Abstract
Ionically crosslinked microspheres of acrylamide (AAm) grafted poly (vinyl alcohol) (PVA)/sodium alginate (NaAlg) were prepared by crosslinking with FeCl3 and 5-fluorouracil (5-FU), which is an anticancer drug and was successfully encapsulated into the microspheres. The graft copolymer (PVA-g-PAAm) was characterized by using Fourier transform infrared spectroscopy (FTIR) and elemental analysis. The prepared microspheres were characterized by FTIR and scanning electron microscopy (SEM). Microspheres were also characterized by particle diameter, equilibrium swelling values and release profiles. The release studies were carried out at three pH values 1.2, 6.8 and 7.4, respectively, each for 2 h. The effects of preparation conditions as PVA-g-PAAm/NaAlg ratio, drug/polymer ratio, crosslinker concentration and exposure time to FeCl3 on the release of 5-FU were investigated for 6 h at 37 °C. The highest 5-FU release was found to be as 99.57% (w/w) at the end of 6 h for PVA-g-PAAm/NaAlg ratio of 1:4 (w/w), drug/polymer ratio of 1:8 (w/w), crosslinker concentration of 0.05 M and exposure time of 10 min. The release results were also supported by the swelling measurements of the microspheres. Release kinetics was described by Fickian and non-Fickian approaches.
Introduction
Recently, polysaccharide microspheres have got much attention because of their low toxicity, good biocompatibility and biodegradability, which are of interest for application in biomedical and pharmaceutical industry (Dai et al., Citation2012). Natural polymers like sodium alginate (NaAlg) (Şanlı & Solak, Citation2009), chitosan (Al-Kahtani Ahmed et al., Citation2009) and methyl cellulose (Rokhade et al., Citation2007) have been preferred because of their biocompatibility and biodegradability. However, there are some synthetic polymers that exhibit biocompatibility under the physiological conditions used in controlled release studies (Babu et al., Citation2008). For this purpose, in this study, acrylamide was grafted onto poly (vinyl alcohol) (PVA) and blended with NaAlg to prepare semi-IPN microspheres.
5-Fluorouracil (5-FU) is one of the oldest chemotherapeutic drugs in use. It is commonly used against many cancers such as, colon, stomach, breast and pancreatic cancers. It is a fluorinated analog of pyrmidine base uracil, which is metabolized intracellulary to its active form, fluorodeoxyuridine monophophate (FdUMP). The active form inhibits DNA synthesis by inhibiting the normal production of thymidine (Gupte & Ciftci, Citation2004).
The delivery of chemotherapeutic agents using polymeric microspheres has become one of the most popular areas of research because of the possibilities of reducing toxicities, enhancing controlled release activity and also localizing the drug delivery. For this purpose, attempts have been focused on the development of drug delivery systems containing antineoplastic drugs. Huang et al. (Citation2009) studied in vitro release of 5-FU from genipin-gelatin microcapsules. They reported that uniform genipin-gelatin microcapsules would provide many potential usages for pharmaceutical applications. Sastre et al. (Citation2007) prepared microspheres of 5-FU-loaded poly(D, L-lactide), poly(D, L-lactide-co-glycolide) 75:25 and poly(D, L-lactide-co-glycolide) 50:50 by the spray-drying technique and subcutaneously injected in the back of Wistar rats in order to evaluate the 5-FU release and biodegradation characteristics. Huang et al. (Citation2010) prepared chitosan/chondroitin sulfate complex microcapsules to encapsulate the 5-FU by emulsion-chemical crosslinking method. They reported that the release performance of the microcapsules could be controlled by the degree of crosslinking, drug loading and pH of the release medium. Reddy et al. (Citation2008) synthesized semi-IPN microspheres of glutaraldehyde crosslinked NaAlg and N-isopropylacrylamide, loaded with 5-FU. Drug release from the microspheres at 25 and 37 °C confirmed the thermosensitive nature in vitro dissolution. Ganguly et al. (Citation2011) studied the release of 5-FU to the colon. The coated microspheres were found to be more suitable for colon targeting than the uncoated formulations. Blend microspheres of poly(3-hydroxybutyrate) and cellulose acetate phthalate was prepared by Chaturvedi et al. (Citation2011) for the colon delivery of 5-FU.
