Abstract
The antibacterial and anti-inflammatory potential of rosemarinic acid (ROA), a naturally occurring ester of caffeic acid has been well reported. Antibacterial effect of ROA is attributed to nucleoid damage with an increase in spatial division and condensation of genetic material. ROA has been found dynamic against many human pathogenic bacterial strains but its inhibitory prospective has never been established against skin inflammations caused by Propionibacterium acne. The skin surface in acne prone areas is colonized with Staphylococcus aureus and Propionibacterium acnes which contribute to inflammation and acne. Resistance to current antimicrobial therapies suggested the need to explore new antimicrobial agents against acne. Present work included the preparation of ROA-loaded niosomes and their in vitro antimicrobial evaluation against P. acne and S. aureus. This work also included the development of niosomal gel of rosmarinic acid for sustained delivery to bacteria infected cells. Niosomes of rosmarinic acid were formulated by reverse phase evaporation method using different ratio of span 85 and cholesterol. The prepared formulations were evaluated for its vesicle size, entrapment efficiency, in vitro release study and antibacterial activity. In vivo study of developed formulation was conducted on Swiss albino mice in comparison with solution of plain drug and a marketed formulation of benzoyl peroxide. It was evident that niosomes are novel carrier for delivery of naturally occurring antimicrobial agents, in deeper tissues of skin. The results showed that drug-loaded niosomes dispersed in the gelling agent are an effective delivery system for treatment of acne vulgaris.
Introduction
Acne vulgaris is a dermatological condition that results because of a chronic inflammation of a sebaceous follicle and is characterized by tender inflammatory papules and nodules. Acne vulgaris continues to burden every generation, and is expected to affect >85% of teenagers and >10% of adults. It is found to be a complex chronic disease associated with P. acnes and despite the multitude of the treatments and products in market, there is still no formula that can guarantee a long-term benefit to all acne patients. Antibiotics are most common treatment however they cannot be given for long term. Evidences from preclinical initiation to clinical presentation of active lesions now support the pivotal role played by cellular inflammatory events at all stages of acne lesion development. Therefore emphasis has moved from acne as a primarily hyperproliferative disorder of the sebaceous follicle to that of an inflammatory skin disorder. Drugs possessing antibacterial and anti-inflammatory potential need to be explored for their anti Propionibacterium potential as the release of inflammatory mediators into the follicle and surrounding dermis due to colonization of sebaceous glands by this bacteria is the major cause for development of acne. The present study was carried out with an objective to evaluate In vitro and in vivo inhibitory potential of rosmarinic acid, an established antimicrobial and anti-inflammatory agent, against Propionobacterium acne and Staphylococus aureus. Rosemarinic acid (ROA) is reported to possess notable activity against Bacillus subtilis, Micrococcus luteus and Escherichia coli (Kuhnt & Pröbstle, Citation1995). ROA has also been reported to possess activity against Pseudomonas aeruginosa, S. aereus, Shigella spp and Enterobacter (Ogundare & Salawu, Citation2011). ROA has been found to be active against Candida albicans and Aspergillu sniger (Reza et al., Citation2009). Anti-inflammatory potential of ROA for reducing the severity of diseases induced in an Experimental Murine Model of Japanese Encephalitis has also been established (Swarup et al., Citation2008). The present study was focused on exploring the antiacne potential of rosmarinic acid (ROA) by encapsulating it in niosomes. Lipophilic nature of ROA limits its use in pharmaceutical formulations so it was hypothesized that its incorporation in niosomes will improve its permeability characteristic as Noisome, improve the stratum corneum properties both by reducing transepidermal water loss and skin condition by increasing smoothness via reloading lost skin lipids. Moreover niosomes offer several advantages over liposomes like intrinsic skin penetration enhancing properties, higher chemical stability and lower costs which make the niosome more attractive for industrial manufacturing. This is for the first time that an antiacne formulation of ROA was developed by incorporating ROA into niosomes which improved the amount and time of drug retention within the skin and increased the therapeutic efficacy of the drug.
Material and methods
Materials
Rosemarinic acid was procured from Sigma–Aldrich, USA. Spans and Cholestrol were obtained from Sigma–Aldrich. Carbopol 940 was purchased from HiMedia Laboratories. Chloroform, sodium chloride and sodium methyl paraben were obtained from CDH Analytical Reagents, New Delhi.
