Abstract
Objective: The specific expression of transferrin receptor can represent a diagnostic tool or therapeutic target in solid tumors expressing this antigen. Herein, the human transferrin receptor monoclonal antibody (T9) was investigated as a tumor-targeting group for active targeted-drug delivery systems.
Materials and methods: A tumor-targeted conjugate T9-TNF was synthesized by the attachment of both human transferrin receptor monoclonal antibody (T9) as a tumor-targeting group and human tumor necrosis factor-α (TNF) as an anti-cancer drug to two terminated hydroxyl groups of poly(ethylene glycol). Subsequently, a solvent evaporation technique was adopted to produce anti-cancer magnetic polymer microspheres T9-TNF-PC-M containing T9-TNF and Fe3O4 magnetic ultrafine powders (M) using poly(trimethylene carbonate-co-5,5-dimethyl trimethylene carbonate) (PC, P(TMC-co-DTC)) as a polymeric carrier.
Results and discussion: These magnetic polycarbonate microspheres possessed a steady TNF release rate in phosphate buffer saline solution, strong magnetic responsiveness and high T9-TNF loading capacity. In vitro cytotoxicity assays demonstrated the microspheres T9-TNF-PC-M and conjugate T9-TNF were strongly inhibitory to the human hepatic carcinoma (Bel-7204) cells. In vivo site-specific therapy in nude mice with human hepatic carcinoma indicated that the microspheres T9-TNF-PC-M and conjugate T9-TNF possessed markedly higher anti-tumor activity against Bel-7204 in mice than that of TNF.
Conclusions: These results indicated that the magnetic polycarbonate microspheres were suitable as the potential-targeted treatment for hepatic carcinoma therapeutics.
Introduction
In recent years, passive and active targeted-drug delivery systems have attracted extensive attention because of their potential to achieve selectively targeted delivery anti-cancer drugs directly at the site of interest and then avoid non-specific distribution (Park et al., Citation2009; Pinhassi et al., Citation2010; Stukel et al., Citation2010; Yan et al., Citation2010b). Polymeric microspheres with the targeting molecules that have a high affinity to tumor cells can increase the local concentration of the anti-cancer drugs in tumor tissue and show the tremendous promise in these ideal targeted-drug delivery systems for the treatment of cancers in the body (Yan et al., Citation2001; Chawla & Amiji, Citation2002; Johnson et al., Citation2002; Li et al., Citation2004; Yan et al., Citation2004; Ganta et al., Citation2008; Kim et al., Citation2008; Yan et al., Citation2008; Chen et al., Citation2009).
These tumor-targeting molecules, including monoclonal antibodies, proteins, vitamin and peptides, have been investigated to increase the site-specific accumulation of anti-cancer drugs and magnetic resonance imaging contrast agents in tumor cells (Yan & Zhuo, Citation2001; Yan et al., Citation2002; Yan et al., Citation2003; Yan et al., Citation2005; Daniels et al., Citation2006a; Yan et al., Citation2007a,Citationb; Yan et al., Citation2010a; Yan et al., Citation2011). The transferrin receptor is a membrane-bound protein expressed in the larger amounts in proliferating, e.g. malignant cells, than in quiescent cells. The specific expression of transferrin receptor can represent a diagnostic tool or a therapeutic target in solid tumors expressing this antigen. Therefore, the human transferrin receptor monoclonal antibody (T9) has been investigated as a tumor-targeting group for active targeted-drug delivery systems (Newman et al., Citation1982; Qian et al., Citation2002; Hafeli, Citation2004; Daniels et al., Citation2006b).
Some magnetic materials, such as magnetite and Fe3O4 magnetic ultrafine powders, have also been incorporated into synthetic polymers such as poly(lactic acid), poly(vinyl alcohol) (PVA) and polyalkylcyanoacrylate to produce magnetic microparticles which may act as the targeted-drug delivery systems for specific cancer therapies. Moreover, this delivery can also be more active through the use of a magnet field applied outside the organ which is used to drive the accumulation of drug carrying magnetic microspheres into localized tumors (Asher et al., Citation1991; Lubbe et al., Citation1996; Hu et al., 2008a).
Over the past decades, aliphatic polycarbonates, such as poly(trimethylene carbonate) (PTMC) and poly(5,5-dimethyl trimethylene carbonate) (PDTC), have been widely used as the biodegradable materials in drug delivery, surgical sutures, tissue engineering and gene delivery due to their good biodegradability, high biocompatibility, low toxicity, low immunogenity, superior mechanical properties and weak inflammatory effect raising from the degraded products (Liu et al., Citation2003; Feng & Zhang, Citation2005; Dobrzynski & Kasperczyk, Citation2006; Kaihara et al., Citation2009; Lu et al., Citation2009; Norowski & Bumgardner, Citation2009; Seow & Yang, Citation2009; Tokiwa et al., Citation2009). Some copolymers of PTMC with various functional groups have been synthesized and evaluated for applications in the biomedical field because they have low glass transition temperatures and retain an amorphous state under physiological conditions, which are crucial requirements for drug delivery carriers (Hu et al. Citation2008b,Citationc; Mei et al., Citation2008; Wang et al., Citation2009).