A few studies have examined drug release from Fe3+ crosslinked beads, microspheres and nanospheres (Mi et al., Citation1997; Sungur, Citation1999; Aiedeh & Taha, Citation2001). Mi et al. (Citation1997) Prepared iron (III)-carboxymethylchitin microspheres for sustained release of 6-mercaptopurine, which is an anticancer agent. They reported that carboxmethylchitin might prove useful as a polymer carrier for the sustained release of anticancer drugs in various dissolution media. Sungur (Citation1999) studied the crosslinking carboxymethylcellulose with ferric ions for the controlled release of erythromycin. Aiedeh & Taha (Citation2001) prepared chitosan succinate and hydroxyamated chitosan succinate, and used this semisynthetic polymer in the preparation of theophylline iron (III) crosslinked polymeric beads. They reported that the generated beads proved to be successful in prolonging drug release. Kim et al. (Citation2012) prepared alginate-carboxymethylcellulose beads with Fe3+ ions and studied controlled release of protein therapeutics.
In this study, we have first synthesized PVA-g-PAAm and than blended with NaAlg to produce the semi-IPN microspheres by crosslinking with FeCl3. The microspheres formed have been characterized by variety of techniques to understand their drug release and morphological characteristics as well as chemical interactions. Particle size, microspheres’ yield, entrapment efficiency and equilibrium swelling degree of the microspheres were determined and 5-FU release rates were investigated at pH values of 1.2, 6.8 and 7.4. The effects of PVA-g-PAAm/NaAlg ratio, exposure time, crosslinker concentration, pH and drug/polymer ratio on 5-FU release were researched and discussed to optimize the release of 5-FU from the microspheres.
Experimental
Materials
NaAlg (medium viscosity) was purchased from Sigma Chemical Co. (St. Louis, MO). 5-FU was provided by Sigma-Aldrich (Steinem, Germany). Na2HPO4, NaH2PO4, hydroquinone, DMSO, acetone, benzophenone, FeCl3, PVA and AAm (microspheres of acrylamide) were all supplied from Merck (Darmstadt, Germany) and used as received. The molecular weight and degree of saponification of PVA were 72.000 and >98%, respectively.
Synthesis of the graft copolymer of PVA with AAm
The graft copolymer of PVA and acrylamide was prepared by using ultraviolet (UV) radiation. Briefly, 10 g of PVA was dissolved in 100 mL of water at 60 °C and then AAm (6 M) solution was added to this solution and mixed for 30 min. After that, benzophenone as a photoinitiator (0.1%, w/w) was added to this solution and polymerization was carried out under a slow stream of nitrogen gas for 6 h with constant stirring. The polymerization was terminated by adding saturated hydroquinone solution. Then, the resultant solution was added into excess amount of acetone to precipitate the polymer. The polymer was dried at 40 °C till constant weight. The graft percentage was found as 18% by using elemental analysis results which is presented in .
Table 1. The elemental analysis results.
Preparation of the 5-FU-loaded microspheres
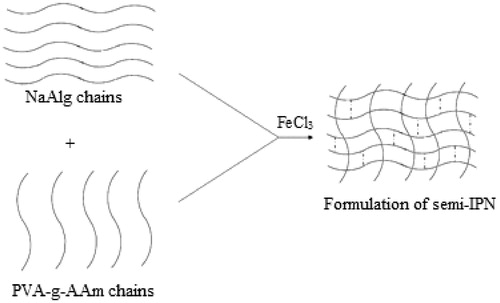
Briefly, NaAlg (2%, w/v) and PVA-g-PAAm (8%, w/v) were dissolved in distilled water by heating. Polymer solution containing 5-FU in various drug/polymer ratios was added dropwise into FeCl3 solution (0.05, 0.1 and 0.2 M) with a peristaltic pump (Masterflex, L/S Digital Economy Drive, Canada and USA). The formed microspheres were removed from crosslinking solution at 5, 10 and 15 min and washed with water. The microspheres were then dried completely in an oven at 40 °C. The microsphere preparation conditions are presented in . A shematic presentation of synthesis of semi-IPN is given .
Table 2. Preparation conditions of the 5-FU-loaded PVA-g-AAm/NaAlg semi-IPN microspheres.
