Abstract
The herbicide glyphosate has undergone multiple safety tests for developmental toxicity in rats and rabbits. The European Commission’s 2002 review of available glyphosate data discusses specific heart defects observed in several individual rabbit developmental toxicity studies, but describes the evidence for a potential causal relationship as equivocal. The present assessment was undertaken to analyze the current body of information generated from seven unpublished rabbit studies in order to determine if glyphosate poses a risk for cardiovascular malformations. In addition, the results of six unpublished developmental toxicity studies in rats were considered. Five of the seven rabbit studies (dose range: 10–500 mg/kg/day) were GLP- and testing guideline-compliant for the era in which the studies were performed; a sixth study predated testing and GLP guidelines, but generally adhered to these principles. The seventh study was judged inadequate. In each of the adequate studies, offspring effects occurred only at doses that also caused maternal toxicity. An integrated evaluation of the six adequate studies, using conservative assumptions, demonstrated that neither the overall malformation rate nor the incidence of cardiovascular malformations increased with dose up to the point where severe maternal toxicity was observed (generally ≥150 mg/kg/day). Random occurrences of cardiovascular malformations were observed across all dose groups (including controls) and did not exhibit a dose–response relationship. In the six rat studies (dose range: 30–3500 mg/kg/day), a low incidence of sporadic cardiovascular malformations was reported that was clearly not related to treatment. In summary, assessment of the entire body of the developmental toxicity data reviewed fails to support a potential risk for increased cardiovascular defects as a result of glyphosate exposure during pregnancy.
Introduction
Glyphosate, the active ingredient in popular herbicide formulations such as Roundup, AquaMaster and Vision branded products, is the most commonly used herbicide in the US (Grube, Citation2011). Specific usage statistics are not readily available for Europe, but are assumed to mirror those of the US. Glyphosate acts by targeting the enzyme enolpyruvylshikamate phosphate synthase in plants (Williams et al., Citation2012). Although this enzyme is important in the synthesis of several essential amino acids in plants, it is not found in animals. For this reason, glyphosate is considered to be generally safe to people and other mammals when used according to the manufacturer’s instructions. Nevertheless, due to its widespread use and the large number of glyphosate manufacturers, glyphosate has been subjected to numerous safety tests to protect health. In a monograph developed to support the European Commission’s Citation2002 review of glyphosate (BBA, 1998–2000; European Commission, Citation2002), the authors discuss specific heart defects observed in individual rabbit developmental toxicity studies of glyphosate, however they describe the evidence for a potential causal relationship as equivocal. Based on data selected from these studies, others have alleged there is evidence of teratogenicity and have called for a new risk assessment of glyphosate (Antoniou et al., Citation2012).
The present critical analysis assesses the glyphosate developmental toxicity database available to European regulatory agencies in order to determine if there is, in fact, a cause for concern for cardiovascular defects or other malformations. Rabbit and rat developmental toxicity studies on glyphosate conducted by member companies of the European Union (EU) Glyphosate Task Force were made available to the authors of this paper for the purpose of this analysis. These included seven developmental toxicity studies conducted in rabbits as well as six developmental toxicity studies conducted in rats. A PubMed search of the peer-reviewed literature through May 2012 was also conducted in an attempt to identify other studies of developmental glyphosate exposure and heart/cardiovascular malformations. No studies were found to be focused on cardiovascular defects as a result of in utero glyphosate treatment. A few published studies examined the effects on the fetal development of in utero exposure to glyphosate-based herbicide formulations (Dallegrave et al., Citation2003, Citation2007; Daruich et al., Citation2001); none of these studies, however, addressed visceral malformations. Therefore, the focus of the present analysis is on developmental toxicity studies of glyphosate that were conducted to fulfill regulatory requirements, particularly those in the rabbit. Each of the seven rabbit developmental toxicity studies has been critically evaluated with attention to whether the database as a whole is of sufficient quality to determine glyphosate’s teratogenic potential in rabbits, particularly for the cardiovascular system. Details of these analyses are found in the Appendix. The findings from six rat developmental toxicity studies conducted with glyphosate for regulatory purposes are also addressed, paying particular attention to heart and cardiovascular defects. Finally, the rabbit and rat data are briefly discussed in the context of the available epidemiological data for glyphosate.
Rabbit developmental toxicity database
A total of seven developmental toxicity studies of glyphosate have been conducted in the rabbit, the designs of which are summarized in . These studies, which are critically evaluated in the Appendix, involved testing in three different rabbit strains (New Zealand white, Japanese white and Dutch belted) and covered a wide range of glyphosate doses, from 10 to 500 mg/kg/day. This range includes doses that caused overt maternal toxicity (150 mg/kg/day and above); in some cases, the maternal toxicity observed was substantial. Two of these studies (Suresh, Citation1993; Tasker, Citation1980a) had insufficient numbers of fetuses available for assessment at the high dose (500 and 350 mg/kg/day, respectively).
Table 1. Maternal and developmental NOAELs from six sufficient rabbit developmental toxicity studies of glyphosate.
The seven rabbit developmental toxicity studies vary considerably in their quality: the numbers of animals per dose group, the spacing of doses, the extent of documentation and detail provided and the specific types of data reported. Five of the studies stated that they followed good laboratory practices (GLP) specific to the time period in which they were conducted (Brooker et al., Citation1991a; Coles and Doleman, Citation1996; Hojo, Citation1995; Moxon, Citation1996; Suresh, Citation1993). Another study was conducted prior to the establishment of GLP requirements, but appears to have generally adhered to GLP principles (Tasker et al., Citation1980a). In the seventh study (Bhide & Patil, Citation1989), it is not clear to what extent GLP practices were followed, but it is unlikely that this study was fully GLP-compliant because the description of study results is extremely limited and inappropriate animals appear to have been included in the calculations for certain endpoints. All these studies were conducted according to developmental toxicity testing guideline requirements current at the time they were initiated and provided quality assurance audits.
As these studies were all done in different laboratories, there is considerable disparity across studies in the classification of various anomalies as major malformations, minor malformations or variations and in the terminology used to describe these findings. Further, three of the studies (Bhide & Patil, Citation1989; Hojo, Citation1995; Suresh, Citation1993) did not report anomalies by individual fetus. Therefore, for these studies, it is not possible to determine whether certain fetuses showed multiple anomalies or if anomalies occurred in combination. The study by Suresh (Citation1993) also used some terminology that is not standard for heart defects in developmental toxicity studies (e.g. seal-shaped heart, dilated heart), which makes interpretation of the findings difficult. Certain cardiovascular changes reported in the Brooker et al. (Citation1991a) study (e.g. retroesophageal right subclavian artery) are considered variations in other laboratories (Appendix), these are discussed in more detail below. Because of inappropriate methods and the poor reporting of data, the Bhide & Patil (Citation1989) study was considered inadequate for assessing glyphosate’s potential for developmental toxicity in rabbits. The remaining six rabbit studies formed the basis for our analysis. While the individual studies may fall short of current guidelines (mainly because the desired number of rabbits per group has increased and the exposure period has been extended beyond GD18), these shortcomings are overcome when one considers the overall database. More specifically, the exposure period in each of these studies extends well before and after the period of organogenesis for the cardiovascular system. Additionally, the studies cover a broad and well-distributed range of 15 different glyphosate exposures ranging from 10 to 500 mg/kg/day. Finally, the combined database from these studies includes evaluation of 347 total litters (99 controls and 247 treated) and 2990 fetuses (834 controls and 2156 treated). Based on these elements, the overall database of six adequate rabbit developmental studies is considered to be robust for the purposes of risk assessment.
To address whether the six adequate studies exhibited evidence of selective offspring sensitivity to glyphosate treatment in utero, the no observed adverse effect levels (NOAELs) for maternal toxicity and developmental effects were determined (). Maternal toxicity was most commonly evidenced in the rabbit studies by diarrhea and reduced food intake, which generally occurred at doses of 150 mg/kg/day or higher. Additionally, maternal weight loss and deaths generally occurred at the highest doses. also shows that offspring effects due to glyphosate, when observed in a particular rabbit developmental toxicity study, always occurred at the same dose or doses as those associated with maternal toxicity. This does not mean that injury to the fetus necessarily occurred as a direct result of maternal toxicity, but rather, when exposures to glyphosate were kept below the doses that cause maternal toxicity, the developing offspring did not exhibit any adverse effects. Therefore, selective offspring sensitivity to glyphosate is not apparent from these studies.
