicity and regenerative hyperplasia. Based on the original 2-year bioassay performed at the National Toxicology Program (NTP), the chemical was considered to be a possible human carcinogen and listed in 1989 on the congressionally mandated List of Carcinogens by the National Toxicology Program. However, based on the mechanistic considerations described above for forestomach tumors induced in rodents, it was determined that it did not pose a risk to humans, that the mechanism was specific to rodents, and ethyl acrylate was removed from the List of Carcinogens by the NTP in 2000 (NTP, 2000).
Numerous other examples have been identified, including a variety of antioxidants, flavoring substances, and numerous naturally occurring chemicals in foods. Thus, the forestomach tumors in mice induced by folpet are not considered relevant to human risk based on a qualitative assessment.
5.2. Evaluation of quantitative differences
Since the key events that lead to the development of tumors in mice could possibly occur in humans qualitatively, a quantitative assessment is necessary. In the IPCS Human Relevance Framework (CitationBoobis et al., 2006), this is again posed as a question: Can human relevance of the MOA be reasonably excluded on the basis of quantitative differences in either the kinetic or dynamic factors between experimental animals and humans? Again, it is likely that exposure to a sufficiently high level of folpet could lead to this sequence of events in humans. The key is to determine what the likely threshold is for human exposure. The best that we can do at the present time is to indicate whether humans are likely to be more or less susceptible or have similar susceptibilities as the mouse.
Differences in the responsiveness in the rat versus the mouse clearly indicate that exposure levels are critical, and it is likely that the degree of generation of thiophosgene is one determinant. In that respect, it is likely that humans will be similar to the mouse and the rat, since generation is likely to occur in the duodenum similar to what occurs in the rodent. However, generation of thiophosgene is unlikely to occur to a great extent in the human stomach because of the strong acid environment.
The studies that have been performed in dogs provide some indication regarding comparative susceptibility of humans to the rodent, since the dog has a stomach similar to humans, without a forestomach. The 1-year study in Beagle dogs showed no evidence of gastrointestinal toxicity, preneoplastic changes, or neoplasia (CitationWaner, 1985). Routine histologic evaluation of tissues showed no evidence of an abnormality in the gastrointestinal tract, including esophagus, stomach, duodenum, other portions of the small intestine, and large intestine. The bolus (capsule) doses that were administered to the dogs were as high as 1300 mg/kg/day, certainly comparable to even higher than the doses administered to the rats and mice. This suggests that the effects in an animal species with a glandular stomach without a forestomach is less likely to lead to toxicity in the gastrointestinal tract beyond the stomach and therefore is less likely to be susceptible to the tumorigenic effects of the chemical and its hydrolysis product. The doses used in the dog study were certainly at levels that could produce toxicity, since there was a decrease in weight gain at the two highest doses of 650 and 1300 mg/kg/day. The NOEL in this study was 325 mg/kg/day.
Thus, on a quantitative basis, the human is likely to be less susceptible to the cytotoxic and carcinogenic effects of folpet than rodents. Nevertheless, one cannot exclude the possibility of toxicity and carcinogenicity at extremely high doses in humans. Therefore, one cannot exclude the possibility of a carcinogenic effect either on a qualitative or quantitative basis. However, extrapolating from rodents to humans, an interspecies uncertainty factor of 10 would be expected to be extremely conservative, since in reality, the interspecies uncertainty factor would more likely be less than 10, and, therefore, the total uncertainty factor would be less than 100.
6. Summary and conclusions
Folpet, like captan, produces duodenal tumors in mice and produces forestomach changes in mice and rats that progress to forestomach tumors at a lower incidence and only in mice. The sequence of key events includes ingestion of a sufficiently high quantity of the chemical to lead to cytotoxicity, which is produced by the reaction of parent (folpet or captan) and thiophosgene, the parent’s initial degradation product, with thiol groups and other cellular components. The cytotoxicity leads to regenerative proliferation with associated inflammation, and ultimately tumor formation. Qualitatively the forestomach tumors in mice are considered to be specific to mice and are not considered relevant to human risk. Qualitatively and quantitatively this mode of action of duodenal tumors can not be excluded in humans, although based on the studies in dogs, the human is likely to be less susceptible to the cytotoxic effects of folpet than the mouse or rat. A DNA-reactive mode of action is excluded based on the absence of mutagenic activity in vivo. This absence of mutagenicity is due to the rapid degradation of folpet and thiophosgene before these compounds can reach DNA or DNA-related targets. The elegant in vitro study showing the elimination of mutagenicity with increasing thiols is consistent with this mode of action (CitationMoriya et al., 1978).
