Abstract
Context: Gallic acid had been reported to possess antidepressant like activity, which may be attributed to its CNS effects like increase in reduced glutathione levels, increased catalase activity and decreased malonaldehyde levels in brain.
Objective: This study was designed to enhance the antidepressant-like activity of gallic acid (GA) using nanoparticulate delivery system in swiss male albino mice and to explore the possible underlying mechanisms for this activity.
Methods: GA loaded chitosan nanoparticles (GANP) and corresponding tween 80 coated batch (cGANP) were formulated for brain targeting of GA and characterized for physicochemical parameters, morphology, differential scanning calorimetry and in vitro drug release. GA, GANP, cGANP (dose equivalent to GA 10 mg/kg, i.p.) and positive control drug, Fluoxetine (10 mg/kg, i.p.) were administered for successive seven days to male swiss albino mice. Then, the in vivo antidepressant-like activity was evaluated using Despair Swim Test (DST) and Tail Suspension Test (TST); along with the evaluation of MAO-A activity, reduced glutathione, malonaldehyde level, catalase and locomotor activity in mice.
Keyfindings: cGANP (equivalent to 10 mg/kg, i.p.) significantly decreased immobility period of mice in DST and TST, indicating significant antidepressant-like activity. There was no significant effect on locomotor activity of the mice by GA and its nanoparticle formulations. cGANP (10 mg/kg, i.p.) significantly decreased Monoamine oxidase-A (MAO-A) activity, malondialdehyde levels, and catalase activity in mice.
Conclusions: GA possess significant antidepressant like activity and ligand coated nanoparticle approach with improved brain targeting may serve as an effective approach to enhance such effect.
Introduction
Neuropsychiatric disorders are expected to rise sharply in current decade (Bloom et al., Citation2011; CitationAccess Economics, 2009; Naismith et al., Citation2012). The lifetime prevalence of depression, anxiety, and stress among adolescents and young adults around the world is estimated to range from 5% to 70%. Almost 25% women and 12% men are affected by this highly chronic disorder (Gelenberg, Citation2010).
Depression is one of the serious mental disorders characterized by consistent depressed mood, feeling of low self-worth, substantial impairment in an individual’s ability to take care of his or her everyday responsibilities and anhedonia (inability to take pleasure from normally enjoyable events) (Bondy, Citation2002; Naismith et al., Citation2012). Drugs acting on the central monoaminergic systems, especially 5-Hydroxy Tryptamine (5-HT), and nor-adrenergic synaptic neurotransmissions; selective serotonin reuptake inhibitors (SSRI) (e.g. Fluoxetine, sertraline, escitalopram, paroxetine, and citalopram) and serotonin and norepinephrine reuptake inhibitors (SNRIs) (e.g. venlafaxine and duloxetine) are usually prescribed for the treatment of such disorders (Millan, Citation2004; CitationNIH, 2011). Only one-third of the patients undergoing monotherapy with an antidepressant drug are able to gain complete remission of depressive symptoms and achieve functional recovery (Skolnick, Citation2002; Pae et al., Citation2008). Thus, in order to facilitate the development of antidepressants with new modes of action, exploration of mechanisms of action of antidepressant is important.
Gallic acid (GA), a natural phenolic compound, is mostly found in gallnuts, sumac, witch hazel, grapes, oak bark, and green tea. It has been reported that steady consumption of polyphenols may attenuate the accumulation of Aβ peptides that contribute to the process of neurodegeneration (Hardy & Selkoe, Citation2002; Terry, Citation2001; Klein, Citation2002; Bastianetto et al., Citation2011). Chemically, it is 3,4,5-trihydroxybenzoic acid and possess antioxidant, antityrosinase, antimicrobial, anti-inflammatory, anticancer and neuroprotectant activity (Phiriyawirut & Phaechamud, Citation2012; Krogh et al., Citation2000; Shahrzad et al., Citation2001; Levites et al., Citation2002; Bastianetto et al., Citation2011). Due to its promising antioxidant activity, GA is often used as a standard in assay for antioxidant potential and possesses free radicals scavenging activities for example: superoxide anion, hydroxyl radicals, singlet oxygen or peroxyl radicals, and thus protect cells from damage induced by UV-B or ionizing irradiation (Phiriyawirut & Phaechamud, Citation2012; Sawa et al., Citation1999). Therefore, GA is used in skincare products (O’Neil et al., Citation2001; Hansen & Just, Citation2001). Moreover, it possesses antibacterial property against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumonia (Rodrguez et al., Citation2007).
The rationale of developing drug delivery systems is to promote the therapeutic efficacy of a drug and minimize its toxic side effects, which is achieved by optimizing the amount as well as the duration of drug in the vicinity of the target cells and reducing the drug exposure to non-target cells. Blood–brain barrier (BBB) is an efficient protective barrier which does not allow easy passage of therapeutic moieties to brain and many drugs acting on CNS are required to be administered at much higher doses than that required for therapeutic response. Since, only a fraction of administered drug will be able to circumvent BBB; higher dose may lead to unnecessary drug load on other tissues which can precipitate unwanted side effects. Strategies such as the development of prodrugs, magnetic drug targeting, or drug carrier systems such as antibodies, liposomes, or nanoparticles (NPs) have been opted to overcome BBB (Kreuter, Citation2001). Among the various drug delivery systems, polymeric NPs have attracted great attention in the brain targeted drug delivery because they have the ability to deliver a wide variety of drugs to targeting areas of the body for controlled and site-specific drug delivery (Sahni et al., Citation2011). A number of CNS acting drugs such as hydrophilic drugs, hydrophobic drugs, proteins, vaccines, and biological macromolecules have been delivered using NPs as carriers (Hans & Lowman, Citation2002). Furthermore, as compared to larger bulk materials, NPs have a higher surface-to-volume ratio and therefore the dose and frequency of administration is reduced leading to improved patient compliance (Wilson et al., Citation2010).
A recent study documented antidepressant effect of GA in mice (Chhillar & Dhingra, Citation2012). In order to improve its therapeutic efficacy, targeted delivery of GA to brain is the pre-requisite for achieving, an effective; better tolerated anti-oxidant as well as anti-depressant like activity.
Materials
Chitosan (degree of deacetylation 84.97%, molecular weight 1,22,978 viscosity molecular weight units) was obtained as a gift sample from Central Institute of Fisheries Technology, Kochi, India. Gallic acid monohydrate was procured from Hi-Media, Mumbai, India. Other chemicals used during the study were of suitable analytical grade and were used as received.
Swiss male albino mice (25–30 g) were procured from Disease Free Small Animal House, LLRUVAS, Hisar (Haryana) India. The animals were kept in groups of three, in plastic cages with soft bedding, under standard conditions of light and dark cycle ad libitum. All the experiments were carried out between 08:00 am and 04:00 pm. The experimental protocol was approved by Institutional Animals Ethics Committee (IAEC) and animal care was taken as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India (Registration no. 0436). Efforts were made throughout to minimize animal discomfort and to use minimum number of animals (n = 6) for statistical significance. All animals were acclimatized to laboratory environment for seven days before testing.
