Abstract
Bacterial infections continue to be one of the major causes of morbidity and mortality. Although many methods for diagnosing and treating of infectious diseases currently exist, there is an urgent need for new and improved approaches for bacterial destruction. The present study focuses on the conjugation of gold nanorods (GNRs) with gentamicin via the Nanothink acid linker and its application in delivery of gentamicin to infection foci due to Staphylococcus aureus. The interaction between gentamicin and gold nanorods was confirmed by FT-IR spectroscopy. The high performance liquid chromatography (HPLC) and atomic absorption spectroscopy analyses showed that 2050 gentamicin molecules were attached to each gold nanorod. The minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of gentamicin–GNRs conjugate showed the enhancement of antibacterial effect of gentamicin. The biodistribution study demonstrated localization of the complex at the site of Staphylococcal infection with high sensitivity in mouse model.
Introduction
The widespread problem of antibiotic resistance in pathogens such as Staphylococcus aureus has prompted the researchers for innovating new antimicrobial approaches (Zolfaghari et al., Citation2009). Infectious diseases are clinically evident disorders resulting from the presence of pathogenic agents that can either be a pathogenic virus, bacterium, fungus or parasite (Tallury et al., Citation2010). Today, poor pharmacokinetics and biopharmaceutical properties of new potential therapeutics have become an obstacle against their practical usage. Therefore, there is a need to develop suitable drug delivery systems not only to overcome the physicochemical barriers, but also to deliver the therapeutically active drug molecules preferably to the site of infection, without any harmful effects on healthy organs and tissues (Brayden, Citation2003).
In the rapidly emerging field of nanobiotechnology, noble metal nanoparticles are extensively used in drug delivery systems, biosensors, bioimaging, antimicrobial activities, food preservation etc. (Keun et al., Citation2008; Fayaz et al., Citation2009). The conjugation of gold nanoparticles (GNPs) with biological important molecules like oligosaccharides, DNA and proteins has made a great deal of impetus in recent days (Grace & Pandian, Citation2007). By conjugation of drugs to nanoparticles, the pharmacokinetics and therapeutic index of drugs can be significantly improved in contrast to free drug counterparts (Tallury et al., Citation2010). In this regard, gold nanoparticles are an obvious choice due to their amenability of synthesis and functionalization, less toxicity and ease of detection. The chemical, optical and electronic properties of GNPs made them well suited for applications in biosensing and therapeutic delivery (Tiwari et al., Citation2011). Because of large surface area, more number of drug molecules gets adsorbed on gold surfaces (Grace & Pandian, Citation2007). Rosemary et al. (Citation2006) found a significant improvement in efficacy of ciprofloxacin-gold nanoshells conjugate when they were used against Escherichia coli in comparison with free ciprofloxacin. This study focuses on the use of GNPs with rod shapes as carriers and its association and advantages in combination with gentamicin molecules for potential biomedical applications. We chose gentamicin because gentamicin sulphate is an aminoglycoside with a wide spectrum of antibacterial activity (Prior et al., Citation2004; Burygin et al., Citation2009). Previously, Ahangari et al. (Citation2013) used gentamicin conjugated to gold nanoparticles in spherical shape against S. aureus. In this study, we report high efficacy of gold nanorods for gentamicin delivery to infection foci due to S. aureus and the antibacterial effect enhancement of gentamicin.
Material and methods
Materials
Gold nanorods (41 × 10 nm), sulfo-NHS, 1-ethyl-3-(3-imethylaminopropyl) carbodiimide (EDC), Nanothinks acid and gentamicin sulfate were purchased from Sigma Aldrich, Saint Louis, MO, USA. Nutrient broth medium was purchased from Merck, Germany. BALB/c mice (7–8 weeks old and 20–25 g weight) were purchased from Razi Vaccine and Serum Research Institute, Karaj, Iran. Staphylococcus aureus strain ATCC 29213 was obtained as a lyophilized culture from the Pasteur Research Institute, Tehran, Iran.
