Abstract
Folate has been used as a targeting moiety of various anticancer agents to increase their cellular uptake within target cells since folate receptors(FR) are vastly overexpressed in many tumors. In this study, amphiphilic block copolymers composed of methoxy poly (ethylene glycol)(MPEG) and poly(L-Alanine)(PALA) were synthesized and then conjugated with folate to produce a folate receptor-targeted drug carrier for tumor-specific drug delivery. The structure of the copolymers was confirmed by 1HNMR. The CMC values of MPEG-PALA(PLAM) and FOL-PALA-MPEG(FOL-PLAM) were 0.678 × 10−5 mol/L and 0.864 × 10−5 mol/L, respectively. The paclitaxel loaded micelles prepared from PLAM and FOL-PLAM both exhibited spherical shapes and nano-scale dimensions. The average diameter, encapsulation efficiency(EE), drug loading efficiency(LC) were 55nm, 80.6%, 20.2% for the PLAM micelles and 75nm, 69.7%, 17.4% for FOL-PLAM micelles. Furthermore, in vitro release study indicated that the release rate of paclitaxel from both drug-loaded micelles was slow and sustained.
1. Introduction
Current anticancer drug therapies have significant side effects due to nonspecific uptake by normal healthy tissues, which limits the dose that can be administered to patients as well as leading to relapse of the tumor and development of drug resistance(CitationRussell-Jones et al., 2004, CitationXu et al., 2005). Therefore, targeting delivery of anticancer drugs has been widely investigated because it would not only improve therapeutic efficiency but also reduce the dose of drug that must be administered to achieve a therapeutic response, thus minimizing negative side effects(CitationPark et al., 2005). Recently, ligand-mediated targeting of anticancer drugs to tumor cells that express selectively or overexpress receptors has become a promising strategy for improving the therapeutic effect(CitationSAPRA and ALLEN, 2003, CitationMajumdar et al., 2004, CitationQian et al., 2002, CitationNa et al., 2003, CitationDe et al., 2008).
Folate or folic acid, an essential vitamin for the biosynthesis of nucleotide bases and one-carbon metabolism, has high binding affinity for the folate receptors (Kd ∼10–10M). The two pathways for entry of folate derivatives into cells are through facilitated transport by a membrane transport protein and endocytosis. Endocytosis is a natural mechanism by which cells transport essential molecules, such as vitamins and hormones, across the cell membrane and into the cell interior. This process involves specific binding of a ligand to a membrane-bound receptor, with subsequent inward folding of the cell membrane to form and release an intracellular vesicle termed an endosome(CitationKe et al., 2003). Folate receptor is a 38 kDa glycosylphosphatidylinositol(GPI)-anchored cell surface glycoprotein that exists in three isoforms, FR-α, FR-β and FR-γ. Folate receptor is expressed lowly in adult tissues and high levels of expression have been found only in a few tissues such as placenta, kidney, lung, and choroids plexus. It is also elevated in many human cancers, including malignancies of the ovary, brain, kidney, breast, lung and choroids plexus(CitationZhang et al., 2004, CitationSUDIMACK and LEE, 2000). The expression level of the folate receptor in tumors could be 100–300 times higher than that observed in normal tissues(CitationPan and Feng, 2008). Thus, folic acid has been used as a targeting ligand to deliver therapeutic agents to cancer cells due to its high binding affinity to folate receptors, low immunogenicity, ease of modification, small size and functional stability(CitationPark et al., 2005). Several folate–drug conjugates have been reported in the literatures, such as folate-low molecular weight drugs(CitationLee et al., 2002, CitationAronov et al., 2003, CitationSatyam, 2008), folate-liposomes(CitationGabizon et al., 2004, CitationShi et al., 2002, CitationWu et al., 2006, CitationXiang et al., 2008), folate-gene vectors(CitationKim et al., 2007, CitationLiang et al., 2008, CitationYoshida et al., 2006), folate-nanoparticles(CitationPan and Feng, 2008, CitationChen et al., 2008), folate-micelles(CitationPark et al., 2005, CitationAlemdaroglu et al., 2008, CitationKim et al., 2008, CitationYang et al., 2008, CitationChu et al., 2009), folate-prodrugs(CitationHenne et al., 2006, CitationSuzuki et al., 2007, CitationCavallaro et al., 2006), folate-imaging agents(CitationKe et al., 2004, CitationMathias et al., 2003).
