Abstract
Drug delivery systems are a promising technology to increase poor solubility and bioavailability of compounds. Therefore we have developed PLGA-PEG encapsulated amphotericin B nanoparticles (NPs) drug delivery technology to increase the solubility of amphotericin B and target the macrophage of infected tissues during visceral leishmaniasis. The structural characterization by transmission electron microscopy and dynamic light scattering revealed the nano-size of the particle (30–35 nanometers). Fourier transform infrared spectroscopy confirmed the PLGA-PEG encapsulation. The mean cytotoxic assay (0.0803 + 0.0253) of extracellular promastigote of PLGA-PEG encapsulated amphotericin B is significantly lower than that of amphotericin B (0.1134 + 0.0153) and inhibition of amastigotes in the splenic tissue was significantly more than with conventional amphotericin B (93.02 + 6.63 versus 74.42 +14.78). Amphotericin B encapsulated PLGA-PEG nanoparticles were found to be more effective than free amphotericin B in terms of therapeutic efficacy during in vitro and in vivo study.
Introduction
Leishmaniasis is a group of diseases with a wide range of clinical manifestations ranging from self-healing cutaneous ulcers to more severe form visceral leishmaniasis and even causes death. (Handman & Bullen Citation2002) Leishmaniasis is an infectious disease caused by the protozoan of the genus Leishmania. It is an obligate intracellular parasite of mammalian macrophage. Each and every form of leishmaniasis is transmitted to the mammalian macrophages by the bite of certain species of female sand fly during blood meal and responsible for cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) or visceral leishmaniasis (VL). Parasites of the genus Leishmania are digenetic and complete their life cycle in two different types of hosts, sand flies are the primary invertebrate host vectors and secondary final host vector is mammals. Leishmania species within sand flies are flagellate, motile promastigotes in alimentary canal, the flagellated infective stage of the parasite the metacyclic promastigotes form is transmitted to the mammalian host during the biting of sand flies. In the mammal, the parasites are in macrophage where they develop into non-motile amastigotes. (Späth et al., Citation2003) Indian Kala-azar or Dum-Dum fever is caused by parasite which belongs to the Leishmania donovani, donovani, L.d infantum and L.d dum fever archibaldi in the old world and L. d chagasi in the new world. Various clinical symptoms of Kala-azar are high fever, weight loss, splenomegaly and hepatomegaly. (Iqbal et al., Citation2002; Mc Conville et al., Citation2002) Kala azar is the most severe and lethal form of leishmaniasis, especially to those patients in which, the patient is particularly co-infected with AIDS mentioned by world health organization. (World health organization, Citation1999) The treatment option of visceral leishmaniasis, potentially caused by Leishmania donovani is limited and unsatisfactory. Availability of drugs is mostly parenteral and has serious toxicity. This problem is further intensified in Bihar (India) where widespread resistance to pentavalent antimonials persists. The first-line of treatment of visceral leishmaniasis has been by amphotericin B in these regions, however, adverse drug reactions (ADRs) are a major limiting factors. A total dose of 15–20 mg/kg has to be given as intravenous infusions either daily or on alternate days, necessitating prolonged hospitalization. ADRs are universal, which can occasionally be serious. (Sundar et al., Citation2000) The development of lipid formulations of amphotericin B, especially liposomal formulations, has alleviated this problem up to some extent. (Muller et al., Citation2001) However, amphotericin B has shown poor gastrointestinal absorption and low bioavailability due to the hydrophobicity of the polyene structure. (Gershkovich et al., Citation2009) There is also report about its interaction with the mammalian cell membrane causing cellular dysfunction. (Bolard et al., Citation1991) Formulation of deoxycholate complexes AmB micelles (Fungi zone) most commonly used drug is highly toxic to patients, often causing decreased renal function, anaphylaxis, chills, high fever, nausea, phlebitis, anorexia and other adverse effects. Usefulness as in anti-infective therapy in general is limited by this adverse reaction coupled with long therapeutic regime. (Hartsel & Bolard, Citation1996) Lipid formulations of amphotericin B reduce toxicity to non-target tissues but the development of resistance cannot be degraded by body. (Croft et al., Citation2006) The prohibitive cost of these formulations puts them beyond the reach of most of the patients in the endemic areas of VL, which represents the poorest areas of the world. A different approach to drug formulations need to solve the problem; this could be done through nanonization of the drug. The nanoparticles are recognized as foreign bodies and phagocytosed by the macrophages leading to target specific delivery, as Leishmania harbors inside the macrophage phagocytic system. Furthermore, the solid nanoparticles are characterized by high weight per volume, which is an ideal situation for sustained drug release by gradual diffusion from the depot. Thus, the drug can be delivered in higher doses and over a shorter duration to achieve cure of the disease. (Muller et al., Citation2001) The present study is aimed at evaluating this formulation of PLGA-PEG nanoparticle and this nano-formulations used against visceral leishmaniasis infection as this nano-formulations improved the efficacy and bioavailability of bioactive drug. Further CD14 increased localization in macrophages predominantly infected with Leishmania parasite. In vitro antileishmanial activities of amphotericin B encapsulated PLGA-PEG nanoparticles (poly (d, l-lactide-co-glycolide)-block-poly (ethylene glycol)) were found to be more effective than that of free amphotericin B in terms of therapeutic efficacy as ED50 (effective dose 50). The promastigotes of Leishmania parasite significantly decreased and additionally inhibition of amastigotes in the splenic tissue with amphotericin B encapsulated PLGA-PEG nanoparticles was significantly more than that with conventional amphotericin B.
