Abstract
Polysaccharide microparticles for the oral administration of gentamicin were designed in order to obtain an increased drug absorption by means of microparticle transport across the intestinal epithelia. Alginate/chitosan microparticles with a size of ∼ 2 μm were developed by spray-drying a water solution containing the drug complexed with the polyanionic alginate and subsequent alginate cross-linking process by calcium ions and chitosan. The pre-formulation study, performed by changing the concentration of both cross-linkers, led to the selection of the most suitable formulation which was assayed for its capacity to be translocated across intestinal epithelia, via both M cells contained in Follicle Associated Epithelium (FAE) of Peyer’s patches and enterocytes of the mucosal epithelium. An ex vivo perfusion technique of rabbit and rat intestinal tissues containing Peyer’s patches combined with an in vitro method by using Caco-2 cell monolayers demonstrated the microparticulate carrier ability to be taken up by both M cells and enterocytes. However, only the endocytosis by M cells appeared to provide the microparticle transport from the epithelium toward deeper sub-epithelial regions.
Introduction
Gentamicin (GM) is an aminoglycoside antibiotic widely used in the treatment of severe infections, caused by many Gram-negative and Gram-positive bacteria, such as meningitis, nephritis, and post-operative infections. Because of poor absorption following the oral administration, GM is commonly administered as injections, topical and ophthalmic dosage forms. The well known poor gastrointestinal membrane permeability and the consequent low bioavailability (class III of the biopharmaceutical classification system) are likely connected to the high polarity of this cationic compound. Various approaches have been investigated in order to increase GM oral bioavailability, including the co-administration of absorption-enhancing agents such as surfactants (CitationHu et al., 2001; CitationIto et al., 2005), bile salts and glucosteroids (CitationAxelrod et al., 1998), and liposaccharides (CitationRoss et al., 2004). Although good gastrointestinal absorption-enhancing effects were demonstrated, cytotoxicity and damage to the mucosa have been reported (CitationSwenson et al., 1994; CitationAungst, 2000; CitationRoss et al., 2004).
Another strategy aiming to promote GM oral bioavailability could involve the use of microparticulate carriers. In fact, inert particulate matter in the micrometer range have been shown to be taken up by the intestinal epithelia either at the level of Peyer’s patch (PP) follicle-associated epithelium (FAE) via micro-fold cells (M-cells), specialized cells acting over organized lymphoid tissue, or at the level of the mucosal epithelium through normal enterocytes (CitationMcClean et al., 1998; CitationMoyes et al., 2007). In any case, the uptake of particulate matter appeared to be mainly transcellular and size-dependent (CitationHussain et al., 2001; Citationdes Rieux et al., 2006).
Several studies dealing with micro- or nanoparticles intestinal uptake were focalized on PP and, consequently, on drug targeting to the Gut Associated Lymphoid Tissue (GALT). In fact, PP contain epithelial M cells, displaying marked phagocytic activity, macrophages on top of the follicle towards the gut lumen, a dense plexus of lymphatic microvessels, having a porous endothelium, and poor blood capillaries (CitationHussain et al., 2001). Microparticles able to be taken up selectively by FAE containing M cells could represent a good approach to achieve drug targeting into the lymph with advantages including avoidance of the first-pass metabolism as well as of the enzymatic degradation from enterocytes, directly delivering cytotoxic drugs in the treatment of several pathologies spreading in the lymph system (cancers, HIV). Moreover, drug delivery into the lymph is an attractive way for peptides and immunostimulating molecules for oral vaccination (Citationdes Rieux et al., 2006). However, some controversial data and opinions were reported on the effectiveness of particle translocation across M cells. In fact, M cells represent a very small proportion of the intestinal epithelium (5% of the human FAE, i.e. ∼ 1% of the total intestinal surface), and the proportion differs widely according to the experimental animal model. Otherwise, more than 80% of all small intestinal epithelia is occupied by enterocytes. Although they show low endocytic activity, there is evidence that particles can be translocated across enterocytes by means of macropinocytosis or fluid-phase pericytosis processes, delivering the drug into the blood circulation (CitationWin & Feng, 2005; Citationdes Rieux et al., 2006).
Calcium alginate (CaA) is a natural hydrogel known to be suitable for the oral delivery owing to its biocompatibility, bioadhesiveness, resistance to the gastric environment, and mild gelation conditions (CitationStockwell et al., 1986; CitationTønnesen & Karlsen, 2002). In the CaA complex, the calcium ions interact mainly with oligopolyguluronic sequences of the alginate, producing an ‘egg-box’ structure (CitationGrant et al., 1973). As alginates are negatively charged polymers, they form preferentially at the level of the mannuronic sequences complexes with cationic compounds (chlorpheniramine, doxorubicin, propranolol) (CitationStockwell et al., 1986; CitationSegi et al., 1989; CitationRajaonarivony et al., 1993), as well as with polycations (polysaccharides, polypeptides, or synthetic polymers) (CitationBystricky et al., 1990; CitationThu et al., 1996; CitationGombotz & Wee, 1998; CitationCoppi et al., 2004). An alginate with high mannuronic content was used in a previous work to design a biodegradable intra-operative system releasing gentamicin for bone infection treatment. The electrostatic interaction between the cationic gentamicin and the polyanion alginate led to a significant associated antibiotic fraction within calcium alginate implant offering a reservoir prolonging the release of the drug (CitationIannuccelli et al., 1996). However, the polymer network is sensitive to the pH, ionic strength, and ionic composition of the medium, swells, and disintegrates above pH 4.5 in the presence of calcium chelators (CitationGombotz & Wee, 1998; CitationTakka & Acarturk, 1999). Contrary to this, complexes between alginate and chitosan (CS), resulting from the electrostatic interaction between carboxylic groups of alginate, for which mannuronic residues of alginate chains are mainly involved, with the amino group of CS (CitationLertsutthiwong et al., 2009), do not dissolve in the presence of calcium chelators, so they have been extensively used to stabilize the gel in media simulating the intestinal environment and to reduce the porosity of the Ca-alginate network (CitationThu et al., 1996; CitationGombotz & Wee, 1998; CitationTakka & Acarturk, 1999). Moreover, CS has received considerable attention for its biocompatibility, mucoadhesiveness, and intestinal permeation enhancing properties for the paracellular route (CitationFelt et al., 1998; CitationSinha et al., 2004). The permeation enhancing property of CS was suggested to involve a combination of mucoadhesion, by increasing the contact time with the mucosa, and of a transient opening of epithelial cell tight junctions (CitationSilva et al., 2006), even if this hypothesis was ruled out for chitosan nanoparticles likely transported by adsorptive endocytosis (Citationdes Rieux et al., 2006).