Recently, the micelles prepared by amphiphilic block copolymers have become the most promising drug delivery system for anticancer drugs. These polymer micelles usually have a hydrophobic core serving as a reservoir for poorly soluble anticancer drugs and a hydrophilic shell that could help the micelles escape from the reticuloendothelial system (RES) and prevent the micelles from being phagocytized by the macrophages to give them an enhanced permeability and retention (EPR) effect. It could be expected that their ability of recognizing tumor cells will be further enhanced by the attachment of folate to the polymer micelles. Several micelles prepared by folate-conjugated polymers such as PLGA-PEG-FOL(poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol)-folate) and DSPE-PEG-FOL(distearoylphosphatidylethanolamine-poly(ethylene glycol)-folate) have been proved that they have higher cytotoxicity and cellular uptake on FR-positive cancer cells compared to micelles without folate(CitationLee et al., 2003, CitationHan et al., 2009, CitationZHAO and YUNG, 2008).
In this study, folate is conjugated to an amphiphilic block copolymer, poly (L-alanine)-monomethoxy poly (ethylene glycol)(PLAM) to obtain folate-poly (L-alanine)-monomethoxy poly (ethylene glycol)(FOL-PLAM). After that, the folate- conjugate polymers are used to form polymeric micelles for encapsulating paclitaxel. Then, the properties of the drug-loaded micelles including drug loading content, encapsulation efficiency and in vitro release would also be evaluated.
2. Experimental
2.1 Materials
Methoxy poly (ethylene glycol) (MPEG, Mw = 2000) was purchased from Sigma–Aldrich. Triphosgene was received from Lianyungang Chaofan chemical Co., Ltd., Lianyungang, China. Pyrene was purchased from J&K and used as received. Paclitaxel with a purity of 99.5% was obtained from Tianfeng Bioengineering Technology Co., Ltd., Shenyang, China. Other reagents were commercially available and were used as received.
2.2 Synthesis of FOL-PLAM
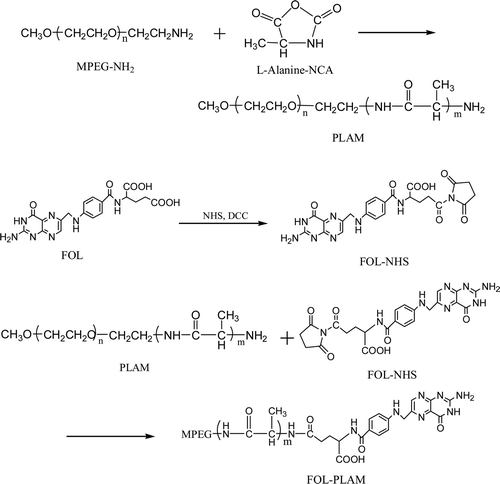
The synthesis of folate-conjugate followed a two-step reaction: synthesis of PLAM and conjugation of folate with PLAM to form FOL-PLAM. A synthetic scheme was shown in .
2.2.1 Synthesis of PLAM
PLAM amphiphilic copolymers were prepared via NCA ring opening polymerization method. First, Amino-monomethoxy poly (ethy1ene glycol)(MPEG-NH2) was synthesized based on the principle of Gabriel synthesis. The detailed reaction and purification conditions could be found in Pillia’s manuscript(CitationPillai et al., 1980). Then, L-alanine-N-carboxvanhvdride(L-Alanine-NCA) was prepared by the following method: L-Alanine(5g) was put into a three-neck flask and dissolved in tetrahydrofuran (50ml). The flask was placed in a water bath (50°C) and started stirring for 5min. Then, triphosgene (8g) was added to the solution and the reaction preformed at 50°C until the solution became clarified. The solution was concentrated and poured into petroleum ether to obtain needle-like crystals at −18°C. The product, L-Alanine-NCA, was filtered and washed by petroleum ether. Finally, L-Alanine-NCA was polymerized to form PLAM using MPEG-NH2 as macroinitiator: MPEG-NH2(10g), L-Alanine-NCA(17g) and dichloromethane(50ml) were put into a three-neck flask and stirred at 25°C for 72 h with N2 protection. After that, the solution was poured into a large excess of ether and formed white precipitate. The precipitate was purified by washing with ether and vacuum drying to obtain the final product.