Post-implantation loss was quite variable across studies. Four of the six adequate studies (Hojo, Citation1995; Moxon, Citation1996; Suresh, Citation1993; Tasker, Citation1980a) reported no statistically significant increase in post-implantation loss in three different strains of rabbits at exposure levels as high as 500 mg/kg/day. In comparison, Coles & Doleman (Citation1996) reported an increase in post-implantation loss at 200 mg/kg/day, but not at 400 mg/kg/day; consequently, a dose–response pattern was not established in this study. Brooker et al. (Citation1991a) reported increased post-implantation loss at doses of 50 mg/kg/day and above (mean = 19.5 ± 19.8%, 15.3 ± 17.2% and 21.0 ± 11.8% for the 50, 150 and 450 mg/kg/day dose groups, respectively), but noted that post-implantation loss in the concurrent control group (5.7 ± 7.2%) was lower than in historical controls (mean: 12.9%; range: 6.5–17.5%), while post-implantation loss in treated litters was within or slightly higher than the historical control range. Post-implantation loss has a high degree of variability as demonstrated by the standard deviations around this endpoint in the six studies reviewed. This variability is common in the rabbit. Other historical control databases have reported mean percent post-implantation loss in the rabbit of 8.1% (range: 2.8–17.7%) and 9.1% (range: 0.6–23.4%) (Holson et al., Citation2006 and MARTA, Citation1997, respectively). Consequently, without a clear dose–response pattern established across the six studies reviewed, it is unlikely that these findings are biologically significant.
As previously noted, the rabbit developmental toxicity data for glyphosate have been previously described as equivocal with regard to cardiovascular defects (BBA, 1998–2000; European Commission, Citation2002). To address this issue, data were extracted from each study for malformations and variations (Appendix). Two of the studies (Brooker et al., Citation1991a; Suresh, Citation1993) suggested a possible association of cardiovascular anomalies with treatment, but the data were not clear-cut; these are discussed in more detail in the Appendix. In addition, two studies (Hojo, Citation1995; Moxon, Citation1996) reported an increase in skeletal defects at the high dose of 300 mg/kg/day. These anomalies appeared to be the result of reduced ossification, which is likely related to delayed development (evidenced by reduced fetal body weights observed at the high dose), or were not clearly dose-related. Based on this information and our evaluation of the combined data, we concluded that glyphosate treatment was not associated with an increase in malformations in rabbits. The remaining discussion focuses on cardiovascular defects only.
Examination of the data from the six rabbit studies showed a variety of malformations of the heart and great vessels. These included: dilated aorta/narrow pulmonary artery; narrow aorta/dilated pulmonary artery; hypoplasia of the pulmonary artery; interventricular (IV) septal defect; cardiomegaly; single ventricle, thickened ventricle walls; dilated ventricle; retro-esophageal right subclavian artery; interrupted aorta; right subclavian artery arising from aortic arch; “seal-shaped” heart. If glyphosate treatment was associated with congenital heart defects and malformation of the great vessels in rabbits, then the prevalence of these defects would be anticipated to increase with dose and the overall malformation rate would also be anticipated to increase. However, as can be seen from the malformation incidence tables in the Appendix, cardiovascular malformations generally occurred in the rabbit studies at a low incidence across all dose groups. Further, in most studies, they did not exhibit a positive dose–response, and oftentimes, clusters of malformations occurred in the same fetuses.
In order to further discern whether there might be an association between exposure of rabbits to glyphosate and cardiovascular malformations, the following conservative assumptions were made so that the malformation data from the six adequate studies could be combined. First, all three rabbit strains (Japanese white, New Zealand white and Dutch belted) were assumed to be equally sensitive to glyphosate. Second, small differences in treatment duration across studies were assumed not to affect the incidence of cardiovascular malformations because all treatment paradigms covered the critical period of heart and great vessel development (i.e. GD 8–17; DeSesso, Citation2012). Third, cardiovascular malformations were categorized depending on the type of cardiovascular defect and what is known about the underlying morphogenetic processes. For instance, several defects are related to development of the aorticopulmonary septum and are grouped together. As an example, Brooker et al. (Citation1991a) reported that many fetuses with IV septal defects exhibited other cardiovascular defects that included enlarged aorta/stenotic pulmonary artery or the converse (stenotic aorta/enlarged pulmonary artery). During formation of the outflow tract from the ventricles, neural crest cells migrate from the hindbrain region into the truncus arteriosus where they contribute to and direct the growth of the aorticopulmonary septum (Hutson & Kirby, Citation2003; Kirby et al., Citation1983; Sadler, Citation2011). The aorticopulmonary (spiral) septum () grows as a pair of ridges that divide the truncus arteriosus into equally sized halves: the aorta and the pulmonary artery (DeSesso & Venkat, Citation2010). At its inferior end, the aorticopulmonary septum forms the upper portion (membranous portion) of the IV septum. Consequently, malformations relating to a disproportionately sized aorta and pulmonary septum, as well as IV septal defects of the upper region, are all related to displacement of the developing aorticopulmonary septum (DeSesso & Venkat, Citation2010).
Figure 1. Division of the outflow tract by the aorticopulmonary (spiral) septum. In the top diagram, the aorticopulmonary septum is forming by the growth and merging of the conotruncal ridges in the walls of the outflow tract. This process divides the outflow tract into the atrioventricular canals (precursors of the aorta and pulmonary artery). In the lower diagram, the spiral septum has completed the separation of the outflow tract into the equally sized aorta (for systemic circulation) and pulmonary artery (for the pulmonary circulation). The most inferior part of the spiral septum will contribute to the upper membranous portion of the IV septum. (Modified from DeSesso & Venkat, Citation2010).
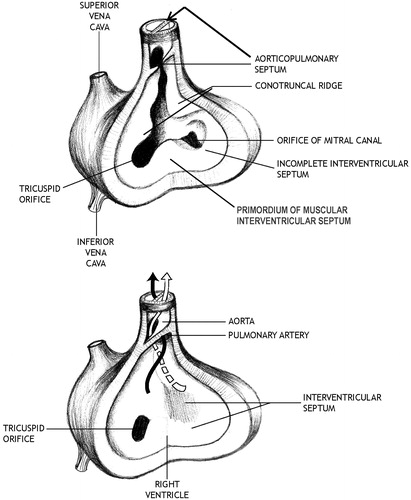
Based on this information, those cardiac defects that involved perturbations of aorticopulmonary septum development were combined based on the premise that glyphosate might cause all or any of these defects by acting on a single developmental process. Data from all numerically similar dose groups (e.g. data from all three studies that treated rabbits at 100 mg/kg/day) were combined into a single entry.
Evaluation of the resulting tabulation () shows that there was no increase in cardiovascular malformations at doses that were not overtly toxic to the pregnant rabbits (i.e. generally at doses over 150 mg/kg/day). The two most commonly observed malformations involved the aorticopulmonary septum and dilated heart. The incidence of aorticopulmonary septum-related defects in the combined control groups was 1/770 (0.1%); in the combined glyphosate-treated groups the incidence was 6/1939 (0.3%). More than half of these affected fetuses were found in litters exposed to one of the highest doses (450 mg/kg/day). Doses of 150 mg/kg/day and above were generally associated with maternal toxicity, including severe weight loss and death. If doses of 300 mg/kg/day and above are not considered because of the confounding maternal toxicity issues, then the incidence of the defects in glyphosate-treated animals is 2/1388 (0.1%). Thus, these data show that the overall incidence of aorticopulmonary septum-related defects in offspring from mothers exposed to glyphosate at doses below those that cause severe maternal toxicity is similar to that seen in non-exposed rabbits.
Table 2. Combined and grouped (number and percentage) cardiovascular malformations from six rabbit developmental toxicity studies.
The other prevalent cardiovascular malformation reported was dilated heart. All observations of this finding occurred in a single study (Suresh, Citation1993). There was also one case of cardiomegaly at 100 mg/kg/day in the same study. None of the other five adequate studies reported dilated hearts or cardiomegaly. Furthermore, neither the criteria used to diagnose dilated heart nor measurements of the hearts were provided in the study report, so it is not possible to directly compare the dilated heart findings to the hearts of the more than 2500 fetuses in the other studies.
Finally, an examination of the overall rate of cardiac malformations across the six studies did not support a dose–response correlation with glyphosate exposure. Based on this analysis, it appears that prenatal glyphosate exposure is not associated with increased cardiovascular defects in rabbits.
Rat developmental toxicity database
The six developmental toxicity studies of glyphosate conducted in the rat are discussed in the Appendix and summarized in . These studies involved testing in two different rat strains (Wistar and Sprague–Dawley) and covered a wide range of glyphosate doses up to 3500 mg/kg/day, which is well above the current limit dose for toxicity studies of 1000 mg/kg/day. With the exception of Tasker et al. (Citation1980b), all studies conformed to internationally accepted general principles of GLPs and were conducted according to OECD 414 (1981) and US EPA 83-3 guideline requirements. The study by Tasker et al. (Citation1980b) predated the establishment of US EPA and OECD guidelines, but it received quality assurance audits by the testing facility and appeared to be well-conducted and essentially guideline-compliant. As with the rabbit studies, the rat developmental toxicity studies of glyphosate varied in the numbers of animals per dose group, the spacing of doses, the extent of documentation and detail provided, and the specific types of data reported. Nevertheless, for the purposes of this evaluation, all six rat studies were considered adequate for assessing the developmental toxicity potential of glyphosate.
