Abstract
Pulmonary drug delivery has become a promising route in the treatment of lung diseases because of better local retention and lower systemic penetration. In this study, etoposide-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres were designed with potential pulmonary delivery properties. The microspheres were prepared via improved emulsion-solvent evaporation method. Physicochemical characteristics, micromeritics properties and in vitro drug release behavior of the microspheres were then evaluated. Results showed that etoposide-loaded PLGA microspheres were spherical in shape with smooth surface with size (11.8 ± 1.25) μm. Particles remained stable without any changing in size and morphology after dried by the freeze-drying method. Etoposide was loaded into PLGA microspheres in an amorphous state with high drug loading ((7.7 ± 0.3)%) and encapsulation efficiency ((84.2 ± 2.9)%). Results of micromeritics properties also demonstrated that etoposide-loaded PLGA microspheres were very suitable for pulmonary delivery. In addition, in vitro drug release study indicated a sustained release profile fitted with the Ritger–Peppas equation for up to 20 days. In conclusion, the etoposide-loaded PLGA microspheres were promising for pulmonary delivery, and etoposide could be sustained released from the PLGA microspheres.
Introduction
Small-cell lung cancer has become a notable healthcare issue because of its high incidence and mortality worldwide. Nowadays, combination chemotherapy is still the mainstay first-line treatment for it (van Meerbeeck et al., Citation2011). Etoposide, an important antineoplastic agent and a so-called standard in therapies for small-cell lung cancer, has been widely studied on many aspects and used in other cancers for many years (Aisner & Lee, Citation1991). As the first epipodophyllotoxin agent recognized as a topoisomerase II inhibitor, etoposide acts with topoisomerase II and DNA to form a ternary complex, causing the breakage of DNA strand and the death of cell. Etoposide has also been used as an efficient agent for the treatment of other tumors, including Testicular, Kaposi’s sarcoma, non-Hodgkin’s lymphoma and non-lymphocytic leukaemia (Hande, Citation1998). Besides, its antibacterial effect has also been confirmed (Calame et al., Citation1988).
The formulation of etoposide has long been a challenging issue because of its poor solubility and lipophilic properties. Adding solubilizers is one of the approaches to increase the solubility, but the following adverse effects limit its application (Patlolla & Vobalaboina, Citation2005). Molecule modification has also been essayed for overcoming the problem. Etoposide phosphate is one of etoposide prodrugs, but it should not be any more effective than etoposide (Hande, Citation1998). Besides, several pharmaceutical formulation techniques have been carried out to improve the formulations of etoposide, including parenteral emulsion (Patlolla & Vobalaboina, Citation2005), long circulating nanoparticles (Yadav et al., Citation2010), pH-sensitive strontium carbonate nanoparticles (Qian et al., Citation2012), positively charged liposomes (Sengupta et al., Citation2000) and microspheres (Schaefer & Singh, Citation2002).
In recent years, poly(lactic-co-glycolic acid) (PLGA) microspheres have gained much attention for pulmonary delivery (Giovagnoli et al., Citation2007; Lee et al., Citation2010; Doan et al., Citation2011; Diab et al., Citation2012). Drugs-loaded PLGA microspheres are inhaled directly into lungs without undergoing first pass metabolism, maintaining high concentration at local site, which brings about satisfactory therapeutic effects and reduces side effects at lower dosage of administration. Pulmonary delivery has been focused on for many years due to its aforementioned great advantages and is desirable for treating both pulmonary and systemic disorders. Insulin (Patton et al., Citation1999), rifampicin (Doan et al., Citation2011), therapeutic siRNA (Lam et al., Citation2012) and quercetin (Silva et al., Citation2013) have been studied for pulmonary delivery. In addition to pulmonary delivery, microspheres can also be administrated through other routes, including peroral administration (Haznedar & Dortunc, Citation2004), intramuscular injection (Han et al., Citation2010) and intra-articular injection (Zhang & Huang, Citation2012) for different bioactive agents and therapeutic applications. Microspheres can be designed into an optimal drug delivery system through the choice and formulation of various drug–polymer combinations, such as chitosan (Sinha et al., Citation2004), PLGA and so on. Besides, microspheres can control or prolong the release of active constituents (Yen et al., Citation2001; Xu & Czernuszka, Citation2008), and in some cases, they can also target specific sites to provide desirable effects (Zhang & Huang, Citation2012). PLGA, a FDA-qualified polymer, has been widely used in biodegradable polymer microspheres, thanks to its great advantages of biodegradability and biocompatibility (Anderson & Shive, Citation2012). PLGA, composed of lactic and glycolic acids, would undergo hydrolysis and be metabolized to CO2 and water in the Krebs cycle (Tamber et al., Citation2005), suggesting good safety with limited toxicity or tissue reaction.