2.2.2 Synthesis of FOL-PLAM
Folate was reacted with dicyclohexylcarbodiimide(DCC) and N-hydroxy-succinimide(NHS) in DMSO at stoichiometric molar ratio of FOL/DCC/NHS = 1:1.2:2 for 24 hours at room temperature. The product was filtered to remove the byproduct, N,N-dicyclohexylurea (DCU). Then PLAM amphiphilic copolymer was added to the solution and reacted with the activated folate for 6 hours at 50°C, followed by dialyzing against water for 48 hours to remove the unreacted folate. The molecular weight cutoff(MWCO) of this dialysis bag was 3500. Finally, FOL–PLAM conjugate was obtained by depositing in a large excess of anhydrous ether and vacuum drying.
2.3 Characterization of polymers
1H nuclear magnetic resonance (1HNMR) spectra were measured on a Bruker AVANCE 300-MHZ instrument using CDCl3 or DMSO-d6 as solvent and TMS as an internal standard.
Fluorescence spectra were recorded on a Shimadzu RF-5301 PC spectrofluorophotometer. Pyrene was used as a hydrophobic fluorescent probe. Aliquots of pyrene solutions (2 × 10−6 M in acetone, 1 mL) were added to containers, and the acetone was allowed to evaporate. Aqueous polymer solutions(10 mL) at different concentrations were then added to the containers containing the pyrene residue. It should be noted that all the aqueous sample solutions contained excess pyrene residue at the same concentration of 2 × 10−7M. The solutions were kept at room temperature for 24 h to reach the solubilization equilibrium of pyrene in the aqueous phase. Excitation was carried at 334 nm, and emission spectra were recorded ranging from 360 to 450 nm. The excitation bandwidth and the emission bandwidth were 3 nm. From the pyrene emission spectra, the intensity of the peak at 384 nm, I384, was analyzed as a function of the polymer concentration. A CMC value was determined from the intersection of two tangents to the parts of the curve corresponding to the left and right sides of the inflection point(CitationWei et al., 2007).
2.4 Preparation of drug-loaded micelles
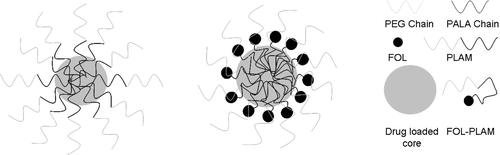
Dialysis is one of the most widely used methods to prepare drug-loaded micelles. In this technique, a block copolymer and drug are dissolved in a common water miscible organic solvent followed by dialysis of this solution against water; a gradual replacement of the organic solvent with water, which is a non-solvent for the core-forming block, triggers self-assembly of hydrophobic blocks accompanied by the entrapment of drug in the micelle cores(CitationRapoport, 2007). In this paper, paclitaxel-loaded polymeric micelles based on PLAM and FOL-PLAM was successfully prepared via dialysis method, respectively. Briefly, 20 mg copolymer and 5 mg paclitaxel were dissolved in 5mL DMSO, and the solution was slowly added to 20 mL distilled water under vigorous stirring. The mixture was encapsulated into a dialysis bag (molecular weight cutoff, MWCO = 3500) and dialyzed against water for a week. After that, the micelle solution was centrifuged (4000 r/min) for 15 min to remove the unloaded drug and was diluted with distilled water to obtain desired concentrations. The possible structure of drug-loaded micelles was shown in .
2.5 Characterizations of micelles
2.5.1 Encapsulation efficiency (EE) and drug loading content (LC)
The drug encapsulation efficiency (EE) was expressed as the percentage of the drug encapsulated in micelles with respect to the initially added drug amount. The drug loading content (LC) was expressed as the percentage of the drug encapsulated in micelles with respect to the total amount of drug-loaded micelles.
To determine the drug encapsulation efficiency (EE) and drug loading content (LC), the unloaded drug was collected and dissolved in ethanol. The paclitaxel concentration was determined from its absorbance at 228nm on an ultraviolet–visible spectrophotometer (UV-2201, Shimadzu) and the standard calibration curve. The calculation formula of EE and LC can be written as:
Where Mi was the initially used drug weigh, mg; Mk was the unloaded drug weigh, mg; Mp was the weigh of total micelles, mg.