Methods
Preparation of chitosan nanoparticles
The chitosan nanoparticles were prepared by ionotropic gelation method (Calvo et al., Citation2001; Tiyaboonchai & Limpeanchob, Citation2007) with minor modifications. The chitosan and tri-polyphosphate pentasodium (TPP, Central Drug House, Delhi, India) were combined electro-statically entrapping the drug (GA) followed by coating with tween 80. It has been reported that in order to produce high yields of stable and solid nanometric structures, the CS/TPP weight ratio should normally be within the range 3:1–6:1 (Prabaharan & Mano, Citation2005). The effect of chitosan to TPP weight ratio on particle size was also very prominent and was found to follow a linear increase in size with increasing chitosan to TPP weight ratio within the tested chitosan to TPP ratio range, i.e. 3:1–7:1 (Gan et al., Citation2005). Thus to get the minimum particle size with maximum yield, chitosan to TPP ratio of 3:1 was selected for the formulation of chitosan TPP nanoparticles.
In this method, 0.1% w/v chitosan (CS) in 2% acetic acid solution was prepared and pH was adjusted to 5.6 by adding aqueous solution of sodium hydroxide or acetic acid solution. A constant amount of GA (50 mg) was added to CS solution followed by drop wise addition of an aqueous solution of TPP (Chitosan:TPP::1:3) with constant stirring for 1 h at 1000 rpm using magnetic stirrer (GANP batch). For coating (cGANP batch), 1.5% tween 80 (SD fine Chem. Ltd., Mumbai, India) was added after 1 h with continuous stirring at low speed (500 rpm). Thereafter, in order to separate nanoparticles, centrifugation of the dispersion was carried out at 15,000 rpm for 60 min at 10°C which yielded nanoparticles containing pellet. To achieve the reproducible results, all the pellets were washed, re-dispersed in constant volume (20 mL) of HPLC grade water (Sisco Research Lab. Pvt. Ltd., Mumbai, India) and sonicated using probe sonicator for 2 min. 2 mL of this suspension was further diluted 10 times, sonicated (2 min) and analyzed for particle size, size distribution and zeta potential. The undiluted nanoparticles were lyophilized using lyophilizer (Alpha 2–4 LD Plus CHRIST, Germany) after adding D-mannitol (Hi-Media Lab. Pvt. Ltd., Mumbai, India) as cryoprotectant to avoid particle agglomeration and adsorbed nanoparticles were obtained. The batch specification is shown in . All experiments were carried out in triplicate and reported as mean ± S.E.M.
Table 1. Batch specification and characterization of GANP and cGANP (mean value ± S.E.M., n = 3).
Physicochemical characterization of nanoparticles
Particle size, size distribution and zeta potential
The average hydrodynamic diameter, polydispersity index (PDI) and zeta potential of the formulated nanoparticles were determined by dynamic light scattering (DLS) analysis using ZetaSizer Nano ZS90 (Malvern Instruments Limited, UK) equipped with a 4.0 mW He-Ne laser operating at 633 nm. 1 mL sample of nanoparticle dispersion were placed in disposable cuvettes (equal volume was used to get reproducible results) for particle size measurements. All measurements were carried out after dispersing the nanoparticles in appropriate volume of HPLC grade water at 25°C, at detection angle of 90° (for size and PDI) and 120° (for zeta potential). Zetasizer applies electric field via electrodes and measures the velocity of particles via Doppler effect (frequency shifting) and by using the relation E = µ × v. The electrophoretic mobility (µ) of the particles which is ratio of applied electric field (E) and particle velocity (v), is measured. For determination of zeta potential, a combination of Laser Doppler velocimetry and Phase Analysis Light Scattering (PALS) were utilized by means of the limiting cases of Smoluchowski and Huckel of the Henry equation through inbuilt software of equipment. Each experiment was conducted in triplicate and the values were reported as mean ± S.E.M.
Drug entrapment efficiency
The supernatant of formulations after centrifugation were collected and filtered through 0.45 µm filter, and amount of drug present was determined by UV spectrophotometer (Cary 5000, Instrument version no. 1.12). A standard calibration curve of concentration versus absorbance was plotted for this purpose. The amount of drug in supernatant (w) was then subtracted from the total amount of drug added (W, 50 mg in this case) during the preparation of nanoparticles (Doung & Jiang, Citation2007). All the prepared nanoparticulate formulations were characterized for percentage drug entrapment efficiency (DEE), using the formula as:
Electron microscopic examination
The nanoparticles were examined under a transmission electron microscope (Morgagni 268 D, Fei Co., The Netherlands, operated at 60 KV) to study the morphology of NPs. The Karnovsky’s fixed samples (Karnovsky, Citation1965) were washed in phosphate buffer (0.1 M, pH 7.4, 6°C) and post fixed for 2 h in 1% osmium tetroxide in the same buffer at 4°C. The specimens were then sonicated to prevent aggregation and dehydrated with graded acetone. The NPs were then stained with uranyl acetate and lead acetate and examined under transmission electron microscope.
Differential scanning calorimetry
The thermogram of pure chitosan, TPP, pure GA, mannitol, their physical mixture and freeze dried cGANP was carried out using differential scanning calorimetry (DSC). The sample (5 mg) was placed onto standard aluminum pan, crimped and heated at the speed of 10°C/min from 40 to 450°C with continuous purging of nitrogen (20 mL/min). An empty aluminum pan was used as a reference.
In vitro release study of formulated nanoparticles
In vitro drug release from cGANP and GANP was carried out using the equilibrium dialysis technique at 37 ± 1°C. Nanoparticles (equivalent to 1 mg GA) were suspended in phosphate buffer saline (PBS) (pH 7.4) and placed in a dialysis membrane bag (Himedia, MWCO, molecular mass cut off 12000–14000, pore size 2.4 nm). The membrane bag containing nanoparticle suspension was placed in 300 mL PBS in beaker placed on magnetic stirrer. The rpm was set at 50 in order to avoid the excessive torque produced at higher rpm which may otherwise lead to rupture the dialysis membrane. At regular intervals, 5 mL of the aliquots were collected and replaced with equal volume of fresh PBS to maintain the sink condition. The samples were centrifuged and the supernatant was used for determination of the concentration of GA by addition of 0.1% w/v EDTA using UV Visible Spectrophotometer (SPECTROD 200-Analytik Jena, 07745 Jena). A standard calibration curve of GA in PBS was prepared with addition of 0.1% EDTA w/v in order to prevent the complexation of GA in PBS (λmax = 261 nm; equation of calibration curve is: y = 0.0943x + 0.0054; R2 = 0.9995).
Pharmacodynamic study
Study design
The experimental animals (male swiss albino mice) were randomly divided in following groups having 6 mice in each group ():
Groups for Despair Swim Test (DST)
Group 1: Control Group; normal saline (i.p.) was administered once daily for 7 consecutive days.
Group 2: Fluoxetine (10 mg/kg, i.p.) (Chhillar & Dhingra, Citation2012), an antidepressant drug was administered once daily for 7 consecutive days as the standard drug (positive control).
Group 3: Pure drug (GA) solution (10 mg/kg, i.p.) (Chhillar & Dhingra, Citation2012) was administered once daily for 7 consecutive days. Gallic acid was dissolved in hot normal saline (60–62°C).
