Abstract
Context: In our recent studies, Brugia malayi molecules have shown interesting immune-stimulating and immune-suppressive properties. Among these, F6 a pro-inflammatory (54–68 kDa) SDS-PAGE resolved fraction of the parasite when administered with Freund’s complete/incomplete adjuvant in animals, elicited both Th1 and Th2 type immune responses and protects the host from filarial parasite.
Objective: The present study was aimed at developing biodegradable microspheres for filarial antigenic protein molecules and to investigate the immunoadjuvanticity of microspheres (Ms)-loaded F6 molecules.
Materials and methods: Poly-lactide microspheres (DL-PLA-Ms) were prepared using double emulsification and solvent evaporation method; and studied their size, shape, antigen adsorption efficiency, in-process stability, and antigen release profiles. F6 and B. malayi adult worm (BmA: ∼17 to 180 kDa) protein molecules adsorbed on the Ms were administered in a single shot into Swiss mice, subcutaneously, and investigated their immunoadjuvant effect and compared with one/two doses-schedule of plain F6/BmA.
Results: Immunization with F6/BmA-loaded DL-PLA-Ms resulted in upregulation of cellular proliferation, IFN- γ, TNF-α and NO release from host’s cells stimulated with F6/BmA or LPS/Con A, IgG, IgG1 and IgG2a levels. These responses were well comparable with the responses produced by two doses of plain BmA/F6.
Discussion and conclusion: In conclusion, a single dose of DL-PLA-Ms-F6 induced predominantly Th1 immune responses and well comparable with two doses of plain F6. This is the first ever report on potential of DL-PLA-Ms as adjuvant for filarial immunogen.
Introduction
Human lymphatic filariasis, a mosquito-borne disease of the tropics, is caused by the nematode parasites Wuchereria bancrofti, Brugia malayi and B. timori. The disease is not fatal but responsible for considerable morbidity leading to huge economic loss. The World Health Organization ranks it as the second most common cause of long-term disability and over 1.25 billion people are at risk of the infection and approximately 125 million are infected with more than 40 million are seriously incapacitated and disfigured by the disease (WHO, Citation2005; Murthy et al., Citation2009; Zeldenryk et al., Citation2011).
The methods to control and prevent the filarial infection include administration of antifilarials alone or combination of diethylcarbamazine (DEC)/ivermectin and albendazole and exposure control programs. In recent years, identification of several filarial antigens/proteins or molecules raised hopes for developing vaccines (Gregory et al., Citation2000; Ramachandran et al., Citation2004; Krithika et al., Citation2005; Babayan et al., Citation2006; Vedi et al., Citation2008; Sahoo et al., Citation2009; Shakya et al., Citation2009; Joseph et al., Citation2012) against lymphatic filariasis.
Novel adjuvants have been developed for enhancing antigen delivery and reducing the vaccine delivery to a single injection. For future human use, it is however necessary to use an adjuvant that is safe, biodegradable and which does not require repeated administration to produce the desired result. Currently, alum is the only adjuvant approved by the U.S. FDA for clinical use to increase immune responses to protein-based vaccines (Schmidt et al., Citation2007) but regrettably, alum is a poor stimulator of cellular (Th1) immune responses, which are important for protection of parasitic diseases including filariasis (Petrovsky & Aguilar, Citation2004; Schmidt et al., Citation2007).
Recently, we reported that F6, a SDS-PAGE resolved 54–68 kDa fraction of B. malayi adult worm extract (BmA) stimulates proinflammatory mediators’ release and protects host from the parasite via Th1/Th2 type responses (Sahoo et al., Citation2009). We have also shown that a single injection of F6 adsorbed on polymeric lamellar substrate particles of poly (l-lactide) (PLSP) and poly (dl)-lactide-co-glycolide (PLGA) upregulated both Th1 and Th2 responses and NO release (Saini et al., 2011, Citation2013). In addition anti-PLGA-Ms-F6 antibodies elicited antibody-dependant cellular cytotoxicity (ADCC) response to infective larval and microfilarial stages of the filarial parasites (Saini et al., Citation2013) and this encouraged us to explore the suitability of a related poly (dl-lactide) as adjuvant. In the present study, we have studied the immune responses of animals produced by the single-shot filarial antigens adsorbed on to DL-PLA microspheres. For this purpose, the B. malayi adult worm extract (BmA) and F6 was adsorbed separately, onto DL-PLA microspheres (Ms), characterized and administered in a single-shot to Swiss mice. Specific IgG, its subclasses and IgE, lymphocyte proliferation, interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and nitric oxide (NO) release were determined. The data were compared with one/two doses of plain BmA and F6 to find out the immunoadjuvanticity of filarial antigen molecules bearing microspheres.
Methods
Polymers
Poly (dl-lactide) (PDL 02, Molecular weight 17 kDa, inherent viscosity (IV):0.2 dl/g) was a gift sample of Purac Biochem, Netherland. All other chemicals and reagents were of analytical grade and purchased from local suppliers.
Preparation of BmA, its fractionation and isolation of a fraction of interest
Methods of isolation of the parasites from the filarial infected jirds (Meriones unguiculatus) Murthy et al. (Citation1997), preparation of BmA and isolation of F6 fraction from BmA were essentially same as described by and Saini et al. (2011b).
