Abstract
Erythropoiesis-stimulating agents (ESAs) are approved to treat anemia in patients with non-myeloid malignancies receiving myelosuppressive chemotherapy. ESAs reduce transfusion rates, but some clinical studies suggest that ESAs may reduce survival or increase disease progression. This study-level meta-analysis examined the effects of darbepoetin alfa, epoetin alfa or epoetin beta on mortality, disease progression and transfusion incidence in patients with lymphoproliferative malignancies, using randomized, controlled trials of patients receiving chemotherapy and ESAs or standard of care. The odds ratio (OR) for mortality was 1.04 (95% confidence interval [CI], 0.81–1.34, random-effects model, 10 studies); the risk difference was − 0.01 (95% CI, − 0.03–0.02). The OR for disease progression was 1.02 (95% CI 0.81–1.30, random-effects model, five studies). A lower proportion of ESA-treated patients than controls received transfusions (seven studies). In this meta-analysis, ESAs reduced transfusions with no clear effect on mortality or disease progression in patients with lymphoproliferative malignancies receiving chemotherapy.
Introduction
Erythropoiesis-stimulating agents (ESAs) such as epoetin alfa, epoetin beta and darbepoetin alfa are licensed for use to treat anemia in patients with non-myeloid malignancies receiving myelosuppressive chemotherapy [Citation1,Citation2,Citation4–6]. In placebo-controlled trials in patients with non-myeloid malignancies, ESAs improved health-related quality of life, increased hemoglobin levels and reduced transfusion rates [Citation7–10] and thus reduced potential exposure to transfusion-associated risks [Citation11–13].
ESA use in patients with cancer has been reported to be associated with certain risks, including an increased incidence of thromboembolic events (a well-characterized risk that is described in the ESA product labeling) [Citation1,Citation2,Citation4,Citation14] and increased mortality, disease progression, or both under some circumstances [Citation15,Citation16]. However, published evidence regarding the effect of ESA use on mortality in individual cancer types is limited. Individual trials in patients with lymphoproliferative malignancies have provided some data on how ESA use affects mortality in this specific patient population. An initial analysis of long-term follow-up data from a controlled ESA trial [Citation17] (for which the primary data were published by Hedenus et al. in 2003) [Citation10] did not show a statistically significant difference in overall survival between ESA and control groups, but a more recent protocol-specified long-term follow-up analysis of patients in that study suggested that increased mortality was associated with ESA use in patients with lymphoproliferative malignancies. Data from this study have been incorporated in the ESA product labeling [Citation1,Citation2,Citation4]. Reduced survival with ESA use was also reported in patients with multiple myeloma in a retrospective study by Katodritou et al. [Citation19], although these findings were questioned by Ludwig et al. [Citation20].
Large meta-analyses evaluating the effect of ESA use on mortality in patients with various malignancies have reported varying results [Citation15,Citation21–23]. The meta-analysis of 60 controlled trials published by Glaspy et al. [Citation22] indicated that ESA use had no clear effect on mortality in the chemotherapy setting or when all treatment settings (chemotherapy, neither chemotherapy nor radiotherapy, radiotherapy only) were grouped together. The patient-level meta-analysis of 53 controlled trials by the Cochrane Collaboration published by Bohlius et al. [Citation21], which included patients with both hematologic malignancies and solid tumors, also reported that ESA use had no important effect on mortality in the chemotherapy setting when used strictly according to the approved clinical indication. However, when studies of chemotherapy-treated patients were combined with studies of radiotherapy-treated and untreated patients, the analysis indicated that increased mortality was associated with ESA use.
Results from other studies [Citation7,Citation24–28] suggest that ESA use does not have a negative effect on mortality in patients with lymphoproliferative malignancies. In the Cochrane Collaboration analysis [Citation21], hazard ratios for overall mortality (on-study and during follow-up) were also estimated for patients receiving chemotherapy, stratified by patient characteristics such as cancer type, with patients drawn both from studies of multiple tumor types and from studies of single tumor types. In this stratified analysis, the overall mortality hazard ratio for patients with hematological malignancies receiving chemotherapy (n = 1832) was 1.12 (95% confidence interval [CI], 0.95–1.32, p = 0.33), reflecting an overall mortality of 32% for ESAs and 33% for controls, and suggesting that use of ESAs does not have an important effect on mortality in patients with hematological malignancies. Since this analysis, three additional studies conducted in patients with lymphoproliferative malignancies have been reported, including a small study of patients with acute lymphoblastic leukemia, lymphoma and Burkitt-like leukemia/lymphoma [Citation26] and final data from the large HD-15 study in patients with Hodgkin disease [Citation29]. Similarly, the second interim analysis of the reported LNH03-6B study of patients with diffuse large B-cell lymphoma did not show a mortality increase with ESA use [Citation30].