Group 4: GANP (equivalent to 10 mg/kg GA, i.p.) was administered once daily for 7 consecutive days
Groups 5: cGANP (equivalent to 10 mg/kg GA, i.p.) was administered once daily for 7 consecutive days
Groups for Tail Suspension Test (TST)
Groups 6: Control Group; normal saline was administered once daily for 7 consecutive days.
Groups 7–10: These were same as groups 2–5, and immobility period was measured in TST model.
Evaluation of antidepressant-like activity
Despair Swim Test (DST)
In this test, the mouse was individually forced to swim in open chamber of glass (dimensions: 25 × 15 × 25 cm3) containing fresh water (26 ± 1°C). The height of water level was maintained at 15 cm (Porsolt et al., Citation1977). Water in the chamber was replaced after subjecting each animal to this test as “used water” may change the behavior of experimental animals (Abel & Bilitzke, Citation1990). When the mouse was placed in the chamber for the first time, it was initially highly active. After 2 min, this activity began to subside and interspersed with phases of immobility or floatation for longer duration. The immobility period was recorded after treatment during the next 4 min and the total testing period was 6 min. When the mouse ceased struggling and remained floating in water, making only those movements necessary to keep their head above water, it was considered “immobile”. After the testing period, each mouse was towel dried and returned to its housing conditions.
Tail Suspension Test (TST)
Here, the mouse was suspended on the edge of a table which is 50 cm above the floor with the help of an adhesive tape (approximately 1 cm from the tip of the tail) (Steru et al., Citation1985). Each animal under test was isolated from other animals both acoustically as well as visually in order to avoid disturbances to test animals. The immobility period was recorded after treatment and total testing period was 6 min. When the animal didn’t show any body movement and hung completely motionless, it was considered “immobile”.
Measurement of locomotor activity
Alterations of locomotor activity and exploration are used to study specific processes, such as learning, memory reward, anxiety, etc. and to evaluate the influence of drug on general motor activity. To study the effects of various treatments on locomotor activity, all the groups were tested for locomotor activities using Photoactometer (INCO, Ambala, India). The horizontal movement of the mice was recorded for 6 min after various treatments.
Biochemical estimations
Study design
After behavioral testing on TST, the mice were sacrificed by decapitation. Their brains were isolated, washed with cold 0.25 M sucrose-0.1 M Tris-0.02 M EDTA buffer (pH 7.4) and weighed. The samples were homogenized in 9 volumes of cold 0.25 M sucrose-0.1 M Tris-0.02 M EDTA buffer (pH 7.4) and centrifuged twice (800g, 10 min, 4°C) using cooling centrifuge (C24, Remi instruments, Mumbai, India) and the supernatant was collected for further biochemical estimations.
Measurement of MAO-A activity
The supernatant obtained was centrifuged (12000g, 20 min, 4°C) and the precipitate was washed twice with sucrose-tris-EDTA buffer; suspended in 9 volumes of 10 mM cold sodium phosphate buffer pH 7.4 (containing 320 mM sucrose); mixed well at 4°C for 20 min and centrifuged (15000g, 30 min, 0°C). The pellets obtained after centrifugation were suspended in cold sodium phosphate buffer (100 mM, pH 7.4). Firstly, 2.75 mL sodium phosphate buffer and 100 µL of 4 mM 5-hydroxytryptamine were mixed and the absorbance was recorded using UV-visible spectrophotometer (Varian Cary-5000; Christ, Netherland) (Pan et al., Citation2005). This was then followed by the addition of 150 µL dispersion of pellet obtained from brain homogenate in order to initiate the enzymatic reaction. The change in absorbance before and after the addition of brain homogenate was recorded at 280 nm for 5 min.
Estimation of lipid peroxidation
Lipid peroxidation is simply the oxidative degradation of lipids. Free radicals remove electron from lipids and damage the cell. Malondialdehyde (MDA) amount is a measure of lipid peroxidation and is examined in the form of thiobarbituric acid-reactive substances (TBARS) according to method described by Wills (Citation1965). In this method, postmitochondrial supernatant (S9 fraction) obtained from brain homogenates (0.5 mL) which may contain MDA was incubated with Tris–HCl (0.5 mL) at 37°C for 2 h. After the incubation, trichloroacetic acid (1 mL of 10%, Hi Media Lab Pvt. Ltd., Mumbai, India) was added and centrifuged at 1,000g for 10 min. To 1 mL of supernatant so obtained, 1 mL of 0.67% thiobarbituric acid (Hi Media Lab Pvt. Ltd., Mumbai, India) was added, and the samples were kept in boiling water for 10 min. After cooling, 1 mL of HPLC grade water was added, and absorbance was measured at 532 nm. Thiobarbituric acid-reactive substances were determined quantitatively using an extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as nanomole of malondialdehyde (MDA) per milligram protein. Tissue protein was estimated by making use of Biuret method.
Estimation of reduced glutathione
The content of reduced form of glutathione (GSH) is the measure of antioxidant content of a sample. Reduced glutathione was determined by the method of Jollow et al., Citation1974 and Beutler et al., Citation1963. In this, 1.0 mL of supernatant of brain homogenate (10%) was precipitated with 1.0 mL of 4% sulfosalicylic acid (Hi Media Lab. Pvt. Ltd., Mumbai, India). The samples were kept at 4°C for at least 1 h and then centrifuged at 1,200g for 15 min at 4°C. To 0.1 mL supernatant, 2.7 mL phosphate buffer (0.1 M, pH 7.4), and 0.2 mL 5,5′-dithiobis-(2-nitro benzoic acid) (DTNB, also called Ellman’s reagent, 0.1 mM, pH 8.0, Hi Media Lab. Pvt. Ltd., Mumbai, India) are added so as to make total volume of 3.0 mL and the yellow color developed was analyzed at 412 nm, and glutathione levels were calculated using molar extinction coefficient of 1.36 × 104 M−1 cm−1 and expressed as micromole per milligram protein.
Estimation of catalase activity
Catalase is a primary antioxidant enzyme that prevents the oxidative damage caused by intracellular ROS. Catalase content and activity was evaluated by the method of Claiborne, 1985. The assay mixture contains 1.95 mL phosphate buffer (0.05 M, pH 7.0), 1.0 mL hydrogen peroxide (0.019 M), and 0.05 mL brain supernatant (10%) in a final volume of 3.0 mL and analyzed at 240 nm. Absorbance changes were noted at 240 nm. Catalase activity was then computed using the millimolar extinction coefficient of H2O2 (0.07 mM) and expressed as micromoles of H2O2 decomposed per minute per milligram protein.
Estimation of protein
Total protein concentration was assayed in brain homogenate by a total protein kit (Erba Chem, Mumbai, India), using semi-autoanalyzer (Model C-500, Logitech).
Statistical analysis
All in vitro studies data are reported as mean ± SEM (n = 3), and the in vivo studies data are reported as mean ± SEM (n = 6). The difference between the groups were tested using analysis of variance (ANOVA) followed by Tukey’s post-hoc test for biochemical parameters at the level of p < 0.05. The difference greater than p < 0.05 was considered significant and p < 0.001 was considered extremely significant and was calculated using GraphPad Instat 3 (GraphPad Software, Inc. San Diego, CA).