Vesicle preparation
Niosomes containing cholesterol and span were prepared by Reverse phase evaporation technique according to method given by Guinedi et al., Citation2004. The method involved cholesterol and surfactants (1:1) dissolved in chloroform. An aqueous phase containing the drug was added, and the dispersion was sonicated. After sonication, the mixture was subjected to vortex shaking to get niosomal suspension.
Optimization of Span 85: cholestrol and span 85: cholesterol ratio to drug concentration
Ratio of surfactant to lipid and surfactant-lipid to drug ratio was optimized by changing their concentrations as shown in Table 1.1.1.
Characterization of niosomes
Niosomal formulations containing ROA were characterized for following attributes.
Surface morphology
The niosome vesicles were characterized for their for structural attributes such as lamellarity, uniformity of size, shape and physical stability characteristics, i.e. aggregation and/or irregularity shape using transmission electron microscope (TEM) Hitachi (H-7500, Japan). A drop of niosomal dispersion was smeared to a carbon film-covered copper grid and was stained with a 1% phospho-tungstic acid. Then samples were examined and photographed with transmission electron microscope at an accelerating voltage of 100 kV.
Particle size and zeta potential
The mean vesicle size and its distribution were estimated by using particle size analyser (Beckman coulter counter) based on photon correlation spectroscopy (PCS) at temperature 25 °C. Zeta potential of the vesicles directs the stability of the niosomes and was measured by Zetasizer (Beckman coulter counter), working on the principle of electrophoretic light scattering (ELS).
Entrapment efficiency
The free drug was removed by passing the niosomal formulation through a sephadex G-50 column. The vesicles were centrifuged at 5000 rpm for 15 min and absorbance of supernatant was checked at 324 nm using UV-VIS spectrophotometer. The entrapment efficiency of the niosomal formulation was calculated from the following equation.
where:
DL = Initial drug added (mg)
DF = Free drug (mg)
In vitro drug release
In vitro release kinetics of ROA-loaded niosomes was performed as per the following procedure. Incubator shaker (for constant temperature 37 °C, 100 rpm), open ended dialysis tubing made up of cellulose (retains substances with molecular weight >12 000 Da) having flat width 35 mm and inflated diameter 21 mm. The release profile of niosomes was performed in phosphate buffer pH 5.5. The wet sac was gently opened and was washed with phosphate buffer pH 5.5. The sac was filled with 3 ml of gel formulation and was suspended in a conical flask containing 200 ml of phosphate buffer pH 5.5. The temperature was maintained at ∼37 °C in the shaking incubator, with 100 rpm. At predetermined time intervals, 5 ml aliquots were withdrawn from the receptors compartment and were equally replenished with phosphate buffer pH 5.5 fresh and subjected to analysis. Spectroscopical analysis was carried out immediately after withdrawal of samples with help of UV-Spectrophotometer (Varshosaz, Citation2003).
Preparation of Carbopol 940 gel
As a vehicle for incorporation of niosomes for skin delivery, a carbopol gel was made. Carbopol 940 (450 mg) was dispersed in distilled water (60 ml) by stirring at 800 rpm for 60 min. The mixture was neutralized by drop wise addition of triethanolamine. Mixing was continued until a transparent gel appeared, while the amount of the base was adjusted to achieve a gel with pH 5.5 (Simonoska & Dodov, Citation2005).
Characterization of gel formulation
The prepared gel formulations were inspected visually for their color, homogeneity and consistency. The pH values of the prepared gels were measured by a pH (pico) meter (Lab India instruments Pvt ltd.).
Viscosity measurements
Viscosity measurements were carried out at temperature (25–27 °C) using a Brookfield DV-1 viscometer with shear rate ranging from 10 to 100 s−1. A specific amount of the formulation (20 ml) was used and the speed of the spindle was adjusted to 100 rpm.
Content uniformity
Content uniformity was determined by following procedure: gel formulations (100 mg) was dissolved in phosphate buffer pH 5.5 and filtered and the volume was made to 100 ml with phosphate buffer. The resultant solution was diluted with phosphate buffer and absorbance was measured at 324 nm using Shimadzu-1700 UV Visible spectrophotometer.
Spreadibility of the gel
Spreadibility of the gel was analyzed using texture analyzer (Brookfield, Germany). Standard female and male cone attachments were used. For the analysis, female cone was filled with the optimized gel formulations and male cone was inserted into it.