Therefore, CaA/CS microparticles for the improvement of GM oral absorption were obtained by spray-drying technique and cross-linking procedures. The current paper involved, at first, a pre-formulation study focusing on the influence of the cross-linkers on microparticle morphological and dimensional properties, GM loading level, and release behavior in simulated gastrointestinal (GI) media. Then, a selected microparticle formulation was investigated for its suitability to be transported across either FAE via M cells or mucosal epithelium via enterocytes. An ex vivo model based on perfused rabbit and rat intestinal tissues was performed and combined with interferential and confocal microscopy to detect microparticle bioadhesion and translocation by M cells at Peyer’s patches, whereas an in vitro model by means of Caco-2 cell lines was adopted to evaluate microparticle uptake by enterocytes.
Materials and methods
Materials
Gentamicin sulfate (GM), chitosan (CS) (low Mw ∼ 70,000, acetylation degree ∼ 85–90%), and fluorescein isothiocyanate (FITC) were purchased from Fluka Chemie (Buchs, Switzerland). O-Phthaldialdehyde (OPA) was purchased from Sigma-Aldrich (Milan, Italy). Sodium alginate (NaA) (Mw ≅ 147,000), containing 62% mannuronic acid and 38% guluronic acid, was kindly donated by ISP Alginates (Girvan, UK). Culture reagents for intestinal Caco-2 cell lines were purchased from Euroclone-Celbio (Milan, Italy) and Hoechst 33258 stain from Hoeschst (Frankfurt, Germany). All the other chemicals were of analytical grade.
Microparticle preparation
Un-cross-linked microparticles were obtained by spray-drying (Buechi 190 Mini Spray-Dryer, Buechi Laboratoriums, Technik AG, Flavil, Switzerland) a 1.0% (w/v) NaA water solution containing GM (NaA/GM ratio = 10/1) under the following operating conditions: inlet temperature 140°C, outlet temperature 60°C, pump setting 15 ml/min, spray flow 500 Nlh−1, aspirator setting 10, and nozzle cup diameter 0.5 mm. Polymer and drug concentrations were chosen to obtain solutions with low viscosity and to avoid the precipitation of the insoluble alginate/GM complex.
Cross-linked microparticles were obtained by pouring 250 mg un-cross-linked microparticles into 25 ml aqueous calcium chloride solution at different concentrations (1.0, 1.5, and 2.5% w/v) under stirring at 8000 rpm by means of an Ultra-Turrax homogeniser (Ultra-Turrax, IKA, Labortechnik, Staufen, Germany) for 10 min.
Co-cross-linked microparticles have been prepared by stabilizing the cross-linked microparticles obtained with 1.0% calcium chloride solution by the treatment with a pH 5.2 acetic acid solution of CS (25 ml) at different concentrations (0.5, 1.0, and 2.0%) for 10 min.
Both the cross-linked and co-cross-linked microparticles were recovered by centrifugation (4000 rpm for 10 min), rinsed with distilled water, collected by centrifugation, and freeze-dried (Lyovac GT2, Leybold-Heraues GMBH, Köln, Germany). Microparticle formulation variables are listed in .
Table 1. Formulation variables and dimensional properties of the microparticles.
The formulation A1 was selected to prepare a fluorescent sample (A1F) to be used in the microparticle translocation studies. The sample A1F was prepared by incubating overnight a 100/1 water/acetone solution of 1/0.01 GM/FITC. The GM/FITC conjugate solution, mixed with NaA water solution in a 10/1/0.01 NaA/GM/FITC ratio, was spray-dried as described above. The obtained FITC-labeled microparticles were cross-linked with 1.0% CaCl2 and co-cross-linked with 0.5% CS according to the same technique described above.
Morphological and dimensional analysis
Morphological and dimensional analyses were performed using scanning electron microscopy (SEM, XL-40 Philips, Eindhoven, The Netherlands). Microparticle samples were placed on the aluminum stub using double-side adhesive tape, coated with a thin film of gold-palladium, and imaged at 8 kV.
Microparticle size and size distribution were determined by analyzing SEM photomicrographs by image processing software (IMG-VIEW, CIGS, University of Modena and Reggio Emila). All analyses were performed on at least 100 microparticles from different batches.
Calcium content determination
Calcium content was determined by suspending exactly weighted microparticle samples in water or dissolving them in 3% (w/v) sodium citrate aqueous solution acting as calcium ions chelating agent. After 24 h, the filtered solutions were analyzed by flame atomic absorption spectroscopy using a model 3030 spectrophotometer (Perkin-Elmer). The calibration graph was obtained by standard solutions ranging from 1–5 mg Ca2+/l.
The calcium values obtained by the sample solutions and suspensions represent the total calcium amount and the free (non-associated to alginate) calcium amount, respectively. The differences between these values were considered as the fraction associated to alginate. All data were averaged on three determinations.
EDX analysis
Microparticle samples, soaked in water for 24 h to remove the free calcium and dried, were analyzed for calcium X-ray emission by EDX (EDAX 9900, Edax International, Prairie View, IL) coupled to SEM. The peak intensities of Ca (3.69 keV) and Na (1.04 keV) were obtained with the following experimental settings: accelerating voltage 12 kV, detection limit ∼ 0.3%, and spatial resolution 0.1 μm. The reported data represent the average of six points detected on three images for each sample.
