Abstract
A transdermal drug delivery system has been reported that can increase the bioavailability, reduce the administration duration, and maintain the concentration of drug in blood. In the present study, drug-in-adhesive transdermal patches of α-asarone using Eudragit E100 as pressure-sensitive adhesives and oleic acid plus isopropyl myristate as penetration co-enhancers were developed. In vitro permeation, in vivo pharmacokinetics in rabbits, and efficacy in asthmatic rats were evaluated. The results showed that co-enhancers could induce a synergistic effect on α-asarone permeability. In vivo study suggested that the patch can keep a relatively certain blood level of drug within 10–30 h in rabbits. Furthermore, the patch with the size of 4 cm2 containing drug 3 mg/cm2 showed a noticeable treating effect on asthmatic rats which is equivalent to the effect of dexamethasone, while avoiding the side-effect induced by the corticorsteroid. This suggests that the drug-in-adhesive transdermal patch is a promising delivery system containing α-asarone to be used for asthma treatment.
Introduction
Asthma is a chronic inflammatory pulmonary disease that is characterized by airway hyper-responsiveness to allergens, airway edema, and increased mucus secretion (CitationO’Byrne & Inman, 2000). Inflammation occurs due to the infiltration of eosinophils, neutrophils, macrophages, and lymphocytes into the bronchial lumen and lung tissue. Asthma is amongst the common chronic disorders affecting humans and accounts for considerable morbidity and mortality worldwide (CitationAsher et al., 2006) Corticosteroid inhalation treatment remains the first preference of treatment with the disease, however these therapies are not always completely effective and are associated with side-effects and steroid resistance. Apart from it, since asthma is a chronic disease, continuous treatment with inhalation corticosteroid agents are unlikely to exert full effects when treatment compliance is poor (CitationCochrane et al., 2000).
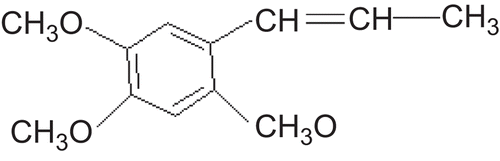
α-Asarone is one of the main efficient ingredients in Acorus gramineus Soland (). It demonstrates many pharmacological effects including anti-hperlipidemic, anti-inflammatory, and antioxidant activity (CitationManikandan & Devi, 2005). By now, there are many products, such as tablets, capsules, and injections of α-asarone, which are available in the market. However, the oral administration of asarone induces very low bioavailability, while invasive administration, such as injection, is not welcomed by patients. Furthermore, the absolute bioavailability of the capsule and tablet are only 2.75% and 5%, respectively (CitationWu, 2003). Therefore, how to improve the bioavailability of the drug through a non-invasive route remains a big challenge.
Being a non-invasive, systemic therapeutic method, the transdermal drug delivery (TDD) system has been extensively studied in the past 20 years. The advantages offered by this route of administration include, but are not limited to, avoidance of the hepatic first pass effect, zero-order absorption, controlled drug release, non-invasive drug delivery, and improved patient compliance (CitationMerkle, 1989). Thus, transdermal administration is a potential approach to overcome the problem with α-asarone treatment.
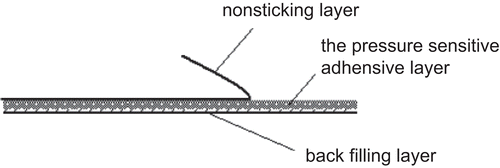
Drug-in-adhesive (DIA) transdermal delivery systems are patches consisting of a polymeric adhesive layer where the drug is directly embedded in a solubilized form. When contacting with human skin, the drug is released from the transdermal patch in a controlled rate and diffuses into the skin (). DIA is the simplest form of TDD, as the drug is directly loaded or dispersed into the pressure-sensitive adhesive. The critical component in DIA transdermal patches is pressure-sensitive adhesive. In addition to meeting the general requirements, the functional adhesive properties used for TDD should have good biocompatibility with the skin and provide sustained drug release. Polyacrylates (Eudragit E100) is one of the most commonly used adhesives in DIA transdermal patches (CitationCui & Frank, 2006). In spite of many advantages of the drug-in-adhesive patches, only limited drugs have been successfully developed in this type of patch due to the excessive barrier function of the skin. In order to reach a therapeutically active concentration in the systemic circulation, it is often a pre-requisite to add a chemical permeation enhancer into the formulation (CitationHadgraft, 1999; CitationKakubari et al., 2006).