In vitro drug release
An in vitro drug release study was performed using modified Franz diffusion cell of capacity 60 ml. Dialysis membrane (molecular weight − 12 kD) was placed between receptor and donor compartments. Niosomal gel equivalent to 1 g was placed in the donor compartment and because of the very low solubility of ROA in water; methanolic phosphate buffer (pH 5.6) was used as receptor compartment. The diffusion cells were maintained at 37 ± 2 °C with magnetic stirring at 100 rpm throughout the experiment. About 1 ml of aliquots were withdrawn at different time intervals up to 24 h from receiver compartment and replaced with the same amount of fresh methanolic PBS to maintain the sink conditions. The samples were analyzed using UV spectrophotometry at wavelength 324 nm.
In vitro skin permeation
Skin of male Swiss albino mice was selected for permeation studies. Skin of male Swiss albino mice was selected for permeation studies. Hairs on the skin were carefully trimmed short (<2 mm) with scissors and the full thickness of skin was removed from abdominal region.
Franz diffusion cell was used for in vitro skin permeation studies. The skin was mounted between the two compartments of diffusion cell with stratum corneum facing the donor compartment. Effective diffusional area was 3.56 cm2. The volume of receiver compartment was 60 ml.
An amount equivalent to 3 mg of ROA (ROA) containing gel was placed in the neck of Franz diffusion cell. Phosphate buffer pH 5.5 was added to the receiver compartment. Cell was placed on magnetic stirrer with temperature maintained at 37 ± 0.5 °C.
At predetermined time intervals, 2 ml aliquots were withdrawn from the receiver compartment with equivalent fresh Phosphate buffer solution replenishment and subjected to analysis. Analysis was carried out spectrophotometrically at 324 nm immediately after withdrawal. The permeation studies were carried for 24 h. Percentage of cumulative amount of drug permeated versus time of different formulation was reported. All the experiments were performed in triplicate (Mean ± SD).
Skin retention studies
Skin retention study was done by following procedure: After permeation study, skin was removed carefully from diffusion cell. Skin was cleaned with cotton soaked in phosphate buffer (pH 5.5).Cleaned skin piece was mashed, and 50 ml of methanolic PBS was added to the meshed mass. Above solution was mechanically shaken in a water shaker bath at 37 °C for 1 h for the complete extraction of the drug. Solution was removed and the drug content in filtrate was determined spectrophotometrically at 324 nm using a UV spectrophotometer. %Skin retention of niosomal gel of ROA was determined.
In vitro evaluation
Antimicrobial assay
Rosemarinic acid, niosomes and niosomal gel was tested against two microbial strains S. aureus (MTCC 96) and Propionobacterium acne (MTCC 1951) procured from Microbial Type Culture Collection (IMTECH, Chandigarh, India). The microbial cultures were maintained in their appropriate agar slants at 4 °C throughout the study and used as stock cultures. The antibacterial activity of ROA, niosomes and gel was determined in triplicate. Pre-warmed Nutrient agar plates were used to test the activity of samples against S. aureus and blood agar was used for testing the compound and formulation against P. acne. ROA was dissolved in DMSO (1 mg/ml), equivalent amount of niosomes and gel was dispersed in DMSO. About 60 µL of each was pipetted onto sterile paper discs (8 mm diameter) placed on the surface of inoculated agar plates. Plates were incubated at 37 °C for 48 h. Antimicrobial potential was determined in terms of the diameter of zone of inhibition exhibited by the test compound.
Minimum inhibitory concentration
For the measurement of minimum inhibitory concentration (MIC) fresh culture were grown in 5 ml of respective broths. Separate broth tubes were impregnated with 15.62, 31.25, 62.5, 125 and 250 µg of ROA and different volume of niosomal suspension for equivalent concentrations. Each of the tube was inoculated with 0.1 ml of freshly growing cultures. Tubes were incubated at 37 °C and the growth of cultures was observed at 24 h for S. aureus and after 48 h for P. acne, of incubation. Minimum concentration that did not show any growth was taken as MIC.
In vivo study
Swiss albino mice of either sex were employed for the in vivo study. In vivo studies of final formulation were carried out against P. acne only. Protocol for studies was approved by Committee for the purpose of control and supervision of experiments on animals (CPCSEA)/Institutional Animal Ethical Committee (IAEC). The experiments were conducted with as per CPCSEA (Committee for Prevention, Control and supervision of Experimental Animals) guidelines.