Table 3. Maternal and developmental NOAELs from six sufficient rat developmental toxicity studies of glyphosate.
The NOAELs for maternal toxicity and developmental effects as assessed for the six rat developmental toxicity studies are shown in . Maternal body weight was not affected in any of the studies at exposure levels lower than 3500 mg/kg/day. Further, there were no dose-related effects on intrauterine parameters at doses of 1000 mg/kg/day and below. Maternal NOAELs were determined to be ≥1000 mg/kg/day for all studies except Hatakenaka (1991) (), which reported loose stools in a few dams at that exposure. No treatment-related effects were observed in the offspring at doses of 1000 mg/kg/day and below. Consequently, the offspring NOAELs for these studies were ≥1000 mg/kg/day and equal to or greater than the maternal NOAELs in each study (). Further, no treatment-related effects of glyphosate on structural development of the offspring were observed (). Generally, malformations (including cardiovascular malformations) were limited to 1–3 fetuses in 1–2 litters in the exposed groups and occurred at incidences as low as or lower than those in the control group. Overall, the rat developmental toxicity studies do not show any evidence of cardiovascular or other types of malformations as a result of glyphosate exposure at doses of up to 3500 mg/kg/day.
Discussion and conclusions
The 13 developmental toxicity studies summarized above and discussed in detail in the Appendix have been submitted to regulatory agencies in support of the registration of glyphosate. Analyses by the regulatory agencies have not supported the claim that glyphosate causes cardiovascular defects or other developmental effects (BBA, 1998–2000; EPA, 1993; European Commission, Citation2002). At the time of the US EPA’s assessment, only the studies by Tasker et al. (Citation1980a,Citationb) were available for evaluation. The European Commission’s review (European Commission, Citation2002), however, included the examination of four of the rabbit studies (Bhide & Patil, Citation1989; Brooker et al., Citation1991a; Suresh, Citation1993; Tasker et al., Citation1980a) and three of the rat studies (Brooker et al., Citation1991b; Suresh Citation1991; Tasker et al., Citation1980b) discussed herein. In a related monograph (BBA, 1998–2000), the results from two of the rabbit studies reviewed by the European Commission were characterized as equivocal for cardiovascular developmental effects. None of the three rabbit developmental toxicity studies that were not evaluated by the European Commission (Coles & Doleman, Citation1996; Hojo, Citation1995) showed a potential for cardiovascular defects.
Based on our assumptions underlying the integrated assessment of data across studies (equal strain sensitivity, insignificant differences in timing of exposure and shared morphogenetic processes of certain defects), the overall conclusion of our analysis of the potential for glyphosate to cause malformations, and cardiovascular defects in particular, is that there is no increased risk at the levels of exposure below those that caused maternal toxicity. This conclusion is in agreement with that of regulatory agency reviews as well as the limited data available from epidemiology studies showing no increased risk of congenital defects with exposure (Bell et al., Citation2001a,Citationb,Citationc; Garry et al., Citation2002; Rull et al., Citation2006; reviewed in Williams et al., Citation2012). It should be noted, however, that these studies investigated exposures to several pesticides and were not specific to glyphosate. More recently, a detailed review of epidemiology studies of glyphosate and non-cancer endpoints found no evidence of a causal relationship between glyphosate exposures and malformations (Mink et al., Citation2011). Finally, a review of the available biomonitoring data demonstrates that human exposure as a result of normal glyphosate application practices is extremely low, often below the limits of analytical detection (Williams et al., Citation2012). In conclusion, this analysis of the developmental toxicity data available for glyphosate exposure confirms that there is no evidence of an increased risk of cardiovascular defects as a result of glyphosate exposure.
Acknowledgements
The authors would like to acknowledge member companies of the EU Glyphosate Task Force who shared their unpublished developmental toxicity study reports with us for the purposes of this assessment. Member companies on the task force are listed at www.glyphosatetaskforce.org.
Declaration of interest
The authors affiliation is as shown on the cover page. Exponent is a consulting firm that provides scientific analysis and advice in areas that include toxicology and risk assessment; Georgetown University School of Medicine is a provider of medical education. Funding for this work was supplied by the European Glyphosate Task Force. Although none of the authors on this assessment have previously consulted for the European Glyphosate Task Force, two authors (A. L. Williams and J. M. DeSesso) have addressed other issues related to glyphosate in work funded by Monsanto. The authors are solely responsible for the analyses and preparation of this manuscript; the opinions and conclusions are those of the authors and are not necessarily those of the sponsoring entity.
Notes
1The authors state that this animal was replaced, but this does not appear to be the case from Appendix 1 in Brooker et al. (Citation1991a).
2Brooker (Citation1991b) & Suresh (Citation1991) refer to it as Day 0 of Pregnancy.
References
- Antoniou M, Habib MEM, Howard CV, et al. (2012). Teratogenic effects of glyphosate-based herbicides: divergence of regulatory decisions from scientific evidence. J Environ Anal Toxicol, Suppl 4, 1–13
- BBA (Federal Biological Center for Agriculture and Forestry – Germany). (1998–2000). Monograph on the active substance glyphosate and its IPA-, Na- and NH4-salts. Annex to the European Commission report for the active substance glyphosate
- Bell EM, Hertz-Picciotto I, Beaumont JJ. (2001a). A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology, 12, 148–56
- Bell EM, Hertz-Picciotto I, Beaumont JJ. (2001b). Case-cohort analysis of agricultural pesticide applications near maternal residence and selected causes of fetal death. Am J Epidemiol, 154, 702–10
- Bell EM, Hertz-Picciotto I, Beaumont JJ. (2001c). Pesticides and fetal death due to congenital anomalies: implication of an erratum (letter). Epidemiology, 12, 595–6
- Berko NS, Jain VR, Godelman A, et al. (2009). Variants and anomalies of vasculature on computed tomographic angiography in adults. J Comput Assist Tomogr, 33, 523–8
- Bhide MB, Patil UM. (1989). Rabbit teratology study with glyphosate technical. Study No. IIT Project No. 1086
- Brooker AJ, Brennan C, John DM, et al. (1991a). The effect of glyphosate on pregnancy of the rabbit (incorporates preliminary investigations). Study/Project No. CHV 45 & 39 & 40/901303
- Brooker AJ, John DM, Anderson A, Dawe IS. (1991b). The effect of glyphosate on pregnancy of the rat (incorporates preliminary investigation). Study No. CHV 43 & 41/90716
- Coles RJ, Doleman N. (1996). Glyphosate technical: oral gavage teratology study in the rabbit. SPL Project No. 434/020
- Dallegrave E, Mantese FD, Coelho RS, et al. (2003). The teratogenic potential of the herbicide glyphosate-roundup in wistar rats. Toxicol Lett, 142, 45–52
- Dallegrave E, Mantese FD, Oliveira RT, et al. (2007). Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol, 81, 665–73
- Daruich J, Zirulnik F, Gimenez MS. (2001). Effect of the herbicide glyphosate on enzymatic activity in pregnant rats and their fetuses. Environ Res, 85, 226–31
- DeSesso JM. (2012). Comparative gestational milestones in vertebrate development. In: Hood RD, ed. Developmental and reproductive toxicology: a practical approach. 3rd ed. New York: Informa Healthcare, 93–138
- DeSesso JM, Venkat AG. (2010). Cardiovascular development and malformation. In: Kapp RW, Tyl RW, eds. Reproductive toxicology. 3rd ed. Chap 13. New York: Informa Healthcare, 223–48
- Epstein DA, DeBord JR. (2002). Abnormalities associated with aberrant right subclavian arteries: a case report. Vasc Endovascular Surg, 36, 297–303
- European Commission (2002). Review report for the active substance glyphosate, Directive 6511/VI/99-final. 21 January 2002. Available from: http:ec.europe.eu/food/plant/protection/evaluation/existactive/list1_glyphosate_en.pdf [last accessed 12 Dec 2012]
- Fazan VPS, Riberio RA, Riberio JAS, Filho OAR. (2003). Right retroesophageal subclavian artery. Acta Cir Bras, 18, 54–6
- Garry VF, Harkins ME, Erickson LL, et al. (2002). Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Persp, 110, 441–9
- Grube A, Donaldson D, Kiely T, Wu L. (2011). Pesticide industry sales and usage: 2006 and 2007 market estimates. Biological and Economic Analysis Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency. February 2011. Available from: www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf [last accessed 12 Dec 2012]
- Hatakenaka N. (1995). HR-001: teratogenicity study in rats. Study No. IET 94-0152
- Hoffman JIE, Kaplan S. (2002). The incidence of congenital heart disease. J Am Coll Cardiol, 39, 1890–900
- Hojo H. (1995). HR-001: a teratogenicity study in rabbits. Study No. IET 94-0153
- Holson JF, Nemec MD, Stump DG, et al. (2006). Significance, reliability, and interpretation of developmental and reproductive toxicity findings. In: Hood RD, ed. Developmental and reproductive toxicology: a practical approach. 2nd ed. Boca Raton: CRC Press, 329–424