GM content determination
GM content was determined by suspending exactly weighted microparticle samples in water or dissolving them in 3% (w/v) sodium citrate aqueous solution displacing the drug associated to alginate. After 24 h, ∼ 2 ml were removed and centrifuged by using an ultrafiltration concentrator with a regenerated cellulose membrane (2 ml, MWCO 30,000 RC, Vivaspin, Vivascience AG, Hannover, Germany) in order to remove alginate. The concentration of GM derivatized by reaction with OPA (CitationAl-Amoud et al., 2002) was determined on an aliquot of 1 ml from the filtered solution by reversed-phase high performance liquid chromatography (HPLC). Chromatography was performed by an Agilent Technologies (Waldbronn, Germany) modular model 1100 system consisting of a vacuum degasser, a quaternary pump, an autosampler, a thermostatted column compartment, and variable wavelength detector. A Lichrospher RP-18 column (125 × 4 mm I.D., 5 μm, Agilent Technologies), with a Lichrospher RP-18 guard column (4 × 4 mm I.D., 5 μm, Agilent Technologies) was used at room temperature with a flow rate of 0.6 ml/min and a total running time of 12 min. The mobile phase consisted of methanol, glacial acetic acid, and water (800/20/180), containing 0.02 M sodium heptanesulfonic acid (pH 3.4). The detector monitored the eluent at 340 nm. Three injection volumes of 50 μl were used for each sample. Peaks were identified on the basis of their retention time values and UV spectra by comparison with that of the standard solution of GM-OPA derivative. Peak identity was also confirmed by spiking the samples with the pure standard (standard addition method). Quantification was performed by four-point external standard calibration, by plotting the peak area of the GM-OPA derivative solution at each level prepared vs the corresponding concentration.
GM fraction obtained by water extraction was considered as non-associated drug, whereas that obtained by dissolving the samples in sodium citrate solution as the total drug. The differences between these values were considered as the drug fraction associated to alginate. The percentage encapsulation efficiency (EE) was calculated by relating the practical drug entrapment to the theoretical one (9.09% w/w). The reported data were averaged on at least three determinations.
GM antimicrobial activity determination on microparticles
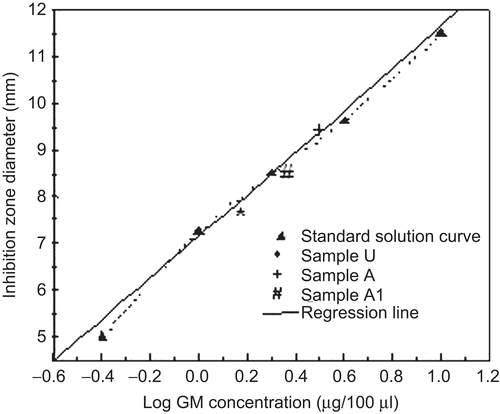
A microbiological agar well diffusion method was performed (CitationGiamarellou et al., 1975) on un-cross-linked (sample U), cross-linked (sample A), and co-cross-linked (sample A1) microparticles in order to investigate whether GM activity was maintained throughout the manufacture steps. Tryptic soy agar (15 ml) and Bacillus subtilis strain ATCC 6633 (105 CFU/ml) were used as growth medium and indicator micro-organism, respectively. The wells in agar plates were filled with microparticle solution (100 μl) obtained by dissolving an exactly weighted microparticle sample in 3% (w/v) sodium citrate aqueous solution and with standards of free GM in 3% (w/v) sodium citrate aqueous solution (100, 40, 20, 10, 4 μg/ml) in the same plate for each sample. The glass plates were incubated at 37°C overnight and the growth of bacteria colonies was monitored. Inhibition zone diameters produced by the microparticle samples containing a known GM amount were plotted on the calibration curve obtained by relating the zone inhibition diameter to GM concentration of standard solutions. The analyses were made in triplicate.
In vitro drug release study
The release of GM from cross-linked and co-cross-linked microparticles was carried out, under sink conditions and at 37°C, in HCl water solution at pH 3.0, which could be well-representative of a gastric average pH value, for the first 2 h and, subsequently, in 0.5 M Tris-HCl buffer solution at pH 7.4 for 3 h, simulating an intestinal pH medium not containing calcium binding ions.
A weighted amount of microparticles (100 mg) was suspended in a glass bottle containing 50 ml of the release media under stirring at 100 rpm. At fixed time intervals, 2 ml of solution were withdrawn, centrifuged at 4000 rpm for 10 min by using a centrifugal concentrator (MWCO 30,000 RC) and analyzed by reversed-phase HPLC after derivatization with OPA as described above. An equal volume of the same dissolution medium was replaced to maintain a constant volume. The experiments were performed in triplicate.
Fluorescence detection
Sample A1F was examined for FITC content by placing exactly weighted amounts of microparticles in 3% w/v sodium citrate aqueous solution. After centrifugation, FITC concentrations in supernatant were assayed spectrophotometrically (λ = 488 nm) (Lambda 35, Perkin-Elmer, Norwalk, CT). Microparticle capacity to maintain the fluorescence was evaluated on the sample A1F, exposed to the same conditions as those of the release assay, by optical videomicroscope coupled with epifluorescence using a FITC filter (N-400FL, Optika Microscopes, MAD Apparecchiature Scientifiche, Bergamo, Italy).
Evaluation of microparticle bioadhesion and uptake by M cells
An ex vivo study was performed on intestinal tracts of rabbits and rats containing Peyer’s patches which are easily observable as elongated or round thickenings of the intestinal epithelium measuring ∼ 1 cm in length. Tracts of the small intestine were excised from just sacrificed adult rabbits (white New Zealand, ∼ 2.5 kg) and rats (Sprague Dawley, ∼ 0.2 kg) in accordance with procedures adhering to the European Community Regulations (CEE Council 89/609) and maintained in oxygenated Tyrode at 37°C. The intestinal content was drained out, the lumen was cleaned with physiological solution, and the intestine was dissected to give 15 cm segments containing 4 or 5 PP, placed in oxygenated Tyrode at 37°C, connected with a peristaltic pump and perfused with a water suspension of the sample A1 and A1F (50 mg/25 ml) for rabbit and rat tissue, respectively, at the flow rate of 1 ml/min. After 30 min, microparticle suspension was removed, and the perfused tissue was longitudinally cut and washed with physiological solution.
For the mucoadhesion and uptake studies in rabbits, the Bouin’s-fixed gut, dehydrated with a progressive ethanol series, embedded within paraffin, was sectioned using a cryomicrotome (HM 500 OM, Micron, Walsdorf, Germany) giving ∼ 7 μm thick sections and viewed by interferential microscope (Axiophot, Zeiss, Oberkochen, Germany).