Preparation and characterization of microspheres
Blank microspheres (Ms) were prepared by double emulsion solvent evaporation method water/oil/water (W/O/W) at room temperature as described earlier (Saini et al., Citation2009, Citation2010, 2011a). These microspheres were characterized for the average size, polydispersity index, zeta-potential [by photon correlation spectroscopy (PCS) using Malvern Zeta Sizer U.K.], shape [Scanning Electron Microcopy (SEM; Leo 430 Germany], adsorption efficiency and in vitro release profile studies (Singh et al., Citation2000; O’Hagan et al., Citation2004; Oster et al., Citation2005; Stivaktakis et al., Citation2005).
Immunization of animals
Swiss mice weighing 20–22 g (3–4 weeks old) were used in the present study. All the experiments were conducted in compliance with the Institutional Animal Ethics Committee (CSIR-Central Drug Research Institute, Lucknow UP, India) guidelines for use and handling of the animals (IAEC No. 43/08/Para/IAEC/Renew 02 (25/10); dated 22.1.2010.). Throughout the study, the animals were housed at 23 ± 2 °C; RH: 60% and photoperiod (12 h light–dark cycles) controlled animal quarters. They were fed standard rodent pellet supplemented with gram and had free access to drinking water. The study included seven groups each consisted of 5–6 animals from two experiments. Animals of Group 1 were treated with PBS and served as control. Groups 2, 3 and 4 received plain BmA protein (10 μg on day 0), plain BmA double dose (10 μg on day 0 and 15) and 10 μg of BmA-loaded microspheres (Ms-BmA) (single dose on day 0), respectively. Groups 5, 6 and 7 received plain F6 (5.0 μg on day 0), plain F6 (5.0 μg on day 0 and 15) and 5.0 μg of F6-loaded microspheres (DL-PLA-Ms-F6) (single dose on day 0), respectively. All the injections were given through subcutaneous (s.c.) route. Animals were killed on day 35 post first administration of the antigen (p.f.a) for determination of immunoglobulins, lymphocyte proliferation and release of IFN-γ, TNF-α and NO.
Collection of sera
Sera were isolated from blood drawn from animals on days 7, 21 and 35 p.f.a. of antigen.
Determination of filaria specific IgG, its subclasses and IgE
Filaria specific IgG on days 7, 21, 35 p.f.a and its subclasses (IgG1,IgG2a, IgG2b) on day 35 p.f.a were determined in sera of animals (Saini et al., 2011b). Briefly, ELISA strips (Nunk, Rosklide, Denmark) were coated with BmA (1.0 μg protein/ml) and F6 (0.5 μg protein/ml) prepared in carbonate buffer (0.06 M; pH 9.6). Optimally diluted sera (diluent: 1% BSA in PBS with 0.05% T20) were added (1:200 for BmA and 1:100 for F6 specific IgG and 1:50 for BmA and 1:25 for F6 specific IgG subclasses) to the wells and incubated. The wells were then washed and probed with HRP-conjugated rabbit anti-mouse-IgG and its subclasses (Sigma-Aldrich, St. Louis, USA) at 1:1000 and 1:15 000 dilution, respectively. Orthophenylenediamine (OPD) was used as substrate and absorbance was read at 492 nm in an ELISA reader (Power Wave X, BioTek, USA).
IgE in sera of animals was determined on day 35 p.f.a. according to the method described by Joseph et al. (Citation2011). Antigen concentrations used were same as mentioned above. Optimum dilution of primary sera and HRP-conjugated goat anti-mouse-IgE were used at 1:4 and 1:1000 dilution, respectively. Rest of the procedure was same as described above.
Lymphocyte transformation test
To assess cell mediated immune (CMI) response lymphocyte transformation test (LTT) was carried out broadly following the method of Klei et al. (Citation1990) with some modifications to suit our conditions. The cells at 4 × 105 in 200 μl RPMI-1640 medium/well in 96-well plate (Nunk, Roskilde, Denmark) were charged with BmA or F6 (1 μg protein/ml) or Con A (10 μg/ml) and incubated at 37 °C in 5% CO2 atmosphere; cells incubated in medium only served as control. 3H-Thymidine (1 µCi/well) was added after 72 h post-stimulation (PS) followed by 16–18 h of incubation, cells were harvested and suspended in scintillation fluid to quantify β-emission by a scintillation counter (LS Analyzer, Beckman Inc., Fullerton, CA). The results are expressed as count per minute (cpm).
Cytokine assay
IFN-γ and TNF-α releases were determined in the supernatants of splenocytes of immunized and non-immunized animals. Splenocytes isolated as above were plated in sterile 24-well plates (Nunc, Rosklide, Denmark) at 2 × 106 ml−1 conc. and stimulated with BmA or F6 (1 μg protein/ml) or LPS (1 μg/ml) for 48 h under the same incubation condition. The cytokines were determined in the 48 h PS culture supernatants. For the assay mouse monoclonal antibodies of IFN-γ (Pierce Endogen, Rockford, IL) and TNF-α (BD, Pharmingen™, New Jersey, USA) were used in a paired antibody sandwich ELISA method following the manufacturer’s instructions with some changes to suit our conditions (Murthy et al., Citation2000).