- Hutson MR, Kirby ML. (2003). Neural crest and cardiovascular development. Birth Defects Res C: Embryo Today, 69, 2–13
- Kirby ML, Gale TF, Stewart DE. (1983). Neural crest cells contribute to normal aorticopulmonary septation. Science, 220, 1059–61
- MARTA (Middle Atlantic Reproduction and Teratology Association). (1997). Historical control data. In: Hood RD, ed. Appendix B in Handbook of developmental toxicology. Boca Raton: CRC Press, 713–33
- Mink PJ, JS Mandel, JI Lundin, Sceurman BK. (2011). Epidemiologic studies of glyphosate and non-cancer health outcomes: a review. Regul Toxicol Pharmacol, 61, 172–84
- Moxon ME. (1996). Glyphosate acid: developmental toxicity study in the rabbit. Report No. CTL/P/5009
- Moxon ME. (2002). Glyphosate acid: developmental toxicity study in the rat. Report No. CTL/P/4819/Amendment – 001
- Rull RP, Ritz B, Shaw GM. (2006). Neural tube defects and maternal residential proximity to agricultural pesticide applications. Am J Epidemiol, 163, 743–53
- Sadler TW. (2011). Selective serotonin reuptake inhibitors (SSRIs) and heart defects: potential mechanisms for the observed associations. Reprod Toxicol, 32, 484–9
- Stump DG, Nemec MD, Parker GA, et al. (2012). Significance, reliability, and interpretation of developmental and reproductive toxicity study findings. In: Hood RD, ed. Developmental and reproductive toxicology: a practical approach. 3rd ed. Chap 9. New York: Informa Healthcare, 229–301
- Suresh TP. (1991). Teratogenicity study in wistar rats – test compound: glyphosate technical (FSG 03090 H/05 March 90). Study No. TOXI: ES.883.TER-R
- Suresh TP. (1993). Teratogenicity study in rabbits – test compound: glyphosate technical (FSG 03090 H/05 March 1990). Study No. TOXI: 884-TER-RB
- Tasker EJ, Rodwell DE, Jessup DC. (1980a). Technical glyphosate: teratology study in rabbits. Report No. IR-79-018
- Tasker EJ, Rodwell DE, Jessup DC. (1980b). Technical glyphosate: teratology study in rats. Report No. IR-79-016
- US Environmental Protection Agency (US EPA). (1993). Re-registration Eligibility Decision (RED) Glyphosate. Report No. EPA-738-R-93-014. Washington, DC: U.S. Environmental Protection Agency, Office of Pesticide Programs and Toxic Substances, 291pp
- Williams AL, Watson RE, DeSesso JM. (2012). Developmental and reproductive outcomes in humans and animals after glyphosate exposure: a critical analysis. J Toxicol Environ Health, Pt B, 15, 39–96
- Wood E. (1996). Glyphosate technical: oral gavage teratology study in the rat. SPL Project No. 434/018
Appendix
Rabbit developmental toxicity studies
A total of seven developmental toxicity studies of glyphosate have been conducted in the rabbit and are summarized in detail below. The studies vary considerably in their quality, the extent of documentation and detail provided and the specific types of data reported. They have been ordered on the basis of quality, with studies of higher quality, and therefore greater relevance to the overall evaluation, detailed first. Although some of these studies reported the results of preliminary range-finding experiments, only the results of the definitive studies are detailed here for the purposes of this review. Typically, doses for the definitive studies were selected based on maternal toxicity observed in the preliminary range-findings studies. Five of the studies stated that they followed GLP specific to the time period in which they were conducted (Brooker et al., Citation1991a; Coles & Doleman, Citation1996; Hojo, Citation1995; Moxon, Citation1996; Suresh, Citation1993). Another study was conducted prior to the establishment of GLP requirements, but generally adhered to GLP principles (Tasker et al., Citation1980a). In the seventh study (Bhide & Patil, Citation1989), it is not clear to what extent GLP practices were followed, but it appears that this study was not fully GLP-compliant because the description of study results is extremely limited and inappropriate animals appear to have been included in the calculation of certain endpoints. All the studies were conducted according to current testing guideline requirements at the time of the study and provided quality assurance audits. The animal supply and husbandry were described, although detailed husbandry data were not provided in the study reports. No other deviations were detailed by the study authors. In the summaries that follow, we address issues of data quality where appropriate. In two cases (Brooker et al., Citation1991a; Suresh, Citation1993), we have tabulated the malformations reported in some detail. This was done because these two studies reported increases in malformations which appeared to be related to increases in cardiovascular defects. All other studies had very low levels of cardiovascular malformations, so no further details were given.
Moxon (Citation1996)
This study was conducted according to OECD 414 (1981) and US EPA 83-3 testing guideline requirements. Female virgin New Zealand White rabbits (age unknown) were paired with males (day of insemination = gestational day [GD] 1) and delivered to the testing laboratory on either GD 2 or 3. The designation of the day of insemination as GD 1 is different than that for the majority of the rabbit studies, which designated the day of insemination as GD 0. For the purposes of comparing to other studies, the day of mating has been corrected to GD 0 in the following discussion with succeeding gestational days changed accordingly. The maternal animals were assigned by a randomized design to minimize (but not necessarily to prevent) the number of animals in the same group that were sisters or mated to the same male. Glyphosate acid (purity: 95.6%) was formulated in deionized water, was stable over the test period and was shown to have an adequate homogeneity. The achieved concentrations were within 12% of the target concentrations. The does were administered 0, 100, 175 or 300 mg/kg/day by oral gavage on GD 7–19 (20 rabbits per group). The dosing volume was 2 mL/kg body weight; the dosing vehicle was deionized water. The rabbits were evaluated daily for mortality, behavior and clinical signs of toxicity. Body weights were recorded on GDs 3, 7–19, 22, 25 and 29. Food consumption was recorded every 3–4 days from GD 3 to GD 25. Does were sacrificed on GD 29 and the uteri and ovaries were examined for the numbers of corpora lutea, implantations, live and dead fetuses, and intra-uterine deaths (both early and late). The does were further evaluated for any gross pathological changes. Fetuses were weighed and examined for external, visceral (via fresh dissection) and skeletal (by means of alizarin red S staining) anomalies. The degree of bone ossification was scored visually based on the extent of alizarin staining.
Clinical symptoms of toxicity observed in the 175 and 300 mg/kg/day dose groups included diarrhea, few feces and/or staining in the genital area. Dose-dependent reductions in food consumption and body weight gains were observed in these two dose groups as well.
Pregnancy outcome and delivery data are shown in . Two does died or were sacrificed in extremis in each dose group except the control, in which there was a single animal death. Abortions occurred in 1, 2, 1 and 2 rabbits in the 0, 100, 175 and 300 mg/kg/day dose groups, respectively. All animals that aborted or had total litter resorptions died or were sacrificed in extremis. No macroscopic findings related to treatment were found in does at necropsy.
Table A1. Maternal and fetal outcome data for New Zealand white rabbits treated with glyphosate on gestational days 7–19† (Moxon Citation1996).
Glyphosate treatment had no effect on the number of corpora lutea, implantations, viable fetuses per litter or the incidences of pre- and post-implantation loss. Mean fetal body weights were significantly reduced at the high dose of 300 mg/kg/day compared to controls; this difference was attributed to two litters for which fetal weights were particularly low. The fetal sex ratio was skewed toward males at the intermediate dose of 175 mg/kg/day. Since this endpoint tends to be highly variable and no dose response trend was evident, the difference was not considered to be treatment-related.
also shows the number of fetuses and litters in each dose group with major and minor external/visceral and skeletal defects and the incidence of fetuses with variations in each. The only changes that appeared to increase with dose were minor skeletal defects and variants, and these were increased almost exclusively in the 300 mg/kg/day group. The increase in minor skeletal defects and variants can be attributed to reduced ossification in several bones, including transverse processes of cervical and lumbar vertebrae, sternebrae and bones of the hindpaw. These are likely related to the reduced fetal body weights seen at the highest dose level.
The type and incidence of major malformations within individual fetuses did not increase with dose. Only five fetuses in the entire study had major malformations, two in the control group, one at 100 mg/kg/day and one at 300 mg/kg/day. Three fetuses had heart defects involving effects on septation of the heart, one in the controls, one at 100 mg/kg/day and one at 300 mg/kg/day. Thus, none of the malformations noted were associated with exposure to glyphosate.
Based on clinical signs of toxicity and on reduced food intake and body weight gain, the NOAEL for maternal toxicity is considered to be 100 mg/kg/day. Based on reduced fetal weights observed at the high dose, the NOAEL for developmental toxicity is considered to be 175 mg/kg/day.