Results
Physicochemical characterization of nanoparticles
Particle size, zeta potential and size distribution
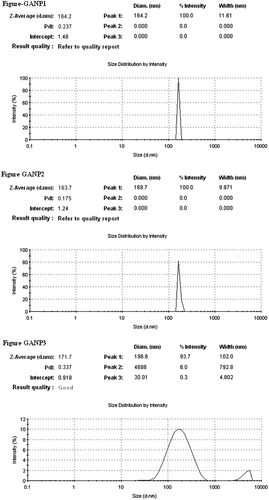
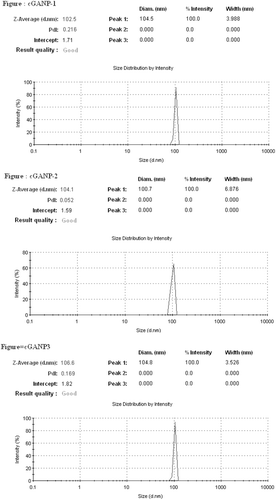
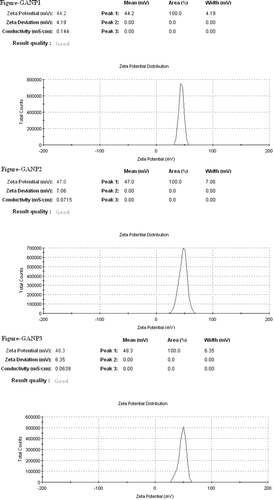
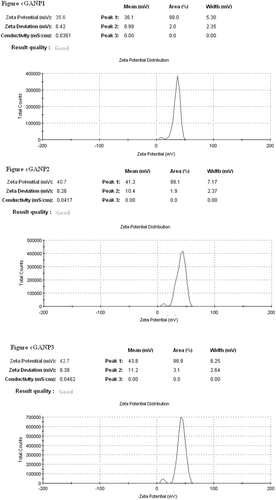
The average particle size (hydrodynamic diameter) of GANP was 166.53 ± 4.48 nm and 104.40 ± 1.69 nm for cGANP ( and ). The decrease in average particle size along with more uniform particles (low polydispersity index) may be attributed to decreased surface free energy by the addition of tween 80. The values of average particle size, average zeta potential and average PDI are shown in . The nanoparticles prepared from ionotropic gelation of chitosan and TPP had an acceptable size (less than 200 nm) and were suitable for pre-clinical studies. The zeta potential and its distribution are represented in and . The zeta potential quantifies the surface charge of particles and can greatly affect the particle stability in suspension through the electrostatic repulsion between the particles. As the zeta potential rises, the particles surface charge also will be increased. Moreover, it determines the in vivo interaction of nanoparticles. For charged particles, the repulsive interactions will be greater between the particles as the zeta potential increases leading to the formation of comparatively stable particles with a more uniform size distribution. The higher zeta potential was obtained for both GANP and cGANP. Although, non-ionic surfactant reduced it to 39.67 ± 3.66 mV (cGANP) from higher zeta potential 46.50 ± 2.09 mV (GANP).
Drug entrapment efficiency
The DEE of a delivery system is crucial for minimizing the amount of carriers and excipients of a drug delivery system used per milliliter of the solvent. The mean percent DEE was found to be above 90% in both GANP and cGANP ().
Electron microscopic examination
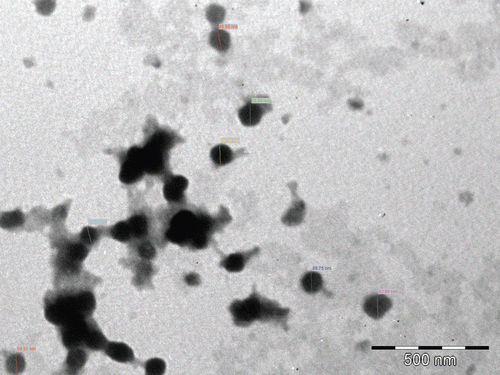
The morphology of the formulated nanoparticles has been studied using transmission electron microscopy (TEM) and the image is shown in . The formulated nanoparticles possessed well defined boundary with spherical shape having diameter in the range of 100 nm.
Differential scanning calorimetry
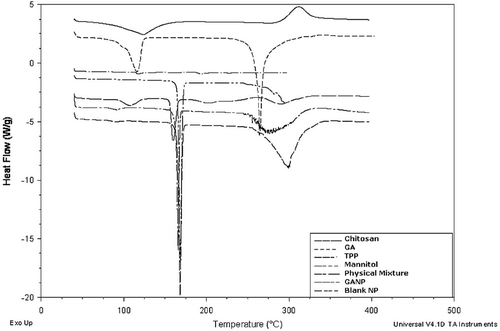
To study the physical state of the GA in the nanoparticles and thermal properties of the polymer nanoparticles, DSC thermograms of polymer (chitosan), GA, TPP, mannitol, their physical mixture, nanoparticles containing GA and NPs without GA were obtained and an overlay plot () was prepared using the in-built software. Sharp peak of drug at 115.82°C and 264.35°C were absent in drug-loaded NPs. During formulation, crystalline GA could have attained an amorphous state of a molecular dispersion or as solid solution in the polymer matrix.
In vitro release study of formulated nanoparticles
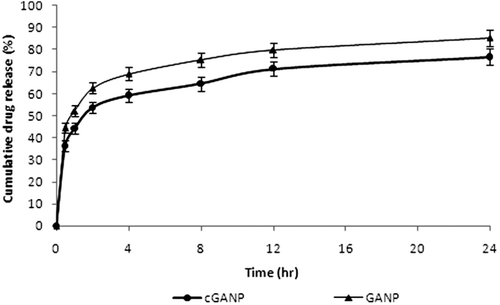
The cumulative percentage release was 85.32 ± 3.76% and 76.77 ± 3.89% from GANP and cGANP respectively after 24 h as shown in . Initial burst release of GA was observed both from GANP and cGANP, which was followed by a slow sustained release. The percentage drug release of GA was 66.62 ± 2.56% from GANP and 53.73 ± 2.65% form cGANP respectively after 2 h. The co-efficient of correlation (R2) of zero order, first order, Korsmeyer-Peppas model, Higuchi model and Baker-Lonsdale model from GANP and cGANP is shown in . Since the co-efficient of correlation (R2) was the maximum for Korsmeyer-Peppas model in case of cGANP and for Higuchi model in case of GANP, these models were observed to be the best fit model.
Table 2. Release kinetics of gallic acid from GANP and cGANP (mean value ± S.E.M., n = 3).
Pharmacodynamic study
Despair Swim Test (DST)
Positive control (Fluoxetine; 10 mg/kg, i.p.) administered for seven successive days decreased the immobility period in mice as compared to the saline treated respective control. Similarly, GA, GANP and cGANP treated group also decreased the immobility period in mice as compared to control group but the effect shown by GANP treated group was not statistically significant. The immobility period (in seconds) in test animal (swiss albino mice) is shown in .
Table 3. Effect of Fluoxetine, gallic acid and its formulations on immobility period for DST, TST and locomotor activity in mice.