For the mucoadhesion study in rats, the intestinal segments were directly observed by fluorescence microscopy (DM IRE2, Leica Microsystems Heidelberg GmbH, Mannheim, Germany). For the M cell uptake, the segments were fixed in paraformaldehyde (3% w/v) for 2 h, embedded in cryostatic medium, sliced in 12 μm thick sections at −20°C by using a microtome cryostat (HM 500 O, Microm International GmbH, Walldorf, Germany), and viewed by fluorescence microscopy and confocal laser scanning microscopy (DM IRE2, Leica Microsystems Heidelberg GmbH) at 1.1 μm step. To examine the actual antibiotic translocation inside the PP and the role of the microparticles on the GM translocation, the rat perfused intestinal segments were evaluated by microbiological activity in comparison with untreated intestinal tissue from the same rat as well as with intestinal tissues perfused with microparticles suspended in pH 7.4 phosphate buffer solution at the same conditions described above. Two isolated PP and equivalent non-PP tissue were homogenated by a glass Potter homogenizer in 3% w/v sodium citrate aqueous solution (5 ml). After centrifugation, the supernatant was discarded and analyzed for microbiological activity according to the method described above for GM antimicrobial activity determination on microparticles.
Evaluation of microparticle uptake by Caco-2 cells
Caco-2 cells, deriving from human colonic adenocarcinoma and producing confluent monolayers of enterocyte-like cells, were cultured at 37°C in Dulbecco’s Modified Eagle’s Minimal Essential Medium (DMEM) with 10% Fetal Bovine Serum (FBS) and 1% non-essential aminoacids. Cells were seeded 48 h before the incubation with the microparticle suspension. The sample A1F, suspended in water (10 mg/5 ml) and diluted 1:1 in DMEM, was applied to the cell monolayer and then incubated at either 37°C for 3 h and 6 h or 4°C for 6 h. Cells were washed twice with Phosphate Buffer Saline (PBS) and then collected by trypsinization for the cytometry analysis. Flow-cytometry evaluation of intracellular uptake was performed by a Coulter Epics XL flow cytometer equipped with a 488 nm argon laser. FITC fluorescent cells were expressed as a percentage of the total cell population. Moreover, after 6 h of incubation at 37°C with the microparticles, the Caco-2 cells were fixed in paraformaldehyde (3%, w/v) for 15 min at room temperature, washed in PBS, and observed under filter set for both green and blue fluorescence by using confocal laser scanning microscopy (DM IRE2, Leica Microsystems, Heidelberg GMbH) at 1.1 μm step after cell nucleus staining by Hoechst 33258 stain (blue) (2 μg/ml) for 20 min at room temperature. To verify the behavior of FITC alone, FITC solutions in DMEM at the concentration corresponding to that present in the sample A1F (0.6 μg/ml) were incubated with Caco-2 cells at the same conditions used for the microparticles. The experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed by using one-way analysis of variance (ANOVA). Significance was indicated by p < 0.05.
Results and discussion
Pre-formulation study
Cross-linked and co-cross-linked alginate microparticles loaded with GM were developed by spray-drying technique and cross-linking by calcium ions and calcium ions/CS, respectively. Co-cross-linked microparticles were obtained by treating with CS the sample A selected among the cross-linked microparticles due to its higher drug encapsulation efficiency ().
Table 2. Calcium and GM content in microparticle samples.
Dimensional and morphological analysis
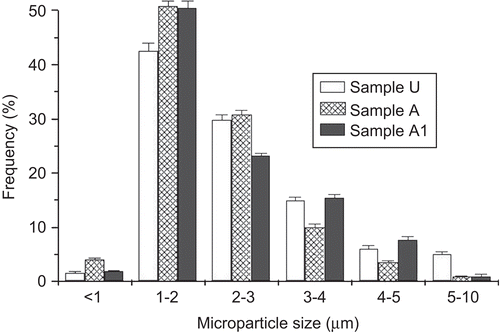
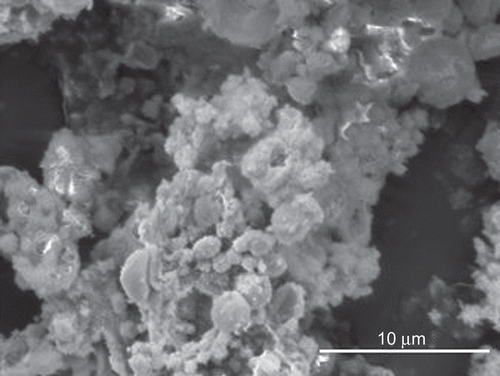
Morphological analysis by SEM showed distinct and well formed un-cross-linked microparticles (sample U) having a nearly spherical shape and smooth surface (). Both the cross-linking processes didn’t modify substantially microparticle morphology with the exception of the sample A3 containing aggregates and irregular shaped particles ().
Figure 1. SEM images of un-cross-linked (sample U) (a), cross-linked (sample A) (b), co-cross-linked (sample A1) (c), and co-cross-linked (sample A3) (d) microparticles.
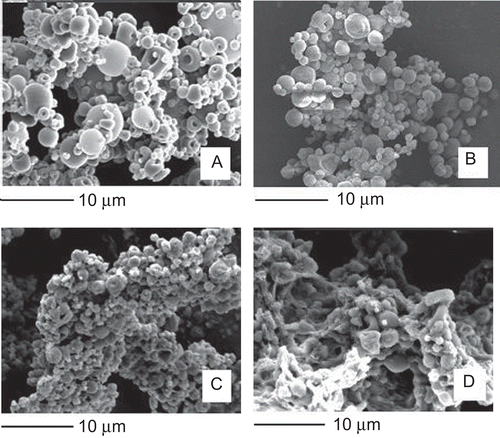
The mean size of the cross-linked and co-cross-linked microparticles was ∼ 2 μm (), with the most population (70–80%) less than 3 μm (). No significant difference (p > 0.05) in the mean size was found between the samples and the batches. The size of particulate matter is considered a critical parameter affecting greatly the translocation process across the intestinal epithelia. Although some discordant results, in general particles with a size up to 2 μm, have been reported to be taken up by enterocytes (CitationSnoeck et al., 2005), and particle sizes less than 3 μm are preferable to be taken up by M cells because particles in the range of 3–10 μm are retained in PP and do not migrate into the mesentheric lymph nodes (CitationFlorence, 1997).
