Abstract
Idelalisib is a first-in-class selective, oral, phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor approved for the treatment of several types of blood cancer. Idelalisib has demonstrated significant efficacy and a tolerable safety profile in clinical trials. However, the US prescribing information contains a black box warning for fatal and/or severe diarrhea or colitis, hepatotoxicity, pneumonitis and intestinal perforation. An expert panel was convened to review the pathology of these treatment-emergent adverse events (TEAEs) to propose key management tools for patients receiving idelalisib therapy. This article provides an overview of idelalisib TEAEs reported in clinical trials, and a summary of the panel's recommendations for identification and management of idelalisib treatment-emergent diarrhea or colitis as well as a discussion of transaminitis and pneumonitis. For idelalisib-related diarrhea or colitis (including unresolved grade 2 and grade ≥ 3), after exclusion of infectious causes, the panel recommends individualized treatment with budesonide or oral or intravenous steroid therapy.
Introduction
Idelalisib (Zydelig®; Gilead Sciences, Inc., Foster City, CA; formerly GS-1101, CAL101) is a selective, oral, phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor [Citation1]. It is approved by the US Food and Drug Administration for the treatment of patients with relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other comorbidities; relapsed follicular B-cell non-Hodgkin lymphoma in patients who have received ≥ 2 prior systemic therapies; and relapsed small lymphocytic lymphoma in patients who have received ≥ 2 prior systemic therapies [Citation2]. The recommended starting dose of idelalisib is 150 mg orally with or without food twice daily (BID) [Citation2]. The European Commission has also granted marketing authorization for idelalisib: (1) in combination with rituximab for the treatment of adult patients with CLL who have received ≥ 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in patients unsuitable for chemoimmunotherapy and (2) as monotherapy in the treatment of adult patients with follicular lymphoma that is refractory to two prior lines of treatment [Citation3]
PI3K–AKT–mTOR pathway and the role of PI3Kδ
Surface-receptor tyrosine kinases activate class I PI3Ks [Citation4], which regulate numerous cellular functions through phosphorylation of lipid second messengers [Citation5]. Class I PI3Ks consist of four catalytic domains (p110α, p110β, p110γ, p110δ) [Citation5]. The α and β isoforms are ubiquitously expressed, and the γ isoform has multiple functions, including roles in T-cell development and signaling [Citation5]. However, the δ isoform is predominantly found in leukocytes [Citation6]. p110δ integrates and transmits signals from surface proteins, including B-cell antigen receptors (BCRs) and chemokine receptors (CXCR4 and CXCR5) responsible for CLL cell homing and retention in tissue compartments, via downstream activation of AKT (also known as protein kinase B) [Citation7]. AKT is a central node in signaling cascades that regulate cell survival, proliferation and migration [Citation4], and it is known to activate the pro-growth mammalian target of rapamycin (mTOR) complex () [Citation4,Citation7]. p110δ plays an essential, non-redundant role in BCR signaling in vitro, and p110δ-deficient mice show decreased numbers of mature B cells and defective humoral responses to immunization with minimal effect on other hematopoietic cells [Citation8]. Hyperactive BCR signaling has been implicated in non-Hodgkin lymphoma and CLL, suggesting that p110δ may represent a useful target in B-cell malignancies [Citation9,Citation10].
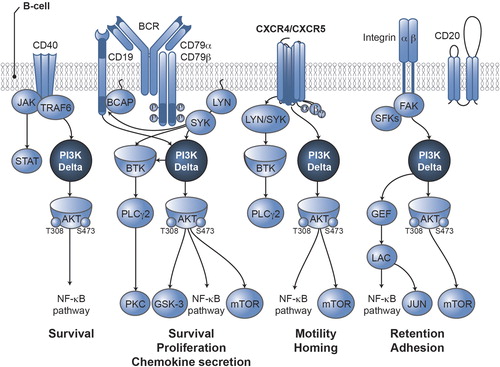
Preclinical studies have shown that inhibition of PI3Kδ-dependent signaling with idelalisib resulted in decreased downstream signaling of BCR, CXCR4 and CXCR5 (i.e. decreased activation of AKT, mTOR and other downstream effectors) [Citation1,Citation7]. Consequently, exposure to idelalisib inhibited proliferation, chemotaxis, motility, adhesion and survival and promoted apoptosis in cell lines derived from B-cell malignancies [Citation1,Citation7].