Tail Suspension Test (TST)
Compared to saline treated control group, all the groups except GANP group significantly decreased the immobility period of mice in TST. The effect was the maximum in case of standard drug, Fluoxetine and was roughly equivalent to that of cGAMP treated group. GA treatment also resulted in decreased immobility period of mice; however the decrease was not as prominent as cGANP. GANP treated animals failed to produce significant decrease in immobility period of mice as compared to saline treated control group. The immobility period in seconds obtained in TST are shown in .
Measurement of locomotor activity
Fluoxetine, GA, GANP and cGANP administered for 7 successive days did not significantly affect the spontaneous locomotor activity in mice as compared to respective saline treated group ().
Biochemical estimations
MAO-A activity
MAO-A activity was studied to explore the mechanism of anti-depressant-like effect of GA shown in behavioral models. As compared to saline treated control group, no statistically significant change in MAO-A activity was observed with positive control (Fluoxetine) treated and GANP treated group. On the other hand, the activity of enzyme reduced by treatment with GA as well as cGANP; but the effect was more significant in case of cGANP group. The data for this test is represented in .
Table 4. Effect of Fluoxetine, gallic acid and its formulations on MAO-A activity, MDA level, reduced glutathione and catalase activity in mice.
Estimation of lipid peroxidation
This is an in vivo test to assess the antioxidant potential of compounds. The results represent the MDA level (). The MDA level decreased to 0.073 ± 0.002 nmol/mg protein as compared with 0.081 ± 0.003 nmol/mg protein with Fluoxetine which were significantly less than normal saline (0.115 ± 0.003 nmol/mg protein) treated group. GANP and cGANP also reduced MDA level and this reduction was comparable to Fluoxetine. Compared to GA, GANP as well as Fluoxetine treated group, the cGANP treated group showed better antioxidant activity.
Estimation of reduced glutathione
Similar to the lipid peroxidation test, this test is an assay for antioxidant activity of a sample. A greater value of GSH indicates better antioxidant activity. It was observed that all the groups except GANP treated group increased GSH level and hence antioxidant potential compared to saline treated control group. The effect was the maximum in case of cGANP treated group and pure GA treated group showed slightly low GSH level and that was not statistically significant (). Thus Fluoxetine, GA and cGANP possess significant antioxidant action.
Estimation of catalase activity
Catalase enzyme is an index that indicates the antioxidant potential. As compared to saline treated control group, an increase in catalase level was observed in case of Fluoxetine, GA and cGANP treated group; but GANP group failed to show significant effect. The level was maximum in case of cGANP treated group which significantly differ from GA (p < 0.01) treatment as well as GANP treatment (p < 0.001). The data obtained is shown in .
Discussion
The aim of present investigation was to develop a novel; biocompatible and biodegradable ligand coated brain targeted delivery system for gallic acid. Chitosan offer many advantages for developing nanoparticles, including the ability to control the drug release and the formulation avoids of the use of hazardous organic solvents because of its solubility in aqueous acidic solution; being a linear polyamine, it contains a number of free amine groups that are readily available for cross-linking (Nagpal et al., Citation2010). Chitosan, is insoluble in water, and was solubilized in dilute acetic acid by the formation of acetate salt. The formation of nanoparticles mainly involves three main steps, i.e. forming emulsion droplets; consolidation; and solidification. The sizes of the nanoparticles so formed depend on the sizes of the emulsion droplets formed at the initial stage (Wilson et al., Citation2010). Chemical cross-linking of chitosan nanoparticles was accomplished using TPP. When chitosan reacts with TPP, ionotropic cross-linking takes place between positively charged chitosan and negatively charged TPP. This leads to the formation of NPs (Calvo et al., Citation1997). The further magnetic stirring in presence of tween 80 after the ionotropic gelation leads to the coating of tween 80 above the nanoparticles so formulated. Centrifugation process at such a high rpm offer selective separation of requisite size range among the heterogeneous particles so obtained in the system during formulation of nanoparticles. Tween 80 coated nanoparticles have been proposed to offer benefits in terms of their targeting efficacy towards brain (Kreuter, Citation2001). The proposed mechanism of brain specificity is their preferential binding with Apo-E (present in the plasma) which would mimic low density lipoproteins (LDL), and thus bind the LDL receptors present on brain micro vascular endothelial cells (BMEC) (Misra et al., Citation2003; Sun et al., Citation2004; Tamai & Tsuji, Citation1996). The cryoprotectant is added owing to its protective effect and thus preventing particle aggregation during lyophillization process for separation of nanoparticles, which upon reconstitution offers improved re-dispersibility, injectability and syringeability. The particle size of nanoparticles affects the endocytosis by brain capillary cells. Therefore, for effective brain targeting of drugs, the size of nanoparticles should be controlled below 200 nm (Calvo et al., Citation2001; Olivier et al., Citation2002). The size of both the formulated batches, i.e. GANP and cGANP was below 200 nm (). The reduction in size on adding tween 80 (in cGANP) may be due to the surfactant effect, thereby decreasing surface free energy which leads to the formation of smaller droplets. Zeta potential reflects the physical stability of a colloidal dispersion. In the present investigation, positive zeta potential values () were detected for both the batches. This positive value may be attributed to the presence of residual amino group which remained unreacted during the interaction with negatively charged TPP molecules (Fazil et al., Citation2012; Wang et al., Citation2008). Beside this, the magnitude of measured zeta potential values was high reflecting their excellent physical stability (Misra et al., Citation2003; Calvo et al., Citation1997). The result may be due to the strong repulsion and therefore lack of particle aggregation among the particles present in the dispersion. Higher zeta potential (either positive or negative) require higher energy for bringing two particles in contact with each other, i.e. it possess high energy barrier in between the particles (Olivier et al., Citation2002). In cGANP batch, a comparative reduction in the measured zeta potential was observed. It is not the indication of reduced electrostatic repulsion but probably it may be due to masking of surface charge of nanoparticles by the adsorbed tween 80, a good non-ionic, steric stabilizer. This layer in turn would have shifted the plain of shear far away from the particle surface at which the zeta potential is measured (Tadros, Citation2005; Wilson et al., Citation2010). Consequently the measured zeta potential is reduced on adding tween 80, a brain targeting ligand for the prepared nanoparticles. PDI represents the size distribution of nanoparticles. Its value may vary from 0 to 1; the more it is towards zero, the narrower is the size distribution of dispersion. The lower value of PDI of both the batches () indicates homogenous, unimodal, narrow particle size distribution. The addition of tween 80 in cGANP to the formulation was found to shift the polydispersity index towards zero yielding more uniform particle size distribution, i.e. monodisperse particles. The average % DEE for both the batches was above 90% () indicating better encapsulation of GA by nanoparticles. On comparing %DEE of GANP and cGANP, it was observed that cGANP showed slightly higher %DEE but the changes were not significant.
TEM image of cGANP batch () clearly revealed spherical shape and almost uniform size distribution of nanoparticles. The image further confirmed that the size of the particles has achieved NP dimensions and was around 100 nm. The mean particle size of cGANP using zetasizer was found to be more than that obtained after TEM imaging. This difference between the results may be attributed to the dehydration of nanoparticles during sample preparation for TEM analysis. Zetasizer is based on Photon Correlation Spectroscopy which measures the apparent particle size of nanoparticles, i.e. the hydrodynamic diameter which includes the hydrodynamic layers that form around the hydrophilic particles leading to overestimation of particle size (Fazil et al., Citation2012). Furthermore, lack of sharp edges in nanoparticles in the image () as well as absence of peak of drug in the DSC thermogram () shows polymorphic changes from crystalline to amorphous form of the drug (Joshi et al., Citation2010). It was observed that the melting endotherm of drug was absent in the thermogram of nanoparticles, which evidences the absence of the crystalline state of drug in nanoparticles (Joshi et al., Citation2010) and GA was encapsulated by the polymer of the nanoparticle (Fazil et al., Citation2012).
