Abstract
High mobility group box 1 (HMGB1) mediates inflammation. We investigated the role of serum HMGB1 in 54 patients with hematological malignancies with and without systemic inflammatory response syndrome (SIRS). There was no difference between groups 1 (complete remission of hematological disease: n = 13) and 2 (no remission: n = 16) in serum HMGB1 levels. However, those of group 3 (complicated by SIRS: n = 25) were significantly higher (vs. group 1: p < 0.001 and vs. group 2: p = 0.008, respectively). Seventeen patients in group 3 also developed disseminated intravascular coagulation and received recombinant human thrombomodulin (rhTM). Thirteen of those with SIRS improved, and serum HMGB1 levels significantly decreased (p = 0.047). Seven patients in group 3 who died within 28 days of SIRS onset had significantly higher serum HMGB1 levels than the survivors (p = 0.016). The anti-HMGB1 properties of rhTM might be useful therapy if serum HMGB1 is associated with the development of SIRS in the presence of hematological malignancies.
Introduction
High mobility group box protein 1 (HMGB1) is recognized as a late mediator of sepsis or lipopolysaccharide endotoxin lethality and an inflammatory cytokine. It is a nuclear protein that can be released actively from monocytes and macrophages or passively from damaged cells [Citation1,Citation2]. Increased serum levels of HMGB1 protein are associated with the death of patients with sepsis and disseminated intravascular coagulation (DIC) [Citation3,Citation4].
Recombinant human soluble thrombomodulin (rhTM) is a new endothelial anticoagulant cofactor for treating DIC that promotes the thrombin-mediated formation of activated protein C [Citation5], and has unique anti-inflammatory properties due to its ability to bind to HMGB1 DNA- binding protein.
We investigated the clinical relevance of serum HMGB1 concentrations in 54 patients with hematological malignancies, including 25 who also had systemic inflammatory response syndrome (SIRS).
The treatment outcomes of 17 of the patients who were complicated by SIRS and DIC and treated with rhTM are also described.
Patients and methods
Patients
We enrolled 54 patients (age range, 26–77 years; median age, 61 years; male, n = 32; female, n = 22) with hematological malignancies that required treatment with high dose chemotherapy between July 2009 and Dec 2011. None of the patients had uncontrolled infection or severe renal, heart or liver disease, and all were serologically negative for the human immunodeficiency virus. Patients with acute promyelocytic leukemia were excluded. Patients who achieved complete remission (CR) after two rounds of chemotherapy were assigned to group 1 (n = 13), those who did not were assigned to group 2 (n = 16) and those who developed SIRS during chemotherapy were assigned to group 3 (n = 25). Serum HMGB1 concentrations were compared among the three groups. All patients in group 1 achieved complete remission of the following hematological malignancies: acute myeloid leukemia (n = 4), acute lymphoblastic leukemia (n = 4), non-Hodgkin lymphoma (n = 3) and myelodysplastic syndrome (n = 2). All patients in group 2 without SIRS did not achieve remission of the following hematological malignancies: acute myeloid leukemia (n = 5), acute lymphoblastic leukemia (n = 1), non-Hodgkin lymphoma (n = 4), myelodysplastic syndrome (n = 4) and multiple myeloma (n = 2). Group 3 with SIRS also had acute myeloid leukemia (n = 9), non-Hodgkin lymphoma (n = 6), myelodysplastic syndrome (n = 2), multiple myeloma (n = 3), adult T cell leukemia (n = 1) and post-allogeneic bone marrow transplant (n = 4). In group 3, minimal responses to medium or high doses of chemotherapies were observed in 21 of 25 patients. Main causes of SIRS were infection (11 of 25 patients) or progression of hematological malignancies (10 of 25 patients). Four patients suffered from complications of post-allogeneic bone marrow transplant (acute graft-versus-host disease; n = 2, and intestinal transplant-associated microangiopathy; n = 2, ). Complete remission was defined as < 5% of malignant cells in the bone marrow and absence of lymph node swelling on computed tomography images. Both SIRS and DIC were diagnosed and graded as described [Citation6,Citation7]. Serum HMGB1 concentrations were measured at the time of complete remission, when febrile neutropenia disappeared and at the onset of SIRS in groups 1, 2 and 3, respectively. Patients with SIRS and DIC were treated with rhTM, and then serum HMGB1 levels were re-analyzed immediately after rhTM. Stabilization of the endothelium by rhTM was estimated by measuring serum endothelial leukocyte adhesion molecule-1 (ELAM-1) concentrations before and after rhTM administration, when the HMGB1 concentration was also determined. The dose and term of rhTM administration was adjusted according to renal function and the effects of the drug on each patient. Patients in group 3 were followed up for 28 days after SIRS was diagnosed, and outcomes were assessed. The institutional review board at St. Marianna University approved the protocol, and all patients provided written informed consent to participate in study-related procedures.
