?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Natural polymers have attracted a great deal of attention for use as potential carriers in site-specific delivery over past decades. Mucoadhesive microspheres are useful tools for nasal drug delivery.
Objectives: To prepare and evaluate mucoadhesive microspheres as mode for nasal delivery of ondansetron using Caesalpinia pulcherrima galactomannan (CPG).
Materials and methods: Conventional spray-dried CPG nasal microspheres loaded with ondansetron for intranasal drug delivery in order to avoid the first pass metabolism with improved therapeutic efficiency in treatment of nausea and vomiting as an alternative therapy to parenterals. Developed microspheres were evaluated for characteristics like particle size, entrapment efficiency, zeta potential, swelling ability, in-vitro mucoadhesion, in-vitro drug release, DSC, XRD study and histopathological evaluation of tissue. CPG-based ondansetron microspheres were studied in rabbits for screening nasal absorption potential of nasal formulation.
Results: Developed nasal microspheres possess entrapment efficiency of 80–89%, higher mucoadhesion of 72–84% across goat nasal mucosa. In-vivo study showed that microspheres based on mucoadhesive polymer were able to promote quick drug absorption as well as enhanced bioavailability of drug.
Discussion: Histopathological studies evaluated biocompatible and nontoxic nature of CPG in nasal cavity. Developed mucoadhesive microspheres by nasal route showed enhancement of bioavailability as compared to oral route in rabbits.
Conclusion: CPG-based mucoadhesive microspheres can successfully deliver ondansetron intranasally, sustain its effect, avoid first pass effect, an alternative route of administration to injection and thus enhance systemic bioavailability of ondansetron hydrochloride.
Introduction
Ondansetron hydrochloride is a novel and specific antagonist of the 5-HT3 receptor used in the management of chemotherapeutic induced and post-operative nausea and vomiting. Its relative bioavailability is about 60% due to first pass metabolism and its plasma half-life is about 3–4 h (Kohler & Goldspiel, Citation1991). The shorter biological half-life and frequent dosing in chemotherapy-induced nausea and vomiting make it as an ideal candidate for sustained release drug delivery system (Chaudhary et al., 2010). Microencapsulation is the technique to sustain the release rate of the drug (Mahajan & Gattani, Citation2010; Prajapati et al., 2011).
Caesalpinia pulcherrima belongs to Leguminosae (family: Fabaceae and subfamily: Caesalpinioideae). It is a traditional medicine plant having thorny bushy legume locally recognized as Dwarf Poinciana, Dwarf Flamboyan, Pride of Barbados, Paradise Poinciana, Red Bird-of-Paradise. It is generally distributed in tropical as well as sub-tropical regions like India, Myanmar, Vietnam, Sri Lanka and Malay Peninsula. Various pharmacological actions of C. pulcherrima have been reported such as analgesic and anti-inflammatory, antimicrobial activity, antiulcer, antibacterial and antifungal activity, cytotoxic activity, antitumor. The composition of CP gum was found to contain mannose: galactose: glucose: xylose in a ratio of 2.8:1:0.1:0.08 with M/G ratio 2.80 (Thombre & Gide, Citation2013).
Galactomannans are water soluble hydrocolloids, high molar mass, non-ionic polysaccharides forming highly viscous, stable aqueous solutions. Galactomannans are commonly useful in the various industries mainly as thickening, stabilizing agents in a range of applications in the development of edible films or coatings for food applications, excellent thickeners and stabilizers of emulsions, and the nontoxic nature allows their use in the textile, pharmaceutical, biomedical, cosmetics and food (Thombre & Gide, Citation2013; Somasundaram et al., 2011).
Nasal drug delivery is a promising drug delivery option where common drug administrations (e.g. intravenous, intramuscular, or oral) are inapplicable. Recently, it has been shown that many drugs have better bioavailability by nasal route than by oral route (Kumar & Kiran, Citation2012). This has been attributed to rich vasculature and a highly permeable structure of the nasal mucosa coupled with avoidance of hepatic first-pass elimination, gut wall metabolism and/or destruction in the gastrointestinal tract. It is a promising route which offers non-invasive systemic delivery of numerous therapies and drug delivery route to the brain. However, nasal delivery has limitation which has restricted its use due to rapid clearance of the formulation from nasal cavity due to mucociliary clearance mechanism (Upadhyay et al., Citation2011; Saraporn et al., 2006).