Coles & Doleman (Citation1996)
This study was conducted according to OECD 414 (1981) and US EPA 83-3 (1984) testing guideline requirements. Female New Zealand White rabbits (2.7–4.1 kg) of 17–19 weeks of age were mated with “stud” males by the supplier and delivered to the test facility at or before GD 3. The day of mating was considered GD 0. Glyphosate technical (purity: 95.3%) was formulated in 1% carboxymethyl cellulose, was stable over the test period and was shown to have an adequate homogeneity. The averaged achieved concentrations were within 11% the target concentrations over the test period. Although doses were described as “mg/kg” in the study report, based on the dosing description, it is assumed that these are daily doses (i.e. mg/kg/day). The does were administered 0, 50, 200 or 400 mg/kg/day by oral gavage on GD 7–19 (18 rabbits per group). The dosing volume was 5 mL/kg body weight. Individual dose volumes were based on the most recent body weight. Animals were examined at least once daily for mortality and clinical signs. Body weights were recorded on GD 3, 7, 10, 13, 16, 19, 22, 25 and 29 (body weight change was based on BW at GD 7); food consumption was measured using the same time intervals (e.g. GD 3–7, 7–10). All surviving animals were sacrificed on GD 29 and the uteri and ovaries were examined. The numbers of corpora lutea, implantations, and live and dead fetuses were recorded. The does were further evaluated for gross pathological changes. All fetuses were sexed, weighed and examined for external and internal abnormalities. The heads of alternate fetuses were fixed and examined separately. The skeletons were stained with alizarin red and examined.
No dose-related clinical signs were reported except soft/liquid feces and mucus in the feces. This was observed most frequently in the 400 mg/kg/day group, but was also observed at 50 and 200 mg/kg/day. During the treatment period, maternal food consumption was reduced from that of controls at 400 mg/kg/day (GD 10–19). In the post treatment period, food consumption in the treated groups tended to be higher than the controls; however, the differences did not attain statistical significance. There was a statistically significant reduction in body weight gain (GD 7–29) at 400 mg/kg/day, and a non-statistically significant reduction at 200 mg/kg/day.
Pregnancy outcome and delivery data are presented in . The numbers of non-pregnant animals were 3, 0, 2 and 1 in the 0, 50, 200 and 400 mg/kg/day groups, respectively. The numbers of does dead or sacrificed in extremis were 1, 0, 1 and 2 in the 0, 50, 200 and 400 mg/kg/day groups, respectively. At least one maternal death at 400 mg/kg/day appeared to be treatment-related; deaths in the control and mid-dose groups were attributed to dosing technical errors. None of the animals aborted.
Table A2. Maternal and fetal outcome data for New Zealand white rabbits treated with glyphosate on gestational days 7–19 (Coles & Doleman, Citation1996).
The total litters included in the data evaluation were 14, 18, 15 and 15 for the 0, 50, 200 and 400 mg/kg/day groups, respectively. Compared to controls, glyphosate treatment exerted no effects on the numbers of corpora lutea, implantations, pre-implantation loss, fetal sex ratios or fetal weights. There was a statistically significant increase in embryo/fetal death and post-implantation loss at 200 mg/kg/day, and a non-statistically significant increase at 400 mg/kg/day. The standard deviations within these data are considerable and Coles & Doleman (Citation1996) point out that at 200 mg/kg/day, there was a preponderance of “early fetal deaths”, and at 400 mg/kg/day, the increase could be attributed to one animal with nine late deaths or a post-implantation loss of 69.2%. If the one litter with high implantation loss is excluded, the mean ± standard deviation for post-implantation loss in the remaining litters is 8.0 ± 10.2. With no consistent, statistically significant dose-response pattern, the biological significance of these data is questionable. No historical control data were provided in Coles & Doleman (Citation1996) to compare with these results.
also shows the number of fetuses and litters in each dose group with external/visceral and skeletal malformations and variations. There was no apparent increase in morphological findings with increasing dose in any group. There was a variety of malformations seen, but no particular pattern of malformations and no apparent dose–response relationship. Only one case of a heart and great vessel defect was seen in the 200 mg/kg/day group in a fetus with a number of other severe abnormalities. A number of skeletal variations were noted, but there did not appear to be a dose-related increase.
Based on clinical signs and a decrease in maternal weight gain at 400 mg/kg/day, the NOAEL for maternal toxicity is considered to be 200 mg/kg/day. It is possible that similar treatment-related clinical signs were observed at exposures lower than 400 mg/kg/day, but there was no clear dose–response. Assuming that the increase in post-implantation loss discussed above is not biologically significant, the NOAEL for developmental toxicity is ≥400 mg/kg/day.
Brooker et al. (Citation1991a)
This study was conducted according to OECD 414 (1981) and US EPA 83-3 (1984) guideline requirements. Female New Zealand White rabbits of 11–24 weeks of age were used; there did not appear to be a period of acclimatization. The females were mated with proven males, followed by an injection of luteinizing hormone to promote ovulation. The day of mating (sperm positive) was considered GD 0. Glyphosate acid (purity: 95.3%) was formulated in 1% methylcellulose, was stable over the test period and was shown to have an adequate homogeneity. The achieved concentrations were within 6% of the target concentrations, with the exception of a single measurement in Group 2 which was 19% below the target concentration. It is unclear how often samples for analysis were taken during the study. The does were administered 0, 50, 150 or 450 mg/kg/day by oral gavage on GD 7-19 (16–20 rabbits per group). The reason for including different numbers of animals per dose group was not reported. The dosing volume was 5 mL/kg body weight. Individual dose volumes were based on individual body weights on GD 7 and adjusted according to body weights on GD 9, GD 11 and GD 15. Animals were examined daily for mortality and signs of toxicity. Body weights were recorded on GD 1, 7, 9, 11, 15, 20, 24 and 29; food consumption was measured using the same time intervals (e.g. GD 1–7, 7–9). Does that did not survive until the end of the study were weighed and necropsied. All surviving animals were sacrificed on GD 29, and the ovaries and uteri were examined for the numbers of corpora lutea, implantations, and live and dead fetuses. The does were further evaluated for gross pathological changes. All fetuses were weighed and examined for external abnormalities, then dissected to examine for visceral abnormalities and to determine sex. The heads were fixed and examined separately. The skeletons were stained with alizarin red and examined. Structural changes were reported by study investigators as malformations (defined as rare and/or probably lethal changes), anomalies (defined as relatively frequent minor differences from “normal”) and variants (defined as alternative structures occurring regularly in the control population).
There were no dose-related clinical signs except soft/liquid feces; this finding was observed at all exposure levels, but not in the controls, and was substantially increased at the high dose (450 mg/kg/day). During the treatment period, maternal food consumption was reduced from that of controls at 150 mg/kg/day (GD 11–19) and 450 mg/kg/day (GD 7–19). In the post-treatment period, both of these groups demonstrated a rebound and food consumption was greater than that in controls. No dose-related differences in maternal body weights were observed.
Pregnancy outcome and delivery data for this study are shown in . Two does were excluded from the study for non-experimental reasons (one control doe was found with a congenital malformation of the uterus at autopsy; one 450 mg/kg/day doeFootnote1 was found to have a broken leg prior to treatment). The numbers of non-pregnant animals were 0, 6, 1 and 5 in the 0, 50, 150 and 450 groups, respectively; there did not appear to be a correlation between age of the animals (assumed based on body weights) and the occurrence of non-pregnancy. One maternal death occurred at 450 mg/kg/day following abortion, gastrointestinal disturbances, reduced food intake and body weight loss. One doe aborted in the 50 mg/kg/day group.
Table A3. Maternal and fetal outcome data for New Zealand white rabbits treated with glyphosate on gestational days 7–19 (Brooker et al., Citation1991a).
The total litters included in the data evaluation were 18, 12, 15 and 13 for the 0, 50, 150 and 450 mg/kg/day groups, respectively. Compared to controls, glyphosate treatment exerted no marked effects on the numbers of corpora lutea, implantations, pre-implantation loss, fetal sex ratios or fetal weights. There was a statistically significant increase in embryo/fetal death and post-implantation loss at all exposure levels. The study investigators questioned the biological significance of these findings for several reasons: (1) No dose–response pattern was evident; (2) the control value was at the lower end of the historical control range, while those of the exposed groups were at the higher end and (3) the values in all groups were within or slightly above the historical control range. The latter two statements are supported by the historical control data provided in the study report. There was also considerable variance around the mean for post-implantation loss.
A dose-related increase in malformations (fetuses and litters) was observed with 3, 3, 5 and 6 fetuses malformed at 0, 50, 150 and 450 mg/kg/day, respectively. The increase at 450 mg/kg/day appeared to be due to an increase in IV septal and other heart defects, which were seen in 1, 1, 4 and 5 fetuses in the 0, 50, 150 and 450 mg/kg/day groups, respectively ().