Table I. Patient characteristics.
Analysis of blood samples
Whole blood (˜10 mL) collected in non-heparinized tubes was left to clot at room temperature for 30 min before centrifugation at 3000 rpm for 15 min. The serum fraction was collected and stored at − 80°C before analysis by enzyme-linked immunosorbent assays using an anti-HMGB1 polyclonal antibody (Shino-Test Corporation, Tokyo, Japan) or an anti-ELAM-1 monoclonal antibody (Mitsubishi Chemical Medience Co. Ltd., Tokyo, Japan) according to the hematological malignancy.
Data analysis
Serum HMGB1 levels of each group are expressed as mean [95% confidence interval (CI) mini-max], and were compared using the Kruskal–Wallis test with post hoc Dunn. Serum HMGB1 and ELAM-1 levels before and after rhTM administration were compared using the Wilcoxon signed-rank test for continuous variables. Serum HMGB1 levels between survival and non-survival patients with DIC in group 3 were compared using the Mann–Whitney test. All data were analyzed using IBM SPSS Statistics for Windows version 20.0.0 software (IBM Corp., Armonk, NY).
Results
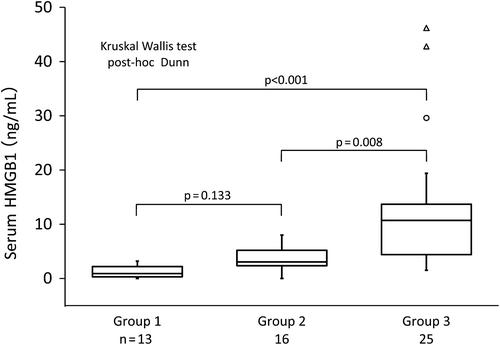
Serum HMGB1 levels did not differ between groups 1 and 2 (mean [95% CI mini-max]: 0.9 [0.5–1.9] vs. 3.1 [2.3–4.8] ng/mL, p = 0.133), but were significantly elevated in group 3 compared with groups 1 and 2 (10.7 [7.7–17.4] vs. 0.9 [0.5–1.9] and 3.1 [2.3–4.8] ng/mL, p < 0.001 and p = 0.008, respectively, and ). The HMGB1 concentrations did not significantly differ among the hematological malignancies in all groups.
Figure 1. Serum HMGB1 levels did not differ between groups 1 and 2, but were significantly elevated in group 3 compared with groups 1 and 2.
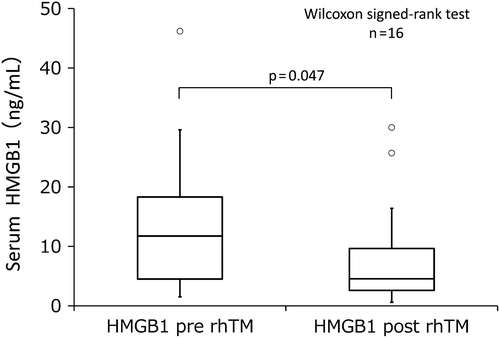
Seventeen of 25 patients with SIRS in group 3 developed DIC that was treated with rhTM, which improved the condition in 13 patients and significantly ameliorated their serum HMGB1 concentrations (p = 0.047, ). Improvement of serum C-reactive protein (CRP) was observed in 12 patients. DIC withdrawal rate was 52.9% (nine of 17 patients). Serum ELAM-1 concentrations tended to decrease (p = 0.38) in 10 of 13 of these patients, with SIRS improved. Adverse events due to rhTM including bleeding did not arise.