In the present study, drug-in-adhesive patches containing α-asarone were constructed after formulation screening. The percutaneous permeability of patches in vitro was evaluated. Furthermore, both the pharmacokinetics in rabbits and the pharmacodynamic characteristics in asthmatic rats of an optimized patch containing α-asarone were investigated.
Materials and methods
Materials
α-Asarone was a gift from Liuzhou pharmaceutical factory (Guangxi, China); Isopropyl myristate (IPM, Sigma, St. Louis, USA), oleic acid (OA, Shuanglin chemical company, Hangzhou, China), Ovalbumin (OVA, Sigma, St. Louis, MO), polyethylene glycol 400(PEG 400 Changqing chemical company, Hangzhou, China); Eudragit® E100 (Rohm, Germany); dexamethasone (DXM, Changzhou Second Pharmaceuticals Company, Changzhou, China); All other chemicals and solvents were of analytical grade quality. For HPLC analysis, methanol (HPLC grade) and double distilled water were used.
Animals
Sprague-Dawley rats (SD rats, weighing 150∼170 g) and New Zealand white rabbits (weighing 2.5∼3.0 kg) were obtained from the Experimental Animal Center of Zhejiang University.
Preparation of drug-in-adhesive transdermal patches
Besides active ingredient, α-asarone DIA patches contained a pressure-sensitive adhesive (PSA) matrix and various penetration enhancers. The PSA matrix was prepared by mixing and homogenization of Eudragit E100, dibutyl sebacate as a plasticizer, and succinic acid as cross-linking agent. The ratio of Eudragit E100, dibutyl sebacate, and succinic acid was 10:5:1, which was selected by preliminary study based on some physical and mechanical characteristics such as initial adhension, adhesive force, and cohesive force. Penetration enhancers used in the patches were oleic acid, isopropyl myristate, and their combinations. The α-asarone DIA patches were prepared using a glass mold solvent evaporation technique (CitationArora & Mukherjee, 2002). Basically, an appropriate amount of α-asarone and various enhancers were dissolved in acetone:isopropanol:ethanol (21:2.3:11.7, v/v) mixing solvent. The solution was then added into the PSA matrix and mixed by stirring on a plate to obtain a final concentration of drug at 1.5 mg/ cm2 or 3.0 mg/cm2. Backing membrane was cast by pouring 4% aqueous solution of polyvingl alcohol in glass molds covered on one side with aluminum foil and then evaporatation at 60°C for 20 min to remove residual solvent. The dried film was then laminated with a polyester release liner for protection.
In vitro permeation experiment
Modified Franz (vertical) diffusion cells (available diffusion area, 0.95 cm2; volume of receiver cell, 5.0 mL) were used for in vitro permeation study. The donor compartment was exposed to room temperature (25°C) while the receptor half was maintained at 37 ± 0.5°C. Excised rat skin was thawed and mounted on the vertical diffusion cells with the stratum corneum facing the donor compartment. The DIA patches were stamped at 0.95 cm2 and applied to the skin on the donor side. The receiver cell was filled with isotonic saline containing 20% PEG 400, which was maintained at 37 ± 0.5°C using a thermostatic water pump and stirred at a constant rate of 600 rpm during the experiment. The amount of permeated drug was quantitated by collecting samples at designated time intervals. The receptor medium of 1 mL was withdrawn and replaced with an equal volume of freshly prepared medium. The samples were analyzed using HPLC at the following conditions: Agilent ZORBAX Eclipse × DB-C18 column (4.6 mm × 150 mm, 5 μm); Temperature 35°C; methol:water (70:30) used as fluid phase; detecting wavelength at 257 nm; fluid speed at 1.0 mL/min.
In vivo percutaneous absorption experiment
The pharmacokinetic characteristics of the DIA patch containing α-asarone were evaluated using New Zealand white rabbits weighing 2.5∼3.0 kg. The DIA patches with optimized formula, covering an area of 20 cm2 (equivalent to 60 mg drug), were applied to the shaved dorsal skin of rabbits with an overlay using adhesive tape and removed 48 h later. The blood samples were collected from the marginal ear vein of these rabbits at 0, 2, 4, 6, 8, 12, 24, 36, and 48 h after the transdermal application of the patch. The blood was transferred immediately into polypropylene tubes containing a small amount of sodium heparin and centrifuged at 3000 rpm for 5 min. The obtained plasma was treated with methanol and ether and then stored at −20°C until analysis using HPLC described above.