Different delivery systems based on mucoadhesive polymers have been developed which are able to enhance the residence time of formulation at site of absorption of drug. The mucoadhesive system as microspheres not only protects the drug from enzymatic degradation but also remains in close contact with the absorption tissue. The mucous membrane releases drug at the site of action which leads to enhanced bioavailability (Mahajan et al., Citation2008). The aim of the present study was to study the probable application of C. pulcherrima galactomannan (CPG) for the preparation of mucoadhesive microspheres for the nasal administration of ondansetron hydrochloride.
Materials and methods
Ondansetron hydrochloride was obtained as a gift sample from Glenmark Pharmaceuticals Ltd., Mumbai, Maharashtra, India. The seeds of C. pulcherrima plant were obtained from Nasik, Maharashtra, India. All other chemicals were of analytical grade and used as received.
Extraction of CPG
Pods of C. pulcherrima were collected from specific shrubs, growing in Nasik, Maharashtra, India. The seeds were separated from the pods, cleaned and suspended in 99% ethanol in ratio 1:3 (seeds: ethanol by volume) at 60–80 °C for 30 min to inactivate the enzymes and separate low-molecular-weight compounds. Then ethanol was poured out. The treated seeds were mechanically crushed. The endosperms were manually isolated from the germ and the husk of the seeds. The distilled water was added in a proportion of 1:5 (endosperm: water in terms of weight) and reserved or approximately 24 h. Further, distilled water was added in a double proportion followed by mixing in a blender for 5 min. The suspension was filtered through nylon net and further centrifugation was continued at 25 000 g (Remi Labs, Thane, India) for 30 min at 25 ± 0.5 °C to eliminate insoluble matter. The galactomannan was precipitated by the addition of 99% ethanol. The ethanol was decanted and the precipitated galactomannan was lyophilized and reserved in a dry place (desiccators) till further use (Thombre & Gide, Citation2013).
Determination of the chemical composition
The moisture content of CPG was detected from the weight loss upon overnight heating at 105 °C in an oven according to the American Association of Clinical Chemistry (AACC, 1995) and the ash content of the GM sample was studied according to AOAC Official Method 923.03. Protein content was calculated by obtaining the total nitrogen content by the Kjeldahl method, as described in the Association of Analytical Communities (AOAC) Official Method of Analysis 981.10. The factor N x 5.7 was used for the estimation of the total protein content of the sample. The elemental analyses were determined with an elemental analyzer (Elementar Vario El, Hanau, Germany) instrument for the determination of carbon, nitrogen, sulfur, oxygen (C, S, N, O) at National Institute of Pharmaceutical Education & Research (NIPER), Mohali, Chandigarh, Punjab, India.
Biocompatibility studies of CPG
Acute toxicity studies were studied according to organization for economic cooperation and development (OECD) guidelines, received draft guidelines 425, received from CPCSEA, Ministry of social justice and empowerment, Government of India, India. Mice weighing 20–25 g in groups of five were used (n = 5) and were fasted for 4 h with free access to water only. The isolated CPG was administered orally in divided doses of 2000 and 5000 mg/kg to different groups of mice and observed over 14 d for mortality and physical/behavioral changes. Acute toxicity study on mice was carried out in compliance with the protocol of Institutional animal ethical committee as per animal ethical committee of Bhujbal Knowledge City, MET’s institute of pharmacy, Adgaon, Nasik, Maharashtra, India (Registration number: 1344/ac/10/2011-2012/CPCSEA under CPCSEA, India).
Preparation of microspheres
Ondansetron hydrochloride microspheres were prepared by the spray drying technique. The formulations of microspheres as presented in consisted of 1:2 and 1:4 drug to polymer ratio. CPG was dispersed in hot water. Ondansetron hydrochloride (Glenmark, Mumbai, India) then mixed with polymer solution. The solution of each batch was spray dried (LU222, Labultima, India) with the process parameters as follows: inlet temperature 160 °C, feed rate 2 ml/min, aspirator rate 55 Nm3/h. The solution was maintained at about 80 °C under magnetic stirring during the spraying process. The total volume of solution used for preparation of microspheres for each batch was 250 ml (Mahajan et al., Citation2008).
Table 1. Formula for different batches of ondansetron hydrochloride-loaded CPG microspheres.