Skin irritation test
The skin irritation test was performed with the objective to confirm the safety of the topical formulation. Van-Abbe et al. mentioned that a value between 0 and 9 indicates that the applied formulation is generally not an irritant to skin. The mean skin irritation score for niosomal gel and plain rosmarinic acid solution was observed to be 1.33 ± 0.31 and 4.27 ± 0.52, respectively. From this it was concluded that the optimized niosomal gel formulation cause very less irritation in comparison to plain rosmarinic acid solution and was considered to be safe for transdermal drug delivery.
Induction of infection
Animals were anaesthetized by intra muscular injection of 6 mg of ketamine per kilogram of body weight and 1 mg of xylazine per kilogram. Animals were shaved with the help of surgical blade. A metal weight 20 mm in diameter heated to 100 °C was pressed on the skin. After 1 h, 1 × 105 CFU/mL of bacterial suspension of P. acne was subcutaneously injected at the burnt site.
Evaluation parameters
The niosomal gel was applied after 24 h of the assessment of P. acne infection. The efficacy was measured on the base of parameters such as inflammation and bacterial multiplication rate (CFU count/ml).
Thickness measurement of the skin
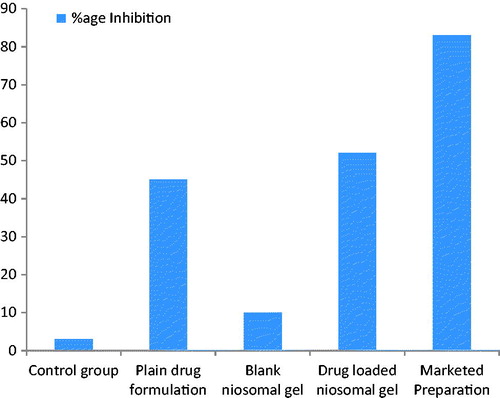
After 24 h of infection the increase in thickness was measured using digital vernier caliper. Then after the assessment of infection the test formulation was applied on respective groups till 3 days except the control group in which formulation was not applied. The percentage inhibition of inflammation after 3 days depicted in the table:
Inhibition of inflammation was found to be quite more in the groups in which drug-loaded niosomal gel was applied. The drug-loaded niosomal gel inhibits the inflammation up to 58.16% in 3 days. Plain rosmarinic acid solution inhibits the inflammation up to 40.9% in 3 days. Based on anti-inflammatory studies, it can be concluded that loaded niosomal gel showed more inhibition of inflammation than the plain rosmarinic acid solution.
Bacterial multiplication rate (CFU/ml)
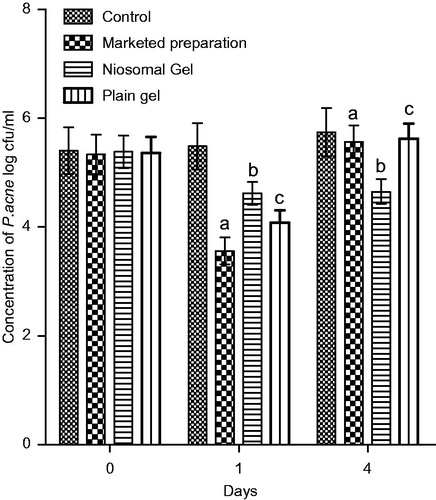
Group 1 was treated with niosomal gel, group 2 was applied topically with an equivalent marketed gel of Benzoyl Peroxide and group 3 served as the control whereas group 4 was treated with plain gel of rosmarinic acid. All animals were sacrificed after 24 h following the last treatment and skin from the infected sites was excised and homogenized in sterile physiological saline. A portion of the homogenate was cultured on Blood Agar plates. Colonies grown on the plate were counted to calculate the number of CFU/mL after 1 and 4 days. There was significant decrease in colony count by the plain gel and the niosomal gel in comparison to the marketed formulation. Furthermore the niosomal preparation was found to be effective till 4th day due to prolong release while plain gel had loss its effect.