Evaluation of calcium and chitosan complexation with alginate
To evaluate alginate cross-linking degree according to the formulation parameters, the content of calcium associated to the polyanionic alginate was determined by flame atomic absorption spectroscopy (). The calcium associated to the polymer increased with increasing CaCl2 concentration used in the preparation (p < 0.05) overcoming in all the samples the theoretical association value (1.1 mol/g polymer, corresponding to ∼ 4.2% calcium), calculated in a previous work for a complete neutralization of the guluronic blocks by calcium ions in a 1:2 calcium/uronic acid molar ratio (CitationIannuccelli et al., 1996; CitationMorris et al., 1978). Such an overcoming confirms the calcium ability to interact also with alginate mannuronic sequences. The cross-linking reaction by calcium would occur quite homogeneously among the microparticles in all the samples, as revealed by the quite similar values of calcium X-ray emission intensity from distinct microparticles (). The addition of CS as a secondary cross-linker provided a slight even if significant (p < 0.05) decrease in the associated calcium amount only in the sample A3. Since calcium ions bind the guluronic sequences strongly, in a highly cooperative way, a competition on the same carboxylic groups between calcium, weakly bound to alginate mannuronic residues, and the polycation CS at this highest concentration could be hypothesized. In any case, CS should be, presumably, localized in the outer layer of the microparticles owing to its low diffusivity through the alginate network, as reported by others (CitationTønnesen & Karlsen, 2002; CitationSarmento et al., 2007; CitationWittaya-Areekul et al., 2006).
Figure 3. EDX analysis coupled with SEM: relative sodium and calcium percentages calculated on element emission intensity from distinct microparticles. The image refers to the sample A1.
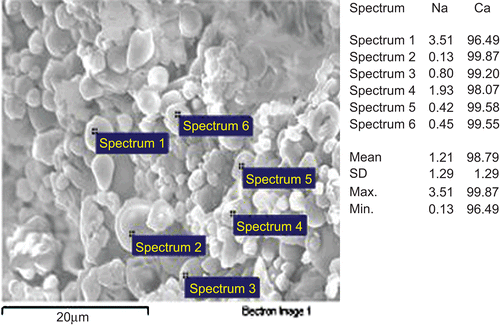
Statistical analysis showed no significant difference (p > 0.05) in calcium content between the batches.
Evaluation of GM content and complexation with alginate
Gentamicin is a cationic drug able to interact selectively with the mannuronic residues of the polyanionic alginate, as previously reported (CitationIannuccelli et al., 1996). Such an interaction could be potentially useful to improve the encapsulation efficiency of hydrophilic cationic drugs and to prevent the drug leaching into the gastrointestinal tract before the microparticle uptake by the intestinal epithelia. shows GM content values obtained by dissolving the samples in a sodium citrate solution containing drug binding anions. Since no drug fraction was extracted by water, the drug loaded in every sample could be considered as complexed with alginate. GM content in the un-cross-linked microparticles (EE = 93.18 ± 7.6%) corresponds to the association capacity of the drug on alginate (0.08 mmoles of gentamicin per gram of polymer, corresponding to ∼ 5.4% gentamicin), as demonstrated in a previous work (CitationIannuccelli et al., 1996). The cross-linking process by calcium ions reduced the amount of GM associated to the polymer and GM loading levels decreased progressively with the increase of calcium concentration (p < 0.05). These results indicate a competitive interaction of GM and calcium for the same carboxylic acid sites on alginate M blocks, confirming that calcium can also interact with the polymannuronic sequences. On the contrary, GM content in co-cross-linked microparticles was not affected by CS concentration (p > 0.05), suggesting the inability of the polycation to displace GM from alginate M residues. Statistical analysis showed no significant difference (p > 0.05) in drug loading levels between the batches.
Evaluation of GM antimicrobial activity
A microbiological assay was performed on un-cross-linked, cross-linked, and co-cross-linked microparticle samples in order to study the effect of the preparation steps on GM antibiotic activity. By comparing the inhibition zone provided by the microparticle samples with that produced by a standard solution of the drug, all the samples produced inhibition zone diameters corresponding to the own GM loading value, providing evidence of GM activity preservation during both spray-drying (sample U) and cross-linking procedures (samples A and A1) (). In fact, statistical analysis showed no significant difference (p > 0.05) in the inhibition diameters between the batches.
In vitro drug release study
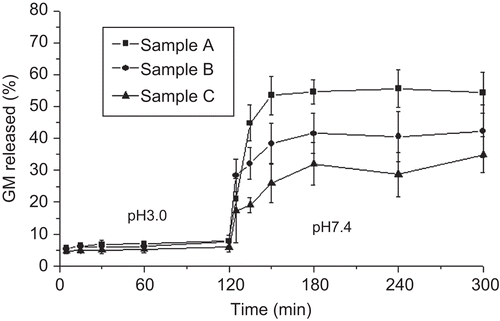
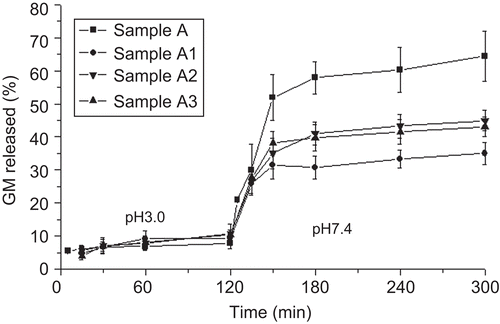
Since sodium alginate is water-soluble in its un-cross-linked state, GM release study was performed on cross-linked and co-cross-linked microparticles capable of maintaining their integrity in aqueous media, which is an essential requisite for microparticle transport across intestinal epithelia. GM in vitro release from cross-linked and co-cross-linked microparticles was evaluated in a pH 3.0 medium for 2 h and, then, in pH 7.4 Tris-HCl buffer for 4 h in order to simulate the pH conditions of the gastrointestinal tract (). By considering that phosphate buffer species, having a chelating action on calcium, are disconnected with the principal physiological species in the human GI tract (CitationDressman & Reppas, 2000; CitationSheng et al., 2009), Tris-HCl buffer was selected instead of phosphate buffer. The cross-linked microparticles provided a three-phase profile showing a negligible GM release at pH 3.0 and a burst effect (∼ 60%) followed by a gradual release phase at pH 7.4. This behavior is in agreement with previously reported data (CitationMumper et al., 1994) where it is assumed that, upon acid treatment, the hydrolysis of alginate takes place resulting in increased drug release at neutral pH. Unlike the acidic medium which did not provide any significant change in drug release (∼ 10% of GM loading) according to the preparative variables, in the neutral pH the burst effect became less relevant as the calcium concentration increased. Similarly to cross-linked microparticles, the co-cross-linked microparticles showed a triphasic profile, having a reduced GM burst release in the neutral pH (30–40% of GM loading) with a slight effect offered by the polycationic concentration increase (). However, the more strengthened microparticle structure provided by CS was not able to remove completely the burst effect in the neutral pH. At this pH value, chitosan has less free amine groups due to the increased deprotonation providing a less strong interaction with alginate (CitationBajpai & Tankhiwale, 2006).