Overall, in this study, we are mainly focusing on the preparation and in vitro evaluation of etoposide-loaded PLGA microspheres for pulmonary drug delivery. Etoposide-loaded PLGA microspheres were prepared via improved emulsion-solvent evaporation method. The optimized etoposide-loaded PLGA microspheres were obtained by orthogonal design and dried by the freeze-drying method. Physicochemical characteristics and micromeritics properties were both investigated to ensure that etoposide-loaded PLGA microspheres have good qualities and are suitable for pulmonary delivery. The in vitro drug release behavior was also studied for etoposide-loaded PLGA microspheres compared with etoposide solution. The objective of this study was to research on the possibility of etoposide-loaded PLGA microspheres dry powder for pulmonary inhalation, so as to enhance the safety and efficiency of treatment on small-cell lung cancer and improve patients’ compliance.
Materials and methods
Materials
Etoposide was kindly provided by Qilu Pharmaceutical Co., Ltd. Poly(lactic-co-glycolic acid) (PLGA:50/50, Mw = 10 000) was obtained from Shandong Institute of Medical Instruments. Polyvinyl alcohol (PVA) (degree of hydrolysis: 87.0–89.0% (mol/mol), CPS:4.6–5.4 mPas) was purchased from Shanghai Jingchun Reagent Co., Ltd. All other chemical reagents used in this study were of analytical or chromatographical grade.
Preparation and optimization of etoposide-loaded PLGA microspheres
The improved emulsion-solvent evaporation method was used to prepare etoposide-loaded PLGA microspheres. Briefly, 0.5 ml of organic phase was prepared by completely dissolving both etoposide and PLGA in chloroform. Then, the organic phase was added to 2 ml of 1% (w/v) PVA solution dissolved in distilled water in a constant rate, stirring for 1 min at a high speed to form oil-in-water (O/W) initial emulsion. Subsequently, the initial emulsion was added to 20 ml of 1% (w/v) PVA aqueous solution used as diluent, and magnetically stirred for 3 h at room temperature to evaporate the organic solvent. The solidified etoposide-loaded PLGA microspheres were collected by centrifugation (10 min, 4000 rpm) and washed three times with distilled water.
The preparation of etoposide-loaded PLGA microspheres was optimized by orthogonal design. Four factors, including drug/PLGA ratio (w/w) (X1), PLGA concentration (mg/ml) (X2), etoposide concentration in diluent (mg/ml) (X3) and shearing speed (rpm) (X4), were chosen as research objects, because of their biggish effects on the qualities of the microspheres. Three levels were selected for each factor separately and nine formulations were prepared and studied according to orthogonal experiment table, so as to determine the optimal formulation. Encapsulation efficiency of the microspheres was used as evaluation index. The factors and their corresponding levels are listed in .
Table 1. Factors and levels of the orthogonal design.
The etoposide-loaded PLGA microspheres powder was obtained by the freeze-drying method. More specifically, 2 ml of 2% (w/v) mannitol solution served as freeze-drying protective additive was added to the solidified microspheres put in penicillin bottles, next pre-freezed at −80 °C for 12 h, and then freeze-dried for 24 h in lyophilizer (Christ Alpha 1-2 LD plus, Germany). Methods of pre-freezed, types of freeze-drying protective additive and concentration of mannitol were studied, in order to determine the optimal freeze-drying process.