Idelalisib clinical data
Idelalisib has shown clinical activity and a tolerable safety profile in phase 2 and 3 trials.
Idelalisib in indolent non-Hodgkin lymphoma
A single-arm, open-label, phase 2 trial (NCT01282424) evaluated treatment with idelalisib 150 mg BID in 125 patients with double-refractory (to rituximab and an alkylating agent), indolent non-Hodgkin lymphoma (iNHL) until disease progression or study withdrawal. Idelalisib treatment resulted in an overall response rate (ORR; primary endpoint and determined by an Independent Review Committee [IRC]) of 57% (95% confidence interval [CI], 48–66%), a complete response rate of 6%, a partial response rate of 50% and a minor response rate of 1% (one patient with Waldenström macroglobulinemia). The median duration of response was 12.5 months (range, 0.03–14.8). The median progression-free survival (PFS) was 11.0 months (range, 0.03–16.6), with a PFS rate of 47% at 48 weeks. The median overall survival (OS) was 20.3 months (range, 0.7–22.0), with an estimated OS rate of 80% at 1 year. The most common adverse events (AEs) were diarrhea, nausea and fatigue. The most common laboratory abnormalities grade ≥ 3 were neutropenia and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevation (). The most common serious AEs (≥ 5%) were pyrexia (10%), pneumonia (7%) and diarrhea (7%). AEs led to treatment discontinuation in 25 patients (20%) and a dose reduction (100 mg BID or 75 mg BID) in 42 patients (34%) [Citation11].
Table I. Overview of adverse events reported in > 20% of patients and key laboratory abnormalities in patients in any idelalisib group in phase 2 and 3 idelalisib studies [Citation11,Citation12].
Idelalisib in CLL
Efficacy and safety of idelalisib were also demonstrated in a randomized, double-blind, placebo-controlled, phase 3 trial (NCT01539512) in 220 patients with progressive CLL and either decreased renal function, previous therapy-induced myelosuppression or major coexisting illness. Patients received 150 mg BID plus rituximab or placebo plus rituximab. At interim analysis, PFS as assessed by IRC demonstrated a statistically significant reduction in the risk of progression or death by 85% (adjusted hazard ratio [95% CI], 0.15 [0.08–0.28]; unadjusted p < 0.0001); median PFS was not reached in the idelalisib group, compared with 5.5 months in the placebo group. At 24 weeks, the PFS rate was 93% in the idelalisib group, compared with 46% in the placebo group. At 1 year, the rate of OS was significantly higher in the idelalisib group (92%) compared with the placebo group (80%; adjusted hazard ratio for death [95% CI], 0.28 [0.09–0.86]; p = 0.02); median OS was not reached in either group at the time of analysis. A significant improvement in ORR was also observed in the idelalisib group compared with the placebo group (81% [95% CI, 71–88%] vs. 13% [95% CI, 6–21%], respectively; p < 0.001); all responses were partial responses. The five most common AEs in idelalisib-treated patients were pyrexia, fatigue, nausea, chills and diarrhea; grade ≥ 3 diarrhea was reported in four patients in the idelalisib group and no patients in the placebo group. The most common laboratory abnormalities grade ≥ 3 were neutropenia, thrombocytopenia, anemia and ALT or AST elevation (). Serious AEs were reported in 40% of patients in the idelalisib group and 35% of patients in the placebo group. The most common serious AEs (≥ 5%) in the idelalisib group were pneumonia (6%), pyrexia (6%) and febrile neutropenia (5%); in the placebo group, only pneumonia (8%) and febrile neutropenia (6%) were reported in ≥ 5% of patients. AEs led to treatment discontinuation in nine patients (8%) in the idelalisib group and 11 patients (10%) in the placebo group [Citation12].
