Abstract
Fatty acids have been widely used as adjuvant, vehicles in drug delivery viz penetration enhancers in topical delivery and in polymeric micelles to provide sustained release. However, the present investigation aims at exploring the potential of fatty acid vesicles for the topical delivery of fluconazole. Vesicles were prepared by film hydration method using oleic acid as a fatty acid principal component. Developed vesicles were characterized for size, size distribution, shape, in vitro release, pH dependent and storage stability, skin irritation study, and ex-vivo skin permeation. Penetration behavior of vesicles was further evaluated and elucidated using confocal microscopic study. Optical microscopy and TEM studies confirmed vesicular dispersion of fatty acid. The vesicles possessed higher entrapment efficiency (44.11%) with optimum vesicle size and homogeneity in regard to size distribution (PDI = 0.234 ± 0.016) at 7:3 oleic acid-to-fluconazole ratio. In vitro drug release study suggested sustained release of drug from the vesicles. The release pattern followed Higuchian kinetics. The vesicles were fairly stable at refrigerated conditions. Ex-vivo skin permeation and confocal microscopic studies suggested that oleic acid vesicles penetrate the stratum corneum and retain the drug accumulated in the epidermal part of the skin. On the basis of sustained release behavior and skin retention it can be inferred that oleic acid vesicles can serve as a potential carrier for the topical localized delivery of bioactives.
Introduction
Skin provides a physical protective barrier to the body against the external environment. The major barrier function is imposed by the upper most layers, consisting of corneocytes surrounded by lipid regions, i.e. stratum corneum. Diseased skin is distinguished and characterized by increased permeability/reduced barrier function or altered lipid composition and organization of the stratum corneum (CitationBouwstra et al., 2003). In the conditions where skin barrier property is not altered, low drugs diffusion across the stratum corneum is the major obstacle for topical delivery. Research in the field of penetration enhancement has presented a number of penetration enhancers which could be successful in effective topical delivery of drug(s) (CitationVarvova et al., 2005; CitationKaushik et al., 2008). The localized topical treatment requires the formulation to be biocompatible; it should serve as a local drug reservoir, and reduce systemic side-effects by decreasing the systemic absorption of drug(s). In treating skin diseases, the primary purpose of applying drugs to the skin is to induce local effects at or close to the site of application. Indeed, cutaneous absorption is desirable and preferred rather than percutaneous absorption. The conventional formulations which include creams, gels, and ointments suffer from the predicament of dermatopharmacotherapy, i.e. limited local activity. To enhance the penetration of bioactive moiety into the skin, and further to localize the drug at the site of action; a number of approaches have been explored including development of vesicular systems such as liposomes and niosomes (CitationSharma et al., 1994; CitationSingh & Vyas, 1996; CitationAgarwal et al., 2001; CitationBoinpally et al., 2003; CitationBhatia et al., 2004; CitationHoneywell-Nguyen & Bouwstra, 2005; CitationBouwstra & Ponec, 2006; CitationSinico & Fadda, 2009). They not only act as penetration enhancers, but simultaneously serve as a depot for the slow and controlled release of dermally applied active substance. In this investigation fatty acid based vesicles were studied for their potential as an alternative carrier system for the topical delivery of bioactives.
It has been documented and reported that unsaturated fatty acids such as oleic acid and linoleic acid have a tendency to form vesicles in the aqueous environment (CitationGebicki & Hicks, 1973). After about a decade saturated fatty acids with carbon atoms in the range of 8–12 were also found to under self-assemblage into vesicles in a pH dependent manner (CitationHargreaves & Deamer, 1978). Fatty acids being highly soluble tend to partition into artificial as well as natural membranes quite rapidly (CitationRobinson et al., 2006). It has also been investigated that fatty acid vesicles enhance the absorption of therapeutic molecules through the GIT, probably by forming mixed micelles or through chylomicron(s), thus increasing the bioavailability of the molecules (CitationMurakami, 1996). It has been reported that free fatty acids act as penetration enhancers for the bioactives through the stratum corneum (CitationNaik et al., 1995). However, skin permeation property of fatty acids varies with the chain length and branching. The penetration enhancement effect of fatty acid increases with an increase in the chain length; however, it follows the relation only up to C18. The skin permeation property of unsaturated fatty acids is higher than the corresponding saturated fatty acid. Further, fatty acid(s) containing Cis double bond exhibited higher penetration potential as compared to Trans form (CitationAungst, 1989; CitationKanikkannan et al., 2000).
The major limiting property of free fatty acids in their use as a penetration enhancer is their skin irritation characteristics. The problem of skin irritation, however, could be addressed by using fatty acid vesicles as drug bearing carriers. It has been shown that bilayer membrane possesses a fusogenic tendency due to its capability to lower the phase transition temperature of the lipids in the biological membrane. The vesicular membrane fuses with skin lipid bilayers, releasing its contents. Thus, it is hypothesized that fatty acid vesicles will act as a suitable carrier to enhance the penetration of bioactive agents through the stratum corneum with reduced toxicity. Moreover, fatty acid vesicles seem advantageous as they are easy to prepare as well as cost effective. The present study involves the use of oleic acid vesicles to encapsulate fluconazole, and evaluates its potential as an alternative drug delivery system for effective topical application.
Materials and methods
Fluconazole was obtained as a gift sample from G. D. Laboraories (Nohar, India). Oleic acid was purchased from CDH (New Delhi, India). Sephadex G-50 and Dialysis membrane were purchased from Sigma chemicals (Saint Louis, USA). Carbopol 940 was purchased from Himedia (Mumbai, India). All other solvents used were of analytical grade, unless otherwise mentioned, and purchased from CDH.