NO determination
NO determination was carried out according to Dixit et al. (Citation2004). Peritoneal cells harvested were washed thoroughly with the medium Dulbecco’s modified Eagle’s medium (DMEM) adjusted to 2 × 106 cells/ml of medium containing 10% fetal bovine serum and dispensed into sterile 48-well tissue culture plates (Nunc-rosklide, Denmark). After overnight incubation at 37 °C in 5% CO2 atmosphere, adherent cells were replenished with fresh medium and stimulants added (BmA or F6: 1 or 0.5 μg protein/ml; LPS: 1 μg/ml) followed by incubation at the same atmosphere. The presence of nitrite in culture supernatants of 48 h PS was quantified (Thomas et al., Citation1997).
Statistical analysis
Results were presented as mean ± SD of two experiments conducted in five to six animals and the data were analyzed using one-way analysis of variance (ANOVA) with Newman–Keuls multiple comparison test as post test in GraphPad prism 3.03. Differences with p < 0.05 were considered significant.
Results
DL-PLA-Ms-BmA/F6 characterization (in vitro characterization)
Blank Ms were spherical with slightly rough surfaces as indicated by morphological examinations using SEM (). The actual size and polydispersity for Ms were found to be 8.42 ± 1.24 μm (mean particle diameter), 0.0920 ± 0.08, respectively. Adsorption efficiency (%) of BmA and F6 adsorbed microspheres was found to be 52.232 ± 2.288 and 68.100 ± 4.224, respectively. Zeta-potential of blank Ms was observed to be −48.66 ± 1.90 mV while zeta potential of BmA and F6 adsorbed microspheres was −36.0 ± 1.40 mV and −32.480 ± 0.840 mV, respectively. Cumulative percentage release of BmA and F6 (in 24 h) from microspheres was found to be 14.0 ± 0.60 and 23.04 ± 0.20, respectively ().
Table 1. Characterization of DL-PLA microspheres bearing BmA and F6 filarial antigen proteins in vitro.
Immune responses of animals to DL-PLA-Ms-BmA/F6 candidates
IgG and its subclass responses
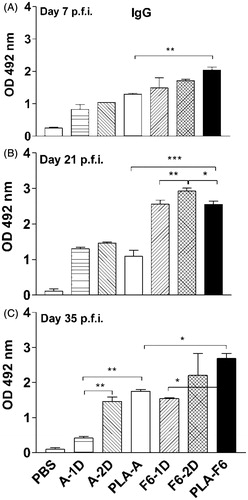
Sera of all immunized animals showed increase in IgG levels (p < 0.05–0.001) at all the time points (days 7–35 p.f.a. of antigens) as compared to those animals given PBS (). IgG levels further increased on days 21 and 35 p.f.a. in animals immunized with DL-PLA-Ms-F6 (p < 0.001; ), however on day 35 p.f.a. the IgG level in DL-PLA-Ms-F6 immunized animals was higher than the other groups except in animals receiving two doses of F6 antigen (). Upon analysis, the level of IgG in DL-PLA-Ms-F6 immunized animals was higher than the level of IgG in DL-PLA-Ms-BmA immunized animals (p < 0.05–0.001) at all time points (), although the level of IgG in DL-PLA-Ms-F6 was increased on later time points (days 21 and 35 p.f.a.).
Figure 2. Levels of specific IgG in immunized Swiss mice. Animals were immunized with For sera tail blood of animals were collected on days 7 (A), 21 (B) and 35 (C) post first administration (p.f.a.) of plain or DL-PLA-Ms adsorbed filarial antigens BmA (B. malayi adult extract) or F6 (SDS-PAGE resolved fraction of BmA). Specific IgG antibodies against BmA (1.0 μg protein/ml) or F6 (0.5 μg protein/ml) were determined by sandwich ELISA in serum of the animals immunized with BmA/F6 adsorbed on DL-PLA-Ms and one (A/F6-1D) or two doses (A/F6-2D) of plain BmA/F6 in PBS or PBS alone; absorbance was read at 492 nm. Abbreviation: p.f.i. = post first immunization; A/F6-1D = plain BmA/F6-one dose; A/F6-2D = plain BmA/F6-two doses; PLA-A/F6 = DL-PLA-Ms-BmA/F6. Values are mean ± SD of data from six animals in two experiments. Statistics: Newman–Keuls multiple comparison tests. *p < 0.5 -- significant; **p < 0.1 -- more significant; ***p < 0.001 -- highly significant.
Analysis of IgG subclasses in animals immunized with DL-PLA-Ms-BmA revealed elevated level of in IgG1 on day 35 p.f.a. of antigens over one dose plain BmA (p < 0.01; ) but not two doses. DL-PLA-Ms-BmA/F6 immunized animals had significantly high IgG1 (p < 0.01; ) compared to animals receiving one or two dose of plain BmA/F6 (p < 0.01; ). DL-PLA-Ms-F6 immunized animals showed increase in IgG2a level (p < 0.001) over DL-PLA-Ms-BmA immunized animals (). The level of IgG2a in these animals was also significantly high (p < 0.01–0.001) as compared to one or two doses of plain F6. There was no difference in response of IgG2b in animals receiving either one or two doses of plain BmA/F6 or DL-PLA-Ms adsorbed BmA/F6 (). In summary, IgG1 and IgG2a levels were increased in animals immunized with one dose of DL-PLA-Ms-F6 as compared to one dose of DL-PLA-Ms-BmA immunized animals. Specific IgE remained undetectable in all the immunized animals (data not shown).