Idelalisib treatment-emergent adverse events
Idelalisib US prescribing information contains a black box warning for fatal and/or severe diarrhea or colitis, hepatotoxicity, pneumonitis and intestinal perforation [Citation2]. Identification and management of diarrhea or colitis, transaminitis and pneumonitis are discussed in the following sections and based on experience from previously conducted studies and the US prescribing information; they do not involve any new studies of human or animal subjects performed by any of the authors.
Additional warnings and precautions from the US prescribing information include severe cutaneous reactions, anaphylaxis, neutropenia and embryo-fetal toxicity. A case of toxic epidermal necrolysis (patient was receiving idelalisib in combination with rituximab and bendamustine) as well as other severe or life-threatening (grade ≥ 3) cutaneous reactions have been reported. The US prescribing information recommends monitoring patients for the development of severe cutaneous reactions and, if they occur, discontinuing idelalisib. Patients who develop serious allergic reactions, including anaphylaxis, should permanently discontinue treatment with idelalisib and institute appropriate supportive measures. Treatment-emergent grade 3 or 4 neutropenia has been reported in 31% (234/760) of patients treated with idelalisib across clinical trials. Drug interruption occurred in 3.6% of patients, and 1.3% of patients required a dose reduction; two patients (< 0.5%) eventually discontinued therapy. The median time to onset of grade ≥ 3 neutropenia analyzed in the New Drug Application (NDA) integrated safety summary (n = 642) was 1.4 months (range 0.0–13.8 months). Granulocyte colony-stimulating factor (G-CSF) was permitted to treat patients with grade 3 or 4 treatment-emergent neutropenia depending on the study protocol. In patients with relapsed CLL in the phase 3 clinical trial and in patients with iNHL in the phase 2 clinical trial, idelalisib was withheld in grade 4 treatment-emergent neutropenia that was not responding to G-CSF after 14 days (phase 3 trial) or 3 days (phase 2 trial). In the phase 3 study, G-CSF was utilized in 24.6% of patients in the idelalisib plus rituximab group and in 19.4% of patients in the placebo plus rituximab group. In the phase 2 trial, G-CSF was used in 15.8% of patients given the 150 mg BID dose. Blood counts should be monitored at least every 2 weeks for the first 3 months of therapy and at least weekly in patients whose neutrophil counts are < 1.0 × 109/L [Citation2]. Intestinal perforation including two fatal cases occurred in 6/1192 (0.5%) patients with hematological malignancies across phase 1, 2 and 3 clinical trials (including ongoing randomized phase 3 clinical trials). Two patients had previously reported having diarrhea or colitis. One patient (idelalisib plus rituximab arm) developed grade 3 colitis on day 119, which subsequently led to a left colon perforation on day 198. One patient developed diarrhea, colitis and duodenitis, followed by microscopic bowel perforation without specified location on day 519. Both patients had concomitant prednisolone use at the time of bowel perforation. Two patients had underlying gastrointestinal malignancies; one patient had colon cancer with tumor perforation confirmed at autopsy, and the other patient had underlying pancreatic cancer with right colon perforation on day 224, preceded by grade 4 colitis. Two patients had perforated diverticulitis; one patient had no preceding diarrhea or colitis, and the other patient developed sigmoid colon diverticulum perforation on day 126 preceded by diverticulitis and diarrhea [Citation13].