Evaluation of microspheres
Morphological examination
Morphological characterization of the microspheres was carried using scanning electron microscopy. The surface of microspheres was determined using scanning electron microscope (SEM TEOL 5400 Model, Tokyo, Japan). The microspheres were kept on the sample holder and the scanning electron micrographs were taken (Jain et al., Citation2012).
Drug content
The various batches of the microspheres were subjected for drug content analysis. Accurately weighed microsphere samples were mechanically powdered. The powdered microspheres were dissolved in adequate quantity of phosphate buffer pH 6.6 and then filtered. The UV absorbance of the filtrate was measured using a UV spectrometer at 248 nm (Yeamin et al., Citation2010).)
Percentage yield
The percentage yield of different formulations was determined by weighing the microspheres after collection from spray dryer. The percentage yield was calculated as per the following equation.
(1)
(1)
Entrapment efficiency
Microspheres containing equivalent to 10 mg of drug was allowed to equilibrate in 100 ml of phosphate buffer pH 6.6 for 24 h. The solution was filtered using Whatman filter paper. The resulting solution was analyzed using a UV spectrophotometric method at 248 nm. The blank sample prepared from microspheres containing all materials except the drug (Khan et al., Citation2010
).
(2)
(2)
Particle size analysis
A microscopic image analysis technique for determination of particle size was applied. The particle sizes were determined using a Motic digital microscope set with a 1/3” CCD camera imaging accessory as well as computer controlled image analysis software(Motic images plus 2). The microspheres were dispersed on a microscope slide and the microscopic field was scanned by video camera. By using software, the images of the scanned field are analysed (Gavini & Hegg, Citation2006; Shah et al., Citation2010).
Zeta potential determination
The zeta potential determination was carried out to detect the charge on the prepared microspheres. The microparticles were dispersed in distilled water and the dispersion was filled in zeta cell. The zeta cell then placed in the zeta sizer (Nano ZS, Malvern instruments, Malvern, UK). The zeta potential study was carried out at a temperature of 25 °C and the measurement position was kept at 2 mm. The zeta potential was determined with the help of software (Shah et al., Citation2010).
In-vitro mucoadhesion
A freshly cut piece of goat nasal mucosa (2 cm2) was obtained and then cleaned by washing with isotonic saline solution. Accurately weighed 100 mg of microspheres were placed on mucosal surface which was fixed over polyethylene support. About 100 µl of simulated nasal electrolyte solution was put on microspheres and the same plate was incubated in desiccators for 25 min at 90% relative humidity to allow polymer to interact with membrane and finally fixed at an angle of 45° relative to horizontal plane. The mucosa was washed with phosphate buffer at the rate of 5 ml/min. After 1 h on administration of microspheres, the concentration of drug in collected perfusate was determined by spectrophotometrically. The amount of microsphere corresponding to the drug amount in perfusate was determined. The amount of adhered microspheres was estimated as the difference between the applied microspheres amount and flowed microspheres amount (Cerchiara et al., Citation2005).
In-vitro degree of swelling
The ability of the spray-dried microspheres to absorb water by capillarity was evaluated using a Franz diffusion cell with a lateral capillary graduated tube in which the volume of liquid absorbed was measured. The tests were carried out in triplicate with accurately weighed samples placed on a Millipore filter 0.22 µm for 3.5 min and with phosphate buffer pH 6.6 as liquid. The following formula was used for calculation of degree of swelling:
(3)
(3)
Where α = degree of swelling, W
0 = initial weight of microspheres, Ws = weight of microspheres after swelling (Juan et al., Citation2001; Jain et al., Citation2012).
Thermal analysis
Differential scanning calorimetry (DSC) was performed on ondansetron hydrochloride, polymer and ondansetron hydrochloride-loaded microspheres. For the structural, crystal and physical state characterization of drug, DSC studies were performed. The DSC measurements were performed on a Shimadzu DSC (DSC 60), with thermal analyzer. Accurately weighed samples (about 3 mg) were placed in a sealed aluminum pan, before heating under nitrogen flow (50 ml/min) at a scanning rate of 10 °C per min from 100 to 400 °C. An empty aluminum pan was used as reference (Tadwee et al., Citation2011).
Powder X-ray diffraction
The crystallinities of ondansetron hydrochloride and ondansetron hydrochloride-loaded microspheres were evaluated by XRD measurement recorded for ondansetron, blank microspheres and ondansetron-loaded microspheres using X-ray diffractometer (BRUKER, Germany D8 Advance). Scanning was done up to 2θ range between 2° and 90° using Ni-filtered (Mahajan & Gattani, Citation2010).