Since folpet is chemically and biologically similar to captan, it is appropriate to classify folpet’s carcinogenicity similar to that of captan for regulatory purposes (CitationBernard and Gordon (2000); EFSA, Citation2009a, Citation2009b; CitationGordon, 2010). The collective folpet data are similar to those data for captan and support a non–DNA-reactive, threshold mode of action (MOA) based on cytotoxicity and regenerative proliferation. The threshold for rodent carcinogenicity is above the NOEL of 450 ppm (approximately 50 mg/kg/day; CitationEast, 1994) and the estimated chronic human exposure to folpet, based on agricultural use, is 0.000039 mg/kg/day, with exposure in children, ages 1–2, estimated at 0.000107 mg/kg/day (CitationUS EPA 2004b). This results in a margin of exposure (MOE) of more than 1,000,000. Occupational dermal exposure to folpet does not present a risk of carcinogenesis because dermal absorption is relatively low and any folpet that is absorbed is rapidly degraded by thiols in the blood; thus, there is effectively no systemic exposure.
We conclude that the classification of folpet with respect to human carcinogenic risk should be similar to that of captan:
Folpet is not likely to be a human carcinogen by the oral route at dose levels that do not cause cytotoxicity and regenerative cell hyperplasia in the proximal region of the small intestine; and
It is possibly carcinogenic to humans following prolonged, high-level oral exposure causing cytotoxicity and regenerative cell hyperplasia in the proximal region of the small intestine.
Acknowledgments
We gratefully acknowledge the assistance of Cheryl Putnam with the preparation of the manuscript.
Declaration of interest
The authors’ employment affiliations are shown on the front page. Dr. Singh is employed by Makhteshim Agan of North America, Inc., a producer of folpet and captan. Drs. Cohen, Gordon, and Arce have served as consultants for Makhteshim Agan of North America, Inc., and Dr. Nyska was the study pathologist on some of the folpet chronic bioassays. The contents of this review reflect solely the view of the authors.
References
- Adams TB, Gavin CL, Taylor SV, Waddell WJ, Cohen SM, Feron VJ, Goodman J, Rietjens IM, Marnett LJ, Portoghese PS, Smith RL. (2008). The FEMA GRAS assessment of alpha beta-unsaturated aldehydes and related substances used as flavor ingredients. Food Chem Toxicol 46:2935–2967.
- Andersen ME, Meek ME, Boorman GA, Brusick DJ, Cohen SM, Dragan YP, Frederick CB, Goodman JI, Hard GC, O’Flaherty EJ, Robinson DE. (2000). Lessons learned in applying the U.S. EPA proposed cancer guidelines to specific compounds. Toxicol Sci 53:159–172.
- Arce GT, Gordon EB, Cohen SM, Singh P. (2009). Genetic toxicology of folpet and captan. Crit Rev Toxicol (In press. Companion article to this one).
- Arndt T, Dohn D. (2004). Measurement of the Half-Life of Thiophosgene in Human Blood. CRO: PTRL West, Inc., Hercules, CA Report No. 1146W-1. MRID 46296101. Captan Task Force, 82 pages, June 3, 2004.
- Bernard BK, Gordon EB. (2000). An evaluation of the common mechanism approach to the Food Quality Protection Act: Captan and four related fungicides, a practical example. Int J Toxicol 19:43–61.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. (2006). IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol 36:781–792.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. (2008). IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol 38:87–96.
- Bullock CH, Cisson CM, Wong ZA. (1982). The eye irritation potential of Phaltan Technical (PN 2623). Richmond, CA: Environmental Health & Toxicology, The Chevron Chemical Company, Ortho Division. Report no. SOCAL 1907 (S-2074); (FLP 178). August 3 1982. MRID 00160444.
- Chasseaud LF. (1991). Comparative metabolic fate and biochemical effects of folpet in male rats and mice. Cambridgeshire, England: Huntingdon Research Centre Ltd. Sponsor: New York: Makhteshim-Agan (America) Inc. Report no. NRC/MBS 32/90110 (Volume I: rats); NRC/MBS 33/90110 (Volume II, mice). March 5, 1991. MRID 42122016.
- Chidiac P, Goldberg M.T. (1987). Lack of induction of nuclear aberrations by captan in mouse duodenum. Environ Mutagen. 9:297–306. MRID 43405103.
- Clayson DB, Iverson F, Nera EA, Lok E. (1990). The significance of induced forestomach tumors. Annu Rev Pharmacol Toxicol. 30:441–463.
- Cohen SM, Ellwein LB. 1990. Cell proliferation in carcinogenesis. Science 249:1007–1011.