Table A4. Types and incidence of malformations by individual fetus (Brooker et al., Citation1991a).
Although the authors indicated retroesophageal right subclavian artery as a malformation in three fetuses at 150 mg/kg/day and in two at 450 mg/kg/day, other laboratories suggest that this is a fairly common variation in rabbits (MARTA, Citation1997; Stump et al., Citation2012) and it occurs in 0.5–2.0% of humans (Berko at al., Citation2009; Epstein & DeBord, Citation2002; Fazan et al., Citation2003). The historical control data provided by Brooker et al. (Citation1991a) indicate that various studies have included 1–3 of such defects in control groups. Removing this defect as a malformation would reduce the total incidence of malformed fetuses to 3, 3, 3 and 5, and the incidence of fetuses with cardiovascular defects to 1, 1, 1 and 4 in the 0, 50, 150 and 450 mg/kg/day dose groups, respectively. Glyphosate treatment had no significant effect on the incidence of fetuses with variations when compared to the control group.
Based on clinical signs and decreased food consumption at 150 and 450 mg/kg/day, the NOAEL for maternal toxicity is considered to be 50 mg/kg/day. There was a slight increase in fetuses with malformations at 450 mg/kg/day. Several of the cardiovascular malformations that were observed, particularly in the high dose group, occurred in the same animals () and are related to a single morphogenetic mechanism (i.e. displacement of the developing aorticopulmonary septum), which may adjust during the postnatal period as some of these improve during the first few months of life in humans (Hoffman and Kaplan, Citation2002). These mechanistically related findings, which often cluster together, include dilated/narrow aorta and narrow/dilated pulmonary artery; IV septal defect and disproportionately sized right and left ventricles. These malformations and the associated morphogenetic mechanism are discussed in greater detail in the integrated assessment below. These findings in the heart were also observed (often in clusters) in the historical control data provided by Brooker et al. (Citation1991a). Overall, the malformation data showed an increase at 450 mg/kg/day (not statistically significant) and all findings in the glyphosate-treated groups were within historical control ranges. Although there were statistically significant increases in embryo/fetal death and post-implantation loss at 50 mg/kg/day and above, this was due to unusually low values in the concurrent control group. Although embryo/fetal death was within the historical control range, post-implantation loss was above historical control values in the high dose group, and both of these parameters were highly statistically significant at the high dose. Based on these data, the developmental NOAEL is 150 mg/kg/day.
Hojo (Citation1995)
This study was conducted according to OECD 414 (1981) and US EPA 83-3 (1984) guideline requirements. Female Japanese White rabbits (3.3–3.8 kg) of 17 weeks of age were acclimatized for 10 days, and then impregnated by artificial insemination with sperm from breeder males of the same strain, followed by 25 units of human chorionic gonadotropin. The day after insemination was considered GD 0; this designation is different than that used in most of the other rabbit studies. Days of gestation have been adjusted for this study by designating the day of insemination as GD 0 to compare with other studies reviewed here. Glyphosate acid (purity: 97.6%, referred to in the report as HR-001) was formulated in 0.5% carboxymethyl cellulose, was stable over the test period and was shown to have an adequate homogeneity. The achieved concentrations were within 5% of the target concentrations. Impregnated does were administered 0, 10, 100 or 300 mg/kg/day by oral gavage on GD 7–19 (18 rabbits per group). The dosing volume was 5 mL/kg body weight, based on the individual body weights on each day of dosing. Animals were examined at least once daily for mortality and clinical signs. Body weights were recorded on GDs 1, 7–19 (daily), 25 and 28. Body weight gains were based on the GD 1 body weight; adjusted weight was not reported, but was calculated herein by subtracting the gravid uterine weight from the body weight on GD 28. Daily food consumption was based on the average consumption over 2-day periods. All surviving animals were sacrificed on GD 28 and the uteri and ovaries were weighed and examined for the numbers of corpora lutea, implantations, resorptions and live and dead fetuses. Uteri without apparent implants were stained to detect possible early resorptions. All fetuses were sexed, weighed and examined for external and internal abnormalities. The skeletons were stained with alizarin red and examined.
The only dose-related clinical sign reported was soft/liquid feces at 300 mg/kg/day. There were no dose-related effects on food consumption, maternal body weight or body weight gain.
Pregnancy outcome and delivery data are presented in . All of the animals on study were reported to be pregnant. One animal in the 300 mg/kg/day group died on GD 21. In the 10 and 300 mg/kg/day groups, one doe in each group aborted and one doe in each group had a premature delivery. The authors reported all of these events as abortions (as shown in ).
Table A5. Maternal and fetal outcome data for Japanese white rabbits treated with glyphosate on gestational days 7–19† (Hojo, Citation1995).
The total numbers of litters included in the data evaluation were 18, 15, 16 and 14 for the 0, 10, 100 and 300 mg/kg/day groups, respectively. Compared to controls, glyphosate treatment exerted no effect on the numbers of corpora lutea, implantations, pre-implantation loss, post-implantation loss, embryo/fetal deaths, fetal sex ratios or fetal weights.
also shows the number and percentage of fetuses and litters in each dose group with external/visceral and skeletal malformations and variations. There was a statistically significant increase in total litters with malformations and variations at 300 mg/kg/day. The increased malformation rate was due to an increase in litters with fetuses showing skeletal malformations, as no external or visceral malformations were noted in fetuses from the high dose group. A change in the number of litters showing defects can be misleading because a litter is counted whether only one or all fetuses are affected. The specific alterations were not available on an individual fetus basis, so it was impossible to determine whether external, visceral or skeletal defects occurred in the same or different fetuses. Even so, the malformations seen were considered to be sporadic in nature rather than related to glyphosate treatment. Further, a dose–response in the number of fetuses showing skeletal malformations was not evident across dose groups. The number of litters with variations was significantly decreased at 300 mg/kg/day, and the incidence of fetuses with skeletal variations was significantly increased at 100 mg/kg/day. Overall, the incidence of fetuses with visceral or skeletal variations did not show a treatment-related change. With regard to malformations of the heart, only one fetus had heart-related defects at 100 mg/kg/day (hypoplasia of the pulmonary artery and ventricular septal defect).
Based on clinical signs at 300 mg/kg/day, the NOAEL for maternal toxicity is considered to be 100 mg/kg/day. The lack of a dose-related increase in fetuses with external, visceral or skeletal defects indicates a lack of biological significance for the total litter finding. Overall, these data support a developmental toxicity NOAEL of ≥300 mg/kg/day.
Tasker et al. (Citation1980a)
Although this study was conducted prior to the establishment of GLPs and EPA or OECD study guidelines, it generally adhered to GLP practices and satisfies the general requirements of OECD 414 (1981). Female Dutch belted rabbits of age 7 months were acclimated for at least 30 days prior to being inseminated on GD 0 using semen from only four proven male rabbits. Glyphosate technical (purity: 98.7%) was formulated in 0.5% aqueous Methocel® solution (Dow Chemical Company, Midland, MI). No additional information on formulation was provided. Impregnated does were administered 0, 75, 175, or 350 mg/kg/day by oral gavage on GD 6–27 (16 rabbits per group). The dosing volume was 1 mL/kg body weight. Doses were based on individual body weights on GD 6. Animals were examined once daily for behavior, mortality and clinical signs of toxicity. Body weights were recorded on GDs 0, 6, 12, 18, 24 and 28. Food consumption rates were not recorded. Does that did not survive until the end of the study were necropsied to determine the cause of death. All surviving animals were sacrificed on GD 28. The uteri and ovaries were examined and the numbers of corpora lutea, implantations, resorptions, live and dead fetuses were recorded. The does were further evaluated for gross pathological changes. All fetuses were weighed, sexed internally, examined for external and visceral malformations (via dissection) and prepared for skeletal examination using alizarin red. External malformations were not reported separately from visceral malformations in this study.
Soft stools and diarrhea were noted in all treatment groups, but showed a dose-dependent rise in incidence in does treated with 175 and 350 mg/kg/day glyphosate compared to controls. Animals at 350 mg/kg/day also demonstrated an increase in nasal discharge. Maternal body weight changes were highly variable across groups throughout the study and no significant differences in body weights or body weight gains were noted compared to controls.
Pregnancy outcome and delivery data are shown in . Abortions occurred in two rabbits from the control group, and in one rabbit in each of the 175 and 350 mg/kg/day treatment groups. The numbers of rabbits that died before the end of study were 0, 1, 2 and 10 in the control, 75, 175 and 350 mg/kg/day glyphosate treatment groups, respectively. Mortality rates were greater than 10% in the intermediate and high dose groups. The causes of maternal death were determined for five of the 13 animals (pneumonia, respiratory disease, enteritis or gastroenteritis), but were not consistent across the groups. No macroscopic findings related to treatment were observed in the does.