Conjugation of gentamicin with GNRs
The conjugation of gentamicin with GNRs was performed by indirect method using Nanothinks acid as a linker. Five milliliters of cetyltrimethylammonium bromide (CTAB)-stabilized GNRs (with OD = 0.8 at 808(were centrifuged twice at 10 000 rpm to get rid of the excess free CTAB molecules in the solution and the pellet was resuspended in 5 ml of deionized water. Then, 50 μl of Nanothinks acid solution was added to GNRs solution, and the solution was sonicated for 15 min at 50 °C to prevent aggregation. The temperature was adjusted to 30 °C while sonication was continued for 2 h. Next, the solution was centrifuged at 10 000 rpm for 10 min, the supernatant was removed, and the pellet was resuspended in phosphate buffer saline (PBS). EDC and sulfo-NHS was added to the reaction solution at the final concentrations of 4 and 1 mM, respectively. The mixture was sonicated for 25 min at 4 °C to produce active GNRs. Finally, gentamicin was added to 5 ml of activated GNRs to the final concentration of 500 μg/ml. The mixture was sonicated at room temperature for 2 h. After sonication, the unbound gentamicin molecules were removed from the solution by centrifuging at 10 000 rpm for 10 min. The final solution was diluted by adding PBS (pH 7.4) and stored at 4 °C (Eghtedari et al., Citation2009).
FT-IR spectroscopy
FT-IR spectrum of the reaction solution was recorded by Perkin-Elmer Fourier transform infrared spectroscopy (model FT/IR-SDC300) to confirm binding of NH2 groups to gold nanorods surfaces. The spectrum ranged from 400 to 4000 cm−1 at the resolution of 4 cm−1, by making a KBr pellet with gentamicin and GNRs–gentamicin complex (Grace & Pandian, Citation2007).
Assessment of bound gentamicin molecules per each GNR
The number of gentamicin molecules attached per each gold nanorod was calculated by dividing the concentration of grafted antibiotic, measured by HPLC, by the number of gold nanorods in the solution, determined by atomic absorption spectroscopy (Hosta-Rigau et al., Citation2010).
Gentamicin concentration assessment
A known volume of the conjugate solution was centrifuged at 10 000 rpm for 10 min. Then, the supernatant was analyzed by HPLC (Waters, model 1525, NY, USA) to determine the amount of non-conjugated antibiotics by interpolation into a calibration curve of the area of HPLC peak. By subtracting the total amount of gentamicin used for the conjugation reaction, the concentration of bound antibiotic was found (Hosta-Rigau et al., Citation2010).
GNRs concentration assessment
A known volume of gentamicin–GNRs solution was centrifuged at 10 000 rpm for 10 min and the pellet was resuspended in PBS. Sulfuric acid was added to the sample and the mixture was incubated at 600–630 °C for 5 min to be changed to ash. The ash was dissolved in a mixture of concentrated hydrochloric and nitric acids. The solution was evaporated to be dried, a necessary amount of 0.5 N hydrochloric acid was added and the sample was analyzed for gold on an atomic absorption spectroscopy (VARIAN, model AA240FS GenTech Scientific, NY, USA). The concentration of gold nanorods in the complex was determined in comparison with the standard curve (Burygin et al., Citation2009).
Antibacterial activity of gentamicin–gold nanorods complex
The antibacterial effect of gentamicin–GNRs complex against S. aureus was investigated by liquid broth dilution method. The serial dilutions (1:2 to 1:2048) of gentamicin–GNRs complex and free gentamicin (for comparison) in the same concentration (500 μg/ml) were prepared in nutrient broth. Then 1.5 × 108 CFU/ml of S. aureus suspension was added to each tube and incubated at 37 °C for 16 h. Then, the tubes were examined for turbidity, indicating the growth of microorganism. The lowest concentration of gentamicin and gentamicin–GNRs complex that inhibited growth of S. aureus, as detected by the lack of visual turbidity, was designated as MIC. The lowest concentration of gentamicin and gentamicin–GNRs complex that allowed survival of less than 0.1% of the original inoculums was assigned as MBC (Srinivasan et al., Citation2001).