Materials and methods
Chemical
Chemicals such as PLGA, COOH-PEG-NH2, methylene chloride, N-hydoxysuccinimide, 1Ethyl-(3-dimethylaminopropyl)-carbodiimide (EDC) was purchased from Sigma-Aldrich (St. Louis, MO). Deionizer water was obtained using a Milli-Q water purification system (Millipore, Billerica, MA).
Parasites
Promastigotes of Indian Leishmania donovani strain MHOM/IN/83/AG83 was obtained from culture bank of Rajendra Memorial Research Institute of Medical Sciences (ICMR), Patna, India. The cryo-cells were revived and grown in RPMI1640 medium (Sigma-Aldrich) supplemented with 10% Fetal Calf Serum (FCS: Sigma-Aldrich) in BOD incubator at 22 °C.
Drug preparation
The designed drugs were obtained from commercial source and stored at −20 °C. The stock solutions were diluted in appropriate medium to required concentrations before drug sensitivity assay.
PLGA-PEG nanoparticles synthesis
Carboxylated-functionalized copolymer PLGA–b–PEG was synthesized by the conjugation of COOH–PEG–NH2 to PLGA–COOH. PLGA–COOH (1 g) in methylene chloride (2 ml) was converted to PLGA-NHS with excess N-hydroxysuccinimide (27 mg) in the presence of 1–ethyl–3–(3–dimethylaminopropyl)-carbodiimide (EDC, 46 mg). PLGA–NHS was precipitated with ethyl ether (1 ml), and repeatedly washed in an ice-cold mixture of ethyl ether and methanol to remove residual NHS. After drying under vacuum, PLGA–NHS (1 g) was dissolved in chloroform (4 ml) followed by addition of NH2–PEG–COOH (250 mg) and N,N-diisopropylethylamine (28 mg). The co-polymer was precipitated with cold methanol after 12 h and washed with the same solvent (3 × 5 ml) to remove untreated PEG. The resulting PLGA–PEG block co-polymer was dried under vacuum.
Chemical carboamide cross-linking of antibody to CD14 to polymeric surface
PLGA–b–PEG NPs (10 μg/ml) were re-suspended in water and were incubated with EDC (400 mM) and NHS (200 mM) for 20 min. NPs were then repeatedly washed in DNase, RNase-free water (30 ml) followed by ultrafiltration. The NHS-activated NPs were reacted with amine terminal of CD14 (1 μg/ml). The resulting NP-CD14 bioconjugate were washed with ultrapure water (15 ml) by ultrafiltration, and the surface-bound CD14 were denatured at 90 °C and allowed to assume binding conformation during snap-cooling on ice. The NP suspensions were kept at 4 °C until use.
Formulation of Amphotericin B encapsulated PLGA-PEG
The nano precipitation method was employed for the formation of drug encapsulated carboxylated PLGA–b–PEG NPs. Briefly, amphotericin B (Sigma-Aldrich) was dissolved in various organic solvents that are miscible with water. Polymer was likewise dissolved and mixed with the drug. NPs were formed by adding the drug-polymer solution to water, a non-solvent. The resulting NP suspension was allowed to stir uncovered for 6 h at room temperature. NPs were purified by centrifugation (10 min, 10 000g). The PLGA–b–PEG NPs were resuspended, washed with water, and collected likewise.
Characterization of nanoparticles
The characterization of nano-encapsulated antileishmanial compound were carried out by transmission electron microscope (TEM), dynamic light scattering (DLS) and Fourier transform Infra red (FTIR).