Diarrhea or colitis
Severe diarrhea or colitis (grade ≥ 3) has occurred in patients treated with idelalisib [Citation2]. It is unknown whether this is a class effect of PI3K inhibitors; however, grade ≥ 3 diarrhea has been reported in a clinical trial with IPI-145, a dual γ/δ PI3K inhibitor [Citation14]. Among idelalisib-treated patients who reported diarrhea or colitis, the median time to onset of any grade diarrhea or colitis was 1.9 months (range, 0.0–29.8), of grade 1 or 2 was 1.5 months (range, 0.0–15.2) and of grade 3 or 4 was 7.1 months (range, 0.5–29.8). Kaplan–Meier curves of time to onset of diarrhea or colitis are shown for all idelalisib-treated patients in [Citation13]. Idelalisib-associated severe diarrhea responds poorly to antimotility agents; however, median time to resolution ranged between 1 week and 1 month across trials following interruption of idelalisib treatment and, in some instances, initiation of corticosteroid treatment [Citation2].
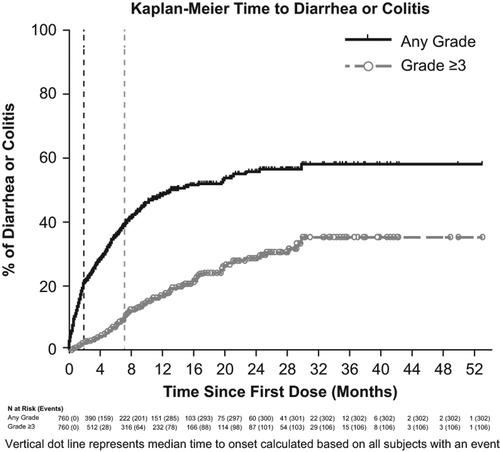
Severe diarrhea or colitis occurred in 106/760 (14%) patients from the NDA 90-day safety update for phase 1 and 2 studies (n = 650; data cut-off, 9 September 2013) plus patients who received idelalisib plus rituximab in the phase 3 CLL study (n = 110; data cut-off, 9 October 2013) [Citation2,Citation12,Citation13]. One fatality was reported among all patients who received idelalisib across all trials (n = 1192). The fatality occurred in an ongoing phase 3 study in a patient who had diarrhea concomitantly with thrush and shingles as well as a positive test for Clostridium difficile, a possible cause of the diarrhea [Citation13].
In patients with relapsed CLL who received idelalisib plus rituximab (n = 110) in the phase 3 clinical trial, any grade diarrhea (includes preferred term colitis) was reported in 21% (placebo plus rituximab [n = 108], 16%), grade ≥ 3 diarrhea was reported in 5% (placebo plus rituximab, 0%) and serious diarrhea was observed in 5% of patients in the idelalisib plus rituximab treatment group. Diarrhea was one of the most common AEs that led to idelalisib dose reduction and treatment discontinuation [Citation2]. Among 146 patients with iNHL who received idelalisib 150 mg monotherapy, any grade diarrhea (includes preferred terms colitis, enterocolitis and gastrointestinal inflammation) was reported in 47%, grade ≥ 3 diarrhea in 14% and serious diarrhea in 11%. Diarrhea was one of the most common AEs that led to idelalisib dose interruption or discontinuation (11% of patients) [Citation2].
For cases of moderate diarrhea (increase of 4–6 stools/day over baseline; grade 2), the idelalisib US prescribing information recommends maintaining idelalisib dose and monitoring at least once weekly until resolved. For cases of severe diarrhea (increase of ≥ 7 stools/day over baseline; grade 3) or hospitalization due to diarrhea, the US prescribing information recommends withholding idelalisib and monitoring at least once weekly until the diarrhea resolves; once resolved, idelalisib may be resumed at a reduced dose of 100 mg BID. If life-threatening diarrhea (grade 4) occurs, idelalisib should be permanently discontinued [Citation2].
Enteric budesonide has been a commonly used treatment for severe diarrhea or colitis, resulting in a relatively shorter time to resolution. Among the 106 previously mentioned cases of severe diarrhea or colitis (grade ≥ 3), 23 were treated with enteric budesonide; however, budesonide use may be underreported in this population. To date, sufficient information is available to calculate time to resolution for 18 cases in which diarrhea or colitis was considered a non-infectious, serious AE attributable to idelalisib. The mean time to resolution after initiation of enteric budesonide was 12.1 days (range, 1–35 days) [Citation13]. All cases of diarrhea or colitis resolved with drug withdrawal. For grade ≥ 3 diarrhea or colitis, 71 of 106 cases were rechallenged with idelalisib, with a success rate of 58% (41/71).