- Cohen SM, Ellwein LB. 1991. Genetic errors cell proliferation and carcinogenesis. Cancer Res 51:6493–6505.
- Couch R, Siegel MR. 1972. Reactions of the trichloromethyl sulfenyl fungicides with histones. Phytopathology 62:802.
- Couch RC, Siegel MR. 1977. Interaction of captan and folpet with mammalian DNA and histones. Pestic Biochem Physiol 7:531–546.
- Couch RC, Siegel MR, Dorough HW. 1977. Fate of captan and folpet in rats and their effects on isolated liver nuclei. Pestic Biochem Physiol 7:547–558.
- Cox RH, Marshall PM, Voelker RW, Vargas KJ, Alsaker RD, Dudeck LE. (1985). Combined chronic oral toxicity/oncogenicity study in rats with Chevron Folpet Technical (SX-1388). Vienna, VA: Hazleton Labs America Inc. Project no. 2107-109. September 9, 1985. MRID 00151560.
- Cracknell S. (1993). Folpet technical (micronized): Acute inhalation toxicity study in the rat. Suffolk, England: Pharmaco-LSR Ltd. Sponsor: Beer Sheva, Israel: Makhteshim Chemical Works. Report no. 93/MAK139B/0507 (FLP 564). October 15, 1993. MRID 44286301.
- Crown S, Nyska A, Waner T. (1985). Folpan carcinogenicity study in the rat. Ness Ziona: Israel Life Science Research Israel, Ltd. Report no. MAK/022/FOL. December 1, 1985. MRID 00157493.
- Crown S, Nyska A, Waner T, Kenan G. (1989). Folpan: Toxicity by dietary administration to rats for two years. Ness Ziona: Israel Life Science Research Israel. Report no. MAK/053/FOL. January 10, 1989. MRID 43640201, 00124023.
- East P. (1994). Folpet: Oncogenicity study by dietary administration to CD-1 mice for 104 weeks. CRO Pharmaco LSR, Ltd., Suffolk, England. Report no. 94/MAK117/0403. MRID 44316501, 1422 pages, July 12, 1994.
- EFSA. 2009a. Conclusions on Pesticide Peer Review. Peer review of the pesticide risk assessment the active substance captan (Question No. EFA-Q-2009-604). EFSA. Scientific Report (2009) 296, 1–90. Conclusion on the peer review of captan. Parma, Italy: EFSA.
- EFSA. 2009b. Conclusions on Pesticide Peer Review. Peer review of the pesticide risk assessment the active substance folpet (Question No. EFA-Q-2009-605). EFSA. Scientific Report (2009) 297, 1–80. Conclusion on the peer review of folpet. Parma, Italy: EFSA.
- FAO/WHO (1996). Pesticide Residues in Food—1995 (Captan; Folpet). Geneva: WHO.
- Ghanayem BI, Sanchez IM, Matthews HB, Elwell MR. (1994). Demonstration of a temporal relationship between ethyl acrylate-induced forestomach cell proliferation and carcinogenicity. Toxicol Pathol 22:497–509.
- Gordon E. (2007). Captan: Transition from ‘B2′ to ‘not likely’. How pesticide registrants affected the EPA Cancer Classification Update. J App Toxicol 27:519–526.
- Gordon EB. (2010). Captan and folpet. In: Krieger RI, ed. Hayes Handbook of Pesticide Toxicoklogy. 3rd ed. Vol. 2. NewYork: Elsevier, 1915–1949.
- Gordon EB, Mobley SC, Ehrlich T, Williams M. (2001). Measurement of the reaction between the fungicides captan or folpet and blood thiols. Toxicol Methods 11:209–223.
- Greaves P. (2007). Histopathology of Preclinical Toxicity Studies. London: Elsevier-Academic Press, 386–392, 397–407.
- Greenfield RE, Ellwein LB, Cohen SM. (1984). A general probabilistic model of carcinogenesis: Analysis of experimental urinary bladder cancer. Carcinogenesis 5:437–445.
- Grice HC. (1988). Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–723.
- Gudi R, Krsmanovic L. (2001). Nuclear aberration test in the mouse duodenum (folpet) and photomicrographs. Rockville, MD: BioReliance Corporation. Report no. AA31SK.123005.BTL. March 15, 2001. MRID 45745105.
- Haseman JK. (1983) A reexamination of false-positive rates for carcinogenesis studies. Fundam App Toxicol 3:334–339.
- Haseman JK, Hailey JR, Morris RW. (1998). Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: A National Toxicology Program update. Toxicol Pathol 26:428–441.