Preparation of fatty acid vesicles
Oleic acid vesicles were prepared by film hydration method, as reported earlier by CitationMurakami (1996) with slight modification. Briefly, oleic acid and fluconazole were dissolved in methanol in a round bottom flask followed by evaporation of solvent under vacuum using a rotary evaporator (Roteva Equitron, Mumbai, India) (600 mm Hg, 100 rpm) to remove even the last traces of organic solvent. The completely dried film in rota evaporator was left overnight for the removal of any possible traces of methanol and also to prevent the formation of emulsion due to the residual organic solvent. The dried film formed was then hydrated at ambient temperature for 1 h with alkaline borate buffer (pH 8.5). The prepared vesicular dispersion was sonicated to form the uniform size vesicular dispersion. Optimization was performed by varying the ratios of oleic acid and fluconazole (). Unentrapped drug was separated from the vesicle dispersion by using gel chromatography on oleic acid vesicle pre-saturated Sephadex G-50 minicolumn using borate buffer as eluant. The encapsulation efficiency was determined by disrupting the eluted vesicles using sodium hydroxide solution (1 M) and subsequence estimation of released fluconazole, as entrapped drug.
Table 1. Size and entrapment efficiency of the prepared oleic acid vesicles.
Vesicle characterization
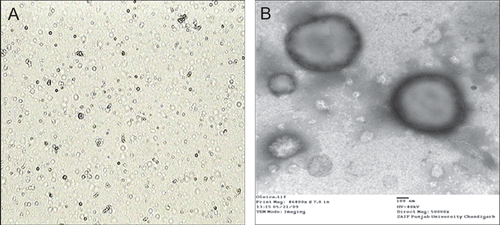
The vesicles were characterized for size and polydispersity index by a dynamic light scattering method (Zetasizer nanoZS, Malvern Instruments Ltd, Worcestershire, UK). Further, their shape and morphology were examined by using a transmission electron microscope (TEM) (Hitachi, Japan) as well as an optical microscope (Motic microscope, Korea). For TEM microscopy, a drop of vesicle dispersion was placed on a carbon-coated grid stained with 1% phosphotungstic acid for contrast enhancement. The samples were examined under a transmission electron microscope at an accelerating voltage of 80 kV and 50,000× magnification (CitationNasr et al., 2008) and photographed.
In-vitro drug release studies
In-vitro drug release study was conducted to evaluate the release rate/kinetics of drug from the fatty acid vesicles. The study was carried out using Franz diffusion cells by following the procedure reported by CitationGillet et al. (2009) with slight modification. Franz diffusion cell consists of two compartments, i.e. donor and receptor compartment, with a polycarbonate membrane (pore size: 50 nm) clamped between these two compartments. The donor compartment contained 1 ml of oleic acid vesicles dispersion, whereas the receptor compartment contained phosphate buffered saline (PBS), pH 7.4, which was stirred with the help of a magnetic stirrer at a constant speed while temperature was maintained at 37°C. Aliquots of samples were withdrawn at specified time intervals and replaced with equal volumes of fresh PBS (pH 7.4). The studies were performed in triplicate.
pH dependent stability
The effect of pH on the stability and on the drug release behavior was monitored by incubating optimized vesicular dispersion with buffers of pH 8.5, 7.4, 6.5, and 5.5. At pre-determined time intervals the samples were withdrawn and centrifuged at 14,000 rpm for 30 min (CitationSingh, 1993). The supernatant was analyzed for released free drug. The amount of drug leached was then calculated by the following formula:
Simultaneously, the incubated vesicles were observed for any change in morphology and size using an optical microscope. The studies were performed in triplicate.
Preparation of formulations for skin permeation study
Oleic acid vesicle dispersion
The oleic acid vesicle dispersion was prepared as described previously. To ensure dermal compatibility on application, the pH of the dispersion was reduced from 8.5 to 7.4 by using 0.1 N HCl. The unentrapped drug was separated from the formulation by size exclusion gel column chromatography using Sephadex G-50 in a mini-column.
Preparation of plain fluconazole gel
Fluconazole containing Carbopol gel was prepared by the method reported by CitationZao et al. (2007). Briefly, 1% w/v of Carbopol 940 was dispersed into purified water with the help of a vortex shaker (Tarsons, Kolkata, India) and allowed to hydrate for 4–5 h. The pH value of the gel was adjusted to 7.4 using triethanolamine. During preparation of the gel, the solution was agitated slowly to avoid any air entrapment. Plain drug gel was prepared by using an equivalent amount of fluconazole solution into the previously made carbopol gel in a 2:1 ratio under gentle mechanical mixing for 5 min.
Skin permeation study
The ex-vivo skin permeation study was conducted using vertical Franz diffusion cells with a diffusion area of 3.3 cm2 and volume 60 ml, as reported by CitationEl laithy and El-Shaboury (2002) with slight modification. The full thickness hairless skin of the dorsal side of the rat was sandwiched between donor and receptor compartments. PBS 7.4 was used as dissolution media and temperature was maintained at 37°C while it was continuously stirred at 300 rpm. Optimized oleic acid vesicle formulation and plain drug gel (equivalent to 6.12 mg of fluconazole) were placed in the donor chamber and samples were withdrawn periodically up to 24 h from the receptor compartment while following each sampling the withdrawn volume was replaced with equal volume of PBS (pH 7.4) in order to maintain the volume and sink condition. Withdrawn samples were analyzed by HPLC method and studies were performed in triplicate.
Similarly, the amount of drug retained within the skin was determined by taking out the skin from the diffusion cell and extracting the drug using a mobile phase. It was stirred for 12 h and then centrifuged at 5000 rpm for 20 min (CitationBhatia et al., 2004; CitationAzeem et al., 2008). The supernatant was filtered through a 0.4 µ membrane filter and analyzed for drug content by HPLC method, as reported earlier by CitationWattananat and Akarawut (2006).