2.5.2 Morphology of micelles
The morphology of the blank and drug-loaded micelles was observed from a JEOL JEM-200CX transmission electron microscope (TEM) operating at an acceleration voltage of 120kV. To prepare TEM samples, a drop of aqueous micelle solution with the concentration of 0.5mg/mL was deposited onto copper grids coated with carbon. Several minutes later, excess solution was gently removed using absorbent paper. The sample was then stained by 0.5wt% phosphortungstic acid aqueous solution, followed by drying at room temperature.
2.6 In vitro release of paclitaxel from the micelles
In vitro drug release study was conducted by placing a dialysis bag (molecular weight cutoff: 14,000) containing 5mL of 1.0mg/mL paclitaxel-loaded micelle solution into 95mL stirred phosphate buffer solution (PBS, pH 7.4) at 37°C.
The released content of paclitaxel was determined using a method described by Jackson(CitationJackson et al., 2007). At scheduled time intervals, 50mL PBS was taken out and replaced by fresh PBS. The paclitaxel in PBS solution was extracted with 10mL dichloromethane. Then, the organic phase was collected and evaporated. The residue was dissolved in 5mL HPLC mobile phase (a mixture of acetonitrile, methanol and water in the volume ratio of 45:20:35) for analysis. Finally, the solution was injected through a 20µL sample loop to an Agilent (HP1100) HPLC system equipped with a DAD detector and reverse-phase C-18 column (5 µm, 4.6 mm×250 mm) to determine the concentration of paclitaxel. The flow rate was 1.0mL/min and the detection wavelength was set at 227 nm.
3. Results and discussion
3.1 Characterization of PLAM and FOL-PLAM copolymers
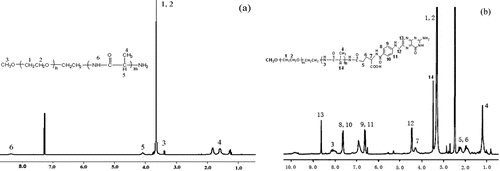
1HNMR was used to confirm the structure of PLAM and FOL-PLAM block copolymers. showed the 1HNMR spectra of PLAM in CDCl3 and FOL-PLAM in DMSO-d6. Furthermore, the molecular weight of PLAM and FOL-PLAM calculated by 1HNMR spectra was 4052 and 4493, respectively. The mean degree of polymerization of poly(L-Alanine) was 28.9.
3.2 Critical micelle concentration of PLAM and FOL-PLAM copolymers
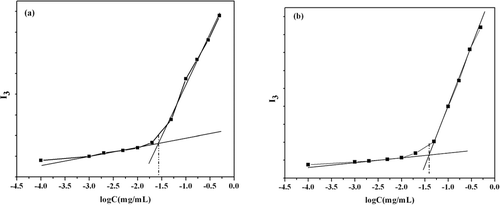
The CMC values of the copolymers were determined by fluorescence probe technique using pyrene as a hydrophobic probe for its sensitivity to surrounding polarity. I384 was plotted with the concentration of the copolymers, as shown in . It showed that the CMC value of PLAM and FOL-PLAM was 0.678 × 10−5 mol/L and 0.864 × 10−5 mol/L, respectively. Because a micelle will be thermodynamically stable relative to disassembly to single chains in pure water if the total copolymer concentration is above the critical micelle concentration(CMC)(CitationAllen et al., 1999), this result indicated that the polymeric micelles had high thermodynamic stability and the potential usage as intravenous injection.
3.3 Preparation and characterization of drug loaded micelles
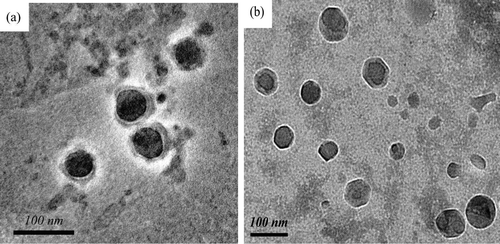
Dialysis method was used to prepare paclitaxel-loaded polymeric micelles based on PLAM and FOL-PLAM, respectively. showed the TEM morphology of the drug-loaded micelles prepared from PLAM and FOL-PLAM. As shown in these images, the micelles exhibited spherical shapes and nano-scale dimensions. The mean diameter from the micelles observed by TEM was 55 nm and 75 nm based on PLAM and FOL-PLAM. They were both small enough to avoid being picked up by RES(CitationMatsumura and Maeda, 1986). The difference in diameter might be that the CMC of FOL-PLAM was higher than that of PLAM, which would increase the aggregated polymer number of every micelle and lead to larger size.
summarized the encapsulation efficiency (EE), drug loading content (LC) and the mean diameter of the micelles prepared from PLAM and FOL-PLAM. It indicated that the EE and LC of PLAM micelles were both higher than that of FOL-PLAM micelles. This may be attributed to the conjugation of hydrophilic folate that prevents the hydrophobic drugs from being wrapped by the polymer. However, compared with the EE and LC of paclitaxel-loaded amphiphilic block copolymeric micelles reported in literature, these EE and LC values of micelles prepared using FOL-PLAM were acceptable.