- Hill BA. (1965). The environment and disease: Association or causation? Proc R Soc Med 58:295–300.
- Ito N, Hirose M. (1989). Antioxidants—Carcinogenic and chemopreventive properties. Adv Cancer Res 53:247–302.
- Jernstrom B, Morgenstern R, Moldeus P. (1993). Protective role of glutathione thiols and analogues in mutagenesis and carcinogenesis. Basic Life Sci 61:137–47.
- Knudson AG Jr. (1971). Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA 68:820–823.
- Lee KS. (1989). [Trichloromethyl-14C]—Captan; Hydrolysis at 25°C. Richmond, CA: ICI Americas Inc. Report no. WRC 89-44. May 26 1989.
- Lin KK. (1998). Formats for submission of animal carcinogenicity study data. Drug Inf J. 32:43–52.
- Liu MK, Fishbein L. (1967). Reactions of captan and folpet with thiols. Experientia 23:81–82.
- Lukens RJ. (1966). The fungitoxicity of compounds containing a trichloromethylthio- group. J Agric Food Chem 14:365–367.
- Lukens RJ, Rich S, Horsfall JG. (1965). Role of the R-group in the fungitoxicity of R-SCCI3 compounds. Phytopathology 55:658–662.
- McConnell EL, Basit AW, Murdan S. (2009). Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol 60:63–70.
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. (2003). A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol 33:591–653.
- Milburn GM. (1997). Folpet: Study of hyperplasia in the mouse duodenum. CRO: Central Toxicology Laboratory, Alderley Park Macelesfield, Cheshire, UK. Report no. CTL/p/5577. R-9688. 56 pages, December 17, 1997. MRID 44866402.
- Moolgavkar SH, Knudson AG, Jr, . (1981). Mutation and cancer: A model for human carcinogenesis. J Natl Cancer Inst 66:1037–1052.
- Moriya M, Kato K, Shirasu Y. (1978). Effects of cysteine and a liver metabolic activation system on the activities of mutagenic pesticides. Mutat Res. 57:259–263.
- Noffsinger A, Waxman I. (2007). Preinvasive neoplasia in the stomach: Diagnosis and treatment. Clin Gastroenterol Hepatol 5:1018–1023.
- NTP (2000). Report on Carcinogens (RoC), Ninth Edition. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program Pursuant to Section 301(b) (4) of the Public Health Service Act as Amended by Section 262, PL 95-622. Available at: http://niehs.nih.gov/. Current report: 11th RoC.
- Nyska A, Waner T, Paster Z, Bracha P, Gordon EB, Klein B. (1990). Induction of gastrointestinal tumors in mice fed the fungicide folpet: Possible mechanisms. Jpn J Cancer Res 81:545–549.
- Potten CS, Loeffler M. (1990). Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110:1001–1020.
- Potten CS, Owen G, Roberts SA. (1990). The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol 57:185–199.
- Proctor DM, Gatto NM, Hong SJ, Allamneni KP. (2007). Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci 98:313–326.
- Provan WM, Eyton-Jones H, Lappin G, Pritchard D, Moore RB, Green T. (1995). The incorporation of radiolabelled sulphur from captan into protein and its impact on a DNA binding study. Chem Biol Interact 96:173–184.
- Quest JA, Fenner-Crisp PA, Burnam W, Copley M, Dearfield KL, Hamernik KL, Saunders DS, Whiting RJ, Engler R. (1993). Evaluation of the carcinogenic potential of pesticides. 4. Chloroalkylthiodicarboximide compounds with fungicidal activity. Regul Toxicol Pharmacol 17:19–34.
- Reno FE, Burdock G, Serota D. (1981). Subchronic toxicity study in rats: Phaltan (folpet). Vienna, VA: Hazleton Laboratories America, Inc. Report no. 2107-100; R-6118. MRID 00115269.
- Riddell RH, Petras RE, Sobin LH, Williams GT. (2003). Epithelial neoplasia of the intestines. Published by Armed Forces Institute of Pathology, Washington, DC. In: Tumors of the Intestines. AFIP Atlas of Tumor Pathology, Third Series, Vol. 32. 85–240.
- Rosen PP, Oberman HA. (1993). Fibroepithelial neoplasms. In: Tumors of the Mammary Gland. Published by Armed Forces Institute of Pathology, Washington, DC. AFIP Atlas of Tumor Pathology, Third Series, Vol. 7. 191–114.
- Rubin Y, Nyska A. (1985). Folpan: Oncogenicity study in the mouse. Life Science Research Israel. Sponsor: Beer Sheva, Israel: Makhteshim Chemical Works, Ltd. Report no. MAK/015/FOL. September 9, 1985. MRID 00151075.