Swelling experiments
Equilibrium water uptake by the microspheres was determined by measuring the extent of the swelling of the matrix in pH 1.2, 6.8 and 7.4. To ensure complete equilibration, samples were allowed to swell for 24 h. Excess surface-adhered liquid drops were removed by blotting. The swollen microspheres were weighted using electronic balance (Precise XB 220 A, USA). The microspheres were then dried in an oven at 40 °C, until there was no change in the dried mass of the samples. The percent equilibrium swelling degree was calculated as follows:
Where Ms and Md were the mass of the swollen microspheres and dry microspheres, respectively.
Determination of 5-FU content of the microspheres
The known mass of microspheres was crushed in an agate mortar with a pestle and then the polymeric powder was taken in a flask. Water (50 mL) was added and refluxed at 25 °C for 1 h, to ensure the complete extraction of 5-FU from the microspheres. At the end of 1 h, precipitated NaAlg was filtered and 5-FU was analyzed by using a UV spectrophotometer (Unico 4802 UV/VIS, UK) at a wavelength of 266 nm using a calibration curve and water as a blank. Percentage of entrapment efficiency was then calculated as follows:
Fourier transform infrared measurements
Infrared spectra of PVA and PVA-g-PAAm, 5-FU and 5-FU-loaded microspheres were taken with Fourier transform infrared (FTIR) spectrometer of Unicam co., Mattson 1000 (UK) and presented in and .
Scanning electron microscopy
Scanning electron microscopy (SEM) photographs were taken with QUANTA 400 F Field Emission SEM (USA) and shown in .
In vitro drug release
In vitro drug release from the semi-IPN microspheres was studied in 250 mL, pH 1.2 HCl solution, pH 6.8 and pH 7.4 phosphate buffer solutions and incubated in a shaking water bath (Medline BS-21, Korea) at 37 °C. At 2-h intervals, medium was changed to: 1.2, 6.8 and 7.4 pH, respectively. At specific time intervals, the 5-FU content was determined using UV spectrophotometer at 266 nm. Equal volume of fresh HCl or phosphate buffer solution was added into the dissolution media to maintain a constant volume. All experiments were performed in triplicate to minimize the variational error. Standard deviations from the average values were calculated.
Results and discussion
Characterization of the graft copolymer of PVA-g-PAAm
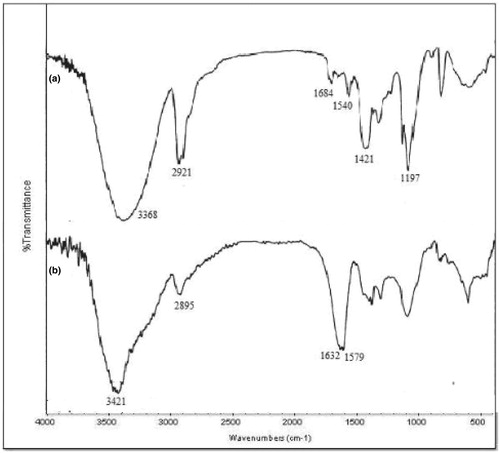
Grafting of AAm onto PVA was achieved in the presence of UV irradiation. FTIR spectra of PVA and grafted PVA are shown in . In case of PVA, a broad band at 3368/cm was seen due to O–H stretching vibrations. Aliphatic C–H stretching vibration was indicated at 2921/cm. Similar C–H stretching could be seen in the grafted copolymer spectra at 2895/cm. The peak due to asymmetric N–H stretching vibration of primary amide overlapped with O–H stretching vibrations. A band at 1632/cm confirms the presence of C = O stretching vibration, which was not observed in PVA. Grafting was also confirmed by the presence of band at 1579/cm corresponding to asymmetric N–H bending. The elemental analysis results and percentage grafting are given in for characterization purposes. Presence of nitrogen in the results also confirms the grafting of AAm onto PVA.