Results and discussion
Optimization and characterization of niosomes
Optimization of niosomes
The niosomes were prepared by varying the ratio of surfactant to cholesterol ratio. Furthermore the selection of cholesterol to surfactant ratio was done on the base of the size of the vesicle. The ratio of drug: surfactant: cholesterol was optimized on the ground of entrapment efficiency, size of the vesicular system and the drug release profile (). The ratio of 3% drug ratio with 6:4 ratio of surfactant: cholesterol and hydration volume of 10 ml yielded the noisome of 814 ± 4.34 nm with entrapment efficiency of 65% ± 3.99.
Table 1. Optimization of surfactant:cholesterol ratio at 1%, 2% drug ratio.
Characterization of optimized niosomal preparation
Surface morphology
Optimized formulation was viewed under TEM for the surface morphology. TEM image depicts the formation of uniform sized vesicular system as shown in .
Size of the vesicular system
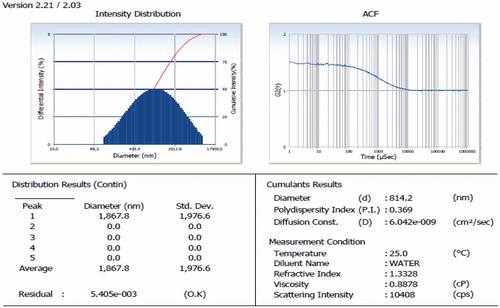
The size of the vesicle was found to be 814.2 nm with the PDI of 0.369 as shown in .
Zeta potential
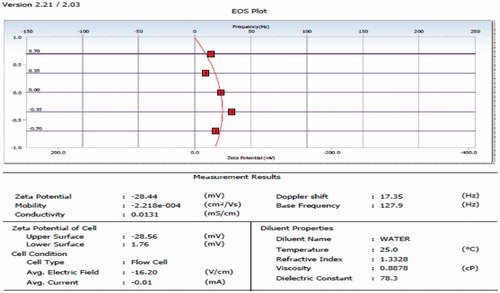
Zeta potential of the optimized formulation was found to be −28 as given in the .
Entrapment efficiency of niosomes
Drug entrapment of the optimized formulation was determined at wavelength of 324 nm with the help of UV spectrophotometer. Entrapment efficiency was found to be 65% ± 3.99.
Characterization of optimized niosomal gel
Physical examination
Physically niosomal gel was examined by placing the gel between thumb and the index finger and the homogeneity and consistency of gel was observed. The colour of the niosomal gel was found to be white translucent and no coarse particles.
pH of the gel
The pH of the niosomal gel was observed to be 6.4 ± 0.159 which was found to be compatible with the pH of the skin.
Content uniformity of the gel
Content uniformity of the prepared gel was found to be 90%.
Spreadibility of the gel
Spreadibility of the gel was found to be 19.6 ± 1.118 (g cm/s).
In vitro drug release of niosomal gel
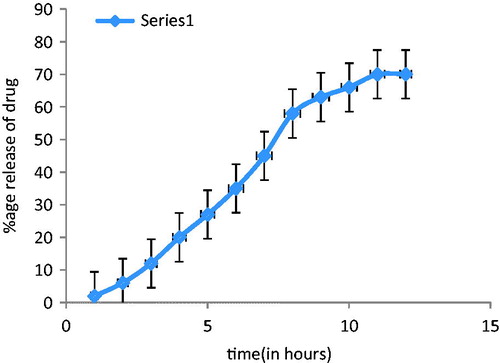
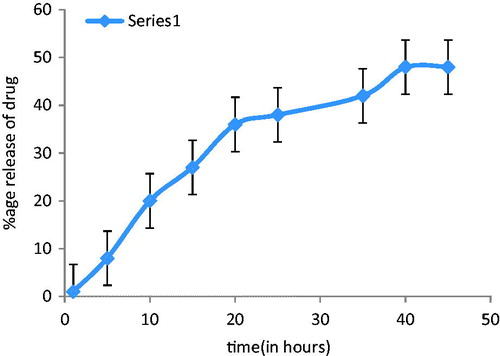
The drug release rate of the rosmarinic acid from the niosomal gel depicted the delivery of drug from the niosomal gel. Phosphate buffer solution was used in receiver compartment. shows the percentage release of ROA from niosomal gel at different intervals. After 24 h, the release was found to be 49.81 ± 1.76%.