Biodistribution study
S. aureus was cultured in aerobic condition in nutrient agar medium for 16 h at 37 °C. Then, a turbid suspension containing 1.5 × 108 CFU/ml of viable bacteria in 0.2 ml of normal saline was injected intramuscularly to the left lateral thigh muscles of four groups of 5 BALB/c mice. The right leg muscles were considered as the controls. For biodistribution assessment in four time intervals, the mice were injected intravenously with 200 μl of gentamicin–GNRs with the concentration of 2.7 mg g−1 GNRs via the tail vein. At 10, 20, 30 and 60 min post-injection, the mice were sacrificed using CO2 gas. The liver, heart, kidney, small and large intestines, stomach, spleen, blood and right and left thigh muscles (normal and infected muscles) were dissected and weighted. The tissues were lysed in a 15 ml tube with aqua regia and subjected to atomic adsorption spectroscopy for quantitative measurement of the conjugate distribution in the infected BALB/c mice (Hainfeld et al., Citation2006; Burygin et al., Citation2009). The results were calculated as a percentage of injected dose per gram of each organ (% ID/g).
Statistical analysis
All the experiments were repeated five times for each test and the results were expressed as mean ± SD. Anova test was used to compare the biodistribution data of getnamicin–GNRs complex in mouse model (p < 0.05).
Results
Conjugation of gentamicin with GNRs
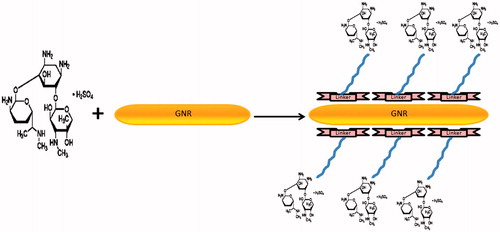
shows a schematic representation of the conjugation procedure used to prepare the gold nanorods–gentamicin complex. It is well known that a covalently coupled antibiotic-GNRs conjugate is much more stable in comparison with electrostatically coupled conjugates. Therefore, gentamicin molecules were attached to GNR covalently using Nanothinks acid as a linker. The size of Nanothinks acid 16 allows for its SH side group to penetrate between CTAB molecules on the surface of nanoparticles to make a dative binding with gold nanoparticles, while the COOH side remains outside of the CTAB layer. On the other side, COOH group is used for bioconjugation with gentamicin.
Gold nanoparticles are attached covalently to gentamicin through Nanothinks Acid 16 as a linker.
FT-IR spectroscopy
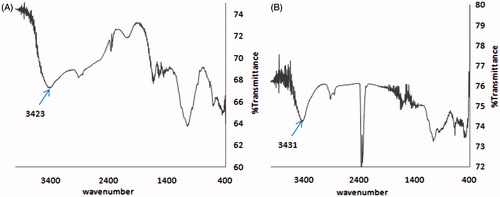
NH2 stretching frequency of the amine groups of gentamicin at 3423 cm−1 was shifted to the higher wavelength at 3431 cm−1 after the conjugation reaction. This pattern means direct binding of nitrogen atoms of free amino groups of gentamicin molecules to gold nanorods ().
Determination of the number of antibiotic molecules attached to each gold nanorod
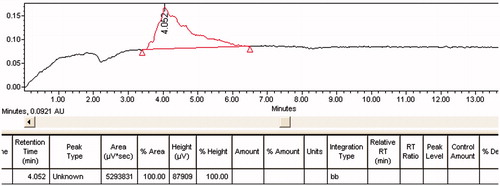
The HPLC analysis determined that approximately 87 ± 7.5% of gentamicin molecules were attached to each gold nanorod ().
The final concentration of gold nanorods at the conjugate measured by atomic absorption spectroscopy was 57 ± 4.7% (). Finally, the calculations indicated that 2050 gentamicin molecules were attached to each gold nanorod.
Table 1. Results of atomic absorption analysis.
MIC and MBC of gentamicin–GNRs complex
MIC and MBC of gentamicin and gentamicin–GNRs against S. aureus are shown in . MIC and MBC of the gentamicin–GNRs were reduced significantly in comparison with free gentamicin. These findings showed enhanced antibacterial effect of gentamicin–GNRs in comparison with free gentamicin.
Table 2. MIC and MBC of gentamicin and gentamicin–GNRs complex against S. aureus.