Physical–chemical characteristics and micromeritics properties
Morphological characterization
The etoposide-loaded PLGA microspheres were observed by optical microscope equipped with photomicrographic apparatus (BH-2, Olympus, Japan). In brief, the solidified or freeze-dried microspheres were dispersed in sufficient distilled water, a drop of which was then dropped on a clean glass slide and covered by a cover glass. The samples were inspected under the optical microscope immediately, and photographs of microspheres were taken during observation.
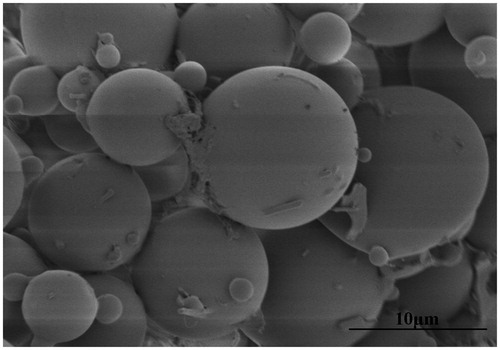
The surface and internal morphology of the microspheres was examined under a scanning electron microscopy (SEM, JEOL 5800LV, Japan). Before analyzing the samples, the freeze-dried microspheres were gold-sputtered in argon atmosphere.
Size and size distribution
The size and size distributions of solidified and freeze-dried microspheres were both measured by laser particle size analyzer (Mastersize 2000, Malvern, Britain). The samples were prepared by dispersing the solidified or freeze-dried microspheres in appropriate amount of distilled water. The results were given by the apparatus automatically.
pH
The pH of the suspensions of etoposide-loaded PLGA microspheres was measured by pH meter (Mettler FE20, Mettler, Switzerland).
Drug loading and encapsulation efficiency
The drug loading and encapsulation efficiency of etoposide-loaded PLGA microspheres were obtained by UV spectrophotometry with using a UV spectrophotometer (UV-2102PCS, Unico, Shanghai). Briefly, 10 mg of freeze-dried microspheres were dissolved in 10 ml DMSO, with ultrasonic processing to attain the complete dissolution of the microspheres. After filtration, the absorbance of the solution was measured at 285 nm, and the concentration was obtained with the etoposide-DMSO solution (60 μg/ml) used as comparison solution. The drug loading and encapsulation efficiency were calculated by using under-mentioned Equations (1) and (2), respectively. It was confirmed that blank microspheres had no interference with the determination of etoposide concentration in etoposide-loaded PLGA microspheres at 285 nm. Etoposide could also be recovered from the microspheres by the above-mentioned ultrasonic method. Standard curve was also done with etoposide dissolved in DMSO in advance.
Here, Mdetermined is the actual amount of etoposide in microspheres determined by UV and Mmicrospheres is the amount of weighed microspheres. Mactual is the actual drug loading calculated by Equation (1) and Mtheoretical is the theoretical drug loading obtained from the quantity added in the preparation process.
DSC analyses
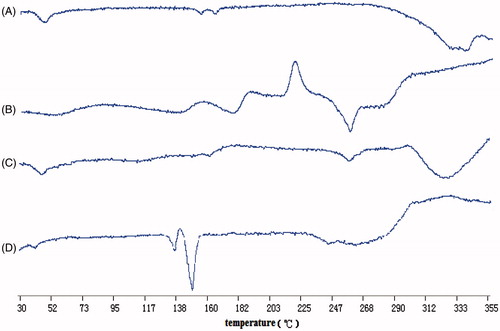
The thermal properties of etoposide-loaded PLGA microspheres were analyzed by differential scanning calorimetry (DSC). It was performed on blank microspheres, etoposide, physical mixture of blank microspheres and etoposide, and etoposide-loaded PLGA microspheres by using a DSC analyzer (DSC822e, METTLER TOLEDO, Switzerland). The samples were heated from 30 °C to 350 °C at a heating rate of 10 °C/min.