To evaluate more thoroughly the available evidence regarding the effect of ESA use on mortality in patients with lymphoproliferative malignancies, we performed a study-level meta-analysis of published ESA trials (placebo-controlled or non-ESA-controlled) reporting mortality data that were conducted in patients with lymphoproliferative malignancies. In this meta-analysis, lymphoproliferative malignancies were defined to include all types of lymphoma, chronic and acute lymphocytic leukemia, and multiple myeloma.
Materials and methods
Literature search
A study-level meta-analysis was conducted of trials evaluating epoetin alfa (Procrit®; Janssen Products LP, Horsham, PA), darbepoetin alfa (Aranesp®; Amgen Inc., Thousand Oaks, CA) and epoetin beta (NeoRecormon®; F. Hoffman- La Roche Inc., Basel, Switzerland) versus placebo or no ESAs in patients with lymphoproliferative malignancies. The starting point for the current meta-analysis was the 2006 Cochrane Collaboration report (Analysis 05.05) [Citation16]. A systematic literature review was performed to identify all placebo- or observation-controlled ESA oncology studies reporting mortality data published since the Cochrane report (from April 2005 through July 2011). A search was also performed for relevant abstracts and associated poster presentations between January 1995 and July 2011 at the American Society of Clinical Oncology, American Society of Hematology, the European Society for Medical Oncology, the European Hematology Association and the Lugano International Conference on Malignant Lymphoma.
Studies identified from the literature search were randomized, placebo- or observation-controlled trials of patients with lymphoproliferative malignancies treated with an ESA (epoetin alfa, epoetin beta or darbepoetin alfa) plus transfusions compared with control patients who received either placebo or best standard of care or prophylaxis for anemia without ESAs. Identified studies reported either percentage of deaths or number of deaths in each treatment group, or had collected mortality data that were available for analysis. Identified studies included interim analyses (when final results were not available), and trials that allowed intravenous (IV) or oral iron use in patients with cancer receiving chemotherapy. Studies were generally excluded if English-language abstracts were not available; if the articles were editorials, letters, clinical guidelines or case studies; or if they allowed ESAs to be administered to the control arm as part of the standard of care. If multiple publications described the same study (e.g., an abstract and a manuscript), the most recent publication (or in some cases, unpublished data from internal databases) was used for survival and disease progression data; adverse event data were obtained from all available sources. Each study was included only once.
For those randomized, controlled ESA trials conducted by Amgen Inc. and Janssen Products LP that also met the search criteria used in the literature search, current data available from company databases were used. Data for epoetin beta studies were collected from only publications, and were not supplemented from internal databases at F. Hoffmann-La Roche Inc. Since the most recent available survival and disease progression data were used, database results did not always match published results (this could occur, for example, if longer-term or final follow-up data became available after publication). Results were narrowed to include only those studies performed in patients with lymphoproliferative malignancies. Data were extracted by a reviewer using a form developed for this meta-analysis and verified by an independent reviewer. Discrepancies were resolved by having a third person extract data or by discussion.
Endpoints assessed in this study-level meta-analysis were the effects of ESA use on mortality, disease progression and the incidence of transfusion. To examine the complete body of evidence regarding the effect of ESA use on mortality in patients with lymphoproliferative malignancies, a study-level meta-analysis of 10 studies was performed. The criteria for disease progression differed among the five studies that reported disease progression [Citation7,Citation10,Citation26,Citation27,Citation29]. In the studies reported by Österborg et al. in 1996 and 2005 [7,27], the determination of progression was based on tumor response assessment just after the completion of chemotherapy, whereas other disease progression data were based on investigators’ assessments. Nonetheless, we believed it would be of interest to examine the possible impact of ESA on disease progression in those studies identified in our literature search.
Reporting of thromboembolic events was also inconsistent among studies. A review of the thromboembolic events reported is included in this manuscript, but no meta-analysis was performed.