Transmission electron microscope
Transmission electron microscope (TEM) measurements were performed using Philips CM 200 TEM in SAIF department of Indian Institute of Technology, Bombay.
Dynamic light scattering
The average size distribution of the nanoparticles was determined by Dynamic light scattering (DLS) (Beckman Coulter instrument) in IIT, Bombay.
Fourier transform infrared spectroscopy
The synthesis and encapsulation of amphotericin B nanoparticles was confirmed by Fourier transform infrared (FTIR) (Shimadzu instrument) in IIT, Bombay.
Extracellular promastigotes study
In vitro assay on L. donovani promastigotes working solutions of drugs, nano-amphotericin B and amphotericin B, at concentrations of 0.8 mg/l were prepared. Aliquots of 200 µl of the drugs in duplicate were dispensed in the first row of a 96-well plate (Corning Inc., COSTAR). In all the remaining wells of selected columns, 100 µl of medium was dispensed. From the drug wells, 100 µl of drugs was aspirated and transferred to the successive well of the second row using a multi-channel pipette. The process was continued to obtain 2-fold dilutions, and the last row was left as the control row. The parasite culture in the stationary phase was washed with RPMI-1640 medium and resuspended to obtain 1 × 106/ml in the same medium containing 15% HIFBS, pH 7.2. Aliquots of 100 µl of the parasite suspension were dispensed in all the medicated rows and the non-medicated row so as to obtain drug concentrations from 0.4 to 0.025 mg/l and the control in duplicate. The plate was incubated at 25 °C for 24 h in a cold incubator.
Hamster studies
Female hamsters were infected, via the tail vein, with 2 × 10−7 amastigotes of Ag83 L. donovani. After 30 days post infection, drug dose was given and the potency of infection has been checked. The infected mice were randomly divided into three groups (with five mice in each group), amphotericin B (5 mg/kg), PLGA-PEG encapsulated amphotericin B and control group. Each group has received dose 5 mg/kg for 7 days; liver impression smears from specimens obtained by biopsies further stained with Giemsa to confirm the infection and activity determined by counting microscopically, the number of amastigotes per 500 liver cells in treated and untreated mice. The percentage of inhibition of parasite replication was calculated by using the following formulae:
where CI is the percentage of inhibition, CP is the number of amastigotes per 500 nuclei in the spleen before treatment, CT is the number of amastigotes per 500 nuclei after treatment.
Statistical analysis
ED50 and inhibition of amastigotes in vivo values were calculated by using Graph Pad Prism 5version (La Jolla, CA).
Results
Nanoparticle synthesis
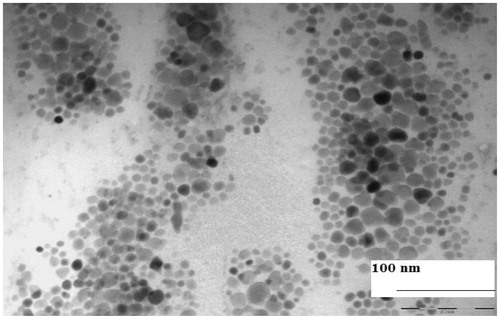
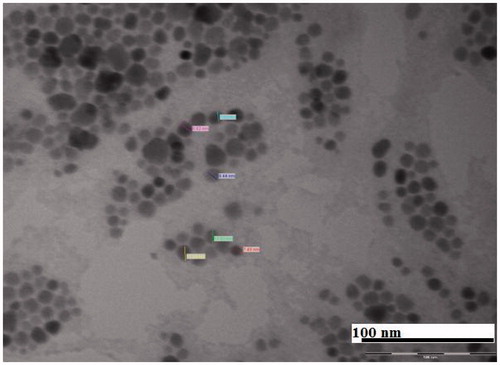
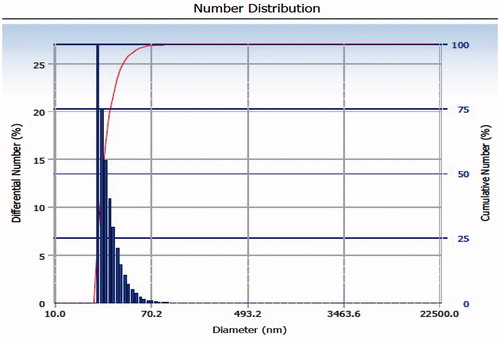
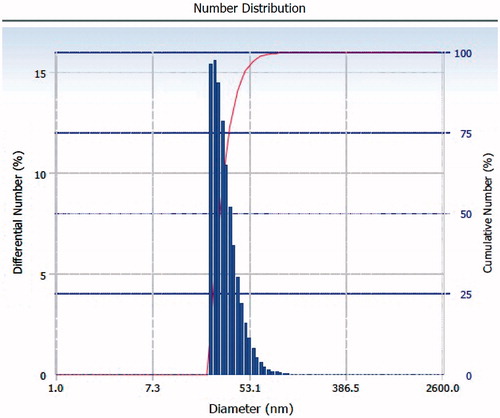