Micromeritics properties
The moisture content of the microspheres was measured by moisture analyzer (MB45, OHAUS, Switzerland), performing on three batches of microspheres and three samples for each batch, with about 0.5 g of samples heated to 105 °C for 15 min. The bulk density of the microspheres was determined by using a 10 ml-measuring cylinder filled with the powder, and the angle of repose of them was obtained by using a funnel. Equation (3) was applied to calculate mass mean aerodynamic diameter (MMAD) of the microspheres (Vanbever et al., Citation1999),
where da is the MMAD, ρ is the bulk density of the microspheres, d is the average size of the microspheres and ρ1 is 1 g/cm3. The deposition ratio in the effective site was determined by the method introduced in Pharmacopoeia of China (Appendix XH, CP2010), which was similar to the twin-stage impinge with 7 and 30 ml distilled water used as absorption solution put into stages 1 and 2, respectively (Louey et al., Citation2003). Stage 2 deposition also referred to the deposition ratio in the effective site or fine particle fraction. Meanwhile, the airflow was formed by using a vacuum pump and the airflow rate was adjusted to (60 ± 5) l/min via a flowmeter for each measurement. During the measurement, etoposide-loaded PLGA microspheres powder (20 mg) was loaded into hard gelatin capsules (size 3) and then placed into the powderhaler (Tianping Pharmaceutical Factory, Shanghai) and 20 capsules were required for the test. More specifically, they were drawn by turns with 10 s for each capsule. The nearby section of stage 2 was rinsed with distilled water, and the rinsing liquid was combined with stage 2 absorption solution. Followed by the combined solution diluted to an appropriate volume, the content of etoposide was measured by UV spectrophotometry at 285 nm as described above. The measured value dividing 20 would be the data for 1 capsule and deposition ratio in the effective site was then obtained by comparing it with the labeled content. The dumping ratio and spray characteristic were determined by in-house devices (Gao, Citation2005; Bi, Citation2008). The dumping ratio could also be determined by using the same device for measuring the deposition ratio in the effective site. Ten capsules filled with etoposide-loaded PLGA microspheres powder were needed. They were accurately weighed (w1) separately, then placed into the powderhaler and drawn into the device one by one. Each capsule was drawn at a (60 ± 5) l/min airflow rate for four times with 1.5 s for each time. Then, each capsule was weighed again (w2). After that, the capsule shell was weighed (w3) after cleaning up the residual contents. The dumping ratio was calculated from the following equation:
The spray characteristic of the microspheres powder was estimated by observing the state of them after they were treated in the same drawn way. The capsules loaded with powder were placed into the powderhaler, and drawn to a 5 l jar. Four levels known as “sprayed very easily”, “sprayed easily”, “could be sprayed” and “sprayed difficultly” were divided, corresponding to different spray phenomena.
In vitro drug release behavior
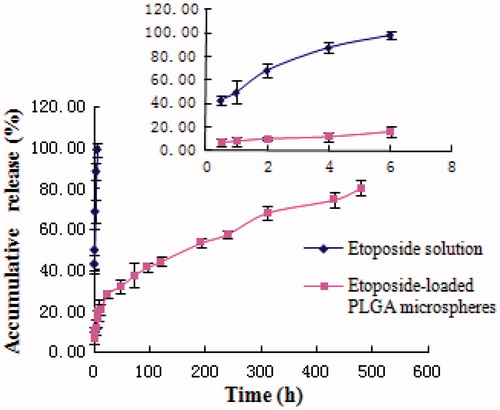
The in vitro release of etoposide-loaded PLGA microspheres was carried out in phosphate buffer of pH 7.4 containing 0.1% polysorbate 80 to increase the solubility of etoposide in water and 0.02% NaN3 known as antimicrobial agent. Etoposide-loaded PLGA microspheres (containing 2 mg etoposide) were added into 2 ml of release buffer in dialysis bags, and then the bags were put into a tube containing 28 ml of pH 7.4 phosphate buffer (containing 0.1% polysorbate 80). All the samples were under the sink condition and kept in a constant shaking bath at 100 rpm maintained at 37 °C. At setted times, 5 ml of dialysis solution was taken for analysis; next, 5 ml of fresh medium was added. After filtration and appropriate dilution, the concentration of etoposide in release medium was measured by UV at 283 nm. Meanwhile, the release of etoposide solution (1 mg/ml) was monitored as control. The accumulative release was calculated, and the results were shown as mean ± SD (n = 3). The mathematical models were adopted to fit and describe the in vitro release of etoposide-loaded PLGA microspheres as well as the etoposide solution both mentioned above.