An expert panel of 10 hematologists and one gastroenterologist was convened in May 2014 to discuss the characteristics of idelalisib treatment-emergent diarrhea/colitis and to propose clinical management recommendations for healthcare providers. The predetermined and primary focus of the panel's discussions and recommendations was diarrhea that responds poorly to antidiarrheal or empiric antimicrobial therapy. During the open discussion, the panel reached unanimous conclusions, which are outlined here. Differences of opinion were thoroughly discussed and in all cases consensus recommendations were subsequently achieved.
First, the two types of diarrhea observed in idelalisib clinical trials were evaluated. The first type of diarrhea tends to be self-limiting. This type generally occurs within the first 8 weeks and is typically mild or moderate (grade 1–2) and responsive to common antidiarrheal agents. The second type of diarrhea, the predetermined panel focus, tends to occur relatively late (although a few cases happened early) and responds poorly to antidiarrheal or empiric antimicrobial therapy; it is considered most likely to be idelalisib related. The idelalisib-related diarrhea may be sudden or gradual in onset and is usually watery, without cramps, devoid of blood or mucus and culture negative. Several colonoscopies in patients with idelalisib treatment-emergent, late-onset diarrhea revealed histologic appearance of the colon consistent with that of lymphocytic colitis. Although a definitive underlying mechanism for treatment-emergent diarrhea is unknown, similar histologic findings were noted in a murine PI3Kδ knock-out model [Citation15]. Anecdotally, these cases were responsive to budesonide and/or systemic corticosteroid (e.g. prednisone) treatment; in some cases, prednisolone 1 mg/kg was administered with tapering off once diarrhea returned to grade 1. The time to resolution of severe diarrhea appeared to be shorter with the initiation of budesonide and/or systemic corticosteroids (1–2 weeks) compared with idelalisib interruption alone (approximately 1 month).
Patients taking idelalisib should be evaluated for any grade diarrhea. For those presenting with diarrhea, a thorough history, physical examination and necessary laboratory testing should be performed during the initial patient examination (). Diagnostic colonoscopy should be reserved for atypical cases (e.g. bloody diarrhea) or those in whom the recommended treatment interventions do not lead to resolution of diarrhea.
Table II. Evaluation and diagnostic testing recommendations for idelalisib-treated patients presenting with diarrhea (any grade).
The expert panel used a previous set of guidelines for the management of diarrhea induced by cancer treatment [Citation16] as a starting point to develop recommendations for community practitioners to implement in cases of idelalisib treatment-emergent diarrhea. Self-limiting, uncomplicated diarrhea (grade 1 and some cases of mild grade 2) can usually be managed with antidiarrheal therapy (e.g. loperamide) and diet modification. However, frequent reassessments are needed to ensure that unresolved diarrhea is managed more aggressively ().
![Figure 3. Management algorithm for grade 1–2 uncomplicated diarrhea. (Adapted in part with permission from reference [Citation16].).](/cms/asset/5a2839a1-c962-489c-8164-89f2dac8026b/ilal_a_1022770_f0003_b.gif)
Idelalisib-related, late-onset, grade 2 diarrhea (including borderline grade 3 diarrhea) in patients taking idelalisib is considered clinically significant and should be managed in a similar manner to grade ≥ 3; this is especially true for any grade 2 diarrhea that is unresponsive to antimotility agents after 24 h. For idelalisib-related diarrhea or colitis (including unresolved grade 2 and grade ≥ 3), initial management should include diagnostic testing to rule out infectious causes (). After exclusion of infectious causes, initiation of budesonide or oral or intravenous steroid therapy is recommended. The duration of budesonide therapy should be based on individual clinical response. In general, it should be continued until complete resolution of diarrhea, but it should not be used for chronic suppression of symptoms associated with ongoing use of idelalisib. Treatment should be individualized based on a patient's age, comorbidities and social/family support. For example, an elderly patient or one who lives alone may need to be hospitalized for observation, whereas a patient with adequate family/caregiver support may be able to have in-home management.