Table 1. Encapsulation efficiency (EE) and loading content (LC) of paclitaxel in polymeric micelles.
3.4 In Vitro Drug Release Study
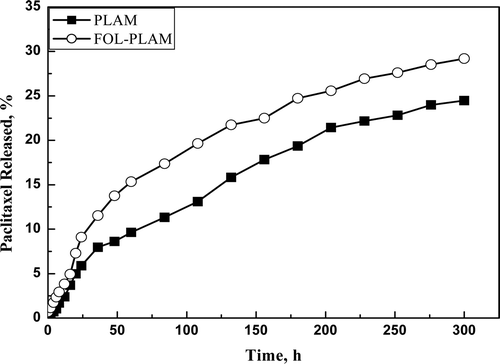
The in vitro drug release study was conducted in phosphate buffer solution (pH = 7.4) at 37°C. showed the in vitro release profiles of paclitaxel from the drug-loaded micelles prepared from PLAM and FOL-PLAM micelles. As seen in , the release rate of paclitaxel from both micelles was slow and sustained. Less than 30% of the incorporated drug was released within 300 hours. The drug release rate from FOL-PLAM micelles was slightly faster than that from PLAM micelles. It may be due to the hydrophilic folate of FOL-PLAM was advantageous to accelerate the diffusion of drug from the micelles core to the external PBS medium. A similar phenomenon was found by Zhao et al. who reported the release of drug loaded micelles prepared from PLGA-PEG and PLGA-PEG-FOL(CitationZHAO and YUNG, 2008).
4. Conclusion
In order to achieve folate targeted delivery of paclitaxel, an amphiphilic block copolymer of folate-conjugated methoxy poly (ethylene glycol)/poly (L-alanine)(FOL-PLAM) has been synthesized. Its low CMC value(0.864 × 10−5 mol/L) indicated that the polymer had high thermodynamic stability and would be the potential drug delivery as intravenous injection. The paclitaxel loaded micelles prepared from FOL-PLAM showed small sizes and regularly spherical shapes. The mean diameter, encapsulation efficiency (EE) and drug loading content (LC) of the micelles was 75nm, 69.7% and 17.7%, respectively. The in vitro release study indicated that the release rate of paclitaxel from the FOL-PLAM micelles was slow and sustained. In conclusion, the micelles system based on FOL-PLAM prepared in this paper could be a potential targeted delivery system for the clinic application of paclitaxel. Further experiments will be under research to raised the release rate of the micelles and confirm their targeting ability for FR positive cells.
Acknowledgement
The authors would acknowledge the financial support of Jiangsu High-tech Project BG2006038.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- ALEMDAROGLU, F. E., ALEMDAROGLU, N. C., LANGGUTH, P. & HERRMANN, A. (2008) DNA block copolymer micelles - A combinatorial tool for cancer Nanotechnology. Advanced Materials. 20:899–902.
- ALLEN, C., MAYSINGER, D. & EISENBERG, A. (1999) Nano-engineering block copolymer aggregates for drug delivery. Colloids and Surfaces B-Biointerfaces. 16:3–27.
- ARONOV, O., HOROWITZ, A. T., GABIZON, A. & GIBSON, D. (2003) Folate-targeted PEG as a potential carrier for carboplatin analogs. Synthesis and in vitro studies. Bioconjugate Chemistry. 14:563–574.
- CAVALLARO, G., MARIANO, L., SALMASO, S., CALICETI, P. & GAETANO, G. (2006) Folate-mediated targeting of polymeric conjugates of gemcitabine. International Journal of Pharmaceutics. 307:258–269.