Biodistribution study
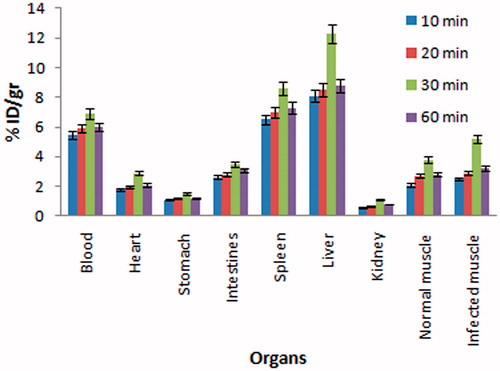
The biodistribution study of gentamicin–GNRs complex in the infected BALB/c mice at 10, 20, 30 and 60 min post-injection showed discrimination between normal and infection muscle. The results revealed that the gentamicin–GNRs complex accumulates significantly at the infected muscle in comparison with normal muscle (p < 0.05) at 30 min post-injection ().
Discussion
In this study, we evaluated the efficacy of gold nanorods as a drug carrier and the antibacterial activity of gentamicin–GNRs conjugate on S. aureus. We developed a new method for indirect binding of gentamicin to GNRs by Nanothink acid as a linker to produce a stable conjugation. Recently, Ahangari et al. have investigated gentamicin conjugated to gold nanoparticles with spherical shape as a drug delivery system and demonstrated the ratio of around 347 gentamicin molecules attached per each gold nanosphere. They found that the gentamicin conjugated with gold nanospheres was significantly more effective against S. aureus in comparison with free gentamicin (Ahangari et al., Citation2013). To develop a more likely effective nanodrug delivery system, we used gold nanorods with 41 × 10 nm diameter as carriers for gentamicin, with this theory that they are able to carry a larger number of gentamicin molecules to Staphylococcal infection sites in comparison with GNPs with spherical shape. The chemical behavior of GNRs is different from that of spherical gold nanoparticles. To target nanorods to infection foci, antibiotics were either electrostatically or covalently linked to the surface of gold nanorods. However, under physiological conditions (buffer and salt conditions), the covalently bound antibiotic–GNRs complex is more likely to be stable than its electrostatic counterpart. In the presence of salts, desorption of electrostatically bound antibiotics are highly probable (Norman et al., Citation2008), and hence for the present study, we focused mainly on using covalently attached antibiotic–gold nanorod complexes. Following successful production of gentamicin–GNRs complex via the Nanothink acid linker, we performed some experiments to determine the number of gentamicin molecules attached to each gold nanorod. On the basis of calibration curve presented in (HPLC analysis) and the data achieved by atomic absorption spectroscopy, the relative concentration of antibiotic covalently bound to each gold nanorod was calculated in a ratio of around 2050 gentamicin molecules per each gold nanorod. The large number of antibiotic molecules/nanoparticle ratio could be attributed to the vast size of nanoparticles surfaces and the rod shape of gold nanoparticles (Hosta-Rigau et al., Citation2010). The higher ratio for carrying more gentamicin molecules per each gold nanoparticle indicated that gold nanorods are more effective drug delivery system for gentamicin delivery in comparison with gold nanospheres. Obviously, when the amount of antibiotic in proximity of a bacterium is more, the antibacterial property may be enhanced.
In one step further, using liquid broth dilution method, we compared the bactericidal activity of gentamicin conjugated with GNRs in comparison with its respective free form. Our findings indicated that gentamicin conjugated with GNRs is more efficient against S. aureus in comparison with free form of gentamicin that might have significant therapeutic implications (). This is really an important parameter, because gentamicin has some important side effects mostly related to nephrotoxicity and ototoxicity that restrict its use. Gentamicin is a highly soluble drug but does not cross the cell membrane efficiently. Gentamicin conjugated with GNRs might have some mechanisms that could enhance the antibacterial efficacy of the antibiotic (Saha et al., Citation2007). Actually each gold nanoparticle surrounded by a number of drug moieties acts as a single group against the microbial organisms (Gargani & Pacetti, Citation1998). The greater antibacterial effect of the antibiotic conjugated with gold nanoparticles may be attributed to their ability to bind to/or penetrate the cell wall and, in doing so they are able to deliver a large number of antibiotic molecules into a highly localized volume (Duncan et al., Citation2010). On the other hand, the studies showed that gold nanoparticles alone do not have any anti microbial activity and only acts as a carrier for gentamicin (Grace & Pandian, Citation2007). Many studies have previously demonstrated that antibiotic molecules conjugated with gold nanoparticles were more efficient in inhibiting the growth of bacteria in comparison with the same dosage of antibiotics used alone (Gu et al., Citation2003; Rosemary et al., Citation2006; Selvaraj & Alagar, Citation2007; Saha et al., Citation2007; Grace & Pandian, Citation2007; Burygin et al., Citation2009).