![Figure 4. Management algorithm for unresolved grade 2 and grade 3/4 diarrhea. IV, intravenous; PO, per os. *Recommended dosage: three 3 mg capsules PO once daily (9 mg total) [Citation23]. †Based on panel members’ experience in clinical trials, prednisolone 1 mg/kg has been used with tapering off once diarrhea returns to grade 1.](/cms/asset/ee03d0e0-f677-4dc2-a2c3-3eb389bb1e95/ilal_a_1022770_f0004_b.gif)
The expert panel concluded that healthcare professionals should advise patients and caregivers that diarrhea of any grade while taking idelalisib is a reportable symptom. The panel agreed that patients should call their doctor at the first sign of diarrhea, stay well hydrated and implement recommendations for diet modification ().
Table III. Diet modification recommendations for patients with idelalisib treatment-emergent diarrhea [Citation21,Citation22].
Transaminitis
Serious, including fatal, hepatotoxicity has occurred in patients treated with idelalisib. Elevations in ALT or AST > 5 × the upper limit of normal (ULN) have been observed, usually occurring within the first 12 weeks of treatment. Most transaminase elevations were reversible with dose interruption for idelalisib-treated patients [Citation2].
Across idelalisib clinical trials, serious hepatotoxicity occurred in 109/760 (14%) patients, with one fatality reported (1/1192) in a patient who was receiving idelalisib in combination with ofatumumab in an ongoing phase 3 clinical trial [Citation13]. The cause of death was determined to be acute liver failure and, at the time of death, the patient also had grade 4 sepsis [Citation13]. Most (74%) patients with ALT/AST elevation resumed idelalisib treatment at a lower dose without recurrence; however, 26% of patients had a recurrence of ALT and AST elevation despite the lower dose [Citation2].
In patients with relapsed CLL treated with idelalisib plus rituximab (n = 110) in the phase 3 clinical trial, any grade ALT and AST increase occurred in 35% (placebo plus rituximab, 10%) and 25% (placebo plus rituximab, 14%) of patients, respectively; grade 3–4 elevations occurred in 8% (placebo plus rituximab, 1%) and 5% (placebo plus rituximab, 0%). Hepatotoxicity was one of the most common AEs that led to idelalisib dose reduction and treatment discontinuation. In patients with iNHL taking idelalisib 150 mg monotherapy (n = 146), any grade ALT increase occurred in 50% of patients, grade 3 in 14% and grade 4 in 5%; any grade AST increase occurred in 41% of patients, grade 3 in 8% and grade 4 in 4%. Elevations in transaminases were one of the most common causes of treatment interruption or discontinuation (10% of patients) [Citation2].
Although the expert panel did not discuss management of transaminitis in patients taking idelalisib, the US prescribing information states that idelalisib should not be used concomitantly with other hepatotoxic drugs. Further, it recommends monitoring ALT and AST every 2 weeks for the first 3 months of treatment, every 4 weeks for the next 3 months of treatment and then every 1–3 months thereafter. The frequency of monitoring should be weekly in any instance of ALT or AST > 3 × ULN until resolved. For ALT/AST elevation > 3–5 × ULN, the US prescribing information recommends maintaining the idelalisib dose and monitoring at least once weekly until levels are ≤ 1 × ULN. For cases of ALT/AST > 5–20 × ULN, it is recommended to withhold idelalisib and monitor ALT/AST at least once weekly until levels are ≤ 1 × ULN; once this occurs, idelalisib may be resumed at a reduced dose of 100 mg BID. If ALT/AST elevation > 20 × ULN occurs, idelalisib should be permanently discontinued [Citation2].