In-vivo studies
All animal studies were performed according to the guidelines compiled by the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA, Ministry of Culture, Government of India). All the study protocols were approved by the animal ethical committee of the ISF College of Pharmacy, Moga (Punjab), India.
Confocal microscopy
Confocal laser scanning microscopy (CLSM) allows visualization of fluorescent probe in the skin specimen at different optical cross-sections without cryofixing. It also provides information about the localization of the fluorescent marker (CitationLópez-Pinto et al., 2005). For CLSM studies, Rhodamine B-loaded oleic acid vesicles dispersion was prepared using the same method as discussed previously. Unentrapped dye was removed by mini column centrifugation method (CitationMarly et al., 1998). The formulation was applied to dorsal skin of the rat. After 6 h the rat was sacrificed by cervical dislocation, the skin was excised, washed, and subcutaneous tissues were removed. The skin was washed with distilled water and preserved in 10% v/v formalin in PBS (pH7.4). The skin was vertically cross-sectioned into pieces of 1 mm2 size and evaluated for probe penetration. The full skin thickness was optically scanned at different increments through the z-axis of a CLS microscope. The excitation wavelength used was 540 nm, while a 625 nm long pass filter was used for emission (CitationMarly et al., 1998).
Skin irritation
The male albino guinea pigs were selected for skin irritation studies of different formulations as their skin is relatively sensitive. The animals were divided into five groups (n = 3) and formulations were topically applied, i.e. Saline solution 0.9% w/v (Group 1, Control), Oleic acid vesicle dispersion (Group 2), Plain oleic acid (Group 3), Plain drug gel (Group 4), and Standard irritant (0.8% v/v formalin) (Group 5) (CitationMutalik and Udupa 2004).
The formulations were uniformly applied over the shaven skin of guinea pigs. The skin was observed for any visible changes such as erythema (redness) or edema at 24, 48, and 72 h after the application of various formulations, and the primary irritation index was calculated (CitationEcobichon, 1997).
In-vivo tape stripping
The drug localization in different skin layers was determined by a reported method (CitationKim et al., 1997) with slight modification. Firstly, the animals were anesthetized with intraperitoneal injection of ketamine hydrochloride (100 mg/kg). Then 1 ml of each formulation (plain drug gel and oleic acid vesicle dispersion) was topically applied over the test area (3 × 3 cm2) on the dorsal skin (after hair removal) with an equivalent quantity of fluconazole.
Determination of amount of drug remaining on skin surface
After 1, 4, 6, and 8 h of application of formulation, the preparations remaining on the skin surface were recovered by rinsing of the skin surface twice with ethanol and water, respectively, using a cotton swab. The drug was extracted from the cotton by immersing the cotton in methanol overnight. The solution obtained was filtered through a membrane filter (0.45 µ). The drug content was finally analyzed by HPLC.
Determination of amount of drug retained in different skin layers
To determine the drug concentration in the SC, 20 tapes (CuDerm® Corporation, Adhesive Tape, Delhi, India) were applied with constant pressure for 2 min and carefully peeled away. The tapes were pooled in a tube containing 1 ml of PBS (pH 6.5) and 1 ml of methanol. Then the tubes were vortexed for 20 min, sonicated for 30 min, and vortexed again for 1 h to extract drug from the tapes. The stripped skin was surgically peeled, cut into small pieces, and homogenized with 5 mL of PBS for 2 min at 10,000 rpm. Then, the suspension was filtered through a membrane filter (0.45 µ) and centrifuged for 10 min at 5000 rpm. The supernatant was separated and the amount of drug was finally estimated by HPLC.
HPLC analysis of fluconazole
High-performance liquid chromatography (HPLC) analysis of fluconazole was performed according to the previously reported procedure (CitationWattananat & Akarawut, 2006) with minor modification. The HPLC system included a Water 515 pump and 2998 photodiode array detector with software empower data station. A thermohypers C-18 column (4.6 mm * 150 mm, 3 µm) was used. The mobile phase consisted of 30:70 (v/v) acetonitrile:10 mM acetate buffer pH 5.0 at a rate of 1.4 ml/min. The column eluant was monitored at 260.8 nm using a photodiode detector.
Storage stability studies
The purpose of the study is to determine the effect of storage at different temperature conditions on stability of oleic acid-based vesicles. Physical stability studies were conducted by monitoring the change in mean vesicle size and the leakage of encapsulated drug from oleic acid vesicles at different time intervals up to 30 days. The dispersions were placed in tightly sealed vials flushed with nitrogen gas and stored at 4 ± 1°C and ambient temperature (28 ± 1°C) (CitationNasr et al., 2008).
Statistical analysis
All the results are expressed as mean ± SD. Statistical analysis was carried out employing the Student’s t-test using Instat 2.1 software. The value of p < 0.01 was considered as significant.
Results
Fatty acid vesicle characterization
Multilamellar oleic acid vesicles were prepared by film hydration method by varying oleic acid-to-fluconazole ratios. Hydration was affected at ambient temperature for 1 h to enable complete hydration. The speed of rotation influences the thickness and uniformity of the film. The rotation speed of 100 rpm was observed to yield a uniform thin lipid film while lower and higher rate of rotation resulted in perceptible non-vesicular aggregated artifacts. The developed formulations were further characterized for size, PDI, and zeta potential studies (). The studies carried out demonstrated no significant difference in the entrapment efficiency; however the mean entrapment efficiency of the vesicles increased with an increase in the molar quantity of fluconazole up to 7:3 oleic acid-to-fluconazole ratio (44.11%). Beyond this ratio no further increase in drug entrapment was recorded. The vesicular sizes were obtained in the range of 500 nm to 1 μm. The oleic acid vesicles dispersions obtained were mostly polydisperse; however, at 7:3 oleic acid-to-fluconazole ratio the dispersity was recorded to be 0.234 ± 0.016. The photomicrographs depict the spherical nature of the oleic acid vesicles (). Further, in addition the TEM study conducted confirmed the ultrastructure of oleic acid vesicles which revealed multilamellarity of vesicles ().