Figure 3. Levels of specific IgG subclasses in immunized Swiss mice. Sera were collected from blood of animals on day 35 p.f.a. of antigens. Specific IgG1 (A), IgG2a (B) and IgG2b (C) antibodies against BmA or F6 were determined by sandwich ELISA in serum of the animals immunized with BmA/F6 adsorbed on DL-PLA-Ms and one (A/F6-1D) or two doses of plain BmA/F6 (A/F6-2D) in PBS or PBS alone; absorbance was read at 492 nm. Values are mean ± SD of data from six animals in two experiments. Group abbreviations: A/F6-1D = plain BmA/F6- one dose; A/F6-2D = plain BmA/F6- two doses; PLA-A/F6 = DL-PLA-Ms-BmA/F6. Statistics were same as described above. p < 0.01. ***p < 0.001.
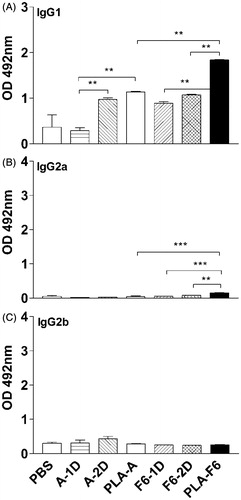
Cellular proliferative response
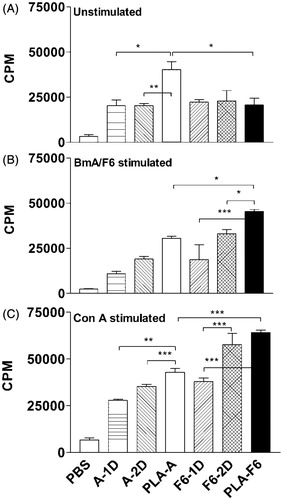
Unstimulated cells of DL-PLA-Ms-BmA immunized animals proliferated maximally (p < 0.001) as compared to cells of other groups of immunized animals (). There was an increasing trend in proliferative response of cells stimulated with BmA in vitro of animals immunized with one or two doses of plain BmA or DL-PLA-Ms-BmA but statistically not significant amongst the groups. Cells of DL-PLA-Ms-F6 (p < 0.05) immunized animals proliferated significantly in response to F6 in vitro compared to cells of one (p < 0.001) or two doses (p < 0.05) of plain F6-immunized animals (), the cellular proliferation of these animals was also greater (p < 0.05) than the response of DL-PLA-Ms-BmA immunized animals. In vitro Con A induced response in cells of DL-PLA-Ms-F6 immunized animals was significantly higher (p < 0.001) than the response of DL-PLA-Ms-BmA or one dose of plain F6 immunized animals (). In summary, the results showed that the cell proliferative response was higher in single shot DL-PLA-Ms-F6 immunized animals than one dose of the plain antigen immunized ones.
Figure 4. Proliferative responses (cpm) of splenocytes of animals immunized with one dose of BmA/F6 adsorbed on DL-PLA-Ms or one or two doses of plain BmA/F6 of B. malayi or PBS alone. The cells were unstimulated (A) stimulated with BmA/F6 at 1.0/0.5 µg/ml, respectively (B) and Con A at 10 µg/ml (C) in vitro. Values are mean ± SD of data of two experiments using six animals. All the animals were killed on day 35 p.f.a. of plain antigens or DL-PLA-Ms adsorbed antigens or PBS alone. Group abbreviations and statistics were same as described above. *p < 0.5 -- significant; **p < 0.1 -- more significant; ***p < 0.001 -- highly significant.
Cytokine release
In this study, we have investigated the effect of immunization with BmA/F6 loaded Ms on the cytokine release by cells from the immunized animals when challenged with the same fraction in vitro. IFN-γ release from unstimulated cells of DL-PLA-Ms-BmA or DL-PLA-Ms-F6 immunized animals was significantly high as compared to their counter parts one dose of plain BmA or F6 immunized animals, however the level of the cytokine release was >3.5 times greater in cells of DL-PLA-Ms-F6 immunized animals () than in cells of DL-PLA-Ms-BmA immunized animals. BmA induced IFN-γ release in cells of DL-PLA-Ms-BmA or 1/2 doses of plain BmA immunized animals was low (). In contrast, there was a remarkable upregulation of F6 induced IFN-γ release in cells of DL-PLA-Ms-F6 immunized animals (p < 0.001) compared to 1/2 doses of plain F6-immunized animals (). LPS induced IFN-γ release in cells of DL-PLA-Ms-F6 immunized animals was >3 times greater (p < 0.001) than given DL-PLA-Ms-BmA ().
Figure 5. IFN-γ release from splenocytes of immunized Swiss mice. The animals were immunized with one dose of BmA/F6 adsorbed on DL-PLA-Ms or one or two doses of plain BmA/F6 of B. malayi or PBS alone. The animals were killed on day 35 p.f.a. of plain antigens or DL-PLA-Ms adsorbed antigens or PBS alone. The cells were unstimulated (A), stimulated with BmA/F6 at 1.0/0.5 µg/ml, (B) or LPS at 1.0 µg/mL in vitro. IFN-γ release in 48 h culture supernatants was determined by ELISA. Values are expressed in mean ± SD of data from six animals. Group abbreviations and Statistics were same as described above. ***p < 0.001.