- CHEN, S., ZHANG, X. Z., CHENG, S. X., ZHUO, R. X. & GU, Z. W. (2008) Functionalized Amphiphilic Hyperbranched Polymers for Targeted Drug Delivery. Biomacromolecules. 9:2578–2585.
- CHU, H. Y., LIU, N., WANG, X., JIAO, Z. & CHEN, Z. M. (2009) Morphology and in vitro release kinetics of drug-loaded micelles based on well-defined PMPC-b-PBMA copolymer. International Journal of Pharmaceutics. 371:190–196.
- DE, P., GONDI, S. R. & SUMERLIN, B. S. (2008) Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules. 9:1064–1070.
- GABIZON, A., SHMEEDA, H., HOROWITZ, A. T. & ZALIPSKY, S. (2004) Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Advanced Drug Delivery Reviews. 56:1177–1192.
- HAN, X., LIU, J., LIU, M., XIE, C., ZHAN, C. Y., GU, B., LIU, Y., FENG, L. L. & LU, W. Y. (2009) 9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: Preparation and evaluation in vitro. International Journal of Pharmaceutics. 372:125–131.
- HENNE, W. A., DOORNEWEERD, D. D., HILGENBRINK, A. R., KULARATNE, S. A. & LOW, P. S. (2006) Synthesis and activity of a folate peptide camptothecin prodrug. Bioorganic & Medicinal Chemistry Letters. 16:5350–5355.
- JACKSON, J. K., HUNG, T., LETCHFORD, K. & BURT, H. M. (2007) The characterization of paclitaxel-loaded microspheres manufactured from blends of poly(lactic-co-glycolic acid) (PLGA) and low molecular weight diblock copolymers. International Journal of Pharmaceutics. 342:6–17.
- KE, C. Y., MATHIAS, C. J. & GREEN, M. A. (2003) The folate receptor as a molecular target for tumor-selective radionuclide delivery. Nuclear Medicine and Biology. 30:811–817.
- KE, C. Y., MATHIAS, C. J. & GREEN, M. A. (2004) Folate-receptor-targeted radionuclide imaging agents. Advanced Drug Delivery Reviews. 56:1143–1160.
- KIM, D., LEE, E. S., OH, K. T., GAO, Z. G. & BAE, Y. H. (2008) Doxorubicin-Loaded Polymeric Micelle Overcomes Multidrug Resistance of Cancer by Double-Targeting Folate Receptor and Early Endosomal pH. Small. 4:2043–2050.
- KIM, Y. K., CHOI, J. Y., YOO, M. K., JIANG, H. L., AROTE, R., JE, Y. H., CHO, M. H. & CHO, C. S. (2007) Receptor-mediated gene delivery by folate-PEG-baculovirus in vitro. Journal of Biotechnology. 131:353–361.
- LEE, E. S., NA, K. & BAE, Y. H. (2003) Polymeric micelle for tumor pH and folate-mediated targeting. Journal of Controlled Release. 91:103–113.
- LEE, J. W., LU, J. Y., LOW, P. S. & FUCHS, P. L. (2002) Synthesis and evaluation of taxol-folic acid conjugates as targeted antineoplastics. Bioorganic & Medicinal Chemistry. 10:2397–2414.
- LIANG, B., HE, M. L., XIAO, Z. P., LI, Y., CHAN, C. Y., KUNG, H. F., SHUAI, X. T. & PENG, Y. (2008) Synthesis and characterization of folate-PEG-grafted-hyperbranched-PEI for tumor-targeted gene delivery. Biochemical and Biophysical Research Communications. 367:874–880.
- MAJUMDAR, S., DUVVURI, S. & MITRA, A. K. (2004) Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Advanced Drug Delivery Reviews. 56:1437–1452.
- MATHIAS, C. J., LEWIS, M. R., REICHERT, D. E., LAFOREST, R., SHARP, T. L., LEWIS, J. S., YANG, Z. F., WATERS, D. J., SNYDER, P. W., LOW, P. S., WELCH, M. J. & GREEN, M. A. (2003) Preparation of Ga-66- and Ga-68-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nuclear Medicine and Biology. 30:725–731.
- MATSUMURA, Y. & MAEDA, H. (1986) A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Research. 46:6387–6392.
- NA, K., LEE, T. B., PARK, K. H., SHIN, E. K., LEE, Y. B. & CHO, H. K. (2003) Self-assembled nanoparticles of hydrophobically-modified polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system. European Journal of Pharmaceutical Sciences. 18:165–173.