Drug release studies
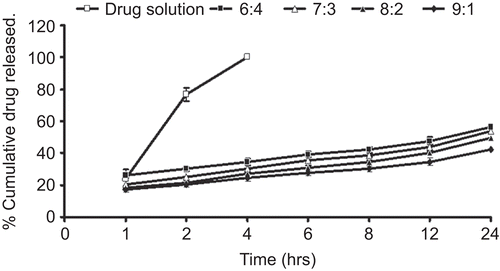
The study provided an insight on the release pattern of the drug from the vesicles. Release studies carried out with all the formulations of oleic acid vesicle dispersions have shown that almost 40–60% of the encapsulated drug was released from the vesicles within 24 h (). The kinetic analysis of the release data was carried out by plotting a graph between cumulative drug release vs square root of time. It suggested a linear correlation of drug release with time. The mathematical evaluation of order of release correlation regression coefficient (from the graph) revealed that the drug release followed Higuchi model > first order > zero order (). Furthermore, in order to evaluate the effect of drug release from vesicles, the release was compared with that of 1% fluconazole solution. It was observed that almost all of the drug was recovered in the acceptor compartment within 4 h.
Table 2. Order of drug release of various formulations determined by the regression coefficients.
pH-dependent stability
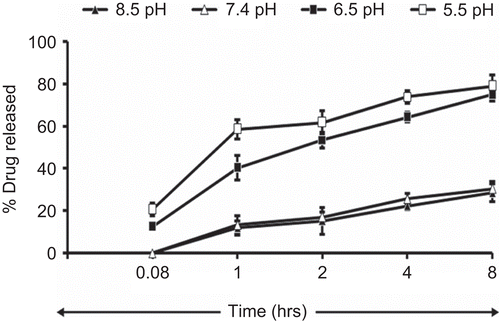
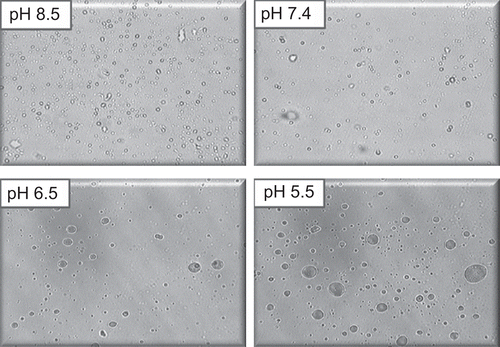
The study demonstrated the pH-dependent nature of the oleic acid vesicles. It also provided useful information on topical drug delivery potential and characteristics of oleic acid vesicles since the pH of skin is 5.5. It was observed that the release from vesicles is highly pH-dependent and on lowering the pH from 8.5 to 5.5, only 20% of the drug remained in vesicles to be released after 8 h of incubation in buffer of pH 5.5 as compared to residual drug estimated, i.e. 71% at pH 8.5 (). The differences in drug diffusion recorded at pH 8.5 and 7.4 were not significant (p > 0.01). Therefore, further studies were continued by adjusting the pH of vesicles suspension to 7.4, since higher pH values may cause skin irritation and may not be acceptable for topical application. Simultaneously, morphological changes in vesicles size and shape were also observed with changing pH. The results displayed an increase in the size of the vesicles at low pH values ().
Skin permeation study
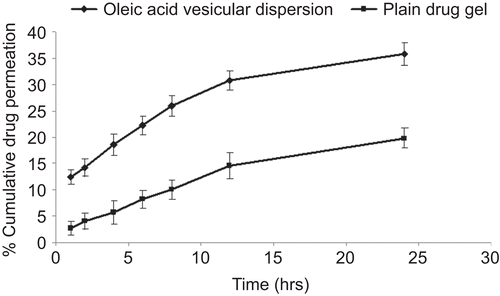
The skin permeation study was conducted on optimized formulation prepared at a 7:3 fatty acid:drug ratio (pH 7.4) (highest entrapment efficiency and more uniform sized vesicles). To normalize the effect of pH on skin permeation, the plain pH of drug gel was also adjusted to pH 7.4. A significant increase in the skin permeation of fluconazole was recorded from oleic acid vesicle dispersion in comparison to plain gel (). The amount of fluconazole permeated from the plain carbopol gel was 19.83%. The drug penetration following application of an equivalent amount of drug in vesicular dispersion was significantly high, i.e. 35.8%. The permeation parameters were calculated by plotting a curve between cumulative amounts of drug permeated per unit area (µg/cm2) vs time. The flux was obtained from the slope of the linear portion of the graph. The transdermal permeation rate constants obtained were higher for oleic acid vesicle dispersions (16 ± 1.5 µg/h/cm2) than the plain drug gel (4.5 ± 0.7 µg/h/cm2). It has also been observed that drug retained in the skin was more in the case of vesicular dispersion (18.95 ± 1.64%) as compared to plain drug gel (5.06 ± 0.84%) ().
Table 3. Skin permeation and retention of fluconazole from various formulations.