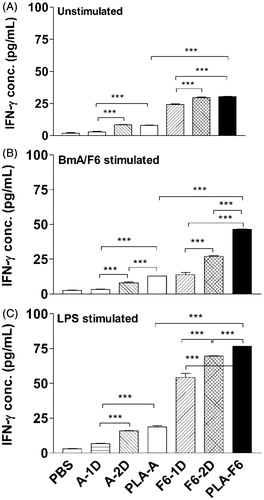
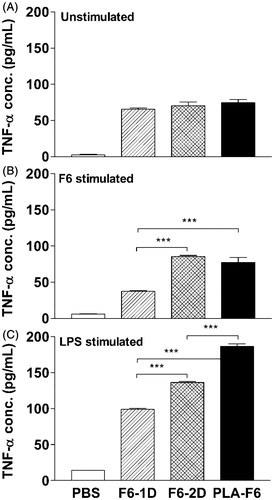
The level of TNF-α release from unstimulated cells of 1/2 doses or DL-PLA-Ms-F6 immunized animals was almost identical (). F6 or LPS induced TNF-α release was upregulated in these animals compared to one and or two doses of plain F6 immunized animals (p < 0.001; ). Taken together, specific IFN-γ and TNF-α release was considerably increased in cells of DL-PLA-Ms-F6 immunized animals.
Figure 6. TNF-α release from splenocytes of immunized Swiss mice. The animals were immunized with one dose of F6 adsorbed on DL-PLA-Ms or one or two doses of plain F6 of B. malayi or PBS alone. The animals were killed on day 35 p.f.a. of plain antigens or DL-PLA-Ms adsorbed antigens or PBS alone. The cells were unstimulated (A), stimulated with F6 at 0.5 µg/ml, (B) or LPS at 1.0 µg/ml in vitro. TNF-α release in 48 h culture supernatants was determined by ELISA. Values are expressed in mean ± SD of data from six animals. Group abbreviations and statistics were same as described above. ***p < 0.001.
Nitric oxide release
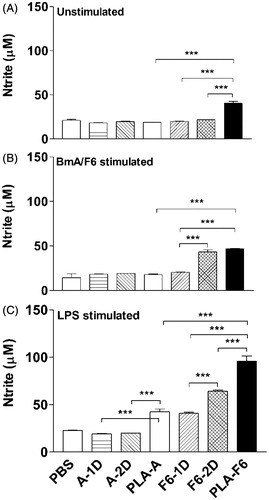
NO release from unstimulated cells of DL-PLA-Ms-F6 was significantly greater (p < 0.001) than that released by cells of DL-PLA-Ms-BmA/1D-F6/2D-F6 immunized animals (p < 0.001; ). Cells of DL-PLA-Ms-F6 immunized animals showed upregulation (p < 0.001) of NO response to F6 stimulation in vitro compared to the response of DL-PLA-Ms-BmA or one dose of plain F6 immunized animals (). LPS stimulated NO release from cells of DL-PLA-Ms-BmA and DL-PLA-Ms-F6 immunized animals was found increased (p < 0.001) as compared to those released by cells of one and or two doses of plain BmA/F6 immunized ones (). In summary, NO response in single-shot DL-PLA-Ms-F6 immunized animals was significantly high.
Figure 7. Nitric oxide release from peritoneal macrophages of immunized Swiss mice. The animals were immunized with one dose of F6 adsorbed on DL-PLA-Ms or one or two doses of plain F6 of B. malayi or PBS alone. The animals were killed on day 35 p.f.a. of plain antigens or DL-PLA-Ms adsorbed antigens or PBS alone. The cells were unstimulated (A), stimulated with BmA/F6 at 1.0/0.5 µg/ml, (B) or LPS at 1.0 µg/ml (C) in vitro. NO release in 48 h culture supernatants was determined by Griess reagent. Values are expressed in mean ± SD of data from six animals. Group abbreviations and statistics were same as described above. ***p < 0.001.
Thus, taken together the above findings show that one dose of DL-PLA-Ms-F6 produced much better immune responses than one and or two doses of plain F6 and that response of IFN-γ was remarkably high.
Discussion
The adjuvantcity of BmA/F6 adsorbed DL-PLA-Ms was investigated in terms of both Th1 and Th2 type of responses. To overcome the problem of BmA/F6 degradation during microcapsulation and to enhance the amount of antigen immediately available to antigen presenting cells (APCs) after cellular uptake of Ms, we have adopted the strategy of presenting antigen onto the surface of Ms. Despite an increased level of adsorption, extensive F6 release occurred from Ms over 24 h in PBS indicating the electrostatic nature of F6 substrate binding. The layer closest to the solid surface binds more strongly than the outer (protein–protein) layer which can be easily removed (burst–effect) by washing (Chesko et al., Citation2005).
Ms play an important role during interaction with phagocytic cells. Particles smaller than 10 μm injected through s.c. route are transported by phagocytic APCs into draining lymph nodes for rapid antigen release and antibody response. Although particles larger than 10 μm act as depot in releasing the antigen in a second step, which are recognized by B cell receptors (Vordermeier et al., Citation1995). The shift in T-cell response to Th1 by administration of the Ms associated antigen may facilitate uptake and presentation of particulate antigen by phagocytic cells, which are considered as APCs for Th1 responses (Saini et al., Citation2009, Citation2010). Adjuvants not only have the ability to selectively modulate the immune response to elicit humoral/cellular immune responses can also modulate MHC class I or II response (Saini et al., 2011b). In the present study, immunization with DL-PLA-F6 resulted in enhanced cell proliferation in response to F6 challenge in vitro compared to one dose of plain antigen. F6 stimulated cells in vitro significantly enhanced the cell proliferation indicating that F6 is a potent stimulator of cell proliferation (Sahoo et al., Citation2009). In filariasis, alterations in survival of the parasites has been shown be due to modulation of lymphocyte proliferation (Maizels & Lawrence, Citation1991; Maizels et al., Citation2004). Therefore, comparatively low immune response observed in immunized animals was due to crude BmA antigen which has mixture of stimulatory and suppressive protein molecules. However, higher cellular proliferative response observed in unstimulated cells of DL-PLA-Ms-BmA immunized animals compared to the response of cells of DL-PLA-Ms-BmA is not clear at present.