Results and discussions
Preparation and optimization
The improved emulsion-solvent evaporation method was used to prepare etoposide-loaded PLGA microspheres. In general, the organic phase was added to the aqueous phase directly. However, in this study, the organic phase was first added to small volume of aqueous phase to form O/W initial emulsion with PVA used as an emulsifier, which was then added to larger volume of aqueous phase to be diluted. Results showed that organic and aqueous phases could be better emulsified via this method. Consequently, the better qualities of the microspheres could be obtained. Four factors, including drug/PLGA ratio (w/w), PLGA concentration (mg/ml), etoposide concentration in diluent (mg/ml) and shearing speed (rpm), were selected for the orthogonal design based on the single factor investigation on several factors of the formulation and preparation process. The results of orthogonal design are shown in .
Table 2. Results of the orthogonal design.
As shown in , average encapsulation efficiency of the three-levels of each factor revealed how the encapsulation efficiency changed as the level of each factor varied. The higher range value represented the bigger effect on the encapsulation efficiency of each factor. The optimized preparation factors were determined according to the results of the orthogonal design and actual studies. The determined levels for each factor were 1:10, 80 mg/ml, 0.1 mg/ml and 4000 rpm, respectively. Among the four factors, PLGA concentration shows the biggest effect on the encapsulation efficiency. Three batches of etoposide-loaded PLGA microspheres were prepared using the optimized factors and the results manifested that the formulation and preparation process were suitable and stable.
The appearance and redispersion time of the freeze-dried microspheres were used to select the optimal freeze-drying process. Compared with saccharose and lactose, mannitol used as freeze-drying protective additive could ensure the good appearance and appropriate redispersion time. Moreover, mannitol is widely used as the excipient for inhalable powder, due to its non-toxic, readily degradable properties after administration (Sham et al., Citation2004).
Physical–chemical characteristics and micromeritics properties
Physical–chemical characteristics
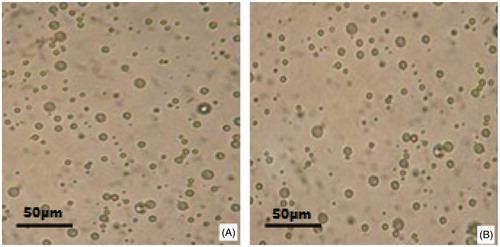
The freeze-dried microspheres were white powder with good appearance. and show the morphological characterization of the microspheres observed under the optical microscope and SEM, respectively. As seen from , the surface morphology of the microspheres had no significant changes after the freeze-dried process. The microspheres were spherical in shape with a smooth surface but without adhesion or aggregation, better illustrated in .
Figure 1. Morphology of etoposide-loaded PLGA microspheres observed under the optical microscope: (A) before freeze-drying and (B) after freeze-drying.
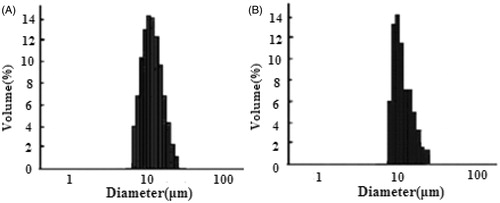
Results of size and size distribution of etoposide-loaded PLGA microspheres are shown in . Size of the microspheres before and after freeze-drying was (11.8 ± 1.25) μm and (11.9 ± 0.40) μm, respectively, both well distributed. The size, also called geometric diameter, could be a little larger than general requirement on diameter (<10 μm, and the majority <5 μm) for porous microparticles with low density and small aerodynamic diameters, because of exhibiting ideal lung deposition profiles (Yang et al., Citation2009).
Figure 3. Size and size distribution of etoposide-loaded PLGA microspheres: (A) before freeze-drying and (B) after freeze-drying.
The pH values of three batches of etoposide-loaded PLGA microspheres suspensions were measured. An average pH of (7.16 ± 0.05) indicated that neutral suspensions were suited for lung administration.