Statistical methods
Data were summarized using odds ratios (ORs) that were generated using Comprehensive Meta-Analysis (V2) software (Biostat, Inc., Englewood, NJ). Data for mortality and disease progression are presented as forest plots of all studies and include an estimated OR and 95% CI. Data for transfusion are presented as bar graphs showing incidence for each study. Patients were analyzed by randomized treatment assignment for consistency with direction from the US Food and Drug Administration. This differs from most published studies, which typically describe death as a safety endpoint that is analyzed by the treatment patients received. If published reports of studies included in this analysis reported mortality or disease progression as safety endpoints, then as-randomized data from company databases were used if available. The primary analysis used ORs to compare results for the ESA-treated patients and control patients. A sensitivity analysis was also performed using the risk difference (the difference between groups in the actual percentages of deaths) rather than the OR (the ratio of the odds of death in the ESA group vs. the odds for controls). A random-effects model was used in all meta-analyses because of known differences in the designs of included studies. Heterogeneity was reported using the I2 statistic.
Role of the funding source
Amgen Inc. (Thousand Oaks, CA) contributed to the study design, performed the literature search and statistical analysis, contributed to the interpretation of data, provided medical writing support for this paper, and supported the decision to submit the paper for publication.
Results
Literature search results
Ten oncology studies (ESA vs. placebo or no ESA) were identified that reported mortality data and were conducted in patients with lymphoproliferative malignancies [Citation7,Citation10,Citation17,Citation24–29,Citation31,Citation32]. All 10 studies were conducted in patients receiving chemotherapy. For each study, the specific diseases examined, the number of patients analyzed and other key study or patient characteristics are listed in . Because there were no deaths in either treatment arm of the study by Hedenus et al. [Citation31], it is not included in the OR estimate but is included in the risk difference analysis.
Table I. Characteristics of controlled ESA trials conducted in patients with lymphoproliferative malignancies that reported mortality data.
Mortality
A study-level meta-analysis was performed of the 10 studies, including a total of 2866 patients. Results using the random-effects model indicated that the OR for mortality was 1.04 (95% CI, 0.81–1.34) with an I2 value of 14.01% (). The risk difference was − 0.01 (95% CI, − 0.03–0.02), with an I2 value of 5.17%.
Figure 1. Study-level meta-analysis of mortality in randomized controlled trials of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls, including odds ratio for death using a random-effects model and results of a risk difference analysis. *The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. reported that the hazard ratio for overall survival was 1.02 (95% CI, 0.55–2.05). Because the Aapro report did not include the numbers or percentages of patients who died, its data for the Österborg 1996 study could not be included in this meta-analysis.
![Figure 1. Study-level meta-analysis of mortality in randomized controlled trials of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls, including odds ratio for death using a random-effects model and results of a risk difference analysis. *The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. reported that the hazard ratio for overall survival was 1.02 (95% CI, 0.55–2.05). Because the Aapro report did not include the numbers or percentages of patients who died, its data for the Österborg 1996 study could not be included in this meta-analysis.](/cms/asset/f05faf5f-3aca-42b2-814f-df97032ba49c/ilal_a_684347_f0001_b.gif)
Disease progression
Of the 10 studies that reported mortality data, five [Citation7,Citation10,Citation17,Citation26,Citation27,Citation29] also reported a disease progression outcome; a meta-analysis of these five studies was performed. In this analysis, the OR for disease progression was 1.02 (95% CI, 0.81–1.30, random-effects model). The I2 value was 0, indicating no heterogeneity among studies ().
Figure 2. Study-level meta-analysis of disease progression in patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls; odds ratio for disease progression using a random-effects model. *In the Österborg 1996 and 2005 studies [Citation7,Citation27], disease progression was based on tumor response assessment. †The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. [Citation35] reported that the hazard ratio for disease progression was 1.43 (95% CI, 0.72–2.86). Because the Aapro report did not include the numbers or percentages of patients with disease progression, its data for the Österborg 1996 study could not be included in this meta-analysis.