In vitro skin permeation study
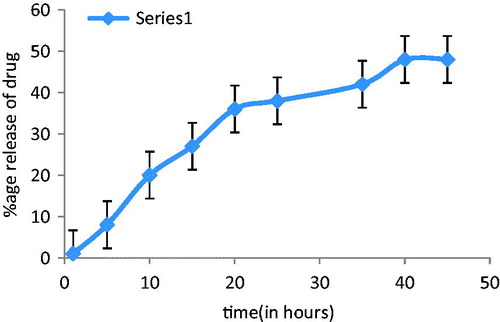
In vitro skin permeation studies were carried out with the objective to determine the permeation rate of rosmarinic acid from niosomal gel using swiss albino mice skin. Phosphate buffer (pH 5.6) was used as receiver compartment and sink conditions were maintained. The in vitro skin permeation of niosomal gel was shown in and . Results stated that in vitro skin permeation was found to be more in case of rosmarinic acid niosomal gel as compared to rosmarinic acid gel of equivalent amount of drug, after 48 h. This increase permeation was the effect of surfactant present in niosomal gel. Size range of niosomes was also the factor responsible for this penetration.
Table 2. Optimization of drug ratio at optimized surfactant: cholesterol ratio: drug.
Table 3. Animal groups for in vivo studies.
Table 4. Skin permeation and skin retention studies.
Table 5. Zone of inhibition (in mm) of different formulations.
Skin retention studies
The percentage skin retention of the drug from niosomal gel was found to be more as compared to the plain gel as shown in . The reason can be the small size of noisome which offers greater surface area in comparison to plain gel.
In vitro antimicrobial activity
Antimicrobial activity of ROA was tested against two microbial strains S. aureus (MTCC 96) and Propionobacterium acne (MTCC 1951).
Zone of inhibition
Zone of Inhibition of ROA against P. acne and S. aureus was determined using disc diffusion method. states the zone of inhibition of test formulations against Staphylococcus aureus and Propionibacterium acne. Zone of inhibition against S. aureus and P. acne revealed that niosome and niosomal gel demonstrated better antibacterial activity profile as compared to plain solution. The small size of niosome results in more penetration of drug inside the bacteria in comparison to plain and hence much more zone of inhibition than plain drug solution.
Table 6. Minimum inhibitory concentration.
Minimum inhibitory concentration
The minimum inhibitory concentration of ROA and niosomes loaded with ROA was calculated against both the test strains ().
In vivo study
Skin irritation studies
The skin irritation test was performed to confirm the safety of the topical formulation. Van-Abbe et al. mentioned that a value between 0 and 9 indicates that the applied formulation is generally not an irritant to skin. The mean skin irritation score for niosomal gel and plain ROA solution was found to be 2.13 ± 0.31 and 4.57 ± 0.62, respectively. From this it was concluded that the optimized niosomal gel formulation was very less irritant as compared to plain ROA solution and was considered to be safe for transdermal drug delivery.
Induction of infection
Infection was observed after 96 h after which the test formulations were applied.
Evaluation parameters
The niosomal gel was applied on the surface of the skin after 96 h of the P. acne injection. The efficiency of the formulation was examined on the parameters such as inflammation and bacterial multiplication rate.
Inflammation rate
After 24 h of the P. acne infection on the skin of the mice, the increase in thickness was measured using digital vernier caliper. Then after 24 h, the test formulations (drug equivalent to 1 mg) were applied on the mice skin of their respective groups up to 3 days. No formulation was applied to the control. The percentage inhibition of inflammation was calculated for all the animal groups by using the formula given as:
The percentage inhibition of inflammation of mice ear after 4 days was shown in table and .
Inhibition of inflammation was found to be quite more in the groups in which drug-loaded niosomal gel was applied. The drug-loaded niosomal gel inhibits the inflammation up to 58.16% in 3 days. Plain rosmarinic acid solution inhibits the inflammation up to 40.9% in 3 days. Based on anti-inflammatory studies, it can be concluded that drug-loaded niosomal gel show more inhibition of inflammation than the plain rosmarinic acid solution.
Bacterial multiplication rate (CFU/ml)
Bacterial count was calculated after 1 day and 4 days of application of marketed preparation, plain gel and niosomal gel in P. acne induced infection as shown in . Comparison was made with control, marketed, niosomal gel and plain gel. Though first day, we found that marketed, plain gel and niosomal gel showed significant difference than control while on fourth day only niosomal gel was able to show a significant decrease in colony count because of prolong release of drug from niosomes. Hence the drug-loaded niosomal gel was more effective in reducing the CFU count/ml after 4 days as compared to plain gel. Furthermore from the in vivo study, it can be concluded that ROA-loaded niosomal gel shows more anti-inflammatory affect and causes more reduction in CFU count of P. acne in mice.