Table A6. Maternal and fetal outcome data for Dutch belted rabbits treated with glyphosate on gestational days 6–27 (Tasker et al., Citation1980a).
Compared to controls, glyphosate treatment exerted no marked effects on the numbers of corpora lutea, implantations, resorptions (early or late), fetal sex ratios or fetal weights. There was also considerable variance around the mean for post-implantation loss. A statistically significant elevation in the number of viable fetuses per doe treated with 75 mg/kg/day was noted, but this result was considered to be a random occurrence because it was not observed in the two higher treatment groups. The total numbers of fetuses with malformations were 0, 3, 2 and 2 in the control, 75, 175 and 350 mg/kg/day dose groups, respectively. External and visceral defects occurred in two fetuses at the high dose level. Only skeletal malformations were observed in the low- and mid-dose groups, with no defects seen in controls. One fetus at the high-dose level had multiple malformations, including acrania with gastro-thoracoschisis, bilateral carpal flexures, fetal anasarca, absent diaphragm, reduced diameter of carotids and associated skeletal changes, while another had a single finding of carpal flexure. Neither the type nor the incidence of these malformations suggests an adverse effect of glyphosate. Although total fetuses and litters with variations were not specifically reported, the types and incidence of fetuses with variations were primarily reduced ossification and there was no indication of a dose-related change. With respect to the heart and cardiovascular system, only the fetus with acrania had carotid stenosis.
Based on mortality and clinical signs at 175 and 350 mg/kg/day, the NOAEL for maternal toxicity is considered to be 75 mg/kg/day. The large number of maternal deaths at the high dose makes interpretation of the overall study data difficult. Since no treatment-related increase in developmental toxicity was observed, ≥175 mg/kg/day is considered the NOAEL for developmental toxicity. Because the study was limited by having too few fetuses available at the high dose of 350 mg/kg/day for adequate morphological assessment, the NOAEL for developmental toxicity could not be established for doses higher than 175 mg/kg/day.
Suresh (Citation1993)
This study was conducted according to OECD 414 (1981). Female New Zealand White rabbits of at least 6 months of age (≥2.5 kg) were acclimatized for at least 10 days, and then mated. The day of mating was considered GD 0. Glyphosate technical (purity: 96.8%) was formulated in 0.5% carboxymethyl cellulose and Tween 80. No additional information on formulation was provided. Doses were described as “mg/kg” in the study report, but based on the dosing description it is assumed that these were daily doses (i.e. mg/kg/day). Impregnated does were administered 0, 20, 100 or 500 mg/kg/day by oral gavage on GD 6–18 (16–26 rabbits per group). The reason for including different numbers of animals per dose group was not reported. The dosing volume was 2 mL/kg body weight. Individual dose volumes were based on animal body weights. Animals were examined twice daily for mortality and clinical signs. Body weights were recorded on GDs 0, 6–18 (daily) and 27. Body weight gain was based on the intervals between body weights (e.g. GDs 0–6, 6–18). Absolute body weight was not reported by the authors, but was calculated here by subtracting the gravid uterine weight from the body weight on GD 28. Food consumption was calculated for GDs 0–6, 6–19, 19–28 and 0–28. All surviving animals were sacrificed on GD 28 and the uteri and ovaries were weighed and examined for the numbers of corpora lutea, implantations, resorptions, and live and dead fetuses. Uteri without apparent implants were stained to detect possible early resorptions. All fetuses were sexed, weighed and examined for external and internal abnormalities. The skeletons were stained with alizarin red and examined.
The major dose-related clinical signs included soft/liquid feces and mucus in the feces; these were observed in 0, 0, 1 and 14 does in the 0, 20, 100 and 500 mg/kg/day groups, respectively. No dose-related effects on maternal food consumption or body weight gain were reported. Maternal body weight, however, was statistically significantly decreased in the 500 mg/kg/day group on GD 0, 6 and 28, indicating that the animals in this group were below the weights of animals in other groups at the beginning of the study.
The pregnancy outcome and delivery data are presented in . The numbers of non-pregnant animals were 4, 4, 0 and 1 in the 0, 20, 100 and 500 mg/kg/day groups, respectively. Animals that died or were sacrificed in extremis were 2, 0, 4 and 8 in the 0, 20, 100 and 500 mg/kg/day groups, respectively. Various findings at gross necropsy were noted in the lungs and trachea for the 100 and 500 mg/kg/day dose groups; these findings suggest possible gavage errors to which the deaths at these doses may be attributed. The number of animals that aborted in each group was not reported.
Table A7. Maternal and fetal outcome data for New Zealand white rabbits treated with glyphosate on gestational days 6–18 (Suresh, Citation1993).
The total numbers of litters included in the data evaluation were 20, 13, 12 and 5 for the 0, 20, 100 and 500 mg/kg/day groups, respectively. Compared to controls, glyphosate treatment exerted no effect on the numbers of corpora lutea, implantations or pre-implantation loss. Although there was no effect on pre-implantation loss, it seems high across groups and especially high in the controls (48%). There were no historical control data provided for this endpoint. There was no effect on post-implantation loss, embryo/fetal death or fetal sex ratios. Although fetal body weights in the 20 and 100 mg/kg/day dose groups were reported to be significantly different from control, the weights were increased, the changes were less than 10% of control values and no dose–response across treatment groups was evident. Thus, the fetal body weight differences observed in these two dose groups are biologically inconsequential with respect to adverse effects.
There were no significant treatment-related increases in minor malformations or variations (). The incidence of visceral malformations appeared to increase with dose, but only 28 fetuses were available for examination in the high-dose group and the incidence in the low, mid and high dose groups was similar.
Major visceral malformations primarily affected the heart, but occurred in single incidences and showed no dose-response (). The exception was dilated heart, which was reported in 0, 4, 4 and 5 fetuses (0, 3, 2 and 2 litters) in the control, 20, 100 and 500 mg/kg/day dose groups, respectively. The terminology used to describe the heart malformations in this study is difficult to interpret (e.g. dilated heart, seal-shaped heart, cardiomegaly). For example, “dilated heart” was not defined in the study report, and how this malformation might relate to other heart defects (i.e. dilated right ventricle, seal-shaped heart, cardiomegaly) was not reported. Neither the criteria used to diagnose dilated heart nor measurements of the hearts were provided, so it is not possible to directly compare the dilated heart findings to the hearts of the fetuses in other studies. It is possible that the observation of dilated hearts was due to overly stringent inspection compared to criteria used by other laboratories. Only two litters exhibited major visceral malformations in the high dose group: one fetus in one litter and an unknown number in another (individual fetus data were not reported). It should be noted that the high-dose group findings were seen in the presence of extensive maternal toxicity, evidenced by clinical signs and a substantial number of maternal deaths.
Table A8. Types and incidence of individual malformations† (Suresh, Citation1993).
This developmental toxicity study in rabbits had several weaknesses including a small number of litters available for examination due to low pregnancy rates and maternal deaths in the mid- and high-dose groups; these weaknesses severely limit the conclusions that can be drawn at these dose levels. It is especially difficult to extract data from the report to confirm the findings. Based on clinical signs and deaths at 500 mg/kg/day, it appears that the high dose in this study significantly exceeded the maximum tolerated dose. Therefore, the NOAEL for maternal toxicity is considered to be 100 mg/kg/day. Since no apparent developmental toxicity was observed at any dose, ≥100 mg/kg/day is considered the NOAEL for developmental toxicity. Because the study is limited by having too few fetuses available at the high dose of 500 mg/kg/day for adequate morphological assessment, the NOAEL for developmental toxicity could not be established for doses higher than 100 mg/kg/day.
Bhide & Patil (Citation1989)
This study was conducted according to OECD 414 (1981). It is not clear to what extent this study followed GLP practices, but it appears to be only partially GLP-compliant at most. Female New Zealand white pregnant rabbits of age 24–28 weeks (1.5–2.0 kg) were used; they were acclimatized for six days. The females were mated with “adult vigorous males”. The day of mating was considered GD 0. Doses were described as mg/kg doses in the study report, but based on the dosing description it is assumed that these were daily doses. Impregnated does were administered 0, 125, 250 or 500 mg/kg/day glyphosate technical (purity: 95%) by oral gavage on GD 6–18 (15 rabbits per group). The dosing volume was 5 mL/kg body weight; the test material was suspended in 0.1% gum acacia in water. Animals were observed twice daily for clinical signs, general behavior and body weight gain. Body weights were recorded on GDs 0, 6, 12, 18, 23 and 29. Food consumption was measured using the weight day intervals (e.g. GD 0–6, 6–12). The females were “delivered by caesarian section 1 day before expected delivery”. The does were sacrificed on GD 29 and the uteri and ovaries examined for the numbers of corpora lutea, uterine weight, implantations, live and dead fetuses. Uteri from non-gravid animals were stained to examine for implantation sites (early resorptions). The does were further evaluated for gross pathological changes. All fetuses were weighed and examined for external abnormalities, then processed by a fresh visceral dissection technique to determine sex and to examine for visceral abnormalities, including those of the heart and great vessels. The heads were removed, decalcified, fixed in Bouin’s solution and examined separately. The remainder of each skeleton was prepared for the examination of osseous tissue using alizarin red.