![Figure 2. Study-level meta-analysis of disease progression in patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls; odds ratio for disease progression using a random-effects model. *In the Österborg 1996 and 2005 studies [Citation7,Citation27], disease progression was based on tumor response assessment. †The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. [Citation35] reported that the hazard ratio for disease progression was 1.43 (95% CI, 0.72–2.86). Because the Aapro report did not include the numbers or percentages of patients with disease progression, its data for the Österborg 1996 study could not be included in this meta-analysis.](/cms/asset/7ed8d6a1-b71e-404d-a709-1f60979e5734/ilal_a_684347_f0002_b.gif)
Transfusions
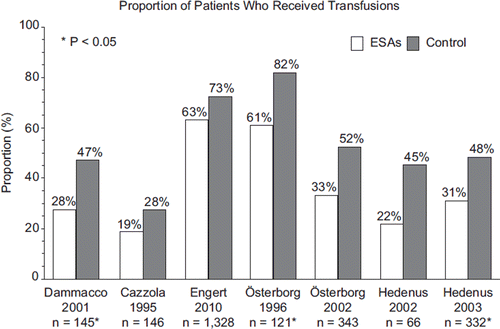
Placebo-controlled trials have shown that ESA use reduces the number of transfusions needed to treat anemia [Citation8,Citation9]. Of the 10 studies of patients with lymphoproliferative malignancies that reported mortality data, seven also reported the incidence of transfusions in each study arm [Citation7,Citation10,Citation25,Citation29,Citation31,Citation33,Citation34]. The incidence of transfusions ranged from 19 to 63% in the ESA treatment group and from 28 to 82% in the control group (). The study by Cabanillas et al. did not report the incidence of transfusion, but found that the mean number of red blood cell units transfused was significantly lower for the ESA group: 10.63 vs. 13.11 (p = 0.035); the mean number of transfusions was 6.22 for the epoetin alfa group and 7.44 for the control group (p = 0.089) [Citation26].
Figure 3. Proportion of patients (%) who received transfusions in studies of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls in randomized controlled trials. n represents the number of patients for whom transfusion data were available in each study.
Thromboembolic events
A comprehensive presentation of thromboembolic events is not possible because of the limited availability of meaningful data; reporting of thromboembolic adverse events was inconsistent among the studies in this analysis. Additionally, the completeness of reporting adverse events has changed over the time period of these studies. Although the Hedenus 2003 article [Citation10] and some reports of more recent studies provide more detail than earlier publications, the available data from studies included in the meta-analysis are not consistent with current reporting practices. Available data are summarized in .
Table II. Thromboembolic or cardiovascular events reported* in studies included in meta-analysis of patients with lymphoproliferative malignancies.
Rose et al. did not report on the incidence of thrombotic events, and Pangalis et al. stated that no side effects were observed [Citation27,Citation28,Citation32]. Cazzola et al. reported that no increased incidence of thromboembolic events was observed in the ESA group versus the controls [Citation24]. Österborg et al. reported fatal heart failure in 5% of ESA-treated patients and 4% of controls, as well as serious adverse events of pulmonary embolism in 3% of ESA patients, but not in control patients [Citation7]. Detailed safety data were available for two studies by Hedenus et al. [Citation10,Citation31]. In these studies, ESA-treated patients had a higher incidence of thromboembolic events and cerebrovascular accidents than did controls (). Additionally, two studies published since the emergence of recent concerns about thromboembolic complications of ESA therapy provided data on these events: Engert et al. reported that the total rate of thromboembolic events for both treatment groups was 10.1%, with an OR of 1.2 (95% CI, 0.7–1.9) [Citation29], and Cabanillas et al. reported that five ESA-treated patients (9%) and two control patients (4%) had thrombotic events, a difference that was not statistically significance (p = 0.44) [Citation26].
Discussion
This study-level meta-analysis examined the effect of ESA use on mortality and other outcomes in controlled ESA trials conducted in patients with lymphoproliferative malignancies receiving chemotherapy. Analyses using both random-effects OR modeling and risk-difference estimates suggest that ESA use does not have a clear effect on mortality in this patient population (). These results are consistent with those reported in the meta-analysis by Bohlius et al. that examined whether ESAs use affects on-study mortality in all treatment settings for patients with hematological malignancies [Citation21]. In the meta-analysis of the five studies that reported disease progression data, the OR for disease progression did not show an increased risk with ESA use (). In all the studies for which transfusion data were available, the use of ESAs reduced the incidence of transfusions.
However, a long-term follow-up analysis of the original Hedenus 2003 study indicated an increased mortality risk associated with ESA use [Citation10], and an analysis of the data used in the Rose study also revealed an increased mortality risk among patients with chronic lymphocytic leukemia; these data were not included in the original published abstract [Citation32]. In addition, the risk of disease progression appears to have increased with ESA use in the Hedenus 2003, Österborg 1996 and Cabanillas studies [Citation7,Citation10,Citation26]. The conclusions that can be drawn from these individual studies are limited. Grouping together progression data may be problematic because of differences in how progression was determined. Moreover, the studies were not designed or powered to prospectively evaluate long-term survival or disease progression, and merely present safety data.