The human tumor necrosis factor-α (TNF) is a chemotherapeutic agent by virtue of its anti-tumor antibiotic activity. However, the broader application and efficacy of TNF has been hampered by high dose used, nearly close to lethal concentrations, the insufficient selectivity and elimination easily by the immune systems. Therefore, the tumor-targeting TNF-attached drugs can straightly be targeted to tumor cells, improve the efficiency toward malignant cells and reduce the drug dose of TNF (Jain, Citation1990; Blankenstein et al., Citation1991; Hino et al., Citation1996; Segura & Shea, Citation2001; Gaur & Aggarwal, Citation2003; Wajant et al., Citation2003; Tewis et al., Citation2004). In this work, TNF was chosen as an anti-tumor drug model and the tumor-targeted conjugate T9-TNF was synthesized by the attachments of both TNF and human transferrin receptor monoclonal antibody (T9) as a tumor-targeting group to two terminated hydroxyl groups of poly(ethylene glycol) (PEG, Mn: 2000). Subsequently, a biodegradable aliphatic carbonate copolymer PC, P(TMC-co-DTC) was synthesized () and further used to prepare the tumor-targeted microspheres T9-TNF-PC-M containing the conjugate T9-TNF and Fe3O4 magnetic ultrafine powders (M) by a modified water-in-oil-in-water solvent evaporation technique. In vitro drug release study, in vitro cytotoxicity assay to hepatocellular carcinoma cells and in vivo magnetic-targeting therapy to hepatic carcinoma in nude mice was evaluated herein.
Methods and materials
Instruments and reagents
The compounds were characterized using a Nicolet IS10 Fourier transform-infrared spectrophotometer, a UV–Vis spectrophotometer (UV-2800 series), a Varian Mercury-VX300 NMR spectrometer and an automatic contact angle meter (SL200A/B/D Series). Molecular weight determination was undertaken using a gel permeation chromatography (GPC) (Waters 2965D separations module, Waters 2414 Refractive Index Detector, Shodex K802.5 & K805 with Shodex K-G Guard Column, Polystyrene Standard, DMF solvent, 1.0 mLmin−1 flow rate, 323 K column temperature and 318 K detector temperature). The glass transition temperature (Tg) of the copolymer was determined using a differential scanning calorimeter (DSC) (NETZSCH DSC 200 F3). The morphology of microspheres was studied using a scanning electron microscope (SEM, Hitachi-X650) and specimens were coated with gold in SEM coating equipment. The recombinant human TNF (5 × 105 units/mL, 6 × 106 µ/mg activity), human transferrin receptor monoclonal antibody (T9, 1 mg/mL) and human serum albumin were provided by the Chinese Academy of Preventive Medicine. The male BALB/C nude mice (5–7 weeks old, weight: 20.40 ± 1.60 g) were provided by the Department of Pharmacy (Tongji Medical College, Huazhong University of Science and Technology, China) and were raised according to the procedure described in the literature (Shi, Citation1990). The human hepatic carcinoma cell line (Bel-7204) was provided by the China Center for Type Culture Collection of Wuhan University, China. The optical densities (OD570) were measured with a DG-3022A ELISA-Reader. The ethical approval was obtained for in vivo experiments in rabbits from the Department of Science and Technology of Hubei Province, China and the Animal Center of Tongji Medical College, Huazhong University of Science and Technology, China.