In-vitro drug release study
An in
-
vitro drug release test of the microspheres was performed using modified Franz diffusion cell with cellophane membrane. The membrane was equilibrated before carefully dispersing the sample equivalent to 15 mg of drug on the donor compartment. The donor compartment contained 3 ml of simulated nasal electrolyte solution and receiver compartment was filled with phosphate buffer solution, pH 6.6 that was within the pH of nasal cavity and maintained at 37 ± 0.5 °C. The samples were withdrawn from the receptor compartment, replaced with the same amount of fresh buffer solution and assayed by a spectrophotometer at 248 nm (Cerchiara et al., Citation2005).
(4)
(4)
Histopathological examination
The histopathological evaluation of tissue incubated in phosphate-buffered saline (pH 6.6) for 6 h after collection was compared with tissue incubated in diffusion chamber with formulation. Tissue was fixed in 10% buffered formalin (pH 7.2) and embedded in paraffin. The paraffin sections were cut on glass slides and stained with eosin. The sections were examined under a light microscope to detect any damage to the tissue (Rita et al., Citation2006; Tadwee et al., Citation2011).
In-vivo studies
In - vivo study on rabbit was carried out in compliance with the protocol of Institutional animal ethical committee as per animal ethical committee of R.C.Patel, Institute of Pharmaceutical Education and Research, Shirpur, India (Registration number: 651/02/C/CPCSEA under CPCSEA, India). Two New Zealand white rabbits with mean weight of 1.5 ± 0.3 kg were used. The rabbits were kept in single cages and fasted for 12 h before the study with free access to water during experiment. A cannula was inserted into the marginal ear vein for blood sampling and flushed with heparinized normal saline solution.
Study design
The white New Zealand rabbits were chosen as an experimental model because it provides a fine controlled animal model for screening the nasal absorption potential of nasal formulations. The rabbit received 5 ml of oral drug solution by an oral tube as well as the nasal formulation was deposited in both the nostrils. Drug was administered to rabbit at the dose of 2 mg/kg. The blood samples of 0.5 ml were collected at preset intervals to analyze the concentration of ondansetron hydrochloride in plasma by using high-performance liquid chromatography (HPLC) method (Shein-Chung & Jen-Pet, Citation2009).
Sample collection and analysis
After administration of the different formulations, blood samples (0.5 ml) were collected at time intervals of 15, 30, 60, 90, 120, 150, 180, 210, 240, 270 and 300 min from the marginal ear vein of the rabbits. Blood samples were mixed with anticoagulant (heparin) as well as centrifuged at 3000 rpm for 15 min. The obtained plasma samples were deep frozen at −20 °C for pending HPLC analysis. Plasma samples (0.1 ml) were mixed with 10 μl of working internal standard (Terazocin HCl 30 μg/ml) and alkalized with 20 μl of 2 mol/l sodium hydroxide solution. Then this mixture was added to 5.5 ml tetra butyl methyl ether, vortex mixed for 7 min and centrifuged for about 5 min. The organic phase (5 ml) was back extracted into 100 μl of 0.05% of phosphoric acid by vortex mixing for 3 min. Then the aqueous phase of 20 μl was injected directly into the HPLC column (C-18, Reverse phase column, Eclipse XDB, 5 μm, 4.6 × 150 mm, Agilent 1200 series, Singapore). The mobile phase consisted of acetonitrile, methanol, water and triethyl amine (25:10:65:0.1 v/v) whose pH was adjusted to 4 by phosphoric acid and delivered at a flow rate of 1 ml/min (Quaternary Pump, Model G1354A, Agilent 1200 series). The volume of injection was 20 μl. The detection wavelength was 302 nm (ultra violet variable wavelength detector, Model G1315D, Agilent 1200 series Diode Array Detector) (Eunsook et al., Citation2008).
Data treatment and statistical analysis
Results obtained from HPLC analysis were plotted as drug concentration in plasma versus time. Non-compartment pharmacokinetic parameters including T
max, C
max and area under curve (AUC) were calculated by Kinetica 5.0 R computer program. The AUC values for each curve were determined from time zero to the last data point using the trapezoidal rule with extrapolation to infinity. The AUC0–∞ values obtained from curve were used to determine the relative bioavailability.