- PAN, J. & FENG, S. S. (2008) Targeted delivery of paclitaxel using folate-decorated poly(lactide) - vitamin E TPGS nanoparticles. Biomaterials. 29:2663–2672.
- PARK, E. K., KIM, S. Y., LEE, S. B. & LEE, Y. M. (2005) Folate-conjugated methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. Journal of Controlled Release. 109:158–168.
- PILLAI, V. N. R., MUTTER, M., BAYER, E. & GATFIELD, I. (1980) New, Easily Removable Poly(Ethylene Glycol) Supports for the Liquid-Phase Method of Peptide-Synthesis. Journal of Organic Chemistry. 45:5364–5370.
- QIAN, Z. M., LI, H. Y., SUN, H. Z. & HO, K. (2002) Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological Reviews. 54:561–587.
- RAPOPORT, N. (2007) Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Progress in Polymer Science. 32:962–990.
- RUSSELL-JONES, G., MCTAVISH, K., MCEWAN, J., RICE, J. & NOWOTNIK, D. (2004) Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. Journal of Inorganic Biochemistry. 98:1625–1633.
- SAPRA, P. & ALLEN, T. M. (2003) Ligand-targeted liposomal anticancer drugs. Progress in Lipid Research. 42:439–462.
- SATYAM, A. (2008) Design and synthesis of releasable folate-drug conjugates using a novel heterobifunctional disulfide-containing linker. Bioorganic & Medicinal Chemistry Letters. 18:3196–3199.
- SHI, G. F., GUO, W. J., STEPHENSON, S. M. & LEE, R. J. (2002) Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. Journal of Controlled Release. 80:309–319.
- SUDIMACK, J. & LEE, R. J. (2000) Targeted drug delivery via the folate receptor. Advanced Drug Delivery Reviews. 41:147–162.
- SUZUKI, T., HISAKAWA, S., ITOH, Y., SUZUKI, N., TAKAHASHI, K., KAWAHATA, M., YAMAGUCHI, K., NAKAGAWA, H. & MIYATA, N. (2007) Design, synthesis, and biological activity of folate receptor-targeted prodrugs of thiolate histone deacetylase inhibitors. Bioorganic & Medicinal Chemistry Letters. 17:4208–4212.
- WEI, H., ZHANG, X. Z., CHEN, W. Q., CHENG, S. X. & ZHUO, R. X. (2007) Self-assembled thermosensitive micelles based on Poly(L-lactide-star block-N-isopropylacrylamide) for drug delivery. Journal of Biomedical Materials Research Part A, 83A, 980–989.
- WU, J., LIU, Q. & LEE, R. J. (2006) A folate receptor-targeted liposomal formulation for paclitaxel. International Journal of Pharmaceutics. 316:148–153.
- XIANG, G. Y., WU, J., LU, Y. H., LIU, Z. L. & LEE, R. J. (2008) Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. International Journal of Pharmaceutics. 356:29–36.
- XU, Z. H., GU, W. W., HUANG, J., SUI, H., ZHOU, Z. H., YANG, Y. X., YAN, Z. & LI, Y. P. (2005) In vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel delivery. International Journal of Pharmaceutics. 288:361–368.
- YANG, X. Q., CHEN, Y. H., YUAN, R. X., CHEN, G. H., BLANCO, E., GAO, J. M. & SHUAI, X. T. (2008) Folate-encoded and Fe3O4-loaded polymeric micelles for dual targeting of cancer cells. Polymer. 49:3477–3485.
- YOSHIDA, T., OIDE, N., SAKAMOTO, T., YOTSUMOTO, S., NEGISHI, Y., TSUCHIYA, S. & ARAMAKI, Y. (2006) Induction of cancer cell-specific apoptosis by folate-labeled cationic liposomes. Journal of Controlled Release. 111:325–332.
- ZHANG, Y. H., GUO, L. L., ROESKE, R. W., ANTONY, A. C. & JAYARAM, H. N. (2004) Pteroyl-gamma-glutamate-cysteine synthesis and its application in folate receptor-mediated cancer cell targeting using folate-tethered liposomes. Analytical Biochemistry. 332:168–177.
- ZHAO, H. Z. & YUNG, L. Y. L. (2008) Selectivity of folate conjugated polymer micelles against different tumor cells. International Journal of Pharmaceutics. 349:256–268.