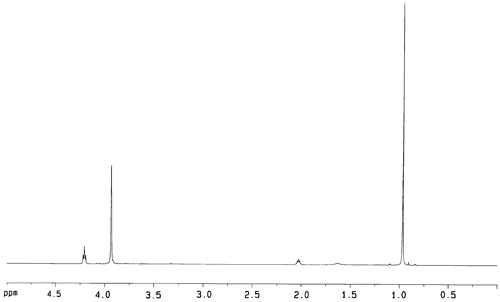
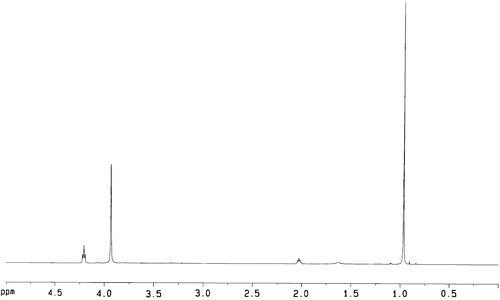
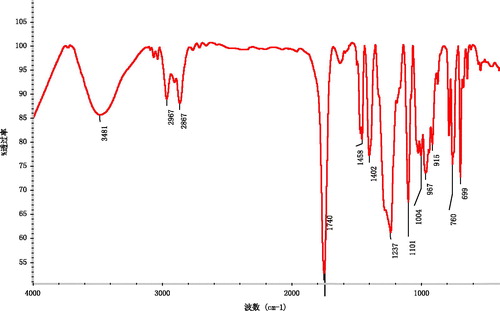
All chemicals and solvents were of analytical grade. Tin (II) 2-ethylhexanoate (Sn(Oct)2) and Fe3O4 magnetic ultrafine powders (average diameter 19.1 nm, surface area ≥ 97 m2/g, material coercive force 250Q) were purchased. The growth medium employed was the usual RPMI-1640 media (10% fetal bovine serum, 100 units/mL Penicillium, 100 µg/mL streptomycin). The solution of phosgene in toluene (3.4 M) was prepared according to the method described in the literature (Huang & Chen, Citation1985). DTC, TMC and aliphatic carbonate copolymer PC, P(TMC-co-DTC) were prepared according to the method described in the literatures (Zurita et al., Citation2006; Mei et al., Citation2008; Lu et al., Citation2009). P(TMC-co-DTC): 1H NMR (CDCl3, δ, ppm): 4.25 (s, CH2OC=O), 3.95 (t, CH2O), 2.04 (m, C–CH2-C),1.0 (s, CH3); 13C NMR (CDCl3, δ, ppm): 154.91, 155.28 (C=O), 72.44 (C–CH2–OCO), 64.30 (CH2–CH2–OCO), 35.12 (C–C–C), 28.05 (C–CH2–C), 21.36 (–CH3); IR (KBr, cm−1): 2982, 2877 (C–H), 1740 (C=O), 1465–1404 (CH3) 1261 (C–O–C=O), 1111 (C–O); Mn 7.615 × 104, Mw/Mn 3.10, repeat structure unit TMC/DTC in copolymer (mol/mol) was 42.9/57.1, glass transition temperature (Tg) was 42.5 °C; melting temperature (Tm) was 141 °C; water contact angle was 77°; water absorption ratio was 0.67%.
Synthesis of the conjugate of TNF and T9 (TNF-T9)
PEG (Mn: 2000, 30 g, 15 mmol) was dissolved in 150 mL of anhydrous toluene and kept boiling for 2 h to separate water from toluene under an argon atmosphere. The solvent was further evaporated to remove and the residue was dissolved in 100 mL of a solvent of anhydrous toluene and chloroform (100 mL, v/v: 3:1). The phosgene solution in toluene (18 mL) was added slowly with rapidly stirring at −5 °C to 0 °C. The reaction mixture was stirred for 2 h at this temperature and 24 h at room temperature under an argon atmosphere.
The solvent was evaporated to remove again and the resultant solid was dried under vacuum for 5 h, and then dissolved in 100 mL of a solvent of anhydrous toluene and dichloromethane (100 mL, v/v: 2:1). N-hydroxysuccinimide (SuOH, 5.25 g, 15 mmol) was added and triethylamine (4.2 mL, 30 mmol) was added slowly to the reaction solution with rapidly stirring at room temperature. The reaction mixture was continued stirring for 3 h and filtered. The filtrate was evaporated to remove the solvents and the resultant solid was recrystallized using iso-propanol twice and dried under vacuum for 48 h to yield a white powder of SuO-PEG-OSu (27.8 g, 83%, m.p.: 48–50 °C).
The recombinant human TNF (600 µL) and 0.1 M phosphate buffer saline solution (PBS, 1 mL, 0.1 mol/L, pH 7.4) were slowly added to the solution of SuO-PEG-OSu in PBS (200 µL, 50 mg/mL). The reaction mixture was stirred for 4 h at 4 °C. After dialysis for 12 h at 4 °C, the dialyzed solution was added the human transferrin receptor monoclonal antibody (T9, 1 mL) and 1 mL of PBS. The reaction mixture was stirred for 4 h at 4 °C and then dialyzed for 6 h at 4 °C. The dialyzed solution was separated by the Sephadex G-100 gel column (2 × 60 cm, velocity of flow: 0.4 mL/min, UV-detected wavelength: 280 nm) and the solution of the first UV absorption peak (70 mL) was collected and concentrated using PEG to the product T9-TNF (1.5 mL). The product solution was sterilized by filtration using the 0.22 µm syringe colander and kept at −20 °C.