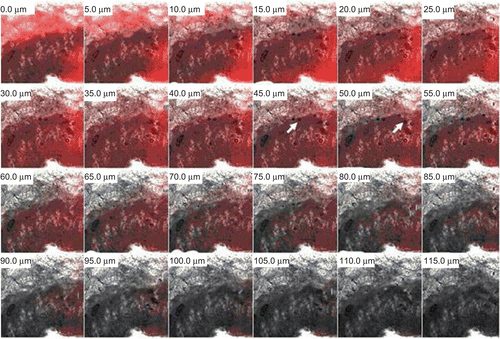
The CLSM study was also conducted to confirm the skin penetration of the fatty acid vesicles. The bright fluorescent intensity of oleic acid vesicles was detected in the stratum corneum and epidermis up to a thickness of 55–60 µm (). However, as the thickness of skin increases, the fluorescent intensity tends to decrease.
Figure 6. Confocal microscopic image of skin showing penetration behavior of oleic acid vesicles along the thickness of skin in vivo. It can be observed that the fluorescence intensity is detectable up to a thickness of 55–60 μm. In the upper part of the epidermis some intact vesicles were also observed.
Skin irritation studies
The oleic acid vesicle dispersions were tested for skin irritation as they have been reported to cause skin irritation owing to free acidic group present in the structure. The skin irritation test revealed negligible irritation scores in the case of oleic acid vesicle dispersion, while when the free oleic acid was applied it produced erythematic events, as shown by the primary irritation scores in . The effect of formulations of the guinea pig skin after 24, 48, and 72 h of application was also visualized. It was found that oleic acid vesicles demonstrated skin tolerance of fatty acids may be because active groups of acid engaged in self-assembly, forming a non-invasive continuous membrane in the form of vesicles.
Table 4. Skin irritation response scores to oleic acid vesicles (Er and Ed are erythemal and edemal irritation index, respectively).
Localization of drug in different skin layers
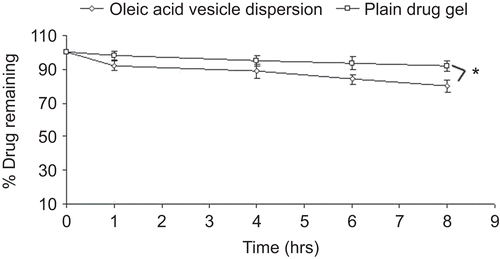
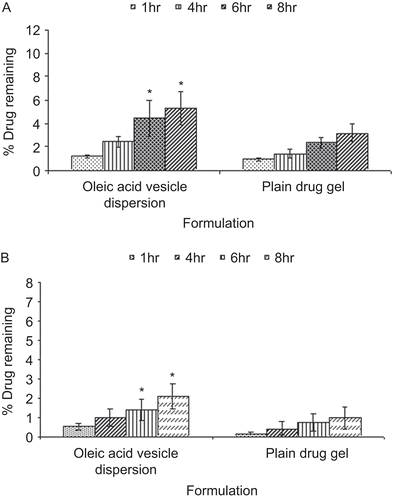
From the amount of fluconazole estimated remaining to be absorbed with time on application of the formulation () it is clear that the percentage of dose unabsorbed decreased with time in both the cases. The absorption of fluconazole was much less in the case of plain drug gel, as only 8% of the drug was absorbed in 8 h whereas in the case of oleic acid vesicle dispersion it was 14%. On comparing the amount of drug retained in SC and viable layers of the skin following the application of plain fluconazole and vesicular dispersion, it could be appreciated that the drug accumulated was 5.32% and 2.1% for oleic acid vesicle dispersion in SC and viable layers, respectively, while in the case of plain drug gel it was 3.2% and 0.98%, respectively (). Moreover, the accumulation of drug within the skin layers further increased with time in the case of both formulations. Nevertheless, the amount of drug accumulated following the application of plain drug gel was significantly low in both the layers of the skin as compared to vesicular formulation.
Stability studies
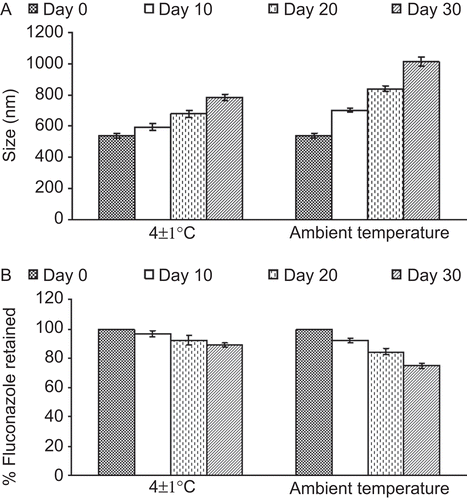
The stability studies demonstrated that the vesicles size increased significantly at ambient temperature after 30 days of storage, whereas when stored refrigerated the increase in size recorded was marginal (20% more than the initial size), as the size increased from 540 to 785 nm (). Further, the amount of drug retained in the vesicles was 88% (at 4°C), indicating better stability of the system. In contrast, ∼ 80% drug content was measured at ambient temperature ().
Discussion
Preparation of vesicles
The fatty acid vesicles were evaluated to assess their efficacy in delivering the bioactives to and through stratum corneum of the skin. The drug fluconazole was used as a model drug.