Characterization of the microspheres
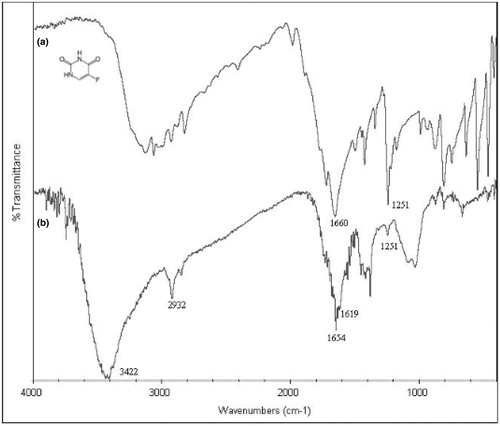
FTIR spectra of the 5-FU and 5-FU-loaded semi-IPN microspheres are shown . A broad band between 3000/cm and 3500/cm is attributed to –NH stretching vibrations in the spectrum of 5-FU, aliphatic C–H stretching band was observed at 2932/cm both in 5-FU and in the microspheres. Bands at 1660/cm and 1654/cm showed C = O stretching vibrations, respectively, due to 5-FU and 5-FU-loaded semi-IPN microspheres. The other peak observed at 3422/cm indicated O–H stretching vibration of the semi-IPN microspheres. In addition, a peak at 1251/cm which represents C–F stretching vibrations was seen in both 5-FU and 5-FU-loaded semi-IPN microspheres, proving the presence of 5-FU in the microspheres.
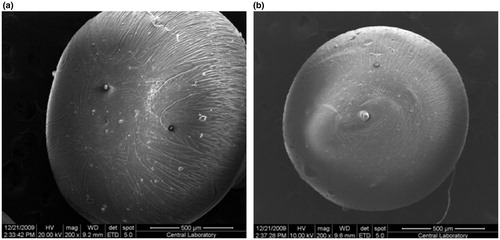
Shapes of dried empty and 5-FU-loaded microspheres are presented in . As it is reflected from the figure, both empty and 5-FU-loaded microspheres almost maintain spherical form at empty and drug-loaded conditions.
The results of entrapment efficiency (%), microsphere yield (%) and microsphere diameter are shown in . As can be seen from the table, the microspheres formed have particle sizes ranging from 0.67 ± 0.003 to 1.28 ± 0.01 mm in diameter. The diameter of the microspheres increased as the amount of NaAlg in the microspheres was increased, whereas it did not change significantly with the increase in crosslinker concentration. Entrapment efficiency percentage increased as the drug content of the microspheres increased. Highest entrapment efficiency obtained was 54% for the drug/polymer ratio of 1:1, PVA-g-PAAm/NaAlg ratio of 1:4. Microsphere yield increased as the crosslinker concentration was increased and the highest microsphere yield obtained was ∼81%.
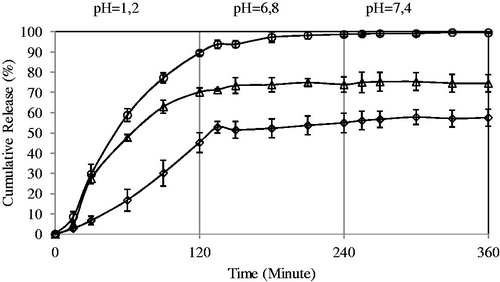
Effect of PVA-g-PAAm/NaAlg ratio on the 5-FU release
To understand the drug release from 5-FU-loaded semi-IPN microspheres of NaAlg and PVA-g-PAAm, in vitro release experiments were performed at pH values of 1.2, 6.8 and 7.4 each for 2 h. Effects of PVA-g-PAAm/NaAlg ratio in the formulations A1, A2, A3 and A4 on the release rates are presented in . The percent cumulative release was found to be higher in case of A1 than A2, A3 and A4. As the NaAlg content of the microspheres increased, release became more controlled at low pH values. Due to the high and more controlled release of 5-FU from the microspheres with a PVA-g-PAAm/NaAlg ratio of 1:4, this ratio was preferred in the rest of the study.
Figure 5. Effect of PVA-g-AAm/NaAlg ratio on the 5-FU release. Concentration of FeCl3: 0.05 M, exposure time to FeCl3: 10 min, drug/polymer ratio: 1:8 (diamond indicates 1:1; square, 1:2; triangle, 1:3; circle, 1:4).
Swelling results of the crosslinked microspheres shown in indicated that as the amount of grafted copolymer in the microspheres decreased, the equilibrium water uptake increased from 530.8% to 1093.2%.
Table 3. Equilibrium swelling degree of microspheres.