In general immunoprotective mechanism is a complex process involving many immune components such as APCs, lymphocytes, co-stimulatory signals (e.g. selectins, integrins, and the Ig super-family) and cytokines. In the present study, immunization with single dose DL-PLA-Ms-F6 produced enhanced F6-specific Th1 response which is in the line with our earlier observations on Mastomys coucha’s responses to three immunizing doses of F6 with Freund’s complete adjuvant Freund’ incomplete adjuvant/(FCA/FIA) (Sahoo et al., Citation2009). It was shown that both type-1 and type-2 responses are altered by individual cytokine’s effect, e.g. TNF-α a type-1 cytokine is required at an early stage of infection to synergize with the Th2-effector mechanism that determines the parasite elimination or persistence. Endemic normals from filaria endemic areas showed predominantly Th1-like anti-filarial immune response which are considered putatively immune individuals (Elson et al., Citation1995; Dimock et al., Citation1996), but chronic lymphatic filarial patients who are constantly exposed to infective mosquito bites showed tolerance and bystander suppression to antigen specific function (Babu et al., Citation2005; Gillan & Devaney, Citation2005). It was also reported that certain fractions/products of adult filarial parasites facilitate survival of the parasite by immunosuppression of host while other products of the parasite facilitate immunostimulation and inflammatory pathology (Ottesen et al., Citation1997; Dixit et al., Citation2004, Citation2006; Murthy et al., Citation2009; Joseph et al., Citation2011). In our earlier study, we have shown that immunization with F6 a strong pro-inflammatory cytokine stimulator, could prevent survival of adult parasites instilled in peritoneal cavity and establishment of L3-induced infection in rodent hosts (Sahoo et al., Citation2009).
Immunoglobulin G (IgG) and its subclasses are well documented in humans with filarial infections. In general, in the murine system, IgG2a antibody isotype is indicative of Th1 response, whereas IgG1 and IgG2b isotypes are associated with Th2 response (Pulendran et al., Citation1999). Antibody-dependent cellular cytotoxicity (ADCC) is a well-known part of adaptive immune mechanism (Th2) by which filarial parasites are killed in vitro and in vivo and this is mediated by the FcR (Chandrashekar et al., Citation1985, Citation1990; Misra et al., Citation1990; Taylor et al., Citation1996; Furchgott, Citation1999). The IgG2a subclass plays a critical role in the pathogenesis of humoral autoimmunity and protection against pathogens. In our recent study, we have shown that a single shot immunization with a pro-inflammatory fraction F6 of B. malayi adsorbed on poly(dl)-lactide-co-glycolide microspheres produced strong IgG, IgG1/IgG2a antibodies and elicited high ADCC response to L3 and mf stages of the parasite in vitro (Saini et al., Citation2013). El Bouhdidi et al. (Citation1994) reported predominance of IgG2a antibody in concert with IgG1 exerted maximum functional activity. In this study, we found elevated level of IgG and IgG1 antibodies along with reasonable increase in IgG2a and higher cellular proliferation. Kanai et al. (Citation2007) reported that during high cellular responses (Th1 responses are usually cellular mediated), lower levels of antibodies are also produced and thereby the antibody responses during Th1 response synergize the cellular components to allow for more effective clearance of the pathogen. Bal & Das (Citation1999) reported that endemic normal individuals have been reported to mount Th1-like anti-filarial immune response with IgG2 and IgG3 isotype. In this study, antibody response produced by DL-PLA-Ms-F6 which was given in a single dose was comparable with the responses produced by one dose (in case of IgG at day 35 p.f.a.) and one or two doses of plain F6 (in case of IgG1 or IgG2a) indicating strong adjuvant activity of DL-PLA-Ms for F6. Thus, DL-PLA-Ms-F6 induced elevated Th1 immune response may likely to be beneficial for parasite killing through ADCC, which is one of the well-known immunological mechanisms by which filarial parasites are killed in vitro and in vivo (Chandrashekar et al., Citation1990).
NO, one of the proinflammatory mediators has been shown to be a major defense molecule of immune cells (Liew, Citation1995). In filariasis, NO has been suggested to play an important role in the elimination or killing of parasites in vitro and L3 in vivo and protect the host through type 1 responses and IFN-γ stimulated toxic mediators’ release (Rajan et al., Citation1996; Taylor et al., Citation1996). Furchgott (Citation1999) reported NO produced by endothelial cells of parasitized lymphatic channels affects the parasite directly. In the present study remarkably enhanced IFN-γ and NO release in DL-PLA-Ms F6 immunized animals indicate that DL-PLA-Ms has potential adjuvanticity for F6. Thus, in the present study elevated level of NO produced by cells of DL-PLA-Ms F6 immunized animals may likely to implicate in the parasites’ removal from the host.