Drug release from nanoparticles and consequent biodegradation are important for the development of a successful formulation. The release rate of drug from nanoparticles depends on (i) desorption of the surface-bound/adsorbed drug; (ii) diffusion through the nanoparticle matrix; (iii) diffusion (in case of nanocapsules) through the polymer network; (iv) nanoparticle matrix erosion: and (v) a combined erosion/diffusion process. Thus, the drug release from polymeric nanoparticles is mainly dependent on diffusion and biodegradation (Costa & Sousa Lobo, Citation2001; Nixon, Citation1983). The cumulative percent release of GA from the nanoparticles in vitro is shown in . During the in vitro release study of the formulated nanoparticles batches, the initial burst release occurs which may be due to easy drug diffusion and desorption of surface bound/ free drug (Costa & Sousa Lobo, Citation2001; Nixon, Citation1983; Wilson et al., Citation2010, Citation2011). Greater percent drug release was observed in case of GANP (85.32 ± 3.76%) than cGANP (76.77 ± 3.89%) after 24 h. Thus coating of nanoparticles with tween 80 slightly decreased the release of drug from the corresponding nanoparticle batch without tween 80 coating which may be attributed to additional barrier layer formed by tween 80 coating although it was not much significant compared to uncoated batches. The release rate in the second phase is supposed to be controlled mainly by the diffusion of drug across the polymer matrix of the nanoparticles so formulated (Costa & Sousa Lobo, Citation2001; Nixon, Citation1983; Wilson et al., Citation2010, Citation2011). The in vitro drug release kinetics were characterized by fitting the data obtained from in vitro–release studies of nanoparticles from various batches to standard drug release kinetics equations (zero order, first-order, Higuchi (Mt/M∞ < 0.6), Korsmeyer-Peppas model (Mt/M∞ < 0.6) and Baker-Lonsdale model (Costa & Sousa Lobo, Citation2001) and are represented in . The best fit model for the drug release data was selected on the basis of correlation coefficient. The results indicated that release of drug from cGANP follow Korsmeyer-Peppas model as indicated by higher R2 values and the n values indicated that the mechanism of drug release from the chitosan nanoparticles was Fickian (Costa & Sousa Lobo, Citation2001; Wilson et al., Citation2010). The GANP batch evidence Higuchi model to be the best fit model for release of GA which indicates the release of drug from the nanoparticles was diffusion controlled. However, a good fit (R2 = 0.978 and 0.957 for cGANP and GANP, respectively) was also observed for Baker-Lonsdale model suggesting the controlled drug release from spherical matrix which was already confirmed by TEM studies.
Some CNS acting drugs are unique compared to drugs acting on any other organ, because of their capability to interfere with brain signaling and to induce specific behavioral effects. This fact upsurge the likelihood of examining whether the compound has a pharmacodynamic effect or not. The effect of the drug in the brain can be studied by monitoring the behavior of the animal with specifically designed behavioral tests. These behavioral tests do not register the whole particle, but only the free drug (that has been released from the nanoparticle) inside the brain, as only the free drug will be able to exert a pharmacodynamic effect. Drug release from nanoparticles at the site of interest is critical; therefore, behavioral tests are of great value (van Rooy et al., Citation2011). In DST, after 1 week, all groups except GANP treated group exhibited significant (p < 0.001) antidepressant like activity compared to saline treated control group. Among GA and its formulations, minimum immobility period was observed in case of cGANP which was comparable to that of positive control, Fluoxetine (). When compared to Fluoxetine (positive control), no statistical significant difference was observed in case of cGANP treated group; whereas GA (p < 0.05) and GANP (p < 0.001) treated groups show significant difference in activity. Moreover, cGANP treated group evidenced decrease in immobility period as compared to both GA treated (p < 0.05) as well as GANP treated (p < 0.001) group. The failure of GANP treated group to show significant antidepressant like activity may be attributed to its comparative failure to reach brain in significant amount to show therapeutic effect. Whereas cGANP treated group exhibited improved antidepressant like activity than pure GA; which supports its improved delivery to brain across BBB. In TST, a similar result to DST model was observed which confirms the ability of ligand coated nanoparticles to overcome BBB, enhance the amount of free drug in brain resulting in improved anti-depressant like activity.
The mechanism for antidepressant-like action is different for various classes of drugs. Monoamine oxidase inhibitor (MAOI), such as phenelzine, inhibits the metabolism of monoamine neurotransmitters and thus increases their level in brain by interfering with their metabolism, whereas selective serotonin reuptake inhibitors (SSRI) such as Fluoxetine blocks the uptake of neurotransmitter from the synapse into pre-synaptic terminal. Monoamine oxidase is a flavin-containing enzyme and is localized in mitochondrial membranes; widely distributed throughout the body in nerve terminals, liver, intestinal mucosa, platelets, etc. The two major molecular species of MAO are MAO-A and MAO-B. MAO-A preferentially deaminates the neurotransmitters epinephrine, norepinephrine, and serotonin. Clorgyline is selective inhibitor for MAO-A. Whereas, MAO-B metabolizes phenethylamine and selegiline is MAO-B inhibitor. Both MAO isozymes metabolize dopamine and tyramine; and are inhibited by phenelzine, tranylcypromine, and isocarboxazid. Inhibition of MAO by MAO inhibitors causes a reduction in metabolism and a consequent increase in the concentrations of biogenic amines. Selective MAO-A inhibitors are reported to be more effective in treating major depression than MAO-B inhibitors (Krishnan, Citation1998). Therefore, MAO-A activity was studied in present investigation so as to explore the mechanism of the anti-depressant-like effect of GA shown in behavioral models. In MAO-A activity study, compared to saline treated control group, both GA (p < 0.05) and cGANP (p < 0.001) treated group showed significantly reduced MAO-A activity; but Fluoxetine and GANP treated groups failed to show such effect. The failure of Fluoxetine to show reduction in MAO-A is because it is a selective serotonin reuptake inhibitor and do not show antidepressant like activity by reducing the activity of MAO-A. The MAO-A activity was minimum in case of cGANP treated group and this effect was statistically significant from that of Fluoxetine (p < 0.001), GA (p < 0.01) as well as GANP (p < 0.001) treated group. These results suggest that reduction in MAO-A activity may be one of the mechanisms of exhibiting antidepressant- like activity of GA and this effect was further improved by augmented brain uptake of GA via ligand coated nanoparticles (cGANP). The failure of GANP treated group to show such activity may be due to the inability of uncoated nanoparticles to cross BBB and hence sufficient free drug was not available to show such effect.