Pharmacodynamic characteristics of α-asarone DIA patch
Male SD rats (150–170 g, Laboratory Animal Center of Zhejiang University School of Medicine, Hangzhou, China) were used, and were fed under a 24-h light/dark cycle with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal experiments met the requirements of Zhejiang Medical Laboratory Animal Administration Committee.
Thirty rats were randomly assigned into five groups (six rats/group): the normal control group, the asthmatic control group, the high-dose of α-asarone group (α-asarone DIA, 4 cm2, 3.0 mg/cm2), the low-dose group (α-asarone DIA, 1.5 cm2, 3.0 mg/cm2), and the positive control group (dexamethasone, DXM). For the asthmatic control group and treatment groups, rats were sensitized with subcutaneous injection of 1 mL saline suspension containing 0.2% ovalbumin (OVA, Sigma, St. Louis, MO) and 10% aluminum hydroxide at footpad, neck, back, groin and abdomen on day 0. Additionally, each animal was primed with an intramuscular injection of 2 × 1010 heat-killed B. pertussis organisms. From day 14 after sensitization, animals were challenged once a day for 7 consecutive days by exposure to aerosolized 1% OVA in saline generated by a jet nebulizer (MASTER, Pari GmbH, Starnberg, Germany; medium diameter of produced droplets: 1–5 μm) for 30 min. The normal control animals were exposed to an aerosol of saline. For drug treatment, DIA (High dose: 4 cm2, or Low dose: 1.5 cm2 ) or DXM (ip. 1 mg/kg, Changzhou Second Pharmaceuticals Company, Changzhou, China) was given, respectively, once a day on days 14–20, for 30 min before challenge. The DIA groups were depilated on the abdominal depilation area (4 cm2 or 1.5 cm2) was administrated by the DIA. At day 21, animals were sacrificed and then bronchoalveolar lavage fluid (BALF) was performed by gently instilling D-Hank’ solution into the lung via a tracheal catheter followed by withdrawal. This process was repeated three times as D-Hank’s solution reached up to 5 mL in total. Ninety per cent of the fluid instilled was retrieved at the end of the lavage procedure. Total BALF cells counts were determined with a hemacytometer. Slides for differential cell counts were prepared by centrifuge at 1500 rpm for 10 min. Slides were stained with Wright stain to determine the percentage of eosinophils/neutrophils, monocytes /macrophages, and lymphocyte. The total numbers of these cells for each sample were then determined according to the volume of BALF recovered (CitationJi et al., 2008; CitationLee et al., 2008).
The lungs were immediately removed and fixed by neutral-buffered formalin (10%, pH 7.0) for 48 h. After fixation, representative samples were cut and dehydrated in 70–100% ethanol/xylene, and embedded in paraffin wax. Sections of 2∼4 μm thickness were sliced, deparaffinized, and stained with hematoxylin and eosin for general morphology study. To assess the pulmonary histological changes, we observed the eosinophil influx, tissue edema, epithelial lesion, and inflammatory changes.
Statistical analysis
An ANOVA test was used to examine the comparisons between the experimental groups and within each test group. All the data are expressed as a mean value ± standard deviation (SD).
Results and discussion
In vitro skin permeability
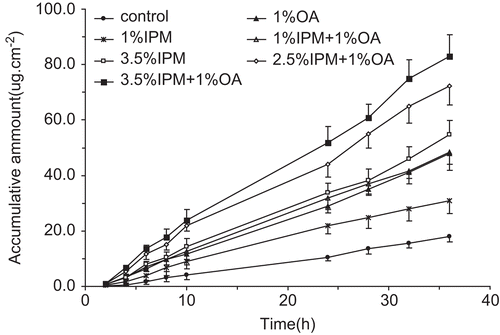
The in vitro skin permeability of α-asarone in DIA patches with different penetration enhancers across excised rat skin was investigated. The profiles of cumulative amount of α-asarone vs time were plotted in . A pseudo zero-order equation was fit to the curves of all these formulations. The slopes were obtained from the linearity regression equation and the flux (µg/cm2 h) was calculated based on the slope. showed the transdermal parameters. Compared to the control, all the penetration enhancers used in DIA patches gave different levels of enhancement of percutanous absorption. Single use of 1% IPM, 1% OA and 3.5% IPM resulted in 1.7-, 2.5-, and 2.8-times improvement of permeability coefficient, respectively. It is expected that the percutanous absorption rate should be increased with the concentration of a single penetration enhancer. However, it is also well-accepted that single chemical penetration with high concentration will cause skin irritation. Limitation of individual chemical enhancers can be potentially overcome by using a mixture of two or more chemical enhancers. Several studies have reported on combinations of one or more enhancers for drug delivery (CitationLevison et al., 1994; CitationZhao & Singh, 1999; CitationLarrucea et al., 2001). In this study, the maximal percutanous absorption rate was achieved by combination use of 1% OA and 3.5% IPM, which was 4.4-times higher than the control group and represented a synergistic effect of enhancing α-asarone transdermal delivery.