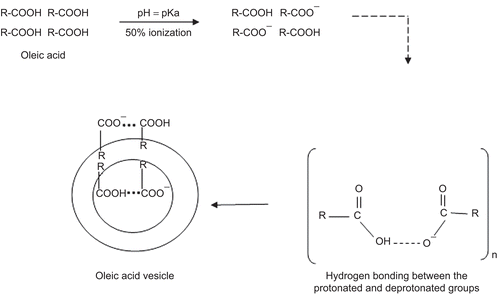
The major consideration in the formulation of fatty acid vesicles is pH of the formulation; this being a critical factor that controls the degree of ionization of fatty acid (CitationHargreaves & Deamer, 1978; CitationCistola et al., 1988) and is hence responsible for the formation of vesicle. The fatty acid (oleic acid) assembled into vesicles only if pH equals the pKa of the acid (8.5). At this pH, ∼ 50% of the carboxylic acid is ionized and transforms into ionized amphiphile(s) with a tendency to form vesicles/aggregates. The acid is present as ionic RCOO− as well as neutral RCOOH species. In such conditions each ionized group is stabilized through a strong hydrogen bond formed with the neutral molecules. The negative charge present on the ionized carboxylic group is shared between two adjacent fatty acid molecules, i.e. ionized and unionized, and thus results in the formation of typical dimers. The hydrophobic hydrocarbon chain of so formed dimers protects itself from the aqueous compartment and thus orients to form an enclosed bilayer structure that minimizes the interaction between the hydrocarbon chain and water. The ratio of protonated and deprotonated group seems also critical in the process of vesiculation. This is possible only if the concentration of the fatty acid in aqueous dispersion exceeds the critical vesicle concentration (cvc), which is reportedly 80 mM for oleic acid (CitationWalde et al., 2007). The stability of the vesicles is attributed to the strong hydrogen bond-based interactions between the protonated and deprotonated groups viz RCOO− … HOOCR (), as suggested and reported by CitationApel et al. (2002), CitationDowling et al. (2009), CitationGebicki and Hicks, (1973), and CitationLuisi et al. (1998).
Figure 10. Schematic presentation showing the mechanism of the fatty acid vesicles formation (R = hydrocarbon chain C18, n = cvc).
The effect of drug:oleic acid ration on the encapsulation of the fluconazole was studied. It was seen that the drug bearing capacity of the oleic acid vesicles depends upon the molar ratio of oleic acid and fluconazole. The entrapment efficiency increased up to oleic acid:drug molar ratio 7:3, beyond this ratio further increase in the amount of drug reduced the degree of drug encapsulation. This may be ascribed to the drug saturation in the bilayer domain. Further addition of drug could have destabilized the vesicle membrane leading to the leakage of content. Since the 7:3 vesicles showed the best physico-chemical characteristics (high homogeneity, high entrapment efficiency), the respective formulation was selected for further studies.
In-vitro performance of oleic acid vesicles
The values of regression coefficients confirmed the diffusion-dependent release of the drug; the drug release from oleic acid vesicles obeyed the CitationHiguchi (1962) model. By comparing the release from various oleic acid vesicles-based formulations (prepared using different oleic acid and fluconazole molar ratios), it was deduced that there was no considerable difference in the release in terms of kinetic pattern, although drug release exhibited a concentration-dependent behavior. These results are in agreement with the release kinetic reported by other research workers who documented that drug release from vesicles is affected by diffusion (CitationHathout et al., 2007; CitationNasr et al., 2008). Further, by comparing the drug release data of oleic acid vesicles with that of fluconazole solution it was concluded that the release of fluconazole from the vesicle was slow, controlled, and uniform as compared to fluconazole solution.
The pH-dependent stability behavior substantiates that drug diffusion across the skin may increase with a decrease in the pH of the vesicles dispersion. Thus, the increased diffusion of drug from the vesicles at low pH may have resulted due to decreased stability of the vesicles at lower pH. This further suggests that vesicles tend to fuse when they are exposed to low pH. This particularly holds for the pH that is lower than physiological pH. Moreover, the vesicles incubated in buffers of different pH were analyzed under an optical microscope. The optical images revealed that the size of the vesicles slumped with the drop in the pH, proving the pH-dependent fusogenic tendency of vesicles.
Ex-vivo and in-vivo assessment of drug accumulation and permeation
The oleic acid vesicles provided good skin permeation property as 36% of the fluconazole permeated across the skin. Comparatively lower permeation from the plain gel can be accounted for by the hydrophilic nature of fluconazole. Moreover, taken together the fusogenicity and lowering of phase transition temperature of SC lipids by oleic acid vesicles and free oleic acid, respectively, may be responsible for permeation enhancement of fluconazole (CitationNaik et al., 1995; CitationThewalt et al., 2006). The permeation parameters such as transdermal flux and the amount of drug retained calculated were higher in the case of oleic acid vesicles as compared to plain drug gel. Thus, fatty acid vesicles have adequate permeation and penetration enhancing property. They may be effective in the treatment of deep localized fungal infections. It has been observed that the permeation of fluconazole across the cellulose membrane was higher than its flux across the skin. This may probably be due to barrier function and restive ability of the skin.
The in-vivo studies suggest that a relatively small amount of drug was absorbed from the plain drug gel. The amount of drug absorbed was the sum total of adsorption as well as percutaneous absorption. Further, the localization or accumulation of drug was studied to reveal the pattern of drug absorption. A significantly high amount of drug was recovered from SC as well as viable tissue, although the amount accumulated and extracted from viable skin was low compared to SC. This may due to the intrinsic high affinity of fluconazole to the dermal part of the skin. It is reported that when administered orally, the drug is retained for longer period of time in the part of skin and elimination from SC is slower compared to plasma clearance (CitationFaergemann & Laufen, 1993; CitationWildfeuer et al. 1994). The prolonged skin retention of fluconazole has been ascribed to the higher affinity of fluconazole to the keratin (CitationKlimke & Schäfer-Korting, 1997). In the case of topical delivery of the drug the major barrier encountered is SC, which may successfully be circumvented by fatty acid vesicle. Thus, the oleic acid vesicle caused a significant increase in fluconazole accumulation in both the layers. This probably explains the deposit effect of drug when applied contained in fatty acid vesicles, since the amount of drug reaching the deeper layers was significantly low, indicating retarded diffusion of the drug into viable tissue. This study is in accordance with earlier reports (CitationWilliams, 2003). The drug accumulation in viable skin is due to percutaneous penetration and diffusion of the drug from the carrier to the deeper skin strata.