Thus, all the above findings indicate that one dose of DL-PLA-Ms-F6 was sufficient to produce a strong response and which may be helpful to participate in suppressing parasite establishment and their survival.
Conclusions
DL-PLA-Ms was prepared and characterized and B. malayi adult worm extract (BmA) and its SDS-PAGE resolved 54–68 kDa fraction F6 were adsorbed on to Ms. Swiss mice received a single injection of DL-PLA-Ms-BmA, DL-PLA-Ms-F6, and one (1D-BmA/F6) or two doses (2D-BmA/F6) of plain antigens. The findings demonstrate that immunization with a single-shot DL-PLA-Ms-F6 produced better immune responses as compared to one dose of plain F6 immunization and well comparable with the responses of two doses of plain F6 immunization. The reasons for better immune responses of the host due to DL-PLA-Ms-F6 formulation over plain F6 molecules indicate that Ms functions as a potent and efficient immunoadjuvant by effectively retaining adsorbed antigen with slow release of the molecules and adjuvant effect. Thus, in terms of convenience and efficacy, single shot immunization with DL-PLA-Ms-F6 may obviate the need for multiple immunization injections. This is the first ever report on potential of DL-PLA-Ms as adjuvant for filarial immunogen.
Declaration of interest
The authors report no declaration of interest.
Acknowledgements
We are thankful to Dr T. K. Chakraborty, Director, CSIR-CDRI for his keen interest and encouragement in carrying out the present work. Mr. V. K. Bose, Division of Parasitology, is duly acknowledged for his technical assistance. We are especially grateful to Dr Kooijman, Esther (Director, Purac Biochemical Limited, Netherland) for gift of DL-PLA polymer. The authors wish to acknowledge to Director NIPER, Chandigarh, for providing facilities of DLS studies. We are also thankful to Director and Mr. V. K. Singh, Birbal Sahni Paleobotany Research Institute, Lucknow India, and also Mr. Sandeep Arya, All India Institute of Medical Sciences, New Delhi, India, for providing facilities of electron microscopy (SEM/TEM).
References
- Babayan SA, Attout T, Harris A, et al. (2006). Vaccination against filarial nematodes with irradiated larvae provides long-term protection against the third larval stage but not against subsequent life cycle stages. Int J Parasitol 36:903–14
- Babu S, Blauvelt CP, Kumaraswami V, et al. (2005). Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J Immunol 175:1170–6
- Bal M, Das MK. (1999). Antibody response to a filarial antigen fraction in individuals exposed to Wuchereria bancrofti infection in India. Acta Trop 72:259–74
- Chandrashekar R, Rao UR, Parab PB, et al. (1985). Brugia malayi: serum dependent cell-mediated reactions to microfilariae. Southeast Asian J Trop Med Public Health 16:15–21
- Chandrashekar R, Rao UR, Subrahmanyam D. (1990). Antibody-mediated cytotoxic effects in vitro and in vivo of rat cells on infective larvae of Brugia malayi. Int J Parasitol 20:725–30
- Chesko J, Kazzaz J, Ugozzoli M, et al. (2005). An investigation of the factors controlling the adsorption of protein antigens to anionic PLG microparticles. J Pharm Sci 94:2510–19
- Dimock KA, Eberhard ML, Lammie PJ. (1996). Th1-like antifilarial immune responses predominate in antigen-negative persons. Infect Immun 64:2962–7
- Dixit S, Gaur RL, Khan MA, et al. (2004). Inflammatory antigens of Brugia malayi and their effect on rodent host Mastomys coucha. Parasite Immunol 26:397–407
- Dixit S, Gaur RL, Sahoo MK, et al. (2006). Protection against L3 induced Brugia malayi infection in Mastomys coucha preimmunized with BmAFII fraction of the filarial adult worm. Vaccine 24:5824–31
- El Bouhdidi A, Truyens C, Rivera MT, et al. (1994). El Bouhdidi A, Bazin H, Carlier Y. Trypanosoma cruzi infection in mice induces a polyisotypic hypergamma-globulinaemia and parasite-specific response involving high IgG2a concentrations and highly avid IgG1 antibodies. Parasite Immunol 16:69–76
- Elson LH, Calvopina M, Paredes W, et al. (1995). Immunity to onchocerciasis: putative immune persons produce a Th1-like response to Onchocerca volvulus. J Infect Dis 171:652–8
- Furchgott RF. (1999). Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 19:235–51
- Gillan V, Devaney E. (2005). Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect Immun 73:4034–42
- Gregory WF, Atmadja AK, Allen JE, et al. (2000). The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun 68:4174–9
- Joseph SK, Verma SK, Sahoo MK, et al. (2011). Sensitization with anti-inflammatory BmAFI of Brugia malayi allows L3 development in the hostile peritoneal cavity of Mastomys coucha. Acta Trop 120:191–205
- Joseph SK, Sambanthamoorthy S, Dakshinamoorthy G, et al. (2012). Protective immune responses to biolistic DNA vaccination of Brugia malayi abundant larval transcript-2. Vaccine 30:6477–82
- Kanai N, Min WP, Ichim TE, et al. (2007). Th1/Th2 Xenogenic antibody responses are associated with recipient dendritic cells. Microsurgery 27:234–9
- Klei TR, McVay CS, Dennis VA, et al. (1990). Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp Parasitol 71:393–405