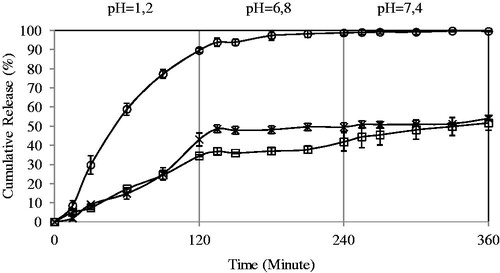
Effect of concentration of FeCl3 on the 5-FU release
The percentage cumulative release versus time curves for varying amounts of FeCl3 (0.05, 0.1 and 0.2 M) at fixed amount of PVA-g-PAAm/NaAlg ratio are displayed in . The percentage cumulative release was quite fast and high at low concentration of FeCl3 (i.e. 0.05 M), whereas the release becomes quite slow on increasing the concentration of FeCl3 (i.e. 0.2 M). At high concentrations of FeCl3, polymeric chains become more rigid due to the contraction of microvoids, thus decreasing the release of 5-FU through polymeric matrices. Similar observations were also found in the literature (Nokhodchi & Tailor, Citation2004; Şanlı & Işıklan, Citation2006; Şanlı et al., Citation2007;). Şanlı et al. (Citation2007) have changed glutaraldehyde (GA) concentration from 1% to 2.5% during the bead preparation and reported that as the GA concentration was increased from 1% to 2.5%, diclofenac sodium release decreased from PVA/NaAlg beads at pHs of 6.8 and 7.4. Nokhodchi & Tailor (Citation2004) have prepared theophylline-loaded NaAlg matrices. Increasing the amount of AlCl3 from 1 × 10−4 to 6.8 × 10−4 moles, the release of theophylline decreased from 95.1% to 29.5%. As the high release was obtained at FeCl3 concentration of 0.05 M, we have continued the studies with this concentration.
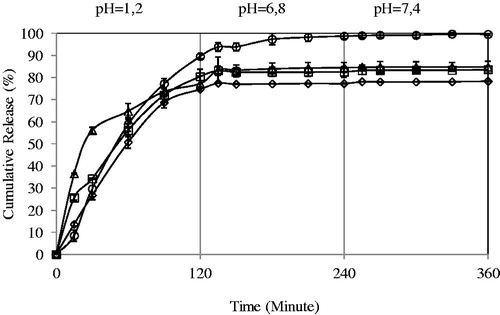
Effect of the exposure time to crosslinker on the 5-FU release
5-FU release from the microspheres was subjected to a number of physical and chemical parameters including those related directly to the release medium, the release conditions (temperature, pH), preparation conditions and those resulting from the change in the characteristics of the microspheres (Şanlı et al., Citation2007). One of the ways of changing drug release from the microspheres is to change the crosslinking density of the matrix by employing various time of exposure to crosslinking agent. The effect of exposure time on the release rate of 5-FU has been investigated by varying exposure time from 10 to 20 min. The maximum 5-FU release was obtained as 99.57% for the microspheres prepared with exposure time of 10 min. In the rest of study, exposure time was selected as 10 min due to the high release at this exposure time. Similar observations were found in some of the studies in previous literature (Yuan et al., Citation2007; Şanlı & Solak, Citation2009). Yuan and co-workers (Yuan et al., Citation2007) prepared protein-loaded chitosan microspheres crosslinking with genipin. They reported that under the same genipin concentration (0.5 mM), the crosslinking degree increased with increasing crosslinking time. The crosslinking degree increased significantly as the crosslinking time changed from 4 to 16 h ().