Although this study-level meta-analysis does not suggest that ESA use has a clear negative effect on mortality or disease progression, this analysis has limitations. Study-level meta-analyses include potential biases that may result from selective reporting and publishing of data. In addition, the lack of access to patient-level data prevents analyses examining the effects of patient characteristics on outcomes. The randomized, controlled trials that enrolled patients with lymphoproliferative malignancies include studies with different study designs and patient characteristics (). These studies spanned nearly two decades, from the early 1990s to 2011; they differed in eligibility criteria, transfusion strategies or recommendations, hemoglobin or hematocrit targets, ESA dosing algorithms and methods for determining disease progression. Patient populations differed in age, in the types of lymphoproliferative malignancies represented and in chemotherapy regimens. The Cabanillas study, which included seven pediatric patients, evaluated patients with acute lymphoblastic leukemia (ALL) and Burkitt type ALL/lymphoma, which are not common indications for ESAs. The point estimates for survival and disease progression in the current meta-analysis should be reviewed within the context of these study and patient differences.
To explore the effects of population differences, we conducted a post hoc analysis excluding patients from the Engert study (Hodgkin disease) [Citation29], Hodgkin patients from the Hedenus 2002 and 2003 studies [Citation17,Citation31], and all patients from the Cabanillas study (ALL, lymphoblastic leukemia and Burkitt type ALL/lymphoma) [Citation26], and thus including only patients with chronic lymphocytic leukemia, non-Hodgkin lymphoma (NHL) and multiple myeloma. Although this analysis had its own limitations, including a smaller, heterogeneous sample and slightly wider confidence intervals, results were essentially the same as the pre-specified analysis, again showing no marked risk difference.
New studies in more homogeneous populations may shed additional light on these issues for patients with lymphoproliferative malignancies. The LNH03-6B study of approximately 600 elderly patients with diffuse large B-cell lymphoma receiving 14-day rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP-14) or R-CHOP-21 chemotherapy and darbepoetin alfa or conventional treatment is in progress [Citation30]. In this study, darbepoetin alfa is administered to patients in the darbepoetin alfa arm to maintain hemoglobin levels at ≥ 13 g/dL; in the conventional therapy arm, anemia is treated according to local policy and can include red blood cells or ESAs according to usual practice. The second interim analysis reported in 2011 indicated that administration of darbepoetin alfa was associated with better progression-free survival and disease-free survival, but not overall survival, compared with conventional treatment. The LNH03-6B study was not included in this meta-analysis because its design is different from those of the studies in the meta-analysis; a high proportion of patients in the conventional treatment arm receive ESAs, making comparisons between as-randomized groups difficult. Nonetheless, the results of the LNH03-6B study are consistent with those found in this meta-analysis.
In summary, in this meta-analysis, the use of ESAs reduced blood transfusions and had no clear effect on mortality or disease progression in patients with lymphoproliferative malignancies receiving chemotherapy. Analyses such as these might provide a useful compilation of the available evidence so that clinicians can evaluate the treatment options available for their patients. Future studies and additional meta-analyses will potentially provide further guidance on the benefits and risks of using ESAs in patients with lymphoproliferative malignancies.
Supplementary Material
Download Zip (1.9 MB)Acknowledgements
This study was funded by Amgen Inc. The authors acknowledge the medical writing support of Linda Rice, PhD and Shawn Lee, PhD of Amgen Inc. and Sue Hudson, whose services were funded by Amgen Inc.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Amgen Inc. Aranesp® (darbepoetin alfa) package insert. Thousand Oaks, CA: Amgen Inc.; 2011.
- Amgen Inc. Aranesp® (darbepoetin alfa) package insert. Breda, The Netherlands: Amgen Inc.; 2011.
- European public assessment reports for authorised medicinal products for human use [Internet]. Available from: www.emea.europa.eu/htms/human/epar/a.htm
- Janssen Products LP. PROCRIT® (epoetin alfa) prescribing information. Horsham, PA: Janssen Products LP; 2011.
- F. Hoffman-La Roche. NeoRecormon® (epoetin beta) summary of product characteristics. Welwyn Garden City, UK: Roche Products Limited; 2010.