Syntheses of PLGA–b–PEG copolymer were carried out by direct conjugation of PLGA–COOH with NH2–PEG–COOH, both having fixed block length, to generate PLGA–b–PEG–COOH. The carboxyl group in the copolymer is located at the terminal end of the hydrophilic PEG block; therefore, upon NP formulation, the PEG should facilitate the presentation of the carboxyl groups on the NP surface making them available for surface chemistry. After preparing the nanoparticle, the size of this nanoparticle was characterized by TEM and DLS. TEM micrograph (the inset shows the particle size distribution) exhibited nearer to spherical nature. Analysis of both TEM and DLS confirmed the size of PLGA-PEG nanoparticles and PLGA-PEG encapsulated amphotericin B particles are between 25 to 30 nm and 30 to 35 nm respectively (). According to TEM and DLS data analysis, with the attachment of amphotericin B, the diameter of the PLGA-PEG encapsulation amphotericin B nanoparticles (than PLGA-PEG NPs alone) exhibited marked increase in diameter.
Chemical carboamide cross-linking of antibody to CD14 to the polymeric surface
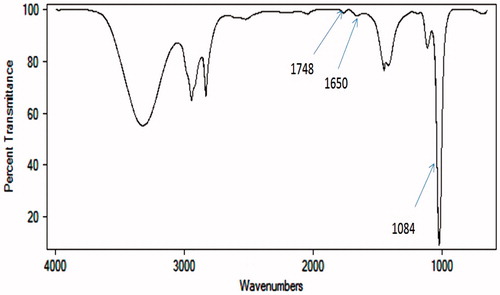
FT-IR spectroscopic analysis of PLGA-PEG NPs CD14 conjugate revealed that CD14 was indeed covalently linked to the PEG via amide bond free carboxyl group on the surface of PLGA nanoparticles through carbodiimide chemistry (). FT-IR spectra of the pure PLGA-PEG NPs confirmed that the PLGA-PEG composite preserved the characteristic peaks of each component. The peak around 1748 cm−1 was attributed to the stretching of carbonyl groups (C = O) from the polymer. The peak at 1084 cm−1 belonged to the stretch band of the C = O bond in PLGA-PEG. Peaks appeared at 1650 cm−l and 1530 cm−l assigned to C = O stretching vibration and NH- bending vibration of amide, respectively. These results suggest that a stable amide bond formed as a result of the reaction of carboxyl groups in PLGA-PEG with CD14.
In vitro study
ED50 for L. donavani amastigotes was determined after three days exposure to different concentration of amphotericin B and PLGA-PEG encapsulated amphotericin B. In all in vitro tests PLGA-PEG encapsulated amphotericin B was significantly more effective than amphotericin B alone. The mean (0.1134 ± 0.0153) of extracellular promastigote of amphotericin B is significantly higher than PLGA-PEG encapsulated amphotericin B (0.0803 ± 0.0253) ().
Table 1. ED50 value for evaluation of efficacy in vitro tests.
In vivo study
Formulations of PLGA-PEG encapsulated amphotericin B against L. donovani infections were significantly more active than amphotericin B alone as measured by parasite load in the liver. The mean values of parasite load were taken for further analysis (). The load of the parasites was found to be significantly lower for group A (93.2 ± 6.7%) compared with group B (74.6 ± 14.8).
Table 2. In vivo activity of amphotericin B and PLGA-PEG encapsulated amphotericin B.