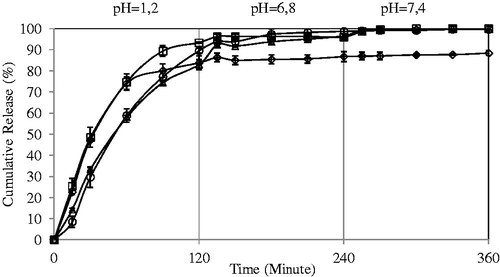
Effect of the drug/polymer ratio on the 5-FU release
shows the release profiles of the microspheres at different amounts of drug loadings. Release data showed that 5-FU release from the microspheres with 1:8 drug:polymer ratio was much higher than that of microspheres 1:4, 1:2 and 1:1 drug:polymer ratio. As the amount of drug increased from 1:8 to 1:1, 5-FU content of the microspheres increases. Low drug content could lead to the easier penetration of solution through microspheres and drug diffusion could be fast from the microspheres. Similar results were obtained in the literature (Zinutti et al., Citation1996; Nokhodchi & Tailor, Citation2004; Yu et al., Citation2008). Zinutti et al. (Citation1996) prepared ethylcellulose microspheres containing 5-FU by an oil-in-oil evaporation/extraction method. They have studied three drug/polymer ratios (1:1, 1:2 and 1:3) and found that as the drug ratio decreased from 1:1 to 1:3, 5-FU release increased.
Analysis of kinetic results
The event of solvent sorption by a bead depends mechanistically on the diffusion of water molecules into the gel matrix and subsequent relaxation of macromolecular chains of the bead (Bajpai & Sharma, Citation2005). The release data of all the systems were further substantiated by fitting the fraction release data >Mt/M∞ to an empirical equation proposed by Peppas (Citation1985).
Where Mt is the amount of 5-FU released at time t and M∞ is the drug released at equilibrium time; k, a constant characteristic of the drug–polymer system; and n, the diffusional exponent which suggests the nature of the release mechanism. Fickian release is defined by initial t1/2 time dependence of the fractional release for slabs, cylinders and spheres. Analogously, Case-II transport is defined by an initial linear time dependence of the fractional release for all geometries (Ritger & Peppas, Citation1987). A value of n = 0.5 indicates the Fickian transport (mechanism), while n = 1 is of Case II or non-Fickian transport (swelling controlled) (Yu et al., Citation2008). The intermediary values ranging between 0.5 and 1.0 are indicative of the anomalous transport. The least squares estimations of the fractional release data along with the estimated correlation coefficient values, r, are presented in . From these data, the n value ranged between 0.2361 and 0.9509, indicating 5-FU release from the semi-IPN microspheres deviates from the Fickian transport.
Table 4. The results of k, n, r and D calculated from Equations (3) and (4).
The values of diffusion coefficients, D, for the transport of aqueous drug solution from the microspheres were calculated using the sorption and desorption results as in Equation (4).
where θ is the slope of the linear portion of the plot of Mt/M∞ versus t1/2, and r is the radius of the microspheres; M∞ is equilibrium sorption. To calculate D from desorption experiments, θ was computed from the initial linear portion of the desorption plot, i.e. ln(1−Mt/M∞) versus time, t. The calculated values of D from Equation (4) for sorption and desorption runs were also presented in . The D values for the desorption were smaller than those observed for sorption, and these ranged from 2.470 × 10−13 to 10.930 × 10−13 cm2/s (Babu et al., Citation2006).
Conclusions
PVA-g-PAAm/NaAlg was synthesized and semi-IPN microspheres were prepared by crosslinking with Fe3+ ions for oral treatment gastrointestinal tract of 5-FU. The release was found to be pH sensitive and 5-FU release was higher at high pH values than at low pH values. Release was found to be low at high concentrations of crosslinker, whereas high at short time of exposure to crosslinker. Decrease in drug content of the microspheres enhanced the 5-FU release. PVA-g-PAAm/NaAlg ratio affected the release. As the amount of NaAlg was increased, release became more controlled. The highest 5-FU release was found to be 99.57% for PVA-g-PAAm/NaAlg ratio of 1:4 and drug/polymer ratio of 1:8, crosslinker concentration of 0.05 M and exposure time of 10 min to crosslinker.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this work.
The authors are grateful to the Gazi University Scientific Research Foundation for support of this study.