Microparticle bioadhesion and translocation studies
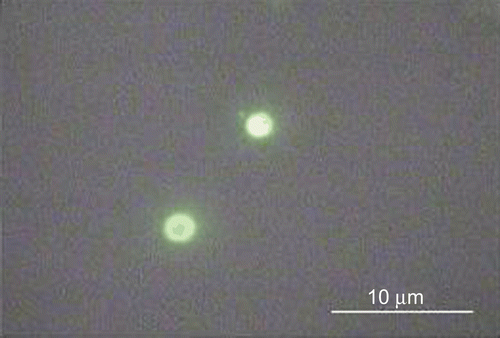
Microparticle capacity to adhere on the intestinal epithelia and to be translocated across the intestinal epithelia was evaluated on the samples A1 and A1F selected from the pre-formulation study owing to their highest ability to retain the antibiotic as shown by the in vitro release assay. The sample A1F, compared with the sample A1, showed similar properties. Moreover, it was still fluorescent upon contact with simulated GI media, as observed by epifluorescence videomicroscopy ().
Figure 7. Epifluorescence microscopy image of the sample A1F recovered from simulated gastrointestinal media.
The study involved an ex vivo method aiming to evaluate the behavior of the microparticles at the level of both FAE containing M cells and mucosal epithelium containing enterocytes as well as an in vitro method aiming to evaluate the behavior of microparticles placed in contact with enterocyte-like cells.
Evaluation of microparticle bioadhesion and uptake by FAE
The study of microparticle bioadhesion and translocation across FAE containing M cells involved two small animal models represented by rabbits and rats having a different proportion of the FAE occupied by M cells, ∼ 50% and 10%, respectively (CitationErmak et al., 1995). The identification of PP in intestinal tracts is a simple procedure since they can be seen by the naked eye as round thickened areas (), located in the lamina propria of the mucosa and extending into the submucosa of the ileum.
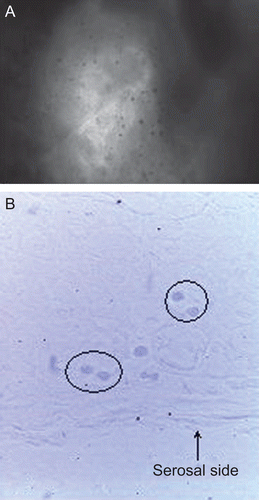
The rabbit perfused intestinal tissue was examined by an interferential microscope for bioadhesion and translocation of the sample A1F and compared with untreated intestinal segments from the same rabbit. Unlike the untreated intestinal segments, the images of perfused segments showed dark dots, reasonably ascribable to the microparticles, on the epithelium exposed to the gut lumen at the level of PP or not-PP tissue (). This finding demonstrates the microparticle’s ability to adhere not only with the mucosal epithelium but also with rabbit FAE containing 50% M cells which are devoid of mucus. By viewing sections of the perfused intestinal segments, spheroidal structures were observed throughout the PP, mainly in the serosal side (). These structures, which are not attributable to cellular components and absent in both the untreated tissue and in perifollicular areas, could be reasonably identified with the microparticles translocated from the intestinal lumen into PP sub-epithelial regions.
Figure 9. Interferential microscopy images of intestinal segments, including PP and perifollicular areas, from rabbit after perfusion treatment with microparticles: gut lumen surface (a), Bouin’s-fixed gut sections (spheroidal structures identified with microparticles are circled) (b). Original magnification ×40.
The rat perfused intestinal tissue was examined by fluorescence microscope for bioadhesion and translocation of the sample A1F and compared with untreated intestinal segments from the same rat. Unlike the untreated tissue, the perfused intestinal segments in correspondence of both PP and non-PP epithelium showed a dotty fluorescence on the gut lumen surface (), reasonably attributable to the microparticles, confirming the polymer material ability to interact with both rat FAE and mucosal epithelium. By viewing perfused segment sections under fluorescence microscopy, a marked dotty fluorescence was observed inside both PP () and non-PP tissue (villous epithelium) (). At the level of PP, the fluorescence was located in PP follicle germinal center and serosal side. Otherwise, at the level of non-PP tissue, the fluorescence was found only at the villus tips.
Figure 10. Fluorescence microscopy images of intestinal segments from rat after perfusion treatment with microparticles: gut lumen surface (a), perfused segment in correspondence of a PP (b), perfused segment in correspondence of intestinal villi (c). Original magnification ×40.
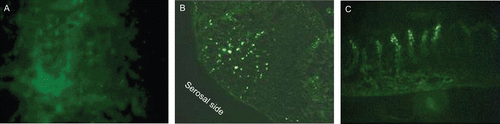
Therefore, microparticles showed their ability to be taken up by both FAE and mucosal epithelium. However, only the translocation across FAE provides microparticle migration into deeper sub-epithelial regions. Such a behavior was not affected by the animal model, i.e. by the different M cell proportion in FAE. These results could be attributed to the intense activity of sub-epithelial and intra-epithelial macrophages included in PP, as already observed by other authors for the transport of bacteria (CitationMamotani et al., 1988). On the contrary, a slight phagocytotic activity was referred for macrophages found at the lamina propria of the villus tips in some mammals (CitationIwanaga, 1995).
Microbiological analysis aiming to assay GM concentrations in rat perfused intestinal tracts containing two PP revealed an antibacterial activity corresponding to ∼ 15 μg of GM. On the contrary, perfused intestinal segments non-containing PP as well as the untreated intestinal tissue did not give evidence of microbial inhibition. This finding could be attributed to the more efficient or faster activity of PP in the transport of microparticles from the gut lumen to the serosal side compared with that of the intestinal regions without PP where microparticles appeared restricted to the epithelial regions, so providing low and undetectable GM concentrations.