The pregnancy outcome and delivery data for this study are shown in . The authors did not describe any statistical methods and there were no designations of statistical significance in their tables. The description of the results provided in Bhide & Patil (Citation1989) was very limited. The numbers of non-pregnant animals were 2, 1, 1 and 3 in the 0, 100, 250 and 500 groups, respectively. There were no maternal deaths. Two does aborted in the 500 mg/kg/day group and were also included in the “No. Litters examined” endpoint. Data are given in the report for fetuses from 12 individual litters, but it is not clear which litters aborted.
Table A9. Maternal and fetal outcome data for New Zealand white rabbits treated with glyphosate on gestational days 6–18† (Bhide & Patil, Citation1989).
Table A10. Maternal and fetal outcome data from the developmental toxicity studies of glyphosate in rats.
The total number of litters included in the data evaluation for various endpoints was not explained in detail in the report and appear to be different for different endpoints. From the tables in the report, it appears that all 15 animals from each exposure group were evaluated for clinical signs, food consumption, maternal body weight gain, corpora lutea counts, implantations, resorptions and embryo/fetal viability. Consequently, these endpoints would appear to include data from animals that were non-pregnant or which aborted. For sex ratio, fetal body weights, and malformations, it appears that only the gravid animals were included (i.e. 13, 14, 14 and 12 in the 0, 100, 250 and 500 groups, respectively).
There were no dose-related clinical signs reported at any exposure level. Maternal food consumption and body weight gain appear to be reduced from that of controls at 500 mg/kg/day (food consumption, GDs 6–29; body weight gain, GDs 12–29). However, with the inclusion of animals that were non-pregnant or aborted, it is not possible to determine if this is a biologically significant result. There were no differences from control for the number of corpora lutea, implantations or pre-implantation loss. Embryo/fetal deaths were slightly higher and viability was slightly lower than controls. Post-implantation loss was not reported.
The incidences of external, visceral and skeletal malformations as well as total malformations are also shown in . Data were not reported for individual litters or fetuses, and the numbers for total fetuses and litters appear to be the sum of the number with external, visceral and skeletal defects. As a result, the authors reported 14 litters with malformations in the high-dose group when only 12 litters were examined. The numbers of fetuses and litters with variations are not reported.
The number of types of malformations reported was low in this study. Several malformations appeared to be increased in the high-dose group, including abnormal tail, missing kidney(s), absent postcaval lung lobe and rudimentary 14th rib. The latter two defects are typically considered variations. The only cardiovascular changes reported as malformations were IV septal defects; these were observed in 0, 1, 1 and 2 fetuses in the 0, 125, 250 and 500 mg/kg/day dose groups. A number of variations were reported, some of which have been included as malformations by other authors; for example, globular heart, small right ventricle, dilated lateral cerebral ventricles and fused thoracic centra. Other variations reported are commonly seen in rabbits (e.g. incomplete septation of lung lobes, irregular palatal rugae, blunt-tipped tail, irregular-shaped liver, globular-shaped kidneys, bilobed vertebral centra, reduced ossification of centra, sternebrae, pubis and skull). Several of these were increased in the high-dose group. In summary, there were a number of changes reported in the high-dose group, but the actual number of fetuses and litters affected is likely to be lower. However, this could not be determined because of the inadequate reporting of data.
This developmental toxicity study in rabbits is limited by the study design (e.g. the number of pregnant does surviving to term in each dose group, especially the high-dose group) and inadequate reporting of data (e.g. the inclusion of inappropriate animals in the calculation of some endpoints, insufficient description of study results). These limitations raise concern about using the results in any evaluation of glyphosate developmental toxicity and NOAELs are not proposed because of these limitations.
Rat developmental toxicity studies
The six rat developmental toxicity studies of glyphosate are summarized below and in . As in the rabbit studies, we have focused only on the results of the definitive studies. Because the impetus for concern regarding cardiovascular development was the rabbit studies and not the rat studies, these studies were not reviewed in the same level of detail as the rabbit studies. Rather, these studies are addressed in a combined discussion.
In the rat studies, the day of finding sperm was designated as GD 0,Footnote2 except in the study by Moxon (Citation2002), which referred to it as GD 1. For the purpose of discussing the timing of exposure and outcome measurements in the rat studies in an integrated fashion, the day of mating and succeeding gestational days have been corrected to GD 0 for Moxon (Citation2002). With the exception of Suresh (Citation1991), all of the studies used a randomized (block) design to assign the impregnated females to treatment groups (n = 20–25 animals/group). In contrast, Suresh (Citation1991) had only one exposure level (1000 mg/kg/day) and it is unclear how the females were assigned to the control and treated groups. Each study included a vehicle control group (0 mg/kg/day). Glyphosate exposure levels ranged from 30 to 3500 mg/kg/day across the studies and were administered as glyphosate technical (i.e. glyphosate acid). The exposure was via oral gavage on GDs 6–15, except in Tasker et al. (Citation1980b), in which animals were exposed on GDs 6–19. Regular observations of the females for mortality, clinical signs and body weight measurements were made in all studies; food consumption was measured in most studies.
In several cases, females were euthanized during the course of the study due to issues that were not associated with glyphosate exposure (e.g. mis-dosing, intubation error). At exposure levels lower than 1000 mg/kg/day, no maternal toxicity was observed over the course of the studies. In Wood (Citation1996), lethargy was reported in two animals on 2 days during treatment; this finding was not considered sufficient evidence of maternal toxicity. Also, in Brooker et al. (Citation1991b), noisy respirations were reported in two animals on a single day. Again, due to the transient nature of the finding, it was not considered evidence of maternal toxicity. At 1000 mg/kg/day and higher, animals in three of the six studies showed signs of lethargy, as well as respiratory and gastrointestinal distress. At 1000 mg/kg/day and lower, there were no maternal deaths. In the two studies that used doses as high as 3500 mg/kg/day (Brooker et al., Citation1991b; Tasker et al., Citation1980b), there were three (two considered treatment-related) and six deaths, respectively.
For the most part, exposure levels less than 1000 mg/kg/day did not affect food consumption except during short intervals at the beginning of treatment (Hatakenaka, Citation1995; Wood, Citation1996) or post-dosing (Hatakenaka, Citation1995). At 3500 mg/kg/day, Brooker et al. (Citation1991b) reported a treatment-related decrease in food consumption during the exposure period (GD 6–15); Tasker et al. (Citation1980b) also tested 3500 mg/kg/day, but did not report food consumption values.
Maternal body weight was not affected in any of the studies at exposure levels lower than 3500 mg/kg/day. At 3500 mg/kg/day, Brooker et al. (Citation1991b) reported that maternal body weight and body weight gain were reduced (GD 6–20); in contrast, Tasker et al. (Citation1980b) did not observe a similar effect at this dose.
Maternal animals were sacrificed on GD 20, except in Moxon (Citation2002) where the animals were sacrificed on GD 21. Standard endpoints of reproductive and developmental toxicity were evaluated (). There were no dose-related effects on the numbers of corpora lutea, implantations, live and dead fetuses, fetal weight or fetal sex ratio at 1000 mg/kg/day and below. At 3500 mg/kg/day, Brooker et al. (Citation1991b) reported reduced mean fetal weight. At this same dose, Tasker et al. (Citation1980b) reported a statistically significant increased number of resorptions, significantly decreased mean numbers of implantations and viable fetuses per dam, and diminished mean fetal body weights compared to controls. Tasker et al. (Citation1980b) also reported statistically significant decreased mean numbers of implantations and viable fetuses per dam in the 300 mg/kg/day treatment group, but this effect was not observed at 1000 mg/kg/day. Consequently, there was no clear dose–response effect for this parameter.
Glyphosate did not produce adverse effects on structural development (). Tasker et al. (Citation1980b) reported 10 fetuses with malformations in three litters at 3500 mg/kg/day. Six of these fetuses were in one litter and showed a syndrome of bent tail, open eyelids, missing kidneys and ureters, and various skeletal defects. Three fetuses in another litter were reported to have dwarfism. All these effects were within the historical control range. With respect to specific cardiovascular malformations, three of the six studies reported no effects (Moxon, Citation2002; Suresh, Citation1991; Tasker et al., Citation1980b). The other three studies (Brooker et al., Citation1991b; Hatakenaka, Citation1995; Wood, Citation1996) reported single incidences of specific defects; in two studies, they were observed in the controls as well as in the exposed fetuses. These results indicate that of glyphosate exposure of pregnant rats at doses of up to 3500 mg/kg/day does not produce any evidence of cardiovascular malformations.