(5)
(5)
Where % F
rel = percent relative bioavailability, AUCOS = area under plasma concentration curve after oral administration and AUCNA = area under plasma concentration curve after nasal administration.
The results of in - vivo experiments were calculated as mean ± SD and analysed by using Graph Pad Instat Version 3.01 software. The pharmacokinetic variables from two dosage forms were compared by unpaired t test. A p value less than 0.05 was considered significant.
Result and discussion
The extraction and purification processes in the present work were found to be effective to achieve the purified galactomannan at the laboratory scale. The purified galactomannan was found to be homogeneous with 32 ± 3% yield of the total weight of the seeds. The obtained values are in good conformity with those reported by Thombre & Gide (Citation2013). The physicochemical characteristics of purified CPG were analysed for moisture content, ash value and protein (Nx5.7) content and were found to be 10 ± 1%, 0.056% and 0.016%, respectively. It has been examined that the purification process was adequate to eliminate ash and proteins in CPG. The elements analysis of the CPG was found to be carbon (39.85 ± 1.52%), hydrogen (7.24 ± 0.31%), nitrogen (0.001909%) and sulfur (0.045%). Thus, the CPG was found to be rich in carbohydrate as per the elemental analysis. Acute oral toxicity was performed by up-down regulation method. It is found that CPG was safe at limit dose 5000 and 2000 mg/kg, with no mortality or abnormal behaviors in studied subjects, respectively.
The microspheres of ondansetron hydrochloride using CPG were prepared by spray drying method. This technique appears to be suitable method for the preparation of ondansetron hydrochloride microspheres. Spray drying process is rapid, easy and single step process which leads to the formation of porous dry microparticles. Optimum aspiration flow rate based on the trial batches was selected for the final experimental batches as 55 Nm3/h to generate optimum moisture content in formulation with proper separation of fine with coarse particles and shows significance in - vitro drug release through in - vitro mucoadhesion of the microsphere.
One of the major advantages of spray drying process is possibility of operation under aseptic conditions. It offers drying of feed as well as embedding of drug into a one-step operation. The spray-dried microspheres appeared as off white powder. The microspheres were found to be discrete, spherical and free flowing powder.
Morphological examination
All microspheres were spherical in shape with smooth surfaces. These microspheres had no hole or rupture on surface, such morphology would result in slow clearance and good deposition pattern in nasal cavity.
Drug content
The determination of drug content shows good uniformity. The drug content was found to be in the range of 17–35.4% ().
Table 2. Characteristics of prepared ondansetron hydrochloride-loaded microspheres.
Percentage yield
The percentage yield of different formulations was determined by weighing the microspheres after collection from spray dryer. The percentage yield of spray-dried microspheres was found to be between 20.74% and 41.93% (). Thus the yield was slightly increased with increase in the concentration of polymer ().
Entrapment efficiency
All microspheres had good entrapment efficiency ranging from 80.70% to 89.41%. Batch B8 shows highest entrapment efficiency while batch B1 shows lowest entrapment efficiency ().
Particle size analysis
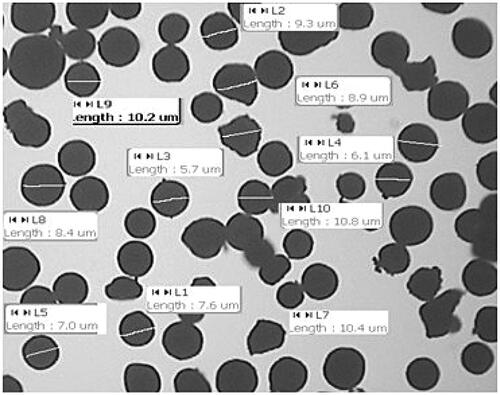
Particle size of microspheres is one of the most important characteristic as a nasal drug delivery. The mean particle size of microspheres ranged from 8.3 to 11.34 µm (). It has been suggested that particle size of 10 µm is most suitable for nasal administration ( and ).
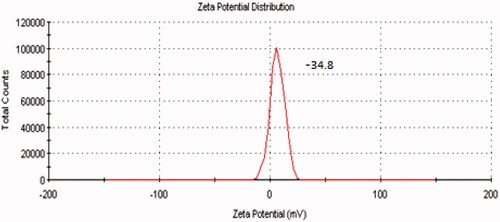
Zeta potential determination
The microspheres prepared were negatively charged, indicating the presence of CPG at the surface of all the microspheres. Studies have mentioned that polymers with charged density can serve as good mucoadhesive agents. shows zeta potential distribution curve of ondansetron-loaded CPG microspheres.