Preparation of microspheres
The P(TMC-co-DTC) copolymer (PC, 1.0 g) was dissolved in a 0.1% solution of Span-80 in dichloromethane (15 mL) and 0.5 mL T9-TNF, 50 µL human serum albumin solution (1 mg/mL) and 300 mg of Fe3O4 magnetic ultrafine powders were added. The solution was homogenized by sonication for 1 min (50 W) whilst cooled in an ice-salt bath and then poured into a 2% solution of PVA in distilled water (60 mL). The mixture was vortexed for 3 min, stirred rapidly for a further 30 min, and then 130 mL of water was added drop-wise under vigorous stirring at room temperature. Subsequently, rapid stirring of the mixture, open to the air, at 30 °C was continued for a further 3 h in order to remove dichloromethane by evaporation. After centrifugation (105 rpm), the precipitate was collected, washed with distilled water and lyophilized to afford a gray power of the microspheres containing T9-TNF and Fe3O4 magnetic ultrafine powder (T9-TNF-PC-M). The empty P(TMC-co-DTC) magnetic microspheres (PC-M) without T9-TNF and non-magnetic microspheres (T9-TNF-PC) containing T9-TNF were prepared by the same method described above. These microspheres were treated through the cobalt-60 radiation sterilization.
In vitro drug release study
The TNF-PC-M microspheres (5 mg) were suspended in 4 mL of the PBS (0.1 mol/L, pH 7.4). The mixture was slowly shaken in a thermostatically controlled water bath at 37 °C. After centrifugation (4000 rpm, 5 min), 15 μL of the solution was taken and the concentration of TNF per 5 h was measured by the TNF ELISA KIT.
In vitro cytotoxicity assay
The human hepatic carcinoma cells (Bel-7204, 1 × 105/mL) were seeded in 96-well plates in RPMI growth medium at a density of 2 × 104 cells/well. The cells were incubated for 24 h in an incubator (37 °C, 5% CO2) and 100 μL of the growth medium containing 0.9% NaCl (Blank test), TNF, T9-TNF, polycarbonate microspheres containing T9-TNF and Fe3O4 magnetic ultrafine powder (T9-TNF-PC-M), non-magnetic polycarbonate microspheres (T9-TNF-PC) containing T9-TNF, or empty polycarbonate magnetic microspheres without T9-TNF (PC-M), respectively, were added. After 48 h incubation, the cells were washed with the growth medium. Twenty microliters of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide solution (5.0 mg/mL) were added and the cells were further incubated for 4 h. The cells were then washed again, this time with 3% fetal calf serum and PBS (3%FCS-PBS) and 100 μL of dimethyl sulfate were added. Subsequently, the cells were shaken for 30 min at room temperature. The optical densities (OD570) were measured at 570 nm and expressed as a percentage relative to control cells.
In vivo inhibition of growth of hepatic carcinoma
An electromagnet with a magnetic flux density of a maximum of 5000 GS was used to produce an inhomogeneous magnetic field. The magnetic flux density was focused onto the region of the tumor with a specially adopted pole shoe that was placed in contact with the surface of the tumor. On the tip of the pole shoe, the gradient has its maximum demonstrating the dependence of the magnetic flux density on the distance to the pole shoe.
The Bel-7204 cells (5 × 106) were injected subcutaneously to the back of six-week-old male nude BALB/c mice (20.40 ± 1.60 g). Mice bearing a tumor of 0.6–0.8 cm in diameter were considered as positive. Thirty-six mice were divided into six groups, each mouse was injected with 0.9% NaCl (Blank test) (0.2 mL), TNF (0.2 mL), T9-TNF (0.2 mL), polycarbonate microspheres containing T9-TNF and Fe3O4 magnetic ultrafine powder (T9-TNF-PC-M, 0.2 mL), non-magnetic polycarbonate microspheres containing T9-TNF (T9-TNF-PC, 0.2 mL), or empty magnetic polycarbonate microspheres without T9-TNF (PC-M, 0.2 mL, 12.5 mg/mL), respectively, via the tail vein. Subsequently, the mouse was anesthetized with urethane (10%, 10 mL/kg), positioned prone and fixed to a polystyrene cradle with adhesive tape to minimize motion. A magnetic flux density of 5000 GS was estimated in the region of the tumor surface and at 10 mm below the tip of the pole shoe immediately after the injection with the microspheres. The mice were exposed to magnetic field for 30 min. This procedure of injection and targeting therapy was repeated every three days. Mice were sacrificed after four weeks and then the tumors were isolated and weighed accurately. The inhibition of the tumor growth was calculated compared with untreated mice.