References
- Aiedeh K, Taha MO. (2001). Synthesis of iron-crosslinked chitosan succinate and iron-crosslinked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release beads. Eur J Pharm Sci 13:159–68
- AL-Kahtani Ahmed A, Bhojya Naik HS, Sherigara BS. (2009). Synthesis and characterization of chitosan based pH-sensitive semi-interpenetrating network microspheres for controlled release of diclofenac sodium. Carbohyd Res 344:699–6
- Babu VR, Hosamani KM, Aminabhavi TM. (2008). Preparation and in-vitro release of chlorothiazide novel pH-sensitive chitosan-N,N′-dimethylacrylamide semi- interpenetrating network microspheres. Carbohyd Polym 71:208–17
- Babu VR, Krishna Rao KSV, Sairam M, et al. (2006). pH sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J Appl Polym Sci 99:2671–8
- Bajpai AK, Sharma M. (2005). Preparation and characterization of binary grafted polymeric blends of polyvinyl alcohol and gelatin and evaluation of their water uptake potential. J Macromol Scı Chem Pure Appl Chem 42:663–82
- Chaturvedi K, Kulkarni AR, Aminabhavi TM. (2011). Blend microspheres of poly(3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind Eng Chem Res 50:10414–23
- Dai C, Wang Y, Hou X. (2012). Preparation and protein adsorption porous dextran microspheres. Carbohyd Polym 87:2338–43
- Ganguly K, Aminabhavi TM, Kulkarni AR. (2011). Colon targeting of 5-fluorouracil using polyethylene glycol cross-linked chitosan microspheres enterc coated with cellulose acetate phthalate. Ind Eng Chem Res 50:11797–807
- Gupte A, Ciftci K. (2004). Formulation and characterization of Paclitaxel, 5-FU and Paclitaxel + 5-FU microspheres. Int J Pharmaceut 276:93–6
- Huang K, Lu K, Yeh C, et al. (2009). Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J Control Release 137:15–9
- Huang L, Sui W, Wang Y, et al. (2010). Preparation of chitosan/chondroitin sulfate complex microcapsules and application in controlled release 5-fluorouracil. Carbohyd Polym 80:168–73
- Kim MS, Park SJ, Gu BK, et al. (2012). Ionically crosslinked alginate–carboxymethyl cellulose beads for the delivery of protein therapeutics. Appl Surf Sci 262:28–33
- Mi F, Chen C, Tseng Y, et al. (1997). Iron(III)-carboxymethylchitin microsphere for the pH-sensitive release of 6-mercaptopurine. J Control Release 44:19–32
- Nokhodchi A, Tailor A. (2004). In situ cross-linking of sodium alginate with calcium and aluminum ions to sustain the release of theophylline from polymeric matrices. Farmaco 59:999–4
- Peppas NA. (1985). Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110–11
- Reddy KM, Babu VR, Rao KSVK, et al. (2008). Temperature sensitive semi-IPN microspheres from sodium alginate and n-isopropylacrylamide for controlled release of 5-fluorouracil. J Appl Polym Sci 107:2820–9
- Ritger PL, Peppas NA. (1987). A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
- Rokhade AP, Shelke NB, Patil SA, et al. (2007). Novel interpenetrating polymer network microspheres of chitosan and methyl cellulose for controlled release of theophyline. Carbohyd Polym 69:678–87
- Sastre RL, Olmo R, Teijón C, et al. (2007). 5-Fluorouracil plasma levels and biodegradation of subcutaneously injected drug-loaded microspheres prepared by spray-drying poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) polymers. Int J Pharmaceut 338:180–90
- Sungur S. (1999). Investigations on drug release systems using CMC crosslinked with ferric ions. Artif Cells Blood Substit Immobil Biotechnol 27:279–90
- Şanlı O, Ay N, Işıklan N. (2007). Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate bled beads. Eur J Pharm Biopharm 65:204–14
- Şanlı O, Işıklan N. (2006). Controlled release formulations of carbaryl based on copper alginate, barium alginate and alginic acid beads. J Appl Polym Sci 102:4245–53
- Şanlı O, Solak EK. (2009). Controlled release of naproxen from sodium alginate and poly(vinyl alcohol)/sodium alginate blend beads crosslinked with glutaraldehyde. J Appl Polym Sci 112:2057–65
- Yu CY, Zhang XC, Zhou FZ, et al. (2008). Sustained release of antineoplastic drugs from chitosan-reinforced alginate microparticle drug delivery systems. Int J Pharmaceut 357:15–21
- Yuan Y, Chesnutt BM, Uttukar G, et al. (2007). The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohyd Polym 68:561–7
- Zinutti C, Kedzierewicz F, Hoffman M, et al. (1996). Influence of the casting solvent on the physico-chemical properties of 5-fluorouracil loaded microspheres. Int J Pharmaceut 133:97–105