- Roche Products Pty Limited. NeoRecormon® (epoetin beta) product information. Dee Why, Australia: Roche Product Pty Limited; 2011.
- Österborg A, Boogaerts MA, Cimino R, . Recombinant human erythropoietin in transfusion-dependent anemic patients with multiple myeloma and non-Hodgkin's lymphoma—a randomized multicenter study. The European Study Group of Erythropoietin (Epoetin Beta) Treatment in Multiple Myeloma and Non-Hodgkin's Lymphoma. Blood 1996;87:2675–2682.
- Littlewood TJ, Bajetta E, Nortier JW, . Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865–2874.
- Vansteenkiste J, Pirker R, Massuti B, . Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002;94:1211–1220.
- Hedenus M, Adriansson M, San Miguel J, . Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003;122:394–403.
- Barbara JA. The rationale for pathogen-inactivation treatment of blood components. Int J Hematol 2004;80:311–316.
- Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med 2007;131:708–718.
- Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest 2004;126:249–258.
- EMEA. electronic Medicines Compendium (eMC) [Internet]. Available from: http://emc.medicines.org.uk
- Bennett CL, Silver SM, Djulbegovic B, . Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914–924.
- Bohlius J, Wilson J, Seidenfeld J, . Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–714.
- Hedenus M, Vansteenkiste J, Kotasek D, . Darbepoetin alfa for the treatment of chemotherapy-induced anemia: disease progression and survival analysis from four randomized, double-blind, placebo-controlled trials. J Clin Oncol 2005;23:6941–6948.
- Amgen Inc. in collaboration with Johnson & Johnson Pharmaceutical Research and Development, L.L.C. Background information for the Oncologic Drugs Advisory Comittee (ODAC) meeting, 13 March 2008 [Internet]. Available from: www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4345b2-00-FDA-index.htm
- Katodritou E, Verrou E, Hadjiaggelidou C, . Erythropoiesis-stimulating agents are associated with reduced survival in patients with multiple myeloma. Am J Hematol 2008;83:697–701.
- Ludwig H, Anderson K, Dammacco F, . ESAs not the culprit: more studies required. Am J Hematol 2008;83:880; author reply 880–881.
- Bohlius J, Schmidlin K, Brillant C, . Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009;373: 1532–1542.
- Glaspy J, Crawford J, Vansteenkiste J, . Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 2010;102:301–315.
- Tonelli M, Hemmelgarn B, Reiman T, . Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ 2009;180:E62–E71.
- Cazzola M, Messinger D, Battistel V, . Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin's lymphoma: dose finding and identification of predictors of response. Blood 1995;86:4446–4453.
- Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol 2001;113:172–179.
- Cabanillas M, Kantarjian H, Thomas D, . Epoetin alpha decreases the number of erythrocyte transfusions in patients with acute lymphoblastic leukemia, lymphoblastic lymphoma, and Burkitt leukemia/lymphoma. Cancer 2012;118:848–855.
- Österborg A, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol 2005;129:206–209.
- Pangalis GA, Poziopoulos C, Angelopoulou MK, . Effective treatment of disease-related anaemia in B-chronic lymphocytic leukaemia patients with recombinant human erythropoietin. Br J Haematol 1995;89:627–629.
- Engert A, Josting A, Haverkamp H, . Epoetin alfa in patients with advanced-stage Hodgkin's lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trial. J Clin Oncol 2010;28:2239–2245.
- Delarue R, Haioun C, Coiffier B, . Survival effect of darbepoetin alfa in patients with diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: the LNH03-6B study. J Clin Oncol 2011;29(Suppl.): Abstract 9048.
- Hedenus M, Hansen S, Taylor K, . Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol 2002;119:79–86.
- Rose E, Rai K, Revicki D, . Clinical and health status assessments in anemic chronic lymphocytic leukemia (CLL) patients treated with epoetin alfa (EPO). Blood 1994;84(Suppl. 1): Abstract 2091.
- Cazzola M, Beguin Y, Kloczko J, . Once-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. Br J Haematol 2003;122:386–393.
- Österborg A, Brandberg Y, Molostova V, . Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002;20:2486–2494.
- Aapro M, Coiffier B, Dunst J, . Effect of treatment with epoetin beta on short-term tumour progression and survival in anaemic patients with cancer: a meta-analysis. Br J Cancer 2006;95:1467–1473.