The drug loading and encapsulation efficiency could be affected by several factors, such as the methods of preparation, the physicochemical characteristics of drug, the concentrations of drug and polymer, phase ratio, the species and concentrations of the stabilizer, solvent evaporation time and stirring speed (Yen et al., Citation2001; Zhang et al., Citation2011). In this study, it was an efficient method to increase the drug loading and encapsulation efficiency by adding etoposide to the diluent. Nonetheless, the concentration of etoposide in the diluent should be considered for the purpose of avoiding the burst release of the drug. An average drug loading of (7.7 ± 0.3)% and encapsulation efficiency of (84.2 ± 2.9)% of etoposide-loaded PLGA microspheres were obtained with the optimized preparation parameters, suggesting that the drug loading and encapsulation efficiency were high enough for further studies.
Results of DSC analyses on blank microspheres (A), etoposide (B), physical mixture of blank microspheres and etoposide (C) and etoposide-loaded PLGA microspheres (D) are shown in . Etoposide had a particular peak at about 260 °C, which also appeared in physical mixture of blank microspheres and etoposide, indicating that etoposide existed as crystal in its natural state and did not have chemical interaction with the blank microspheres. Whereas the disappearance of the particular peak in etoposide-loaded PLGA microspheres revealed that etopoisde existed in the PLGA microspheres in a form of uncrystallization rather than crystallization just as in physical mixture of blank microspheres and etoposide. Etoposide could remain in an amorphous state throughout the entire preparation process, ensuring that phase separation, which would cause the drug released from the microspheres, could not occur (Nath et al., Citation2013).
Micromeritics properties
Etoposide-loaded PLGA microspheres were supposed to be fabricated into powder for inhalation. Therefore, the micromeritics properties of the microspheres were crucial for effective pulmonary delivery. The moisture content was (1.58 ± 0.17)% suitable for pulmonary delivery. The high moisture content can cause the adhesions of the powder, resulting in poor dispersibility, limited flowability and bad spray characteristic of the particles, and affect the stability of the drugs as well (White et al., Citation2005; Li et al., Citation2010). The bulk density calculated by weight and volume of the microspheres powder was (33.4 ± 2.03) mg/ml, easy for redissolution. It is confirmed that the flowability of the microspheres could be evaluated by the angle of repose. The smaller angle of repose reflected the better flowability. It is generally acknowledged that the angle of repose should be smaller than 40° so as to meet with the demand on flowability in industrial processes. An average angle of repose measured for the microspheres was (37.2 ± 3.97)°, suited for industrial processes. Moreover, the MMAD was an important indicator for powder for inhalation. Generally speaking, MMAD should range from 1 to 5 μm for deep lung deposition. An average MMAD was found to be (2.83 ± 0.30) μm in the study, desirable for pulmonary administration. The deposition ratio in the effective site was measured as (38.03 ± 2.30)%, suggesting that most microspheres could deposit at lungs to ensure the drug effects. The deposition ratio in the lungs depends on the powder dispersibility which is controlled by inter-particle cohesive forces. That is, high deposition ratio in the lungs was obtained with good powder dispersibility based on weak interparticulate forces (Steckel & Brandes, Citation2004). The result of dumping ratio was (96.53 ± 1.0)%, larger than 90%, meeting with the requirement as per the Pharmacopoeia of China, and indicated that the microspheres could be emptied sufficiently. The spray characteristic was assessed as “sprayed very easily” defined as “aerosolizing immediately, whole space filled with powder cloud and no granules depositing”, showing that the powder could be dispersed quickly and inhaled into the lung. Taken together, results of the micromeritics properties demonstrated that etoposide-loaded PLGA microspheres were extremely suitable for pulmonary delivery.