In many aerobic cellular metabolic processes, reactive oxygen species (ROS) (such as superoxide and hydrogen peroxide) are produced which react with various intracellular targets, including lipids, proteins, and DNA (Cerutti, Citation1985; Weydert & Cullen, Citation2010). ROS are normal products of aerobic metabolism, but their biological effects on intracellular targets are concentration dependent. Increase in levels of ROS is cytotoxic leading to cell death, mutations, chromosomal aberrations, and carcinogenesis and increased level of ROS is observed in oxidative stress. Oxidative stress is defined as an imbalance between oxidants and antioxidants in favor of the oxidants, potentially resulting in cell damage (Sies, Citation1991, Citation1997) under the influence of adverse physicochemical environment or pathological agents. Free radicals play a major role in the etiology and pathogenesis of many neurodegenerative disorders (Hockenbery et al., Citation1993; Kumar et al., Citation1995; Sun & Chen, Citation1998). Antioxidants are substances that neutralize free radicals by inhibiting or delaying the oxidation of substrate on which free radicals attack (Young & Woodside, Citation2001). The efficacy of such protection depends on the type of ROS, its origin and the severity of the damage (Halliwell, Citation1994, Citation1997; Halliwell & Gutteridge, Citation1995). A number of phytonutrients have shown to exhibit multiple pharmacological effects including antioxidant, anti-inflammatory and anticancer properties and hence can be used in patients with multiple diseases. Gallic acid (GA) is one such polyphenolic phytonutrient found in gallnuts, sumac, witch hazel, grapes, oak bark, and green tea, and has already proven its antioxidant potential (Rasool et al., Citation2010) and in vitro neuroprotective activity (Lu et al., Citation2006). Also, oxidative damage due to acute restraint stress and unpredictable chronic mild stress (UCMS) has been found to be involved in the pathogenesis of depression (Bhattacharya & Muruganandam, Citation2003; Novío et al., Citation2011). Fluoxetine has been reported to attenuate stress-induced increase in oxidative parameters (Zafir & Banu, Citation2007). Since GA has been reported to possess antioxidant and neuroprotective activities, therefore this compound may have potential in the management of depression. Thus, the present investigation was hypothesized to explore the antidepressant-like effect of GA loaded chitosan nanoparticles (GANP) and corresponding tween 80 coated batch (cGANP) in mice.
The concentration of intracellular ROS depends on their production and/or removal by the antioxidant system. Mammalian cells contain numerous antioxidants that not only prevent/repair the damage caused by ROS, but also regulate redox-sensitive signaling pathways. The MDA content is a measure of lipid peroxidation and a reduction in MDA level evidence antioxidant activity (Chhillar & Dhingra, Citation2012). The antioxidant activity evidenced by GA proposes scavenging of reactive oxygen species (such as free radicals and peroxides) as one of the mechanism of antidepressant like activity of GA. The lower mean value of MDA was observed with Fluoxetine treated (p < 0.001); GA treated (p < 0.01) and GANP treated (p < 0.05) groups as compared to saline treated control group. Statistically significant lowering of MDA level was observed with cGANP treated group as compared to both GA treated (p < 0.05) and GANP (p < 0.001) treated groups which confirms the enhanced brain uptake of GA by ligand coated nanoparticulate drug delivery system.
Gltuathione exists in two states, reduced and oxidized state. Its reduced form contains thiol group (GSH) which is able to donate electron to other unstable molecule, such as reactive oxygen species. It has essential role in protecting tissues against electrophillic attack by alkylating agents and other toxic metabolites. Thus the estimation of reduced glutathione (GSH) using DTNB will indicate the antioxidant activity of a compound. In the present study, all groups except GANP treated group showed a significantly higher GSH level compared to saline treated control group. Maximum GSH level was observed in case of cGANP treated group which was significantly higher than Fluoxetine treated group (p < 0.001); GA treated group (p < 0.001) as well as GANP treated group (p < 0.001). This higher GSH level in experimental animal by cGANP treated group further confirmed enhanced uptake of GA via BBB and hence better antioxidant activity.
In all oxygen metabolizing cells, catalase; substrate specific peroxidase; and glutathione peroxidase are primary antioxidant enzymes necessary for life (Weydert & Cullen, Citation2010). Catalase is the enzyme that scavenges toxic oxygen by-products (such as hydrogen peroxide) and an increased level of catalase indicates antioxidant activity. Catalase converts toxic hydrogen peroxide to safer products, i.e. water and oxygen. Catalase activity is largely located in subcellular organelles known as peroxisomes (Weydert & Cullen, Citation2010). Large amount of hydrogen peroxide is produced by inflammatory cells to destroy pathogens. Its high concentration is toxic to cells and the accumulation causes oxidation of cellular targets such as DNA, proteins, and lipids that result in mutagenesis and cell death. Catalase removes H2O2 from the cell which provides protection against oxidative damage to the cell. It was assayed by reacting it with H2O2 and increased catalase activity was observed in all the groups (except GANP treated group) as compared to saline treated group. Complying with the results of other biochemical estimation, cGANP treated group evidenced statistically significant increase in catalase activity compared to GA treated (p < 0.01) and GANP treated group (p < 0.001). The catalase activity was slightly higher than Fluoxetine treated group but was not statistically significant. Thus all the biochemical results reflects better antioxidant activity of cGANP treated group which may be attributed to its improved brain uptake via brain targeted ligand coated nanoparticulate drug delivery system; whereas nanoparticles without ligand coating (GANP) treated group failed to show such effect.
No statistically significant change in locomotor activity of mice was observed with any group. This confirms the assumption that the antidepressant like effect of these treatments was specific and the results were not false positive.
Conclusions
Gallic acid encapsulated nanoparticles were formulated in this study as a novel biodegradable brain drug delivery system and further evaluated for in vitro and in vivo delivery properties. The formation of nanoparticles was confirmed and validated by physicochemical characterization techniques. The significant improvement in vivo pharmacodynamic activity; better MAO-A inhibition; and stronger in vivo antioxidant activity by cGAMP treated group show the success of ligand coated nanoparticulate system for the delivery of GA across brain. However, its long term in vivo toxicity and immunogenicity should be further investigated. Owing to its improved in vivo antioxidant activity, neuroprotectant activities other than antidepressant like effect may also be investigated.
Acknowledgements
The authors are grateful to Haryana State Counseling society for providing CV Raman Scholarship to Ms Kalpana Nagpal as financial assistance.
Declaration of interest
The authors report no conflicts of interest.
References
- Abel EL, Bilitzke PJ. A possible alarm substance in the Forced Swimming Test. Physiol Behav 1990;48:233–239.
- Access Economics. (2009). Keeping Dementia Front of Mind: Incidence & Prevalence 2009–2050. Alzheimer’s Australia, Canberra.
- Bastianetto S, Dumont Y, Quirion R. Catechins and resveratrol as protective polyphenols against beta-amyloid-induced toxicity: possible significance to Alzheimer’s disease. In: Ramassamy C, Bastianetto S, eds. Recent Advances on Nutrition and the Prevention of Alzheimer’s disease. Kerala, India: Transworld Research Network, Inc., 2011:145–154.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–888.
- Bhattacharya SK, Muruganandam AV. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav 2003;75:547–555.
- Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S et al. The global economic burden of noncommunicable diseases. World Economic Forum, Geneva 2011. Available at: www.weforum.org. REF: 080911.