Pneumonitis
Pneumonitis, including fatal cases, has occurred in patients treated with idelalisib [Citation2]. Across clinical trials, pneumonitis occurred in 24/760 (3%) idelalisib-treated patients and of these, 19 cases were reported as serious AEs. Fatalities due to pneumonitis were reported in 3/760 patients (< 0.5%) [Citation13]. Two deaths occurred in elderly patients with previously untreated CLL who received idelalisib concurrently with rituximab. An autopsy in one patient revealed findings consistent with late changes of acute respiratory distress syndrome for which a pulmonary hypersensitivity to idelalisib and/or rituximab was a reasonable possibility. The third fatality occurred in a 52-year-old patient who received idelalisib monotherapy for follicular lymphoma and had been previously exposed to rituximab and bendamustine for 17 months prior to idelalisib exposure. Lung wedge biopsy revealed changes consistent with hypersensitivity pneumonitis [Citation13].
In a phase 3 study of idelalisib plus rituximab in patients with CLL, pneumonitis occurred in 4% (4/110) of patients (placebo plus rituximab, 1/110 [1%]); no grade ≥ 3 pneumonitis was reported in either group [Citation12]. In the phase 2 iNHL study, 3/125 (2%) patients developed serious pneumonitis [Citation11].
The expert panel did not discuss management of pneumonitis; however, the US prescribing information recommends that any patient taking idelalisib who presents with pulmonary symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on a radiologic examination or a decline in oxygen saturation by > 5% should be evaluated for pneumonitis. If pneumonitis is suspected, idelalisib treatment should be interrupted until the cause is determined. Idelalisib should be discontinued with any severity of symptomatic pneumonitis [Citation2]. Appropriate and extensive evaluations should be performed for infectious etiologies of pneumonitis, including testing for Pneumocystis jirovecii pneumonia in patients with CLL. In clinical trials, some patients have been treated with corticosteroids in addition to continuing antibiotics if their pneumonitis had not improved.
Currently there are no data to define the mechanism of idelalisib-related pneumonitis; however, reports of pneumonitis with idelalisib use are consistent with the drug-induced pneumonitis associated with mTOR inhibitors (e.g. everolimus, sirolimus and temsirolimus) and therefore warrant consideration. Although the mechanisms underlying development of drug-induced pneumonitis in patients taking mTOR inhibitors are not fully understood, hypotheses include immune-mediated, host-mediated and dose- dependent processes. An expert panel of nephrologists, urologists, medical oncologists and pulmonologists was convened in 2012 to discuss the management of pneumonitis associated with mTOR inhibition. Primary recommendations included education of patients and healthcare professionals for prompt recognition and proper management of this AE. The panel advised that mTOR therapy should be initiated with caution in patients with pre-existing interstitial lung disease, significant pulmonary fibrosis or severe chronic obstructive pulmonary disease because a poorer prognosis for drug-induced pneumonitis would be expected in these patients. After diagnosis, management of drug-induced pneumonitis should consist of grading the toxicity, establishing a monitoring schedule, reconsidering the dose of the mTOR inhibitor (or interrupting or discontinuing therapy), utilizing other treatments as warranted and determining the need for referral [Citation17]. Although the recommendations of this expert panel cannot be formally applied to patients with idelalisib-associated pneumonitis, they provide a framework for future considerations.
Conclusion
Idelalisib has recently been approved by the US Food and Drug Administration and European Commission. In clinical trials, it has demonstrated significant efficacy and an acceptable safety profile in indolent B-cell malignancies. Clinically, the selectivity of idelalisib for the δ isoform has the potential to reduce off-target effects compared with non-selective PI3K inhibitors [Citation18,Citation19].