In vitro drug release behavior
Drugs released from PLGA microspheres may occur through polymer erosion, diffusion or a combination of those two. Furthermore, polymer properties, including molecular weight, copolymer composition and crystallinity can alter polymer degradation and lead to the change of drug release profiles (Zolnik & Burgess, Citation2007). Besides, physicochemical properties of the drug and properties of the microspheres can also greatly affect the release mechanism and rate of the drug from PLGA microspheres (Schaefer & Singh, Citation2001). The in vitro drug release profiles of etoposide from etoposide-loaded PLGA microspheres and etoposide solution (1 mg/ml) in phosphate buffer (pH 7.4, containing 0.1% polysorbate 80 and 0.02% NaN3) are shown in . While a burst release was observed in the initial 6 h in etoposide solution with 98% free etoposide liberated from the solution into the bulk phase, only 16% free etoposide was released from etoposide-loaded PLGA microspheres in the initial 6 h. Etoposide was graduated released from the microspheres as time lapsed (∼25% released in 1 d, ∼55% released in 10 d and ∼80% released in 20 d), suggesting that etoposide was well entrapped in etoposide-loaded PLGA microspheres. Under certain conditions, the in vitro release can be used for the assessment of bioequivalence (Costa & Sousa Lobo, Citation2001). Several models describing drug release from immediate and modified release dosage forms were adopted. The model that fitted best for etoposide released from the microspheres was Ritger–Peppas equation expressed as lnQ = 0.3593lnt − 2.4992, r = 0.9929, revealing that etoposide could be controlled released from the microspheres. Whereas the fittest model for etoposide solution was first-order kinetics with the equation ln(100 − Q) = −0.3365t + 4.2055, r = 0.9948. Hence, etoposide-loaded PLGA microspheres are thought to have the potential to maintain etoposide concentration within target ranges for a long time, decreasing side effects caused by concentration fluctuation, ensuring the efficiency of treatment and improving patient compliance by reducing dosing frequency.
Conclusion
In this study, we successfully prepared etoposide-loaded PLGA microspheres by improved emulsion-solvent evaporation method. The formulation and processing parameters were further optimized by orthogonal design. The freeze-drying method was then carried out to obtain etoposide-loaded PLGA microspheres dry powder. Etoposide-loaded PLGA microspheres had good physicochemical characteristics and micromeritics properties, both suitable for pulmonary inhalation. Results of in vitro drug release behavior showed that etoposide could be sustained released from the PLGA microspheres, indicating that etoposide-loaded PLGA microspheres could minimize drug concentration fluctuation and prolong drug action time. All the results indicated that etoposide-loaded PLGA microspheres could be administrated through pulmonary inhalation. The following work would be focused on further pharmacokinetic studies in rats to define the release profiles for etoposide from etoposide-loaded PLGA microspheres within lungs after pulmonary administrated. Moreover, more studies should be performed on the safety and effectiveness of etoposide-loaded PLGA microspheres for pulmonary drug delivery as well.
Acknowledgments
The authors wish to thank Qilu Pharmaceutical Co., Ltd for kindly providing the drug and the School of Pharmaceutical Science, Shandong University, for supporting this study on etoposide-loaded PLGA microspheres.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aisner J, Lee EJ. (1991). Etoposide. Current and future status. Cancer 67:215–9
- Anderson JM, Shive MS. (2012). Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev 64:72–82
- Bi R. (2008). Studies on dry powder inhalation of insulin loaded solid lipid nanoparticles. Shandong: Shandong University
- Calame W, van der Waals R, Douwes-Idema N, et al. (1988). Antibacterial effect of etoposide in vitro. Antimicrob Agents Chemother 32:1456–7
- Costa P, Sousa Lobo JM. (2001). Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–33
- Diab R, Brillault J, Bardy A, et al. (2012). Formulation and in vitro characterization of inhalable polyvinyl alcohol-free rifampicin-loaded PLGA microspheres prepared with sucrose palmitate as stabilizer: efficiency for ex vivo alveolar macrophage targeting. Int J Pharm 436:833–9