Results and discussion
Preparation and characterization
Aliphatic carbonate copolymer P(TMC-co-DTC) was synthesized by the polymerization of TMC and DTC using Sn(Oct)2 as a catalyst. The 1H NMR spectra showed the characteristic methylene peaks (2.04 ppm) of the –CH2CH2CH2– group of the TMC repeat unit and the characteristic peaks (1.0 ppm) of the –CH3 group of the DTC repeat unit in copolymer structure (). The data of 13C NMR and IR also supported the expected formation of carbonate copolymer P(TMC-co-DTC) ().
The human TNF is a recognized chemotherapeutic agent by virtue of its anti-tumor antibiotic activity and was chosen as an anti-tumor drug model in this work. Two tumor-targeting polymeric drug delivery systems, including the conjugate T9-TNF and polycarbonate microspheres T9-TNF-PC-M containing T9-TNF and Fe3O4 magnetic ultrafine powders, were prepared, respectively. Polymeric microspheres can show the tremendous promise in the ideal target-specific delivery of TNF for the treatment of cancers in the body. The conjugate T9-TNF was a targeted prodrug synthesized by the attachments of TNF and human transferrin receptor monoclonal antibody (T9) as a tumor-targeting group.
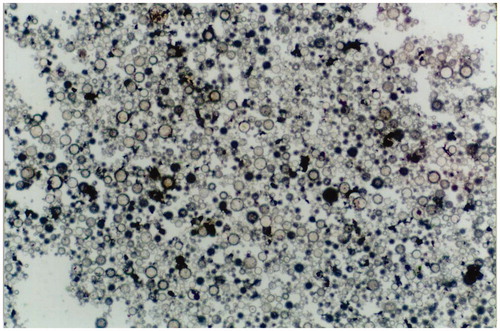
Carbonate copolymer P(TMC-co-DTC) was used to prepare the T9-TNF-PC-M microspheres containing T9-TNF and Fe3O4 magnetic ultrafine powders, the empty magnetic microspheres (PC-M) without T9-TNF, and non-magnetic microspheres (T9-TNF-PC) containing T9-TNF by a solvent evaporation technique. The SEM morphologies of T9-TNF-PC-M microspheres are shown in . The average diameter of the T9-TNF-PC-M microspheres was 1.0 μm (ranging from 0.7 to 2.4 μm). In these microspheres, the average weight of magnetic powder accounted for 35% of the mass and the TNF loading concentration was 102 units/mg. Their mobile velocity was 23.5 cm/min in a magnetic field of 5000 GS. Meanwhile, the TNF loading concentration of non-magnetic T9-TNF-PC microspheres was 106 units/mg. The average weight of magnetic power in empty magnetic microspheres (PC-M) without T9-TNF was 37% of the mass and their mobile velocity was 26.5 cm/min in a magnetic field of 5000 GS. The resultant homogenous T9-TNF-PC-M microspheres possessed high water affinity, good dispersal and mobility in 0.9% sodium chloride solution suiting the experimental purpose. Therefore, the microspheres had the potential to be applied in targeting therapy of human hepatic carcinomas under the influence of a controlling magnetic field.
In vitro drug release study
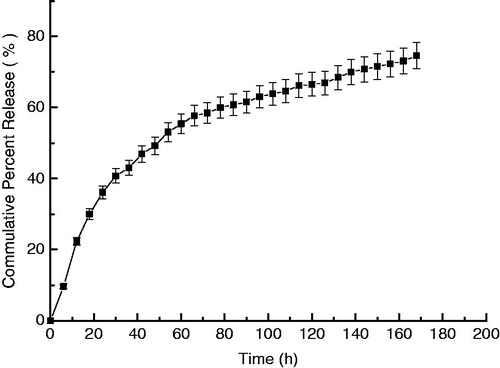
The TNF release profile of T9-TNF-PC-M microspheres in PBS was shown in . A substantial release rate from T9-TNF-PC-M microspheres was sustained over the seven days of measurement. The microspheres had no obvious phenomenon of abrupt release and the initial drug release was fast but trended to gently as lengthening of the time. The cumulative percent releases of TNF from polycarbonate anti-cancer drug microspheres reached 74.62% up to seven days. The T9-TNF-PC-M microspheres released the drug slow, presumably due to the low degradation rate and low water absorption rate of polycarbonate copolymer P(TMC-co-DTC) and the low drug diffusion coefficient of TNF in the T9-TNF-PC-M microspheres.
In vitro cytotoxicity assay
The effects on cell growth and metabolism of human hepatic carcinoma cells (Bel-7204) in vitro of the drug alone, microspheres containing magnetic particles, the conjugate of T9 and TNF, non-magnetic microspheres containing the drug T9-TNF and microspheres containing both the drug T9-TNF and magnetic particles (TNF, PC-M, T9-TNF, T9-TNF-PC and T9-TNF-PC-M) are shown in .