In-vitro mucoadhesion
Mucoadhesion studies were followed to ensure the adhesion of formulation to the mucosa for a prolonged period of time at site of absorption. The mucoadhesion was increased with increase in polymer concentration. This could be because of more presence of hydroxyl functional groups for interaction with mucin. Being carbohydrate, CPG is rich in functional groups contents hence showed higher percentage of mucoadhesion ().
In-vitro swelling studies
It is expressed as degree of swelling. Swelling capacity of microspheres was determined by CPG in the preparation. Microspheres with highest concentration of polymer showed the maximum swelling (). After getting contact of microspheres with nasal mucosa, the microspheres formulations are believed to form viscous gel by withdrawing water from nasal mucosa due to swelling of microspheres. The excess of carboxyl groups present in the network of the polymer holds water to form viscous gel. The resultant gel formation reduces the ciliary clearance rate and the residence time of formulation at nasal mucosa is prolonged.
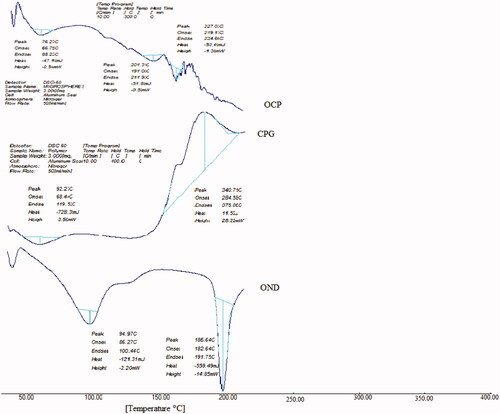
Thermal analysis
DSC was performed on ondansetron hydrochloride, polymer and ondansetron hydrochloride-loaded microspheres. For the structural, crystal and physical state characterization of drug, DSC studies were performed. of DSC thermogram shows the sharp endothermic peak at 186.64 °C due to the melting of drug. In blank microspheres, thermal transition at 340.71 °C is observed which is attributed to melting of polymer. In the DSC, the thermogram of the drug-loaded peak for pure drug-loaded microspheres did not appear. DSC studies had shown that ondansetron was molecularly dispersed inside the microspheres.
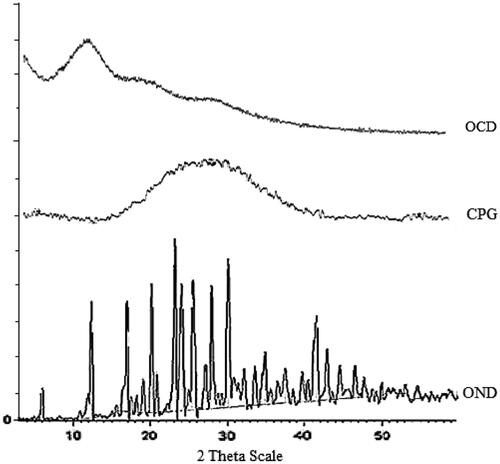
Powder X-ray diffraction study
The X-ray diffraction spectra for pure drug, CPG (blank microspheres) and drug-loaded microspheres are depicted in . Ondansetron had shown characteristic intense peaks between 2θ of 10 and 30, but in case of blank microspheres and drug-loaded microspheres, the intensity of peaks was decreased indicating amorphous nature of drug after entrapment into CPG microspheres by spray drying.
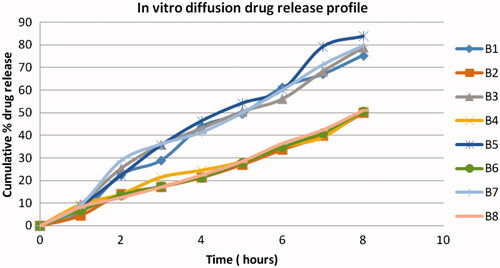
In-vitro drug release study
The drug release profile from various formulations of microspheres is shown in . Microspheres prepared with CPG moderately sustained the drug release to 8 h without any lag time. The rate and extent of ondansetron release from microspheres decreased with an increase in CPG. The drug release from microspheres was at slower rate due to ionic gelation. In order to investigate the drug release mechanism, the release data were fitted to models representation zero order, Korsemeyer, Peppas and Higuchi ().