Diarrhea or colitis, transaminitis and pneumonitis are three clinically significant idelalisib treatment-emergent AEs that warrant further investigation. An expert panel of hematologists concluded that any grade diarrhea while taking idelalisib is a reportable symptom and idelalisib-related, late-onset, grade 2 diarrhea (including borderline grade 3 diarrhea) should be managed in a similar manner to grade ≥ 3. The US prescribing information recommends that idelalisib should not be used concomitantly with other hepatotoxic drugs and serum transaminase levels should be evaluated at regular intervals during idelalisib treatment to identify signs of hepatotoxicity. Any patient presenting with pulmonary symptoms should be evaluated for pneumonitis.
It is worth noting that a number of drug interactions have been associated with idelalisib. Coadministration of idelalisib with the strong cytochrome P4503A (CYP3A) inducer, rifampin, decreased the area-under-the-curve (AUC) and peak concentration (Cmax) of idelalisib. Moreover, coadministration of idelalisib with the sensitive CYP3A substrate, midazolam, increased the AUC and Cmax of midazolam [Citation2,Citation20]. Thus, idelalisib should not be coadministered with CYP3A substrates or strong CYP3A inducers [Citation2].
Overall, although the US prescribing information carries black box warnings for fatal and/or severe diarrhea or colitis, hepatotoxicity and pneumonitis, their risk and severity have the potential to be mitigated through proper identification and management.
ilal_a_1022770_sm1604.zip
Download Zip (16.1 MB)Acknowledgements
Gilead Sciences, Inc. sponsored this research, funded article processing charges and provided funding for professional medical writing and editorial support by Jessica Holzhauer, DVM, of C4 MedSolutions, LLC (Yardley, PA), a CHC Group company.
Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117:591–594.
- ZYDELIG (idelalisib tablets). Full prescribing information, Gilead Sciences, Inc., Foster City, CA, 2014.
- ZYDELIG (idelalisib tablets). Summary of product characteristics, Gilead Sciences International Ltd, Cambridge, UK, 2014.
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007;129:1261–1274.
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 2003;3:317–330.
- Chantry D, Vojtek A, Kashishian A, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem 1997;272:19236–19241.
- Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011;118:3603–3612.
- Jou ST, Carpino N, Takahashi Y, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol 2002;22:8580–8591.
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88–92.
- Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011;117:563–574.
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008–1018.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370: 997–1007.
- Data on file. Gilead Sciences, Inc., Foster City, CA, 2014.
- Flinn I, Oki Y, Patel M, et al. A phase 1 evaluation of duvelisib (IPI-145), a PI3K-δ,γ inhibitor, in patients with relapsed/refractory iNHL. Blood 2014;124(Suppl. 1): Abstract 802.
- Uno JK, Rao KN, Matsuoka K, et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide-3 kinase subunit p110delta. Gastroenterology 2010;139:1642–1653, 1653.e1–6.
- Benson AB 3rd, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 2004;22:2918–2926.
- Duran I, Goebell PJ, Papazisis K, et al. Drug-induced pneumonitis in cancer patients treated with mTOR inhibitors: management and insights into possible mechanisms. Expert Opin Drug Saf 2014;13:361–372.
- Byrd JC, Woyach JA, Johnson AJ. Translating PI3K-delta inhibitors to the clinic in chronic lymphocytic leukemia: the story of CAL-101 (GS1101). Am Soc Clin Oncol Educ Book 2012;32:691–694.
- Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 2010;116:2078–2088.
- Jin F, Robeson M, Zhou H, et al. Drug interaction profile of idelalisib and its major metabolit, GS-563117. J Clin Oncol 2014; 32(Suppl.): Abstract 2593.
- American Cancer Society. Caring for the patient with cancer at home: a guide for patients and families. 2013. Available from: www.cancer.org/acs/groups/cid/documents/webcontent/002818-pdf.pdf
- National Cancer Institute. Managing chemotherapy side effects: diarrhea. 2012. Available from: www.cancer.gov/cancertopics/coping/chemo-side-effects/diarrhea.pdf
- Entocort® EC (budesonide). Full prescribing information, AstraZeneca LP, Wilmington, DE, 2011.