Figure 2. Seventeen patients with both SIRS and DIC in group 3 were treated with rhTM. Serum HMGB1 concentrations, before and after rhTM administration, could be analyzed in 16 patients. Therapy improved the condition in 13 patients and significantly ameliorated their serum HMGB1 concentrations.
Seven of the 25 patients with SIRS died within 28 days of diagnosis due to multiple organ failure caused by hematological disease progression or complications. None of these patients achieved complete remission of the hematological malignancy, and six of seven patients developed DIC. Their serum HMGB1 levels remained above 10 ng/mL or were not decreased by rhTM therapy, and were significantly higher than those of survivors with SIRS and DIC (18.5 [9.4–41.2] vs. 6.10 [3.2–15.0] ng/mL, p = 0.016, , ; cut-off value, 12.65 ng/mL, , ). It has been reported that the mean serum HMGB1 level of a healthy population was 1.65 ± 0.04 ng/mL by the same method using the same anti-HMGB1 polyclonal antibody as in the present study (Shino-Test Corporation) [Citation8].
Figure 3. Pretreatment levels of serum HMGB1 between survival patients and non-survival patients in group 3.
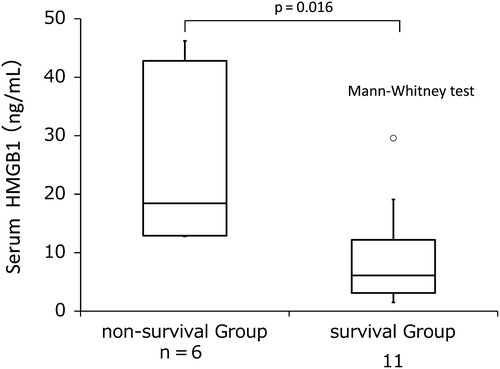
Figure 4. Receiver operator characteristic (ROC) curve generated with serum HMGBI levels in group 3.
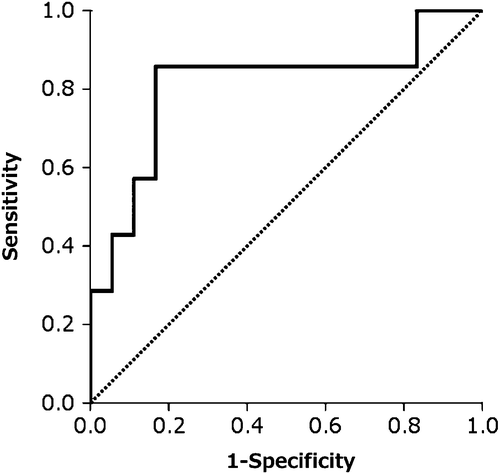
Table II. Treatment outcome of 17 patients complicated by SIRS and DIC in group 3 using rhTM.
Table III. Cut-off values (ng/mL) of serum HMGB1 levels in group 3.
Discussion
The nuclear factor HMGB1 is also a secreted protein. In the nucleus it acts as an architectural chromatin-binding factor that binds DNA and promotes protein assembly on specific DNA targets that play an important role in cancer development. It is also a potent extracellular mediator of inflammation [Citation9,Citation10]. Plasma or serum HMGB1 levels might serve as a prognostic marker of SIRS arising from systemic diseases such as septic shock, severe pancreatitis and DIC [Citation3,Citation4,Citation11,Citation12]. There have been a few studies investigating the role of HMGB1 in hematological malignancies. Nomura et al. showed significantly high serum HMGB1 levels and a decrease after rhTM therapy in patients with DIC associated with hematological malignancies [Citation13]. High levels of serum HMGB1 were indicated in children with acute lymphoblastic leukemia in the initial treatment group [Citation14]. In an in vitro study, HMGB1 released in leukemia cell lines functioned as a positive regulator of autophagy, which enhanced resistance to anticancer therapies [Citation15]. HMGB1 also has something to do with leukemia pathogenesis and chemotherapy resistance. Serum HMGB1 levels have not been investigated in patients with SIRS with hematological malignancies, although it seems relevant to understand them from the viewpoint of managing SIRS when complicated by hematological malignancies.