Moreover, intact vesicles were seen present in the epidermal part of the skin, which may contribute to vesicles/drug retention (accumulation) in deep layers of the epidermis. The oleic acid vesicles traverse the stratum corneum and on contact with sebum fluids (acidic in nature) fuse with the cells to form a depot within the skin (CitationPrestegard and Kantor, 1975). This is also confirmed by the CLSM study. Thus, it can be concluded from the images that fatty acid vesicles can penetrate intact, carrying along the entrapped drug through the stratum corneum and following the penetration may be retained/accumulated in the epidermal part of the skin. However, a low amount of drug reaches the deeper part of the skin following subsequent release and diffusion of drug. Therefore, oleic acid vesicles are seen to be potential carriers for localized delivery of bioactives on topical application and can provide an adequate local drug concentration without systemic absorption.
Safety profile of the formulation
The study was conducted to test the safety and skin irritation caused by formulations. The fatty acid vesicles caused negligible erythematic manifestations. On account of self-assembly the oleic acid in vesicles have reduced exposure of the acidic functions (-COOH) (the triggering factor for the erythematic events) (CitationYamaguchi et al., 2005) with the stratum corneum resulting in reduced erythematic episodes. In fact, the hydrogen bonding between the protonated and deprotonated groups reduces the polarity of the COO− responsible for irritation (CitationCreutz, 1981). It was found that oleic acid vesicles demonstrated skin tolerance, indicating their possible clinical potential in regard to patient acceptance and therapeutic efficacy.
Physical stability
The magnitude of drug retained within the vesicles ultimately governs the shelf-life of the formulation. The fatty acid-based vesicles formulations when stored at ambient and refrigerated conditions have shown that the vesicles are stable at 4 ± 1°C. This is because the vesicles sizes at this temperature remains stable and unchanged, thus the leakage from the vesicles was minimal. The increase in size indicates inter-vesicular fusion. At ambient temperature (28°C), the phase transition temperature of oleic acid is exceeded, hence they tend to fuse (CitationCreutz, 1981; CitationChoppin & Roos, 1985). The drug leakage studies carried out also suggested better stability of fatty vesicles at refrigerated conditions.
Conclusion
From the above preliminary studies it has been concluded that fatty acid vesicles can serve as potential carriers for the delivery of therapeutic molecules for the treatment of skin diseases. They are cost effective and therapeutically viable. Sustained release behavior and drug retention in the deeper part of skin might be beneficial for the long-term effects of drugs. The oleic acid vesicles seemingly fuse with the skin and release the contents. They are seen to penetrate intact and to form drug depots in the skin. The fatty acid in addition may serve as a penetration enhancer, thus by circumventing the stratum corneum barrier potential they may lead to better permeation of the drug molecules.
Acknowledgements
The authors acknowledge the help of Professor S. C. Lakhotia and Professor J. C. Roy (Department of Zoology, Banaras Hindu University (BHU), Varanasi, India) in confocal microscopy study. One of the authors, F. Zakir, wants to acknowledge the help of Mr Praveen Garg, Chairman ISF College of Pharmacy, Moga Punjab for providing excellent research facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agarwal, R., Katare, O.P., Vyas, S.P. (2001). Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 228:43–52.
- Apel, C.L., Deamer, D.W., Mautner, M.N. (2002). Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta. 1559:1–9.
- Aungst, B.J. (1989). Structure/effect studies of fatty acid isomers as skin penetration enhancers and skin irritants. Pharm Res. 6:244–55.
- Azeem, A., Jain, N., Iqbal, Z., Ahmad, F.J., Aqil, M., Talegaonkar, S. (2008). Feasibility of proniosomes-based transdermal delivery of frusemide:formulation optimization and pharmacotechnical evaluation. Pharm Dev Technol. 13:155–63.
- Bhatia, A.K., Kumar, R., Katare, O.P. (2004). Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J Pharm Pharm Sci. 7:252–9.
- Boinpally, R.R., Zhou, S.L., Poondru, S., Devraj, G., Jasti, G.R. (2003). Lecithin vesicles for topical delivery of diclofenac. Eur J Pharm Biopharm. 56:389–92.
- Bouwstra, J.A., Honeywell-Nguyen, P.L., Gooris, G.S., Ponec, M. (2003). Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 42:1–36.
- Bouwstra, J.A., Ponec, M. (2006). The skin barrier in healthy and diseased state. Biochim Biophys Acta. 1758:2080–95.
- Choppin, P.W., Roos, D.S. (1985). Biochemical studies on cell fusion. II Control of fusion response by lipid alteration. J Cell Biol. 101:1591–8.
- Cistola, D.P., Hamilton, J.A., Jackson, D., Small, D.M. (1988). Ionization and phase behaviour of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 27:1881–8.
- Creutz, C.E. (1981). Cis-unsaturated fatty acids induce fusion of chromaffin granules aggregated by synexin. J Cell Biol. 91:247–56.
- Dowling, M.B., Lee, J.H., Raghavan, S.R. (2009). pH-Responsive jello: gelatin gels containing fatty acid vesicles. Langmuir. 25:8519–25.
- Ecobichon, D.J. (1997). The basics of toxicity testing. New York: CRC.
- El laithy, H.M., El-Shaboury, K.M.F. (2002) The development of cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPS PharmSciTech. 3:1–9.