- Krithika KN, Dabir P, Kulkarni S, et al. (2005). Identification of 38 kDa Brugia malayi microfilarial protease as a vaccine candidate for lymphatic filariasis. Indian J Exp Biol 43:759–68
- Liew FY. (1995). Nitric oxide in infectious and autoimmune diseases. Ciba Found Symp 195:234–39
- Maizels RM, Balic A, Gomez-Escobar N, et al. (2004). Helminth parasites-masters of regulation. Immunol Rev 201:89–116
- Maizels RM, Lawrence RA. (1991). Immunological tolerance: the key feature in human filariasis? Parasitol Today 7:271–6
- Misra S, Singh DP, Murthy PK, Chatterjee RK. (1990). Mode of action of antifilarials: modulation of immune adherence to microfilariae in vitro. Trop Med 32:3233–43
- Murthy PK, Dennis VA, Lasater BL, et al. (2000). Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect Immun 68:6663–9
- Murthy PK, Joseph SK, Abbas M, et al. (2009). Pathobiology of lymphatic filariasis: Understanding at molecular level. Proc Nat Acad Sci India 79:177–94
- Murthy PK, Murthy PS, Tyagi K, et al. (1997). Fate of infective larvae of Brugia malayi in the peritoneal cavity of Mastomys natalensis and Meriones unguiculatus. Folia Parasitol 44:302–4
- O’Hagan DT, Singh M, Dong C, et al. (2004). Cationic microparticles are a potent delivery system for a HCV DNA vaccine. Vaccine 23:672–80
- Oster CG, Kim N, Grode L, et al. (2005). Cationic microparticles consisting of poly(lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. J Control Release 104:359–77
- Ottesen EA, Weller PF, Heck L. (1997). Specific cellular immune unresponsiveness in human filariasis. Immunology 33:413–21
- Petrovsky N, Aguilar JC. (2004). Vaccine adjuvants: current state and future trends. Immunol Cell Biol 82:488–96
- Pulendran B, Smith JL, Caspary G, et al. (1999). Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA 96:1036–40
- Rajan TV, Porte P, Yates JA, et al. (1996). Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun 15:619–23
- Ramachandran S, Kumar MP, Rami RM, et al. (2004). The larval specific lymphatic filarial ALT-2: induction of protection using protein or DNA vaccination. Microbiol Immunol 48:945–55
- Sahoo MK, Sisodia BS, Dixit S, et al. (2009). Immunization with inflammatory proteome of Brugia malayi adult worm induces a Th1/Th2-immune response and confers protection against the filarial infection. Vaccine 27:4263–71
- Saini V, Jain V, Sudheesh MS, et al. (2010). Humoral and cell-mediated immune-responses after administration of a single-shot recombinant hepatitis B surface antigen vaccine formulated with cationic poly(l-lactide) microspheres. J Drug Targeting 18:212–22
- Saini V, Jain V, Sudheesh MS, et al. (2011a). Comparison of humoral and cell-mediated immune responses to cationic PLGA microspheres containing recombinant hepatitis B antigen. Int J Pharm 408:50–7
- Saini V, Sahoo MK, Murthy PK, et al. (2009). Polymeric lamellar substrate particles as carrier adjuvant for recombinant hepatitis B surface antigen vaccine. Vaccine 27:2372–8
- Saini V, Verma SK, Murthy PK, et al. (2013). Poly(d,l)-lactide-co-glycolide (PLGA) microspheres as immunoadjuvant for Brugia malayi antigens. Vaccine 31:4183–91
- Saini V, Verma SK, Sahoo MK. (2011b). Sufficiency of a single administration of filarial antigens adsorbed on polymeric lamellar substrate particles of poly (L-lactide) for immunization. Int J Pharm 420:101–10
- Schmidt CS, Morrow WJ, Sheikh NA. (2007). Smart adjuvants. Expert Rev Vaccin 6:391–400
- Shakya S, Kumar Singh P, Kushwaha S, et al. (2009). Adult Brugia malayi approximately 34 kDa (BMT-5) antigen offers Th1 mediated significant protection against infective larval challenge in Mastomys coucha. Parasitol Int 58:346–53
- Singh M, Briones M, Ott G, et al. (2000). Cationic microparticles. A potent delivery system for DNA vaccines. Proc Nat Acad Sci 97:811–6
- Stivaktakis N, Nikou K, Panagi Z, et al. (2005). Immune responses in mice of b-galactosidase adsorbed or encapsulated in poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. Wiley Periodical Inc 73:332--8
- Taylor MJ, Cross HF, Mohammed AA, et al. (1996). Susceptibility of Brugia malayi and Onchocerca lienalis microfilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFN-γ activated macrophages. Parasitology 112:315–22
- Thomas GR, McCrossan M, Selkirk ME. (1997). Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect Immun 65:2732–9
- Vedi S, Dangi A, Hajela K, et al. (2008). Vaccination with 73kDa recombinant heavy chain myosin generates high level of protection against Brugia malayi challenge in jird and mastomys models. Vaccine 26:5997–05
- Vordermeier HM, Coombes AGA, Jenkins P, et al. (1995). Synthetic delivery system for tuberculosis vaccines: immunological evaluation of the M. tuberculosis 38 kDa protein entrapped in biodegradable PLG microparticles. Vaccine 13:1576–82
- WHO. (2005). Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec 80:202–12
- Zeldenryk LM, Gray M, Speare R, et al. (2011). The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl Trop Dis 5:e1366