Table 1. In vitro anti-tumor activity of microspheres to human hepatocellular carcinoma Bel-7402 cells.
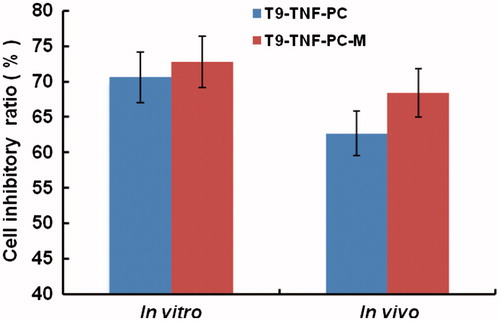
At the same concentration of TNF (5000 U) in the growth medium, the Bel-7204 cells incubated with the targeting conjugate T9-TNF produced over 62.4%, respectively, mortalities relative to control, and higher than those of the cells incubated with TNF (57.3%) and empty magnetic microspheres PC-M (5.2%). Moreover, the Bel-7204 cells incubated with non-magnetic microspheres T9-TNF-PC and magnetic microspheres T9-TNF-PC-M produced over 72.8% and 70.6% mortality at the lower concentration of TNF (500 U) in the growth medium, respectively, relative to control, and significantly higher than those of the cells incubated with TNF (57.3%) at the higher concentration (5000 U). The results supported the concept of that the TNF was released slowly from the microspheres, and hence the steady controlled-release and extended exposure led to increased kill rates for the tumor cells. Both T9-TNF-PC and T9-TNF-PC-M similarly demonstrated strongly inhibitory and high anti-tumor activity to Bel-7204 cells in vitro indicating that the presence of the magnetic particles did not have a detrimental effect on the efficacy whether on the action of TNF or the release rates from the microspheres.
In vivo growth inhibition of hepatic carcinoma
Tumor growths after receiving injections with either TNF, PC-M, T9-TNF, T9-TNF-PC or T9-TNF-PC-M were monitored and the growth inhibitions were measured relative to control. After four weeks, the inhibition by T9-TNF (with high dose of 10 000 U), T9-TNF-PC (with low dose of 1000 U) and T9-TNF-PC-M (with low dose of 1000 U) on the growth of hepatic carcinoma in mice was 60.75%, 62.74% and 68.40%, respectively, and all were significantly higher than that of TNF (26.88%) (with high dose of 10 000 U) (). As a consequence, the increased drug concentration at the targeted site and an enhanced local release of the drugs improved the therapeutic action on the tumors. Under a magnetic field of 5000 GS, magnetic polymeric microspheres T9-TNF-PC-M can be activated by a magnet applied outside the body and the human transferrin receptor monoclonal antibody (T9) as a tumor-targeting group, and then accumulated specifically drugs into localized hepatic carcinomas.
Table 2. In vivo inhibitory effect of microspheres on nude mice-bearing human hepatocellular carcinoma.
Subsequently, TNF is expected to have been released slowly from the magnetic polymer microspheres was further endocytosed and then taken up primarily by the tumor cells. The increased localized levels of TNF, activated the immune system to the Bel-7204 cells and hence suppressed the growth of tumor. The targeting therapy of T9-TNF-PC-M applied to nude mice-bearing Bel-7204 carcinomas, provided markedly inhibited tumor growth (68.40%) which was higher than the effect of single TNF or the activities of the conjugate T9-TNF and non-magnetic microspheres (T9-TNF-PC) (). Therefore, these results indicated that the T9-TNF-PC-M microspheres possessed markedly high anti-tumor activity against human hepatic carcinoma (Bel-7204) in vivo and showcased the potential for this treatment modality. In addition, the results showed that these microspheres T9-TNF-PC and T9-TNF-PC-M, and conjugate T9-TNF protected TNF from the elimination of the immune system in the body. Moreover, the T9-TNF-PC-M microspheres showed the noticely higher tumor inhibitory rate and anti-tumor activity to the human hepatic carcinoma cells (Bel-7204) in vitro and in vivo than that of T9-TNF.
Conclusions
The tumor-targeted conjugate T9-TNF was synthesized by the incorporation of both T9 as a tumor-targeting group and TNF as an anti-cancer drug into PEG. A solvent evaporation technique was adopted to produce anti-cancer magnetic polymer microspheres T9-TNF-PC-M containing T9-TNF and Fe3O4 magnetic ultrafine powders using carbonate copolymers P(TMC-co-DTC) as drug delivery carriers. Compared with TNF, the T9-TNF-PC-M microspheres possessed a steady TNF release rate in PBS, strong magnetic responsiveness, high tumor inhibition, and high anti-tumor activity against human hepatic carcinoma cells (Bel-7204) in vitro and in vivo. Thus, these magnetic polycarbonate microspheres containing T9-TNF and Fe3O4 magnetic ultrafine powders have the potential as targeted-drug delivery systems for hepatic carcinoma therapeutics.