Table 1. Transdermal parameters of α-asarone patches through rat skin (x ± s, n = 3).
In vivo transdermal administration
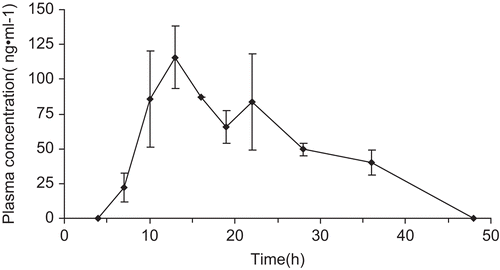
DIA patches containing α-asarone with 3.5% IPM and 1% OA as co-enhancers was selected based on the skin permeation experiment. The maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were compiled from the plasma concentration of drug vs time profile. The area under the plasma concentration-time curve (AUC) up to 48 h was calculated using the linear trapezoidal rule. and showed plasma concentration-time curve and the pharmacokinetics parameters after transdermal administration. Cmax and AUC48 were 132 ng/ mL and 3749 ng/h/mL, respectively. In vivo pharmacokinetics study of the transdermal patch proved that high serum concentration with constant release of drug was obtained by applying transdermal delivery. The patch provided a relatively certain blood level of drug up to 3 days. The experiment using α-asarone oral administration was also carried out and the results demonstrated that Cmax, Tmax, and relative bioavailability of transdermal patch were 20%, 1490%, and 426%, respectively, compared to oral administration (data not shown).
Table 2. Pharmacokinetics parameters of α-asarone patches in rabbits (n = 5).
DIA patch of α-asarone suppressed the infiltration of inflammatory cell in bronchoalveolar lavage fluid
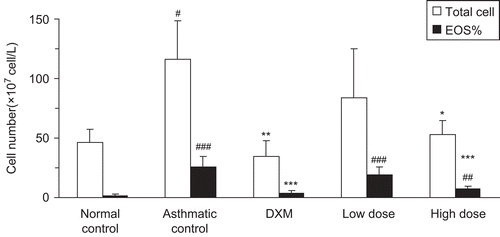
Because eosinophils, neutrophils, and lymphocytes in bronchoalveolar lavage fluid (BALF) have been reported to be significantly increased in OVA-induced asthma (CitationLee et al., 2008), we addressed whether a drug-in-adhesive patch containing α-asarone could decrease the total cell number and eosinophil number or percentage in BALF. As shown in the total cell number and eosinophil number in BALF of OVA-sensitized and challenged allergic rats (asthma control group) were more than 2-folds as high as those of the normal control group after saline inhalation. The total cell number and the eosinophil number were remarkably decreased in rats treated with dexamethasone, compared to the asthma control group (p < 0.01). α-Asarone DIA patch also suppressed the total cell number and the eosinophil number in a dose-dependent manner, where high dose of the DIA treated group had a significantly lower number of the total cell and the eosinophil was similar to the dexamethasone-treated group (p > 0.05).
Figure 5. The number of inflammatory cells in BALF in different groups. BALF cells were separated using Cytospin and then stained with Wright. Differential cell counting was performed using standard morphological criteria. This experiment used six rats per group (n = 6). * p < 0.05, ** p < 0.01, *** p < 0.001 vs model; # p < 0.05, ## p < 0.01, ### p < 0.001 vs control. Total cells: the total cell number; EOS%: Eosinophil number; DXM: dexamethasone; Low dose: 4 cm2 of DIA; High dose: 1.5 cm2 of DIA; DIA: drug-in-adhesive.