- Bondy B. Pathophysiology of depression and mechanisms of treatment, Dialogues. Clin Neurosci 2002;4:7–20.
- Calvo P, Gouritin B, Chacun H, Desmaële D, D’Angelo J, Noel JP et al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res 2001;18:1157–1166.
- Calvo PC, Remunan-Lopez J, Vila-Jato L, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carrier. J Appl Polym Sci 1997;63:125–132.
- Cerutti PA. Prooxidant states and cancer. Science 1985;227:375–381.
- Chhillar R, Dhingra D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fund Clin Pharmacol 2012. DOI: 10.1111/j.1472-8206.2012.01040.x.
- Claiborne A. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC, 1985.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123–133.
- Doung H, Jiang M. Fabrication, characterization and drug loading of pH dependent multi-morphological nanoparticles based on cellulose. Polym Int 2007;56:1206–1212.
- Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci 2012;47:6–15.
- Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces 2005;44:65–73.
- Gelenberg AJ. The prevalence and impact of depression. J Clin Psychiatry 2010;71:e06.
- Halliwell B, Gutteridge JM. The definition and measurement of antioxidants in biological systems. Free Radic Biol Med 1995;18:125–126.
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 1994;344:721–724.
- Halliwell B. Antioxidants: the basics–what they are and how to evaluate them. Adv Pharmacol 1997;38:3–20.
- Hans ML, Lowman AM. Biodegradable NPs for drug delivery and targeting. Curr Opin Solid State Mater Sci 2002;6:319–327.
- Hansen CM, Just L. Prediction of environmental stress cracking in plastics with Hansen solubility parameters. Ind Eng Chem Res 2001;40:21–25.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–356.
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993;75:241–251.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11:151–169.
- Joshi SA, Chavhan SS, Sawant KK. Rivastigmine-loaded PLGA and PBCA nanoparticles: preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur J Pharm Biopharm 2010;76:189–199.
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965;27:137A–138A.
- Klein WL. ADDLs & protofibrils–the missing links? Neurobiol Aging 2002;23:231–235.
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2001;47:65–81.
- Krishnan KRR. Monoamine oxidase inhibitors. In: Schatzberg AF, Nemeroff CB, eds. The American Psychiatric Press Textbook of Psychopharmacology. Washington, DC: American Psychiatric Press, Inc., 1998:239–249.
- Krogh R, Yunes RA, Andricopulo AD. Structure-activity relationships for the analgesic activity of gallic acid derivatives. Farmaco 2000;55:730–735.
- Kumar R, Agarwal AK, Seth PK. Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem 1995;64:1703–1707.
- Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 2002;277:30574–30580.
- Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 2006;48:263–274.
- Millan MJ. The role of monoamines in the actions of established and “novel” antidepressant agents: a critical review. Eur J Pharmacol 2004;500:371–384.
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci 2003;6:252–273.
- Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull 2010;58:1423–1430.
- Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol 2012;98:99–143.
- National Institute of Health (NIH). U.S. Department of Health & Human Services. Publication No. 11–3561, Revised 2011. Available at: http://www.nimh.nih.gov.
- Nixon JR. Release characteristics of microcapsules. In: Lim F, ed. Biomedical Applications of Microcapsulation. Boca Raton, FL: CRC Press, Inc., 1983:19–52.
- Novío S, Núñez MJ, Amigo G, Freire-Garabal M. Effects of Fluoxetine on the oxidative status of peripheral blood leucocytes of restraint-stressed mice. Basic Clin Pharmacol Toxicol 2011;109:365–371.
- O’Neil MJ, Smith A, Heckelman PE, Obenchain JR, Gallipeau JAR, D’Arecca MA. Gallic Acid. In: O’Neil MJ, Smith A, Heckelman PE, Obenchain JR, Gallipeau JAR, D’Arecca MA, eds. The Merck Index. New Jersey, USA: Merck Research Laboratories, Inc., 2001:261.
- Olivier JC, Huertas R, Lee HJ, Calon F, Pardridge WM. Synthesis of pegylated immunonanoparticles. Pharm Res 2002;19:1137–1143.
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed Pharmacother 2008;62:308–311.
- Pan Y, Kong L, Xia X, Zhang W, Xia Z, Jiang F. Antidepressant-like effect of icariin and its possible mechanism in mice. Pharmacol Biochem Behav 2005;82:686–694.
- Phiriyawirut M, Phaechamud T. Gallic acid-loaded cellulose acetate electrospun nanofibers: thermal properties, mechanical properties, and drug release behavior. Open J Polymer Chem 2012;2:21–29.
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977;229:327–336.
- Prabaharan M, Mano JF. Chitosan-based particles as controlled drug delivery systems. Drug Deliv 2005;12:41–57.
- Rasool MK, Sabina EP, Ramya SR, Preety P, Patel S, Mandal N et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacol 2010;62:638–643.
- Rodrguez VMJ, Alberto MR, de Nadra MMC. Antibacterial effect of phenolic compounds from Dif-ferent Wines. Food Control 2007;18:93–101.
- Sahni JK, Doggui S, Ali J, Baboota S, Dao L, Ramassamy C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J Control Release 2011;152:208–231.
- Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem 1999;47:397–402.
- Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr 2001;131:1207–1210.
- Sies H. Free radicals in human diseases. Am J Med 1991;91:31–38.
- Sies H. Physiological role of free radicals. Exp Physiol 1997;82:291–295.
- Skolnick P. Beyond monoamine-based therapies: clues to new approaches. J Clin Psychiatry 2002;63 Suppl 2:19–23.
- Steru L, Chermat R, Thierry B, Simon P. The Tail Suspension Test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370.
- Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci 1998;5:401–414.
- Sun W, Xie C, Wang H, Hu Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004;25:3065–3071.
- Tadros TF. Applied Surfactants Principles and Applications. In: Tadros TF, ed. Weinheim: Wiley-Vch Verlag GmbH & Co. Inc. KGaA, 2005:112–115.
- Tamai I, Tsuji A. Drug delivery through the blood brain barrier. Adv Drug Deliv Rev 1996;19:401–424.
- Terry RD. An honorable compromise regarding amyloid in Alzheimer disease. Ann Neurol 2001;49:684.
- Tiyaboonchai W, Limpeanchob N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int J Pharm 2007;329:142–149.
- van Rooy I, Cakir-Tascioglu S, Hennink WE, Storm G, Schiffelers RM, Mastrobattista E. In vivo methods to study uptake of nanoparticles into the brain. Pharm Res 2011;28:456–471.
- Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm 2008;70:735–740.
- Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 2010;5:51–66.
- Wills ED. Mechanisms of lipid peroxide formation in tissues. role of metals and haematin proteins in the catalysis of the oxidation unsaturated fatty acids. Biochim Biophys Acta 1965;98:238–251.
- Wilson B, Samanta MK, Muthu MS, Vinothapooshan G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer’s disease. Ther Deliv 2011;2:599–609.
- Wilson B, Samanta MK, Santhi K, Kumar KP, Ramasamy M, Suresh B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine 2010;6:144–152.
- Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001;54:176–186.
- Zafir A, Banu N. Antioxidant potential of Fluoxetine in comparison to Curcuma longa in restraint-stressed rats. Eur J Pharmacol 2007;572:23–31.