Because all patients in group 3 developed SIRS during chemotherapy, we compared their serum HMGB1 concentrations with those of patients without SIRS after receiving chemotherapy. Our findings suggest that serum HMGB1 levels were associated more closely with severe inflammation than with the status of remission and the development of malignant cells in patients with hematological malignancies. None of the patients without SIRS had HMGB1 levels of ≥ 10 ng/mL, and the prognosis of patients with SIRS and HMGB1 levels of ≥ 12.65 ng/mL was very poor.
Due to its favorable effects, safety and ease of administration, rhTM has recently been recommended as a first-line anticoagulant with which to treat DIC. Thrombomodulin was originally identified as an endothelium-specific membrane protein. The extracellular portion of TM is composed of an N-terminal lectin-like domain (D1), followed by an epidermal growth factor (EGF)-like domain (D2) consisting of six EGF-like repeats and an O-glycosylation-rich domain (D3). D2 is critical for the anticoagulant cofactor properties of TM, which are thrombin inhibition and the promotion of activated protein C formation [Citation16–18]. Recombinant human TM binds HMGB1 at the level of D1 and suppresses the induction of proinflammatory events [Citation9].
Although targeting HMGB1 ligand or its receptor in vivo as a therapeutic approach to managing SIRS has not yet been established, some studies have investigated the effects of an anti-HMGB1 antibody in animal models of SIRS. The findings showed that anti-HMGB1 antibody improves the survival of mice challenged with a lethal endotoxin and ameliorates murine lung inflammation after intratracheal endotoxin challenge [Citation1,Citation19]. This response is dose dependent, and a higher survival rate correlates with an increased frequency of anti-HMGB1 antibody administration [Citation19]. Serum HMGB1 levels were decreased among the 12 patients with SIRS who improved after rhTM administration in the present study. One of the effects of rhTM on SIRS and DIC complicated by hematological malignancies might be due to its ability to bind HMGB1 DNA-binding protein. Thus, rhTM might serve as the first anti-HMGB1 agent to treat SIRS.
One patient in group 3 developed intestinal transplant-associated microangiopathy (iTAM) after allogeneic hematopoietic stem cell transplant (allo-HSCT) when the HMGB1 level reached 10.7 ng/mL (patient no. 4). He was also complicated by prednisolone-resistant grade 3 acute graft-versus-host disease (aGVHD) and DIC and developed severe diarrhea (> 5 L/day). Notably, rhTM was obviously effective against DIC, iTAM and aGVHD and also ameliorated the diarrhea and high HMGB1 level [Citation20]. Some reports have described successful outcomes of rhTM treatment among patients with veno-occlusive disease (VOD) or iTAM that arises after allo-HSCT [Citation21,Citation22]. Two of our patients with iTAM in group 3 had serum HMGB1 concentrations of > 10 ng/mL. The receptor for advanced glycation end product (RAGE) is also a receptor for HMGB1, and it is expressed on endothelium. Endothelial cells might be activated by HMGB1 through up-regulating surface receptors and inducing the secretion of soluble proinflammatory mediators [Citation23]. Serum HMGB1 might be associated with the development of high cytokine syndrome after allo-HSCT, such as GVHD, VOD and TAM. The anti-HMGB1 effects of rhTM might help to avoid these early complications of endothelial damage or excessive inflammation after allo-HSCT. Endothelial cell vulnerability and dysfunction is related to steroid-refractory GVHD such as VOD and TAM [Citation24]. The tendency for rhTM to ameliorate serum ELAM-1 in the present study indicated a potential improvement in endothelial cell activation.