Table 3. In-vitro release kinetic parameter of ondansetron hydrochloride-loaded CPG from microspheres.
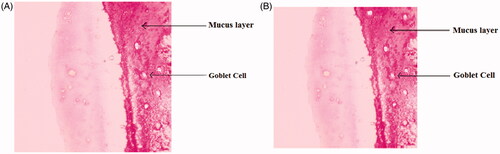
Histopathological examination of nasal mucosa
The histopathological examination of nasal mucosa was carried out for detection of any damage to the tissue. The microphotographs were taken of nasal mucosa following incubation with microsphere formulations for more than 6 h (). Nothing of the severe signs like appearance of epithelial necrosis, sloughing of epithelial cells was detected.
In-vivo study
Rabbits remained conscious during in - vivo experiment, thus it predicts efficient mucociliary transport function. Usual anesthetizing agent could affect the in - vivo absorption of drug so the anesthesia was avoided.
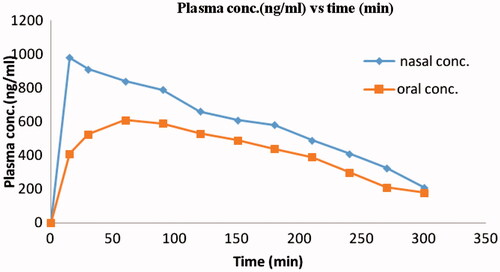
Calibration curve of ondansetron hydrochloride by HPLC was plotted as peak area versus concentration. Results from HPLC analysis were plotted as drug concentration in plasma versus time. The non-compartment pharmacokinetic parameters counting T max, C max and AUC were estimated by using Kinetica 5.0® computer program. AUC values for each curve were calculated from time zero to the last data point using the trapezoidal rule with extrapolation to infinity. To calculate the relative bioavailability the AUC0– ∞ values obtained from curve were used. % F rel is the percent relative bioavailability.
The relevant pharmacokinetic parameters, ondansetron peak concentration (C max, ng/ml), T max (min) and area under the concentration versus time curve (AUC, ng/ml min) illustrating ondansetron hydrochloride level in plasma of rabbits after administration of ondansetron hydrochloride containing nasal mucoadhesive microspheres and oral solutions are reported in . Mean C max of ondansetron hydrochloride after nasal administration was greater than that of oral administration. The AUC0–300 after nasal administration was greater than that of oral administration which was statistically significant (p < 0.05). The AUC0–∞ after nasal administration was greater than that of oral administration which was statistically significant (p < 0.05). The time to reach C max after nasal administration was somewhat slower than that by oral administration although difference was not significant. In - vivo performance of nasal formulation exhibited better bioavailability (AUC0–300 and AUC 0 – ∞ , 40.08 ± 2.48 and 43.04 ± 1.67) for ondansetron hydrochloride microspheres in comparison with oral administration of solution (AUC0–300, 22.46 ± 3.46 and AUC0–∞, 23.98 ± 4.32) that could be due to avoidance of first pass metabolism. These values corresponded to relative bioavailability values (F rel) of oral solution 34.24 ± 2.89% and for nasal microspheres 58.65 ± 4.54% respectively. The ondansetron hydrochloride microspheres by nasal route showed the enhancement of bioavailability as compared to oral route ().
Table 4. Comparative pharmacokinetic parameters of ondansetron hydrochloride following administration of oral solution and nasal microspheres in rabbits.
Conclusion
The results of our present study clearly represents promising capabilities of ondansetron hydrochloride–CPG microspheres for intranasal drug delivery as well as it could be viewed as substitute to conventional dosage form. The microspheres showed the particle size of about 10 µm which is ideal for nasal drug delivery could be prepared by spray-dried method. The microspheres had not only good sphericity but also uniform distribution of particle size. After getting contact with the nasal mucosa, microspheres formulations are supposed to form viscous gel by withdrawing water from nasal mucosa and interaction with cations present in nasal secretions. The resulting gel formation decreases ciliary clearance rate and as significance the residence time of formulation at the nasal mucosa is prolonged. CPG is biocompatible; it does not cause any harmful or toxic effect in nasal cavity as evaluated by histopathological studies. The results of in - vivo studies in rabbits showed that the microspheres system based on mucoadhesive polymer was able to promote absorption through nasal mucosa and to extremely improve the bioavailability of drug.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
Authors are thankful to Glenmark Pharmaceuticals Ltd, Mumbai, India, for gifting sample of Ondansetron hydrochloride. The authors are also grateful to Trustees, Bhujbal Knowledge City, MET’s Institute of Pharmacy, Adgaon, Nasik, Maharashtra, India, for providing the necessary facilities to carry out this work.