Declaration of interest
We thank the National Natural Science Foundation of China (Grant No. 51173140, 51373128), Wuhan Scientific and Technological Project, Hubei Province (No. 2013010501010131), Key Science Foundation of Hubei Province (Grant No. 2009CDA052), China for their financial support.
References
- Asher AL, Mule JJ, Kasid A, et al. (1991). Murine tumor cells transduced with the gene for tumor necrosis factor-α: evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol 146:3227–34
- Blankenstein T, Qin Z, Uberla K, et al. (1991). Tumor suppression after tumor cell targeted TNF alpha gene transfer. J Exp Med 173:1047–52
- Chawla JS, Amiji MM. (2002). Biodegradable poly(epsilon-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm 249:127–38
- Chen H, Yan GP, Li L, et al. (2009). Synthesis, characterization and properties of ε-caprolactone and carbonate copolymers. J Appl Polym Sci 114:3087–96
- Daniels TR, Delgado T, Helguera G, et al. (2006a). The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol 21:159–76
- Daniels TR, Delgado T, Rodriguez JA, et al. (2006b). The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 121:144–58
- Dobrzynski P, Kasperczyk J. (2006). Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. V. multiblock and random copolymers of L-lactide with trimethylene carbonate obtained in copolymerizations initiated with zircon ium(IV) acetylacetonate. J Polym Sci Part A: Polym Chem 44:3184–201
- Feng YK, Zhang SF. (2005). Hydrophilic-hydrophobic AB diblock copolymers containing poly(trimethylene carbonate) and poly(ethylene oxide) blocks. J Polym Sci Part A: Polym Chem 43:4819–27
- Ganta S, Devalapally H, Shahiwala A, et al. (2008). A review of stimuli-responsive nanocarriers for drug and gene delivery. J Controll Release 126:187–204
- Gaur U, Aggarwal BB. (2003). Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol 66:1403–8
- Hafeli UO. (2004). Magnetically modulated therapeutic systems. Inter J Pharm 277:19–24
- Hino N, Higashi T, Nouso K, et al. (1996). Apoptosis and proliferation of human hepatocellular carcinoma. Liver 16:123–9
- Hu B, Yan GP, Zhuo RX, et al. (2008a). Polycarbonate microspheres containing tumor necrosis factor-α genes and magnetic powder as potential cancer therapeutics. J Appl Polym Sci 107:3343–9
- Hu XL, Chen XS, Xie ZG, et al. (2008b). Aliphatic poly(ester-carbonate)s bearing amino groups and its RGD peptide grafting. J Polym Sci Part A: Polym Chem 46:7022–32
- Hu XL, Liu S, Chen XS, et al. (2008c). Biodegradable amphiphilic block copolymers bearing protected hydroxyl groups: synthesis and characterization. Biomacromolecules 9:553–60
- Huang WD, Chen CQ. (eds.) (1985). Protection of amino groups. In: Synthesis of polypeptides. Beijing, China: Science Press Ltd., 13--44
- Jain RK. (1990). Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 50:814–9
- Johnson J, Kent T, Koda J, et al. (2002). The MTC technology: a platform technology for the site-specific delivery of pharmaceutical agents. Eur Cells Mater 3:12–5
- Kaihara S, Fisher JP, Matsumura S. (2009). Chemo-enzymatic synthesis of degradable PTMC-b-PECA-b-PTMC triblock copolymers and their micelle formation for pH-dependent controlled release. Macromol Biosci 9:613–21
- Kim MS, Hyun H, Kim BS, et al. (2008). Polymeric nano-micelles as drug carrier using polyethylene glycol and polytrimethylene carbonate linear and star-shaped block copolymer. Curr Appl Phys 8:646–50
- Li H, Yan GP, Wu SN, et al. (2004). Numerical simulation of controlled nifepine release from chitosan microspheres. J Appl Polym Sci 93:1928–37
- Liu ZL, Zhang JM, Zhuo RX. (2003). Synthesis and characterization of functional polycarbonates. Chem J Chin Univ 24:1730–2
- Lu K, Yan GP, Chen H, et al. (2009). Microwave-assisted ring-opening copolymerization of ε-caprolactone and 2-phenyl-5,5-bis(oxymethyl) trimethylene carbonate. Chin Sci Bull 54:3237–43
- Lubbe AS, Bergemann C, Riess H, et al. (1996). Clinical experiences with magnetic drug targeting: a phase I study with 4'-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 56:4686–93