The perfusion of microparticles suspended in pH 7.4 phosphate buffer solution decreased the extent of antimicrobial activity (6.5 μg of GM) at the level of intestinal tract including PP. Since most microparticles disintegrated upon contact with a medium containing calcium chelating ions (), the maintenance of the microparticulate structure would be essential to allow the transport across intestinal epithelia.
Evaluation of microparticle uptake by Caco-2 cells
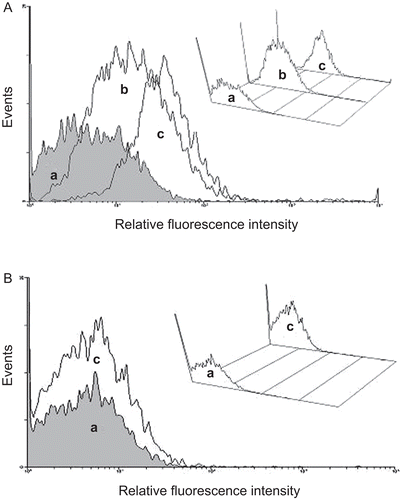
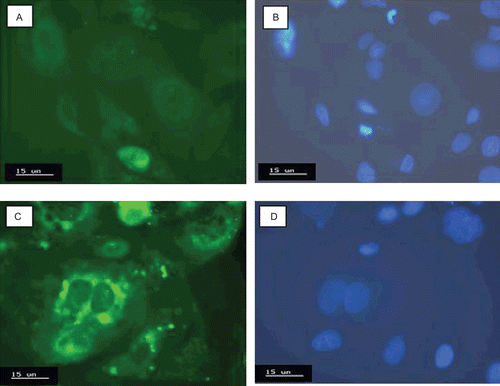
The capacity of the sample A1F to interact with enterocytes was exploited by means of Caco-2 cell monolayers. Such a technique represents the most commonly used cell line to study drug transport across enterocytes, offering the advantages of using an in vitro model as the initial stage of the research (CitationYee, 1997; CitationYamashita et al., 2000; Citationvan der Lubben., 2002; CitationSambuy et al., 2005; CitationMoyes et al., 2007). The interaction between the sample A1F and Caco-2 cells was evaluated by flow cytofluorimetry and showed by cell counts vs fluorescence intensity profiles. Caco-2 cells from control cultures showed a weak fluorescence attributable to the natural fluorescence emission from cells. Following incubation at 37°C for 3 h, a pronounced shift to higher fluorescence intensity was observed, being the shift increased following 6 h incubation (). Reducing the temperature to 4°C, cells did not produce significant fluorescence above control cultures (). Confocal microscopy analysis provided a superimposed montage of 10 confocal optical sections of a Caco-2 cell monolayer, having blue stained nuclei, after 6 h of incubation at 37°C with the sample A1F in comparison with the control, both observed under filter set for green and blue fluorescence (). Caco-2 cells treated with the sample A1F revealed the presence of strong green fluorescence spots (), with diameters corresponding to the microparticle size, around the respective nuclei (). On the contrary, control Caco-2 cells as well as Caco-2 cells incubated with FITC solutions showed a very weak and diffuse fluorescence () around the respective nuclei (). The green spots observed in Caco-2 cells treated with the sample A1F suggest reasonably the presence of microparticles inside the cytoplasm and, then, the occurring of an internalization process at 37°C. The result obtained by using Caco-2 cell lines is consistent with those reported by other authors showing, in general, an intracellular localization for microparticles sized from 0.5–2 μm, with the exclusion for particles > 4 μm (CitationMcClean et al., 1998).
Conclusions
Gentamicin-loaded alginate/chitosan microparticles have been investigated as an oral drug delivery system. The study performed on ex vivo animal models demonstrated the microparticle bioadhesiveness at the level of both mucosal epithelium and FAE encasing Peyer’s patches and their ability to be endocytosed by both epithelial cells, as confirmed by the in vitro model (Caco-2 cell lines). However, microparticles appeared to be delivered into sub-epithelial regions only in correspondence of the lymphoid aggregates, regardless of the animal model used and, consequently, the proportion of M cells in FAE. Therefore, a more efficient microparticle translocation process by M cells compared with that by enterocytes could be supposed even if the entire microparticle pathway toward blood circulation should be examined in vivo to demonstrate the possible drug therapeutic effect. Furthermore, the study demonstrated the usefulness of both ex vivo and in vitro models in the preliminary assessment of microparticulate matter transport across the intestinal epithelia.
Declaration of interest
The authors are grateful to MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca, Rome, Italy) for financial support.
References
- Al-Amoud, A.I., Clark, B.J., Chrystyn, H. (2002). Determination of gentamicin in urine samples after inhalation by reversed-phase high-performance liquid chromatography using pre-column derivatisation with o-phthalaldehyde. J Chromatogr B. 769:89–95.
- Aungst, B.J. (2000). Intestinal permeation enhancers. J Pharm Sci. 89:429–42.
- Axelrod, H.R., Kim, J.S., Longley, C.B., Lipka, E., Amidon, G.L., Kakarla, R., Hui, Y.W., Weber, S.J., Choe, S., Sofia, M.J. (1998). Intestinal transport of gentamicin with a novel glycosteroid drug transport agent. Pharm Res. 15:1876–81.
- Bajpai, S.K., Tankhiwale, R. (2006). Investigation of water uptake behavior and stability of calcium alginate/chitosan bi-polymeric beads: part-1. React Funct Polym. 66:645–58.
- Bystricky, S., Malovikova, A., Sticzay, T. (1990). Interaction of alginates and pectins with cationic polypeptides. Carbohydr Polym. 13:283–94.
- Coppi, G., Iannuccelli, V., Sala, N., Bondi, M. (2004). Alginate microparticles for polymyxin B Peyer’s patches uptake: microparticles for antibiotic oral administration. J Microencapsulation. 21:829–39.
- des Rieux, A., Fievez, V., Garinot, M., Schneider, Y.-J., Préat, V. (2006). Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Contr Rel. 116:1–27.
- Dressman, J.B., Reppas, C. (2000). In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 11:S73–S80.
