Abstract
In this study, to develop a multifunctional targeting nano-carrier drug delivery system for cancer therapy, the novel pH-sensitive ketal based oligosaccharides of hyaluronan (oHA) conjugates were synthesized by chemical conjugation of hydrophobic menthone 1,2-glycerol ketal (MGK) to the backbone of oHA with the histidine as the linker of proton sponge effect. The multifunctional oHA conjugates, oHA-histidine-MGK (oHM) carried the pH-sensitive MGK as hydrophobic moieties and oHA as the target of CD44 receptor. The oHM could self-assemble to nano-sized spherical shape with the average diameters of 128.6 nm at pH 7.4 PBS conditions. The oHM nanoparticles (oHMN) could release encapsulated curcumin (Cur) with 82.6% at pH 5.0 compared with 49.3% at pH 7.4. The results of cytotoxicity assay indicated that encapsulated Cur in oHMN (Cur-oHMN) were stable and have less toxicity compared to Cur suspension. The anti-tumor efficacy in vivo suggested that Cur-oHMN suppressed tumor growth most efficiently. These results present the promising potential of oHMN as a stable and effective nano-sized pH-sensitive drug delivery system for cancer treatment.
Introduction
Hyaluronan (HA), a naturally negatively charged and biodegradable polysaccharide, has been studied as a good targeting moiety in drug delivery system (Morra, Citation2005; Stern et al., Citation2006; Gerecht et al., Citation2007). Because various tumor cells can over-express the HA receptor, CD44, HA and its derivatives can possess good biocompatibility and unique biological characteristics in the use of tumor targeting drug delivery systems (Toole, Citation2004; Jaracz et al., Citation2005; Götte & Yip, Citation2006; Choi et al., Citation2011).
Many studies about HA and its derivatives were reported, which can be divided into two types. One type was HA–drug conjugates, also called prodrugs (Coradini et al., Citation2004; Auzenne et al., Citation2007; Lee et al., Citation2007). The other type was the micro/nanoparticles loading the different drugs, such as siRNA (Lee et al., Citation2007), doxorubicin (Eliaz et al., Citation2004), and mitomycin C (Peer & Margalit, Citation2004). It has been reported that both the types of HA can obtain some enhanced tumor targeting and therapeutic efficacy. However, some new ideas presented that the native high molecular weight hyaluronan (nHA) and oligosaccharides of hyaluronan (oHA) could produce distinct biological effects upon binding to CD44 (Yang et al., Citation2012). It has been reported that nHA binding to CD44 selectively induces CD44 clustering, which could be inhibited by oHA (Yang et al., Citation2012), which is very important and interesting for the future use of HA in drug delivery.
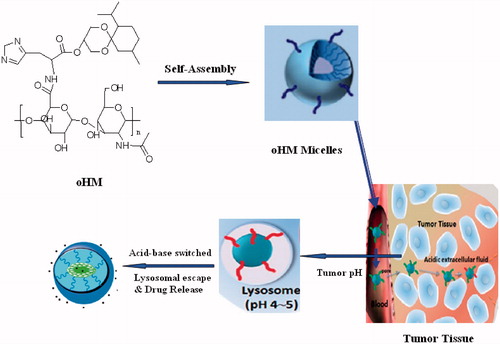
After irritated by highly generated HA, CD44 clustering is a unique character which can influence cell response (Sleeman et al., Citation1996). Due to the “Coating Saccharide” (CD44 clustering) of tumor surface and the slightly acidic condition of tumor, it is difficult to target the chemotherapeutic drugs to the tumor site and achieve satisfying effect for targeting drug. To solve this problem, based on our previous basic work of pH-sensitive nano-carrier (Chen et al., Citation2010, Citation2011, Citation2012, Citation2013; Yu et al., Citation2013; Chen & Wang, Citation2014), we will design a multifunctional oHA-histidine (oHA)–menthone 1,2-glycerol ketal (MGK) nano-drug delivery system with CD44 receptor as target. As shown in , once oHA-histidine-MGK (oHM) nanoparticles meet the CD44 receptor, the coating of CD44 clustering will not appear. Due to the protonation of histidine imidazole group, it is useful for the cellular uptake. After endocytosis, oHM nanoparticles (oHMN) will release the encapsuled drugs under the slightly acidic condition of tumor rapidly with the degration of micellar hydrophobic ketal group. Then, lysosomal membrane will be distroyed via proton sponge effect of histidine imidazole group. The neutral ketone, alcohol degradation products of oHM can also help the escape of drug in lysosome. The encapsuled drugs will escape from lysosome to cytoplasm with good stability, produce efficacy and reduce side effects. The synthesis, characterization, and anti-tumor efficacy of oHMN were under investigation. Because of curcumin (Cur) with low non-specific toxicity to normal cells, it is a good candidate for anti-tumor drug delivery. In our previous study, we have described the Cur. Thus, we choose the Cur as the model drug.