- Faergemann, J., Laufen, H. (1993). Levels of fluconazole in serum, stratum correum, epidemis-dermis (without stratum correum) and ecrine sweet. Clin Exp Dermatol. 18:102–6.
- Gebicki, J.K., Hicks, M. (1973). Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature. 243:232–4.
- Gillet, A., Grammenos, A., Compère, P., Evrard, B., Piel, G. (2009). Development of a new topical system: drug-in-cyclodextrin-in-deformable liposome. Int J Pharm. 380:174–80.
- Hargreaves, W.R., Deamer, D.W. (1978). Liposomes from ionic, single chain amphiphiles. Biochemistry. 17:3759–68.
- Hathout, R., Mansour, S., Mortada, N.D., Guinedi, A.S. (2007). Liposomes as ocular delivery system for acetzolamide: in vitro and in vivo studies. AAPS Pharm Sci Tech. 8:E1–2.
- Higuchi, W.I. (1962). Analysis of data on the medicament release from ointments. J Pharm Sci. 51:802–4.
- Honeywell-Nguyen, P.L., Bouwstra, J.A. (2005). Vesicles as a tool for transdermal and dermal testing. Drug Discov Today. 2:67–74.
- Kanikkannan, N., Kandimalla, K., Lamba, S.S., Singh, M. (2000). Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem. 7:593–608.
- Kaushik, D., Batheja, P., Kilfoyle, B., Rai, V., Michniak-Kohn, B. (2008). Percutaneous permeation modifiers: enhancement versus retardation. Expert Opin Drug Deliv. 5:517–29.
- Kim, M.K., Chung, S.K., Lee, M.H., Cho, A.K., Shim, C.K. (1997). Targeted and sustained delivery of hydrocortisone to normal and stratum corneum-removed skin without enhanced skin absorption using a liposome gel. J Contr Rel. 46:243–51.
- Klimke, K., Schäfer-Korting, M. (1997). Effect of keratin on the efficacy of fluconazole. Mycoses. 40:43–6.
- López-Pinto, J.M., González-Rodríguez, M.L., Rabasco, A.M. (2005). Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int J Pharm. 298:1–12.
- Luisi, P.L., Walde, P., Blocher, M., Blochliger, E. (1998). Matrix effect in the size distribution of fatty acid vesicles. J Phys Chem B. 102:10383–90.
- Marly, E.M.J., Kuijk-Meuwissen, V., Mougin, L., Junginger, H.E., Bouwstra, J.A. (1998). Application of vesicles to rat skin in vivo: a confocal laser scanning microscopy study. J Contr Rel. 56:189–96.
- Murakami, M.Y. (1996). Effect of oleic acid vesicles on intestinal absorption of carboxyfluorescein in rats. Pharm Res. 3:35–41.
- Mutalik, S., Udupa, N. (2004). Glibenclamide transdermal patches: physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J Pharm Sci. 93:1577–94.
- Naik, A., Pechtold, L., Potts, R.O., Guy, H.R. (1995). Mechanism of oleic acid-induced skin penetration enhancement. J Contr Rel. 35:299–306.
- Nasr, M., Mansoor, S., Mortada, M.D., Elshamy, A.A. (2008). Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul. 25:499–512.
- Prestegard, J.H., Kantor, H.L. (1975). Fusion of fatty acid containing lecithin vesicles. Biochemistry. 14:1790–5.
- Robinson, B.H., Walde, P., Rogerson, M.L., Bucak, S. (2006). Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf B Biointerfaces. 48:24–34.
- Sharma, B.B., Jain, S.K., Vyas, S.P. (1994). Topical liposomal system bearing local anesthetic lignocaine. J Microencapsul. 11:229–86.
- Singh, M.S. (1993). Preparation of liposomal fluconazole and their in-vitro antifungal activity. J Microencapsul. 10:229–36.
- Singh, R., Vyas, S.P. (1996). Topical liposomal system for localized and controlled drug delivery. J Dermatol Sci. 13:107–11.
- Sinico, C., Fadda, A.M. (2009). Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 6:813–25.
- Thewalt, J.L., Rowat, A.C., Kitson, N. (2006). Interactions of oleic acid and model stratum corneum membranes as seen by 2H NMR. Int J Pharm. 307:225–31.
- Vavrova, K., Zbytovska, J., Hrabalek, A. (2005). Amphiphilic transdermal permeation enhancers: structure-activity relationships. Curr Med Chem. 12:2273–91.
- Walde, P., Namani, T., Morigaki, K., Hauser, H. (2007). Formation and properties of fatty acid vesicles (liposomes). In: Gregoriadis, G., ed. Liposome technology. 3rd ed. New York: Informa Healthcare, 1–20.
- Wattananat, T., Akarawut, W. (2006). Validated HPLC method for the determination of fluconazole in human plasma. Biomed Chromatogr 20:1–3.
- Wildfeuer, A., Faergemann, J., Laufen, H., Pfaff, G., Zimmermann, T., Seidl, H.P., Lach, P. (1994). Bioavailability of fluconazole in the skin after oral medication. Mycoses. 37:127–30.
- Williams, A. (2003). Transdermal and topical drug delivery. 1st ed. London, UK: Pharmaceutical Press.
- Yamaguchi, Y., Nagasawa, T., Nakamura, N., Takenaga, M., Mizoguchi, M., Kawai, S., Mizushima, Y., Igarashi, R. (2005). Successful treatment ofphoto-damaged skin of nano-scale atRA particles using a novel transdermal delivery. J Contr Rel. 104:29–40.
- Zao, S.S., Du, Q., Cao, D.Y. (2007). Preparation of liposomal fluconazole gel and in vitro transdermal delivery. J Chin Pharm Sci. 16:116–8.