- Mei LL, Yan GP, Yu XH, et al. (2008). Ring-opening copolymerization and properties of polycarbonate copolymers. J Appl Polym Sci 108:93–8
- Newman R, Schneider C, Sutherland R, et al. (1982). The transferrin receptor. Trends Biochem Sci 7:397–400
- Norowski PA, Bumgardner JD. (2009). Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res Part B: Appl Biomater 88B:530–43
- Park K, Lee S, Kang E, et al. (2009). New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater 19:1553–66
- Pinhassi RI, Assaraf YG, Farber S, et al. (2010). Arabinogalactan-folic acid-drug conjugate for targeted delivery and target-activated release of anticancer drugs to folate receptor-overexpressing cells. Biomacromolecules 11:294–303
- Qian ZM, Li H, Sun H, et al. (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 54:561–87
- Segura T, Shea LD. (2001). Materials for non-viral gene delivery. Annu Rev Mater Res 31:25–46
- Seow WY, Yang YY. (2009). Functional polycarbonates and their self-assemblies as promising non-viral vectors. J Controll Release 139:40–7
- Shi FQ. (ed.) (1990). How to copy the disease model of the animals. In: Medical animal experiment method. Beijing, China: People's Medical Publishing House, pp. 226--32
- Stukel JM, Li RC, Maynard HD, et al. (2010). Two-step synthesis of multivalent cancer-targeting constructs. Biomacromolecules 11:160–7
- Tewis B, Angela B, Heinz R, et al. (2004). A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol (England) 6:97–105
- Tokiwa Y, Calabia BP, Ugwu CU, et al. (2009). Biodegradability of plastics. Int J Mol Sci 10:3722–42
- Wajant H, Pfizenmaier K, Scheurich P. (2003). Tumor necrosis factor signaling. Cell Death Differ 10:45–65
- Wang CF, Lin YX, Jiang T, et al. (2009). Polyethylenimine-grafted polycarbonates as biodegradable polycations for gene delivery. Biomaterials 30:4824–32
- Yan GP, Zhuo RX. (2001). Research progress of magnetic resonance imaging contrast agents. Chin Sci Bull 46:531–8
- Yan GP, Daniel B, Bottle SE. (2007a). Synthesis and properties of novel porphyrin spin probes containing isoindoline nitroxides. Free Radic Biol Med 43:111–6
- Yan GP, Liu ML, Li LY. (2005). Studies on polyaspartamide gadolinium complexes containing sulfadiazine groups as MRI contrast agents. Bioconjug Chem 16:967–71
- Yan GP, Li H, Bottle SE, et al. (2004). Preparation, properties and mathematical modeling of microparticle drug delivery systems based on biodegradable amphiphilic tri-block copolymers. J Appl Polym Sci 92:3869–73
- Yan GP, Li Z, Xu W, et al. (2011). Porphyrin-containing polyaspartamide gadolinium complexes as potential magnetic resonance imaging contrast agents. Int J Pharm 407:119–25
- Yan GP, Robinsonand L, Hogg P. (2007b). Magnetic resonance imaging contrast agents: overview and perspectives. Radiography 13:e5–19
- Yan GP, Zhuo RX, Zheng CY. (2001). Study on the anticancer drug 5-fluorouracil-conjugated polyaspartamides containing hepatocyte-targeting group. J Bioact Compat Polym 16:277–93
- Yan GP, Peng L, Jian SQ, et al. (2008). Spin probes for electron paramagnetic resonance imaging. Chin Sci Bull 53:3777–89
- Yan GP, Xu W, Yang L, et al. (2010a). Dextran gadolinium complexes as contrast agents for magnetic resonance imaging to sentinel lymph nodes. Pharma Res 27:1884–92
- Yan GP, Zheng CY, Cao W, et al. (2003). Synthesis and preliminary evaluation of gadolinium complexes containing sulfonamide groups as potential MRI contrast agents. Radiography 9:35–41
- Yan GP, Zhuo RX, Zhang X, et al. (2002). Tumor-selective macromolecular MRI contrast agents. J Bioact Compat Polym 17:139–51
- Yan GP, Zong RF, Li L, et al. (2010b). Anticancer drug loaded nanospheres based on biodegradable amphiphilic ε-caprolactone and carbonate copolymers. Pharm Res 27:2743–54
- Zurita R, Puiggali J, Franco L, et al. (2006). Copolymerization of glycolide and trimethylene carbonate. J Polym Sci Part A: Polym Chem 44:993–1013