Materials and methods
Materials
Cur was supplied by Yantai Science & Biotechnology Co. Ltd. (Yantai, China). oHA was provided by the FREDA Group Co. Ltd. (Jinan, China). MGK was provided by the Adamas Reagent Co. Ltd. (Shanghai, China). 1Ethyl3(3dimethyllaminopropyl) (EDC), 4-(dimethyl amino) pyridine (DMAP), and Pd/C were obtained from Aladdin Chemistry Co. Ltd. All other reagents were of analytical grade and supplied by Sinopharm Group Chemical Reagent Corp. All the animal experiments were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Synthesis of oHM
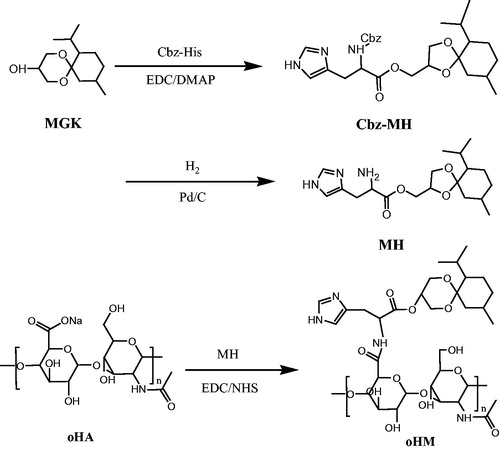
As shown in , water-soluble oHA was modified by chemical conjugation of hydrophobic MGK to the backbone of oHA with the histidine as the linker. oHM was synthesized with three steps. First, Cbz-His (0.5 g, 1.72 mmol), DMAP (0.3 g, 2.58 mmol) and EDC (0.36 g, 1.89 mmol) were dissolved in 15 ml of DMF and stirred for 30 min. Then MGK (0.43 g, 1.88 mmol) was added. After reaction of 8 h, the reaction solution was extracted by 40 ml ethyl acetate and 70 ml water. The yellow oil product of Cbz-His-MGK was obtained under vacuum at 60 °C overnight. Second, Cbz-His-MGK (200 mg), methanol (5 ml) and tetrahydrofuran (1 ml) were added in the 25-ml flask. After reaction of 20 h with the H2 protection and Pd/C, the solvent removed and the residue was concentrated and purified with column chromatography. The purified His-MGK was obtained. Third, oHA (0.012 g, 0.03 mmol), EDCI (0.016 g, 0.08 mmol), NHS (0.010 g, 0.08 mmol) and His-MGK (0.029, 0.08 mmol) were suspended in 5-ml water, stirring at room temperature for 24 h. After dialysis of 4 h and being freeze-dried, a faint yellow oHM powder was obtained.
Preparation of multifunctional Cur-oHMN
The multifunctional Cur-oHMN were prepared by the enhanced thin-film hydration method. Briefly, the oHM conjugate and curcumin (4:1) were dissolved in chloroform. The organic phase was removed at 40 °C on a rotary flask. The flask was evaporated under reduced pressure. The dry lipid formed was hydrated with phosphate buffer saline (pH 7.4). The solution was filtrated through 0.45-µm filters and stored at 4 °C until use.
Characterization of Cur-oHMN
The size and zeta potentials of Cur-oHMN were measured by a Zetasizer 3000 HS instrument. Atomic Force Microscope (AFM) and Transmission electron microscopy (TEM) observation carried out in the Cur-oHMN solution. The concentration solution was kept constant at 1 mg/ml.
In vitro drug release assay
The in vitro release assay of Cur-oHMN was determined using a dialysis bag placed in a sealed glass vial under constant shaking. The powder of Cur-oHMN (containing curcumin 3 mg) and 2 ml PBS (pH 7.4) were put into a dialysis tube. Then, the tube was introduced into 250 ml PBS (0.1 M, pH 7.4 and pH 5.0) containing 0.1% (w/v) Tween 80 with stirring at 120 rpm and 37 °C. The 20-μL sample was filtrated and analyzed by HPLC after different time intervals (Chen et al., Citation2011, Citation2012; Yu et al., Citation2013).