Conclusion
Acne is a pleomorphic disorder characterized by formation o papules, pustules, nodules and cyst. Propionobacterium acne is implicated in the development of inflammatory acne by its capability to activate complements and by its ability to metabolize sebaceous triglycerides into fatty acids, which chemotactically attract neutrophils (Mullika et al., Citation2005). Antibiotics have been used for many years for treatment of acne. However antibiotics resistance is prevalent in acne. To overcome the problem of antibiotic resistance, herbal drugs need to be explored (Bhattacharyaa & Mitra, Citation2007). There are many more problems associated with the drugs currently being used for treatment of acne. The main limitation of benzoyl peroxide is cutaneous irritation or dryness and bleaching of clothes and hair (Bojor et al., Citation1995). The main adverse effects with topical retinoid are primary irritant dermatitis, which can present as erythema, scaling and burning sensation (Rathi, Citation2011). Use of topical antibiotics is also associated with Side effects though minor includes erythema, peeling, itching, dryness and burning, pseudomembranous colitis (Parry & Rha, Citation1986).
The present study was carried out with an aim to explore the inhibitory potential of ROA against P. acnes and S. aureus. ROA a phenolic compound obtained from Rosmarinus officinalis has been reported to inhibit the growth of human pathogens and acts as antimicrobial herb. Moreno et al reported the antimicrobial potential of rosmarinic acid against many Gram positive and gram negative bacteria (Moreno et al., Citation2006). Inspite of so many reports for antimicrobial and anti-inflammatory potential of ROA no pharmaceutical preparation of ROA is found in market. ROA suffers from low water solubility so we incorporated it in niosomes. Niosomes of ROA were found to increase the skin retention of ROA and facilitated prolong release. Niosomes loaded with drugs for dermal application interacts with epidermal tissue without showing strong systemic action. The plain gel of ROA was effective against S. aureus and P. acne on first day while no response was observed on fourth day. The niosomal gel was found to be effective till 4 days of application because of prolong release. So we can conclude that ROA has powerful inhibitory potential against the P. acne and can be used as a possible therapy against this bacteria provided it should reach the dipper layers of skin and for this niosomes can be used as carrier for delivery of naturally occurring antimicrobial agents, in deeper tissues of skin.
Declaration of interest
Authors declare no conflict of interest.
References
- Bhattacharyaa PK, Mitra A. (2007). Antimicrobial activity of leaf extract of Basilicum polystachon(L)Moench. Indian J Exp Biol 45:744–8
- Bojor RA, Cunliffe WJ, Holland KT. (1995). The short term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora. Br J Dermatol 132:204–8
- Guinedi A, Rao BR, Ravi D. (2004). Ascorbyl palmitate vesicles (Aspasomes) formation characterization and applications. Int J Pharm 271:96–113
- Kuhnt M, Pröbstle A. (1995). Biological & pharmacological activities & further constituents of Hyptis verticulata. Planta Med 61:227–32
- Moreno S, Scheyer T, Romano CS, Vojnov AA. (2006). Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res 40:223–31
- Mullika T, Chomnawang A, Suvimol A, et al. (2005). Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol 4:38
- Ogundare AO, Salawu SO. (2011). Antimicrobial activities of phenolic containing extract of some tropical vegetables. Afr J Pharm Pharacol 5:486–92
- Parry MF, Rha CK. (1986). Pseudomembranous colitis caused by topical clindamycin phosphate. Arch Dermatol 122:583–4
- Rathi SK. (2011). Acne vulgaris treatment: the current scenario. Indian J Dermatol 56:7–13
- Reza AG, Saednia S, Shahverdi AR, Yassa N. (2009). Phytochemistry and antimicrobial compounds of Hymenocrater calycinus. Eur Asia J BioSci 3:64–8
- Simonoska M, Dodov MG. (2005). Formulation and characterisation of topical liposome gel bearing lidocaine hcl. Bull Chem 24:59–65
- Swarup V, Ghosh J, Kumar M, Basu A. (2008). Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignin. J Antimicrob Chemother 61:679–88
- Varshosaz J. (2003). Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv 10:25