The biodistribution study of GNRs–gentamicin complex showed the localization of complex at the site of Staphylococcal infection foci with high sensitivity in mouse model. This result showed that functionalization of gold nanoparticles with gentamicin facilitates delivery of these antibiotics to infection foci. With the ongoing efforts in this field, there is no doubt that nanoparticle-based drug delivery systems will continue to improve treatment to bacterial infections, especially in life-threatening diseases such as Staphylococcal infections.
Conclusion
The results demonstrated that gold nanorods were able to facilitate gentamicin delivery to Staphylococcal infection sites much more effective in comparison with gold nanospheres. In addition, enhanced antibacterial activity of gentamicin conjugated with GNRs can minimize dose of gentamicin that consequently results to its side effect reduction and the treatment duration.
Notice of correction
In the version of this article, published on 24 April 2014, there was an error in Table 2. This has now been corrected in this version.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahangari A, Salouti M, Heidari Z, et al. (2013). Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv 20:34–9
- Brayden DJ. (2003). Controlled release technologies for drug delivery. Drug Discov Today 8:976–8
- Burygin GL, Khlebtsov BN, Shantrokha AN, et al. (2009). On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res Lett 4:794–801
- Duncan B, Kim C, Rotello VM. (2010). Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control Release 148:122–7
- Eghtedari M, Motamedi M, Brodwick M, et al. (2009). Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett 1:287–91
- Fayaz A, Balaji K, Girilal M, et al. (2009). Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine 6:103–9
- Gargani G, Pacetti AM. (1998). Sensitivity of 115 strains of the genus Brucella to some antibiotics (cephalosporins, ureidopenicillins and aminoglycosides). Chemioterapia 5:7–13
- Grace NA, Pandian K. (2007). Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles – a brief study. Colloids Surf A Physicochem Eng Asp 297:63–70
- Gu H, Ho PL, Tong E, et al. (2003). Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3:1261–3
- Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. (2006). Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 79:248–53
- Hosta-Rigau L, Olmedo I, Arbiol J, et al. (2010). Multifunctionalized gold nanoparticles with peptides targeted to gastrin-releasing peptide receptor of a tumor cell line. Bioconjug Chem 21:1070–8
- Keun SO, Ree SK, Jinho L, et al. (2008). Gold/chitosan/pluronic composite nanoparticles for drug delivery. J Appl Polym Sci 108:3239–44
- Norman RS, Stone JW, Gole A, et al. (2008). Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett 1:302–6
- Prior S, Gander B, Lecaroz C, et al. (2004). Gentamicin-loaded microspheres for reducing the intracellular Brucella abortus load in infected monocytes. J Antimicrob Chemother 53:981–8
- Rosemary MJ, MacLaren I, Pradeep T. (2006). Investigations of the antibacterial properties of ciprofloxacin at SiO2. Langmuir 22:10125–9
- Saha B, Bhattacharya J, Mukherjee A, et al. (2007). In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res Lett 2:614–22
- Selvaraj V, Alagar M. (2007). Analytical detection and biological assay of antileukemic drug 5-fluorouracil using gold nanoparticles as probe. Int J Pharm 337:275–81
- Srinivasan D, Nathan S, Suresh T, Perumalsamy PL. (2001). Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol 74:217–20
- Tallury P, Malhotra A, Byrne LM, Santra S. (2010). Nanobioimaging and sensing of infectious diseases. Adv Drug Deliv 62:424–37
- Tiwari PM, Vig K, Dennis VA, Singh SR. (2011). Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1:31–63
- Zolfaghari P S, Packer S, Singer M, et al. (2009). In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiology 9:27