In vivo anti-tumor efficacy
The in vivo anti-tumor efficacy of Cur-oHMN was evaluated in tumor-bearing BALB/c mice. The mice model was carried out with inoculation of 1 × 106 SCC7 cells at the right flank of the mice using a 1.0-ml syringe. When the tumor volume grow about 100–300 mm3, 0.1 ml Cur-oHMN (20 mg/kg) were injected by tail vein every other day. Normal saline and free Cur suspension injected for the control groups. Each individual tumor size was measured with a caliper and the tumor volume was calculated every other day using the following equation: (W2 × L)/2, where W is the tumor measurement at the widest point and L is the tumor dimension at the longest point (Chen et al., Citation2010, Citation2011, Citation2012; Yu et al., Citation2013).
Statistical analysis
The results were expressed as the mean ± standard deviation (n = 3) and the statistical analysis was performed using SPSS software (Chicago, IL). p < 0.05 was considered to be statistically significant.
Results
Characterization of oHM
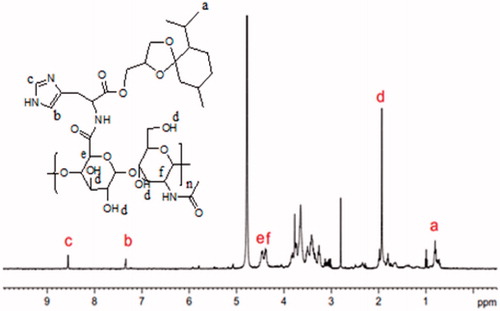
The chemical structures of the oHM determined by 1H-NMR (400 MHz) (). The molar ratio of oHA and ketal group moiety was about 5:1. The oHA of the oHM conjugate was confirmed by 1HNMR with the peak at 4.38–4.40 ppm (2H), 1.77 ∼ 2.13 ppm (4H). The peak at 0.83 ∼ 0.94 ppm of ketal group was confirmed by the MGK conjugated to oHM. The peaks at 7.33, 8.55 ppm were confirmed by the histidine conjugated to oHM.
Preparation and characterization of oHM
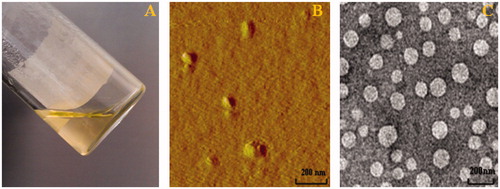
As shown in , the Cur-oHMN could self-assemble to nano-sized spherical shape with the diameters of 128.6 nm at pH 7.4 PBS conditions. The entrapment efficiency (EE) was determined to be about 57.6% (). The zeta potentials of Cur-oHMN were negative and ranged from −28 to −16 mV, indicating that the Cur-oHMN surface were covered by the charged oHM conjugates (). This negatively charged surface of Cur-oHMN might offer a long-term stability in water.
Table 1. The physicochemical parameters of Cur-oHMN before freeze-drying and after freeze-drying.
In vitro releasing assay and in vivo anti-tumor efficacy of Cur-oHMN
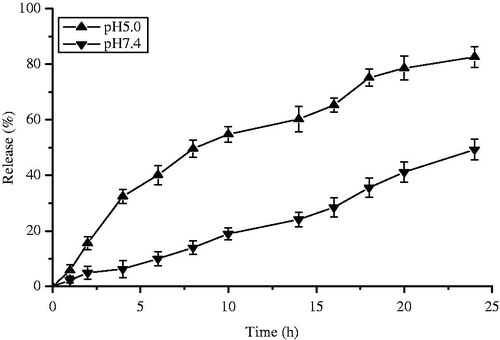
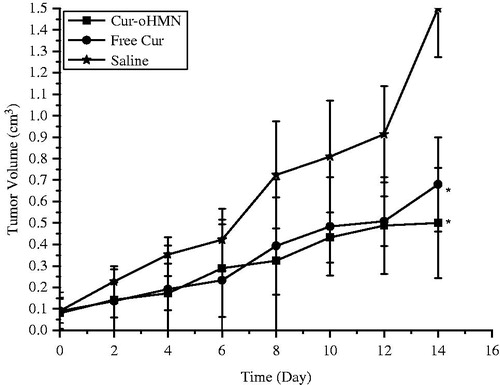
In vitro pH-sensitive test were presented at 37 °C for 24 h in different pH buffers. The Cur-oHMN could release encapsulated curcumin with 82.6% at pH 5.0 compared with 49.3% at pH 7.4, shown in . As shown in , the tumor volume of the saline control group was excessively enlarged (>1000 mm3), while the other groups were much smaller. The Cur-oHMN group suppressed tumor growth most efficiently, followed by the free Cur group (p < 0.05) (Chen et al., Citation2010, Citation2011, Citation2012; Yu et al., Citation2013).