- Russo J, Russo I. H. (1987). Biological and molecular bases of mammary carcinogenesis. Lab Invest 57:112–137.
- Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. (1990). Biology of disease—Comparative study of human and rat mammary tumorigenesis. Lab Invest 62:244–278.
- Ruzo LO, Ewing AD. (1988). Hydrolysis of [14C]-folpet. Richmond, CA: PTRL West, Inc. Report no. PTRL 124. August 4, 1988. MRID 40818801.
- Schnaubelt LJ. (1995). Reregistration of phosmet; literature review of the metabolite phthalimide. Washington, DC: Reregistration Branch, Section II, Special Review and Reregistration Division. May 24, 1995.
- Seed J, Carney EW, Corley RA, Crofton KM, DeSesso JM, Foster PM, Kavlock R, Kimmel G, Klaunig J, Meek ME, Preston RJ, Slikker W Jr, Tabacova S, Williams GM, Wiltse J, Zoeller RT, Fenner-Crisp P, Patton DE. (2005). Overview: Using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol 35:664–672.
- Siegel MR. (1971a) Reactions fo the fungicide folpet (n-trichloromethylthio) phthalimide with a nonthiol protein. Pestic Biochem Physiol 1:234–240.
- Siegel MR. (1971b) Reaction of the fungicide folpet (N-trichloromethylthio phthalimide) with a thiol protein. Pestic Biochem Physiol 1:225–233.
- Sonich-Mullen C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek E, Rice JM, Younes M. (2001). IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34:146–152.
- TERA. (2003). Report of Peer Review Meeting Cancer Assessment for Captan September 3–4, 2003. Cincinnati, OH: Toxicology Excellence for Risk Assessment (TERA). November 21, 2003. Available at: http://www.TERA.org/peer/CAPTAN/Captan%20final%20meeting%20report.pdf.
- US EPA. (1999a). Captan: Reregistration Eligibility Decision (RED). Washington, DC: Prevention, Pesticides and Toxic Substances (7508C). Report no. EPA 738-R-99-015. November 1999.
- US EPA. (1999b). Folpet: Reregistration Eligibility Decision (RED). Washington, DC: Prevention, Pesticides and Toxic Substances (7508C). Report no. EPA 738-R-99-011. November 1999.
- US EPA. (1999c). Guidelines for Carcinogen Risk Assessment (Review Draft). Washington, DC: Risk Assessment Forum. US Environmental Protection Agency. Report no. NCEA-F-0644. July 1999. Available at: http://www.epa.gov/ncea/raf/cancer.htm.
- US EPA. (2003). Folpet; pesticide tolerance. Federal Register 68:10377–10388.
- US EPA. (2004a). Captan: Cancer Reclassification; Amendment of Reregistration Eligibility Decision; Notice of Availability. Federal Register 69:68357–68360.
- US EPA. (2004b). Folpet; pesticide tolerance. Federal Register 69:52182–52192.
- US EPA. (2005). Guidelines for Carcinogen Risk Assessment. Washington, DC: Risk Assessment Forum, US Environmental Protection Agency. Report no. EPA/630/P-03/001F. March 2005.
- Waner T. (1985). Folpan chronic oral study in beagle dogs for 52 weeks. Life Science Research Israel. Report no. LSRI MAK/062/FOL. October 28, 1985.
- Waterson LA. (1995). Folpet: Investigation of the effect on the duodenum of male mice after dietary administration for 28 days with recovery. Cambridgeshire, England: Huntingdon Research Centre Ltd. Sponsor: Beer Sheva, Israel: Maktheshim Chemical Works, Ltd. Report no. MBS 45/943003. R-7794; April 11 1995. MRID 44286303.
- Wester PW, Kroes R. (1988). Forestomach carcinogens: Pathology and relevance to man. Toxicol Pathol 16:165–171.
- Wilkinson CF, Arce G, Gordon EB. (2004). Analysis of the appropriate cancer classification of captan under EPA’s current guidelines. Captan Task Force: San Francisco, CA: Arvesta Corporation, and New York: Makhteshim Agan of North America, Inc. Report no. CTF 0304. 82 Pages, April 9, 2004. MRID 46247701.
- Wong ZA. (1985). Lifetime oncogenic feeding study of Phaltan Technical (SX-946) in CD-1 mice (ICR derived). Richmond, CA: Chevron Environmental Health Center, Chevron Chemical Company. Report no. Socal 1331. July 26, 1985. MRID 00125718.