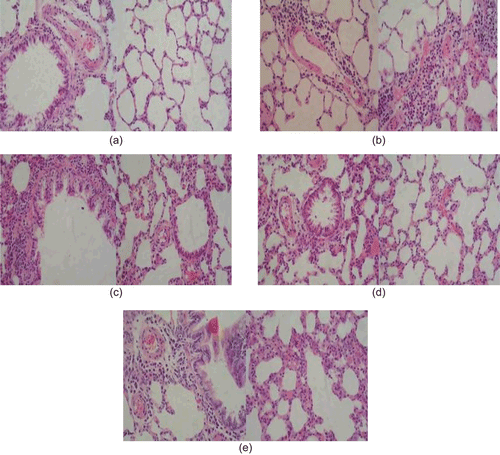
DIA patch of α-asarone inhibited the inflammatory changes and thickening of the bronchiolar wall in small bronchial
Histological analyses revealed a marked eosinophilic infiltration around the airways and blood vessels were observed in the OVA-sensitized and challenged group. As shown in , the airway epithelium was thickened and a marked goblet cell hyperplasia was found in larger airways (vs normal p < 0.05). The normal control group did not produce those obvious inflammation changes in lung. DIA significantly inhibited inflammatory changes and thickening of the bronchiolar wall in small bronchial in a dose-dependent manner. Dexamethasone also significantly reduced eosinophil infiltration and thickening of the bronchiolar wall, alleviated epithelial lesions, and inhibited the goblet cell hyperplasia.
Conclusions
α-Asarone DIA patch was successfully prepared in this present study. A synergistic enhancement effect of α-asarone transdermal permeability was achieved by co-enhancers of 3.5% IPM and 1% OA. In vivo pharmacokinetics study indicated that the optimized α-asarone DIA patch provided a sustained plasma concentration up to 3 days. A positive in vivo efficacy of this patch was observed in asthma model rats. These results indicated that the α-asarone DIA transdermal patch is a promising and alternative route for α-asarone to treat asthma.
Declaration of interest
The study was financially supported by the Science and Technology Key Project of Ningbo Science and Technology Bureau, China (2006C100022).
References
- Arora, P., Mukherjee, B. (2002). Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 91:2076–89.
- Asher, M.I., Montefort, S., Bjorksten, B., Lai, C.K., Strachan, D.P., Weiland, S.K., Williams, H. (2006). Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lance. 368:733–43.
- Cochrane M.G., Bala, M.V., Downs, K.E., Mauskopf, J., Ben-Joseph, R.H. (2000). Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 117:542–50.
- Cui, Y., Frank, S.G. (2006). Characterization of supersaturated lidocain/polyacrylate pressure sensitive systems:thermal analysisi and FT-IR. J Pharm Sci. 95:701–13
- Hadgraft, J. (1999). Passive enhancement strategies in topical and transdermal drug delivery. Int J Pharm. 184:1–6.
- Ji, W., Chen, X., Zhengrong, C., Yumin, H., Huang, L., Qiu Y. (2008). Therapeutic effects of anti-B7-1 antibody in an ovalbumin-induced mouse asthma model. Int Immunopharmacol. 8:1190–5.
- Kakubari, I., Shinkai, N., Kawakami, J., Uruno, A., Takayasu, T., Yamauchi, H. (2006). Formulation and evaluation of ethylene-vinyl acetate copolymer matrix patches containing formoterol fumarate. Biol Pharm Bull. 29:513–6.
- Larrucea, E., Arellano, A., Santoyo, S., Ygartua, P. (2001). Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur J Pharm Biopharm. 52:113–9.
- Lee, C.M., Chang, J.H., Moon, D.O., Choi, Y.H., Choi, I.W., Park, Y.M., Kim, G.Y. (2008). Lycopene suppresses ovalbumin-induced airway inflammation in a murine model of asthma. Biochem Biophys Res Commun. 374:248–52.
- Levison, K., Takayama, K., Isowa, K., Okabe, K., Nagai, T. (1994). Formulation optimization of indomethacin gels containing a combination of three kinds of cyclic monoterpenes as percutaneous penetration enhancers. J Pharm Sci. 83:1367–72.
- Manikandan, S., Devi, R.S. (2005). Antioxidant property of α-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res. 52:467–74.
- Merkle, H.P. (1989). Transdermal delivery systems. Methods Find Exp Clin Pharmacol. 11:135–53.
- O’Byrne, P.M., Inman, M.D. (2000). New considerations about measuring airway hyperresponsiveness. J Asthma. 37:293–302.
- Wu, Y. (2003). Study on the bioavailability of α-asarone tablets. China Clin Hosp Pharm J. 23:597–8.
- Zhao, K., Singh, J. (1999). In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethaol. J Contr Rel. 62:359–66.