Discussion
Treatment of visceral leishmaniasis (VL) involves several limitations such as poor solubility, low bioavailability, specific toxicities, drug resistance and relapses in HIV–Leishmania co-infected patients. Thus targeted drug delivery systems such as PLGA-PEG nanoparticle (NP) bio-conjugate with antibody to CD14 (targeting macrophage) with encapsulation of amphotericin B may prevail over these drawbacks. The encapsulated amphotericin B(with PLGA-PEG) nanoparticles are considerably smaller (25–35 nm) and PLGA-PEG encapsulation will prevent the premature degradation and maintain the characteristic properties of drugs, leading to opsonization of these particles preferentially by the cells of the macrophage phagocyte system leading to targeted drug delivery. (Muller et al., Citation2001; Yoshiaki, Citation2001) When evaluated by in vitro studies, PLGA-PEG nanoencapsulated retained their antileishmanial activities against promastigotes. ED50 values of PLGE-PEG encapsulated amphotericin B nanoparticles are lower than that of amphotericin B alone; it gives higher efficacy at lower dose. The reason behind the encouraging results of these PLGE-PEG encapsulated amphotericin B nanoparticles, especially with regard to lower dose, is probably due to the targeted delivery to tissues due to nanosizing. Hence, PLGA-PEG encapsulated amphotericin B nanoparticles are likely to be taken up by the macrophage phagocytic system, thereby considerably reducing the systemic side effects of amphotericin B akin to that seen with liposomal amphotericin B. (Kreuter, Citation2005) Moreover inhibition of amastigotes in the splenic tissue with amphotericin B encapsulated PLGA-PEG was significantly more than that with conventional amphotericin B, this may be due to the phagolysosomes acidic pH, accelerating the degradation of PLGA-PEG, promoting specific release of the drug in the vicinity of the amastigotes. (Mundargi et al., Citation2008) Hence the study signifies that there is an increased contact surface area of the drug, significant reduction in size and improved efficacy than that of liposomal amphotericin B.
Conclusion
The nano-encapsulated system such as amphotericin B encapsulated PLGA-PEG nanoparticles was developed with an aim to convert poorly soluble, poorly absorbed substances to biologically active substances with promising deliverable drugs against visceral leishmaniasis. The promastigote and amastigote studies revealed that the inhibition efficiency of amphotericin B encapsulated PLGA-PEG nanoparticles is more as compared to amphotericin B alone.
Declaration of interest
The authors are thankful to Indian Council of medical research, for providing financial support for carrying out nanomedicine related research work in our institute. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgment
We acknowledge Indian Council of Medical Research, India for finanicial support. We acknowledge Dr. Geeta Jotwani, Manas Ranjan Dikhit, Dr. Sindhuprava Rana, Md. Yousuf Ansari, Dr. Ravi, Gaurav Kumar, Dr. Vahab Ali, Rani Mansuri, Kalyani Trivedi, Zahra Bandi, Sahil Sinha and Mukta Rani for their scientific and moral supports.
References
- Bolard J, Legrand P, Heitz F, Cybulska B. (1991). One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochem 30:5707–15
- Croft SL, Sundar S, Fairlamb AH. (2006). Drug Resistance in Leishmaniasis. Clin Microbiol Rev 19:111–26
- Gershkovich P, Wasan EK, Lin M, et al. (2009). Pharmacokinetics and biodistribution of amphotericin B in rats following oral administration in a novel lipid-based formulation. J Antimicrob Chemother 64:101–8
- Handman E, Bullen DV. (2002). Interaction of Leishmania with the host macrophage. Tren Parasitol 1:332–4
- Hartsel S, Bolard J. (1996). Amphotericin B, new life for an old drug. Tren Pharmacol Sci 17:446–9
- Iqbal J, Hira PR, Saroj G, et al. (2002). Imported visceral leishmaniasis: diagnostic dilemmas and comparative analysis of three assays. J Clin Microbiol 40:475–9
- Kreuter J. (2005). Liposomes and nanoparticles as vehicles for antibiotics. Infection 19:224–8
- Mc Conville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. (2002). Regulated degradation of ER membrane proteins in a novel tubular lysosome in Leishmania mexicana. Microbiol Mol Biol Rev 66:122–54
- Muller RH, Jacobs C, Kayser O. (2001). Nano-suspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev 47:3–19
- Mundargi RC, Babu VR, Rangaswamy V, et al. (2008). Nano/micro technologies for delivering macromolecular therapeutics using poly (D,L-lactide-co-glycolide) and its derivatives. J Control Release 25:193–209
- Späth GF, Garraway LA, Turco SJ, Beverley SM. (2003). The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci U S A 100:9536–41
- Sundar S, More DK, Singh MK, et al. (2000). Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis 31:1104–7
- World health organization. (1999). Wkly Epidemiol Rec 74:365–76
- Yoshiaki K. (2001). Nanoparticulate systems for improved drug delivery. Adv Drug Deliv Rev 47:1–2