- Doan T, Couet W, Olivier J. (2011). Formulation and in vitro characterization of inhalable rifampicin-loaded PLGA microspheres for sustained lung delivery. Int J Pharm 414:112–7
- Gao J. 2005. Study on thymopentin(Tp5) dry powder inhalations for pulmonary delivery and their pharmacodynamics. Shanghai: Second Military Medical University
- Giovagnoli S, Blasi P, Schoubben A, et al. (2007). Preparation of large porous biodegradable microspheres by using a simple double-emulsion method for capreomycin sulfate pulmonary delivery. Int J Pharm 333:103–11
- Han B, Wang HT, Liu HY, et al. (2010). Preparation of pingyangmycin PLGA microspheres and related in vitro/in vivo studies. Int J Pharm 398:130–6
- Hande KR. (1998). Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34:1514–21
- Haznedar S, Dortunc B. (2004). Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm 269:131–40
- Lam JKW, Liang W, Chan HK. (2012). Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev 64:1–15
- Lee J, Oh YJ, Lee SK, et al. (2010). Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J Control Release 146:61–7
- Li X, Tang Y, Zhu J. (2010). Progress on dry powder inhalers. Chinese J Pharm 41:219–23
- Louey MD, Razia S, Stewart PJ. (2003). Influence of physico-chemical carrier properties on the in vitro aerosol deposition from interactive mixtures. Int J Pharm 252:87–98
- Nath SD, Son S, Sadiasa A, et al. (2013). Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int J Pharm 443:87–94
- Patlolla R, Vobalaboina V. (2005). Pharmacokinetics and tissue distribution of etoposide delivered in parenteral emulsion. J Pharm Sci 94:437–45
- Patton JS, Bukar J, Nagarajan S. (1999). Inhaled insulin. Adv Drug Deliv Rev 35:235–47
- Qian WY, Sun DM, Zhu RR, et al. (2012). pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. Int J Nanomed 7:5781–92
- Schaefer MJ, Singh J. (2001). Effect of additives on stability of etoposide in PLGA microspheres. Drug Dev Ind Pharm 27:345–50
- Schaefer MJ, Singh J. (2002). Effect of tricaprin on the physical characteristics and in vitro release of etoposide from PLGA microspheres. Biomaterials 23:3465–71
- Sengupta S, Tyagi P, Velpandian T, et al. (2000). Etoposide encapsulated in positively charged liposomes: pharmacokinetic studies in mice and formulation stability studies. Pharmacol Res 42:459–64
- Sham JOH, Zhang Y, Finlay WH, et al. (2004). Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int J Pharm 269:457–67
- Silva LFC, Kasten G, de Campos CEM, et al. (2013). Preparation and characterization of quercetin-loaded solid lipid microparticles for pulmonary delivery. Powder Technol 239:183–92
- Sinha VR, Singla AK, Wadhawan S, et al. (2004). Chitosan microspheres as a potential carrier for drugs. Int J Pharm 274:1–33
- Steckel H, Brandes HG. (2004). A novel spray-drying technique to produce low density particles for pulmonary delivery. Int J Pharm 278:187–95
- Tamber H, Johansen P, Merkle HP, Gander B. (2005). Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Adv Drug Deliv Rev 57:357–76
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. (2011). Small-cell lung cancer. Lancet 378:1741–55
- Vanbever R, Mintzes JD, Wang J, et al. (1999). Formulation and physical characterization of large porous particles for inhalation. Pharm Res 16:1735–42
- White S, Bennett DB, Cheu S, et al. (2005). EXUBERA®: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol Ther 7:896–906
- Xu Q, Czernuszka JT. (2008). Controlled release of amoxicillin from hydroxyapatite-coated poly (lactic-co-glycolic acid) microspheres. J Control Release 127:146–53
- Yadav KS, Chuttani K, Mishra AK, Sawant KK. (2010). Long circulating nanoparticles of etoposide using PLGA-MPEG and PLGA-pluronic block copolymers: characterization, drug-release, blood-clearance, and biodistribution studies. Drug Dev Res 71:228–39
- Yang Y, Bajaj N, Xu P, et al. (2009). Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 30:1947–53
- Yen SY, Sung KC, Wang JJ, et al. (2001). Controlled release of nalbuphine propionate from biodegradable microspheres: in vitro and in vivo studies. Int J Pharm 220:91–9
- Zhang Z, Bi X, Li H, et al. (2011). Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv 18:536–44
- Zhang Z, Huang G. (2012). Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Deliv 19:255–63
- Zolnik BS, Burgess DJ. (2007). Effect of acidic pH on PLGA microsphere degradation and release. J Control Release 122:338–44