In conclusion, serum HMGB1 concentrations were significantly higher in patients with hematological malignancies with, as opposed to without, complication by SIRS. We suppose that HMGB1 is involved in the development of inflammation in hematological malignancies. If serum HMGB1 concentrations exceed 10 ng/mL in hematological malignancies complicated by inflammation, then early treatment for SIRS or DIC should be considered. Because HMGB1 is a late reactive protein associated with inflammation, rhTM administration at early onset of SIRS in a background of hematological malignancy might prevent progression to multiple organ failure and achieve a more favorable clinical outcome. Although anti-HMGB1 therapies for inflammation have not yet been established, the anti-HMGB1 effect of rhTM might play an important role in patients with hematological malignancies complicated by SIRS and/or DIC.
This is a limited pilot study and further clinical studies are warranted to estimate the optimal strategy for treating SIRS with rhTM.
Supplementary Material
Download Zip (4.8 MB)Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000;165:2950–2954.
- Yang H, Wang H, Tracey KJ. HMGB1 rediscovered as a cytokine. Shock 2001;15:247–253.
- Hatada T, Wada H, Nobori T, et al. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost 2005;94:975–979.
- Huang LF, Yao YM, Dong N, et al. Association of high mobility group box-1 protein levels with sepsis and outcome of severely burned patients. Cytokine 2011;53:29–34.
- Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 2007; 5:31–41.
- Wada H, Gabazza EC, Asakura H, et al. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC):diagnostic criteria of the international society of thrombosis and hemostasis (ISTH) and of the Japanese ministry of health and welfare for overt DIC. Am J Hematol 2003;74:17–22.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874.
- Fukami A, Adachi H, Yamaguchi S, et al. Factors associated with serum high mobility group box 1(HMGB1) levels in general population. Metabolism 2009;58:1688–1693.
- Abeyama K, DStren M, Ito Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 2005;115:1267–1274.
- Meyer A, Staratschek-Jox A, Springwald A, et al. Non-Hodgkin lymphoma expressing high levels of the danger-signaling protein HMGB1. Leuk Lymphoma 2008;49:1184–1189.
- Gibot S, Massin F, Cravoisy A, et al. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med 2007;33:1347–1353.
- Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas 2006;33:359–363.
- Nomura S, Fujita S, Ozasa R, et al. The correlation between platelet activation markers and HMGB1 in patients with disseminated intravascular coagulation and hematologic malignancy. Platelets 2011;22:396–397.
- Kang R, Tang DL, Cao LZ, et al. High mobility group box 1 is increased in children with acute lymphocytic leukemia and stimulates the release of tumor necrosis factor alpha in leukemic cell. Zhonghua Er Ke Za Zhi 2007;45:329–333.
- Liu L, Yang M, Kang R, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia 2011;25:23–31.
- Esmon CT. The role of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem 1989;264:4743–4746.
- Suzuki K, Kusumoto H, Deyashiki Y, et al. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J 1987;6:1981–1997.
- Zushi M, Gomo K, Yamamoto S, et al. The last three consecutive epidermal growth factor-like structures of human thrombomodulin comprise the minimum functional domain for protein C-activating cofactor activity and anticoagulant activity. J Biol Chem 1989;254:10351–10353.
- Wang H, Bloom O, Zhang M, et al. HMGB-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251.
- Inoue Y, Kosugi S, Miura I, et al. Successful treatment of refractory acute GVHD complicated by severe intestinal transplant-associated thrombotic microangiopathy using recombinant thrombomodulin. Thromb Res 2011;127:603–604.
- Ikezoe T, Togitani K, Komatsu N, et al. Successful treatment of sinusoidal obstructive syndrome after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Bone Marrow Transplant 2010;45:783–785.
- Sakai M, Ikezoe T, Bandobashi K, et al. Successful treatment of transplantation-associated thrombotic microangiopathy with recombinant human soluble thrombomodulin. Bone Marrow Transplant 2010;45:803–805.
- Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003;101:2654–2660.
- Luft T, Dietrich S, Falk C, et al. Steroid-refractory CVHD: T-cell attack within a vulnerable endothelial system. Blood 2011; 118:1685–1692.