References
- Cerchiara T , Luppi B , Chidichimo G , et al . (2005). Chitosan and poly (methyl vinyl ether co maleic anhydride) microparticles as nasal sustained delivery system. Eur J Pharm Biopharm 61:195–200
- Chaudhary A , Jadhav KR , Kadam VJ . (2010). An over view: microspheres as a nasal drug delivery system. Int J Pharm Sci Rev Res 5:8–17
- Eunsook C , Hyesun G , Inkoo C . (2008). Formulation and evaluation of ondansetron nasal delivery systems. Int J Pharm 349:101–17
- Gavini E , Hegg AB . (2006). Nasal administration of carbamazepine using chitosan microspheres in vitro/in vivo studies. Int J Pharm Sci 307:9–15
- Jain N , Gulat N , Kumar D , Nagaich U . (2012). Microspheres: mucoadhesion based controlled drug delivery system. RGUHS J Pharm Sci 3:28–40
- Juan JT , Alfredo GA , Santiago TS , Luis G . (2001). Spray-dried powders as nasal absorption enhancers of cyanocobalamin. Biol Pharm Bull 24:1411–16
- Khan R , Prajapati SK , Singh G . (2010). Formulation and evaluation of mucoadhesive microspheres of flurbiprofen. Pharmacol Online 3:659–70
- Kohler DR , Goldspiel BR . (1991). Ondansetron: a serotonin receptor antagonist for anti-neoplastic chemotherapy induced nausea and vomiting. Ann Pharmacother 25:367--80
- Kumar G , Kiran S . (2012). Strategies and prospects of nasal drug delivery systems. IJPSR 3:648–58
- Mahajan HS , Gattani S , Surana S . (2008). Spray dried mucoadhesive microspheres of ondansetron for nasal administration. Int J Pharm Sci Nanotechnol 1:267–74
- Mahajan HS , Gattani SG . (2010). Nasal administration of ondansetron using a novel microspheres delivery system Part II: ex vivo and in vivo. Pharm Dev Technol 15:653–7
- Prajapati RK , Mahajan HS , Surana SJ . (2011). PLGA based mucoadhesive microspheres for nasal delivery: in vitro/ex vivo studies. Indian J Novel Drug Deliv 3:9–16
- Rita JM , Pradip KG , Manish LU , Rayasa SR . (2006). Thermo reversible mucoadhesive gel for nasal delivery of sumatriptan. AAPS Pharm Sci Technol 7:1–7
- Saraporn H , Lipipun V , Sutanthavibul N , Garnpimol CR . (2006). Spray-dried mucoadhesive microspheres: preparation and transport through nasal cell monolayer. AAPS Pharm Sci Technol 7:1–10
- Shah V , Sharma M , Parmar V , Upadhyay U . (2010). Formulation of sildenafil citrate loaded nasal microspheres: an in vitro, ex vivo characterization. Int J Drug Deliv 2:213–20
- Shein-Chung C , Jen-Pet L . (2009). Design and analysis of bioavailability and bioequivalence studies. : CRC press, 31–56
- Somasundaram J , Duraiswamy D , Kothapalli Bannoth CS . (2011). Drug release studies from Caesalpinia pulcherrima seed polysaccharide. Iran J Pharm Res 10:597–603
- Tadwee I , Shahi SR , Thube MW , et al . (2011). Spray dried nasal mucoadhesive microspheres of carbamazepine: preparation and in-vitro/ex-vivo evaluation. Int J Sci Publ Res Pharm 2:23–32
- Thombre NA and Gide PS . (2013). Rheological characterization of galactomannans extracted from seeds of Caesalpinia pulcherrima . Carbohydr Polym 94:547–54
- Upadhyay S , Parikh A , Joshi P , et al . (2011). Intranasal drug delivery system – a glimpse to become maestro. J Appl Pharm Sci 1:34–44
- Yeamin H , Hyun-Jong C , In-Soo Y , et al . (2010). Preparation and evaluation of spray-dried hyaluronic acid microspheres for intranasal delivery of fexofenadine hydrochloride. Eur J Pharm Sci 40:9–15