- Ermak, T.H., Dougherty, E.P., Bhagat, H.R., Kabok, Z., Pappo, J. (1995). Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer’s patch M cells. Cell Tissue Res. 279:433–6.
- Felt, O., Buri, P., Gurny, R. (1998). Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 24:979–93.
- Florence, A.T. (1997). The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res. 14:259–66.
- Giamarellou, H., Zimelis, V.M., Matulionis, D.O., Jackson, G.G. (1975). Assay of aminoglycoside antibiotics in clinical specimens. J Infect Dis. 132:399–406.
- Gombotz, W.R., Wee, S.F. (1998). Protein release from alginate matrices. Adv Drug Del Rev. 31:267–85.
- Grant, G.T., Morris, E.R., Rees, D.A., Smith, P.J.C., Thom, D. (1973). Biological interactions between polysaccharides and divalent cations: the egg-box model. Febs Letts. 32:195–8.
- Hu, Z., Tawa, R., Konishi, T., Shibata, N., Takada, K. (2001). A novel emulsifier, Labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci. 69:2899–910.
- Hussain, N., Jaitley, V., Florence, A.T. (2001). Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Del Rev. 50:107–42.
- Iannuccelli, V., Coppi, G., Cameroni, R. (1996). Biodegradable intraoperative system for bone infection treatment. I. The drug/polymer interaction. Int J Pharm. 143:195–201.
- Ito, Y., Kusawake, T., Ishida, M., Tawa, R., Shibata, N., Takada, K. (2005). Oral solid gentamicin preparation using emulsifier and adsorbent. J Control Release. 105:23–31.
- Iwanaga, T. (1995). The involvement of macrophages and lymphocytes in the apoptosis of enterocytes. Arch Histol Cytol. 58:151–9.
- Lertsutthiwong, P., Rojsitthisak, P., Nimmannit, U. (2009). Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mat Sci Eng. C29:856–60.
- Mamotani, E., Whipple, D.L., Thiermann, A.B., Cheville, N.F. (1988). Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Vet Pathol. 25:131–7.
- McClean, S., Prosser, E., Meehan, E., O’Malley, D., Clarke, N., Ramtoola, Z., Brayden, D. (1998). Binding and uptake of biodegradable poly-DL-lactide micro- and nanoparticles in intestinal epithelia. Eur J Pharm Sci. 6:153–63.
- Morris, E.R., Rees, D.A., Thom, D., Boyd, J. (1978). Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr Res. 66:145–54.
- Moyes, S.M., Smyth, S.H., Shipman, A., Long, S., Morris, J.F., Carr, K.E. (2007). Parameters influencing intestinal epithelial permeability and microparticle uptake in vitro. Int J Pharm. 337:133–41.
- Mumper, R.J., Hoffman, A.S., Puolakkainen, P.A., Bouchard, L.S., Gombo, W.R. (1994). Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J Control Release. 30:241–51.
- Rajaonarivony, M., Vauthier, C., Couarraze, G., Puisieux, F., Couvreur, P. (1993). Development of a new drug carrier made from alginate. J Pharm Sci. 82:912–7.
- Ross, B.P., DeCruz, S.E., Lynch, T.B., Davis-Goff, K., Toth, I. (2004). Design, synthesis, and evaluation of a lyposaccharide drug delivery agent: application to the gastrointestinal absorption of gentamicin. J Med Chem. 47:1251–8.
- Sambuy, Y., De Angelis, I., Ranaldi, G., Scarino, M.L., Stammati, A., Zucco, F. (2005). The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 21:1–26.
- Sarmento, B., Ribeiro, A., Veiga, F., Sampaio, P., Neufeld, R., Ferreira, D. (2007). Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 24:2198–206.
- Segi, N., Yotsuyanagi, T., Ikeda, K. (1989). Interaction of calcium-induced alginate gel beads with propranolol. Chem Pharm Bull. 37:3092–5.
- Sheng, J.J., McNamara, D.P., Amidon, G.L. (2009). Toward an in vivo dissolution methodology: a comparison of phosphate and bicarbonate buffers. Mol Pharm. 6:29–39.
- Silva, C.M., Veiga, F., Ribeiro, A.J., Zerrouk, N., Arnaud, P. (2006). Effect of chitosan-coated alginate microspheres on the permeability of Caco-2 cell monolayers. Drug Dev Ind Pharm. 32:1079–88.
- Sinha, V.R., Singla, A.K., Wadhawan, S., Kaushik, R., Kumria, R., Bansal, K., Dhawan, S. (2004). Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 274:1–33.
- Snoeck, V., Goddeerls, B., Cox, E. (2005). The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 7:997–1004.
- Stockwell, A.F., Davis, S.S., Walker, S.E. (1986). In vitro evaluation of alginate gel systems as sustained release drug delivery systems. J Control Release. 3:167–75.
- Swenson, E.S., Milisen, W.B., Curatolo, W. (1994). Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res. 11:1132–42.
- Takka, S., Acarturk, F. (1999). Calcium alginate microparticles for oral administration: I: effect of sodium alginate type on drug release and drug entrapment efficiency. J Microencapsulation. 16:275–90.
- Thu, B., Bruheim, P., Espevik, T., Smidsrød, O., Soon-Shiong, P., Skjak-Braek, G. (1996). Alginate polycation microcapsules. I. Interaction between alginate and polycation. Biomaterials. 17:1031–40.
- Tønnesen, H.H., Karlsen, J. (2002). Alginate in drug delivery systems. Drug Dev Ind Pharm. 28:621–30.
- van der Lubben, I.M., van Opdorp, F.A.C., Hengeveld, M.R., Onderwater, J.J.M., Koerten, H.K., Verhoef, J.C., Borchard, G., Junginger, H.E. (2002). Transport of chitosan microparticles for mucosal vaccine delivery in a human intestinal M-cell model. J Drug Targeting. 10:449–56.
- Win, K.J., Feng, S.S. (2005). Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 26:2713–22.
- Wittaya-Areekul, S., Kruenate, J., Prahsarn, C. (2006). Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int J Pharm. 312:113–8.
- Yamashita, S., Furubayashi, T., Kataoka, M., Sakane, T., Sezaki, H., Tokuda, H. (2000). Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 10:195–204.
- Yee, S. (1997). In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res. 14:763–6.