Discussion
CD44 is a major cell surface receptor for the HA. nHA and oHA display different biological effects upon binding to CD44. It has been reported that the biological effect of HA depends on its molecular weight (Camenisch & McDonald, Citation2000; Stern et al., Citation2006; Kothapalli et al., Citation2008). Interestingly, oHA could promote cell proliferation (Slevin et al., 2006), whereas nHA could represent inhibitory effect (Kothapalli et al., Citation2008). However, there is no report about the oHA nanoparticles in drug delivery system, which is interesting and important.
In this work, we have prepared a multifunctional targeting nano-carrier drug delivery system for curcumin, which is novel and interesting. The oHM conjugates were synthesized by chemical conjugation of pH-sensitive MGK to the backbone of oHA with the histidine as the linker. The MGK has the pH-sensitive ketal moietie, which can selectively degrade under tumor microenvironment pH condition and its pH degradation products of ketal could avoid inflammatory problems. Simultaneously, the linker between the oHA and MGK has the proton sponge effect of histidine imidazole group, which can destroy the lysosomal membrane and help the intracellular drugs avoiding destruction. In our previous study (Chen et al., Citation2010, Citation2011, Citation2012, Citation2013; Yu et al., Citation2013; Chen & Wang, Citation2014), novel pH-sensitive mPEG-Chitosan-Ketal (PCK) conjugates were also synthesized and were used to potential pH-sensitive drug delivery system. The similar research was studied in our lab. However, compared to the PCK conjugates, the oHM conjugates possess multi multifunction, such as the CD44 targeting and proton sponge effect of histidine imidazole group.
Conclusion
The oHM could self-assemble to nano-sized spherical shape with the diameters of 120–200 nm at pH 7.4 PBS conditions. The Cur-oHMN could release encapsulated curcumin with 82.6% at pH 5.0 compared with 49.3% at pH 7.4. The results of cytotoxicity assay indicated that encapsulated curcumin in Cur-oHMN were stable compared to curcumin suspension. The anti-tumor efficacy in vivo suggested that Cur-oHMN suppressed tumor growth most efficiently. Much more details are under our research. These results present the promising potential of oHM as a multifunctional targeting nano-carrier in drug delivery system for cancer treatment.
Declaration of interest
This study is financially supported by the National Natural Science Foundation of China (81302718), the National Basic Research Program of China (973 Plan, 2012CB724003), Taishan Scholar Project (To Dr. Fenghua Fu), Open Project Program of State Key Laboratory of Long-acting and Targeting Drug Delivery System (YX12H071).
References
- Auzenne E, Ghosh SC, Khodadadian M, et al. (2007). Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia 9:479–86
- Camenisch TD, McDonald JA. (2000). Hyaluronan: is bigger better? Am J Respir Cell Mol Biol 3:431–3
- Chen D, Jiang X, Liu J, et al. (2010). In vivo evaluation of novel pH-sensitive mPEG-Hz-Chol conjugate in liposomes: pharmacokinetics, tissue distribution, efficacy assessment. Artif Cells Blood Substit Immobil Biotechnol 38:136–42
- Chen D, Liu W, Shen Y, et al. (2011). Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomed 6:2053–61
- Chen D, Sun K, Mu H, et al. (2012). pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int J Nanomed 7:2621–30
- Chen D, Mu H, Sun K, Liu W. (2013). Synthesis, anticoagulant activity and potential drug carrier of biomimetic N-cholesteryl hemisuccinate-O-sulfate chitosan polymer of blood cell membrane. J Contr Rel 172:e25
- Chen D, Wang H. (2014). Novel pH-sensitive biodegradable polymeric drug delivery systems based on ketal polymers. J Nanosci Nanotechnol 14:983–9
- Choi KY, Min KH, Yoon HY, et al. (2011). PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 32:1880–9
- Coradini D, Zorzet S, Rossin R, et al. (2004). Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res 10:4822–30
- Eliaz RE, Nir S, Marty C, Szoka FC Jr. (2004). Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res 64:711–18
- Gerecht S, Burdick JA, Ferreira LS, et al. (2007). Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA 104:11298–303
- Götte M, Yip GW. (2006). Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res 66:10233–7
- Jaracz S, Chen J, Kuznetsova LV, Ojima I. (2005). Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem 13:5043–54
- Kothapalli D, Flowers J, Xu T, et al. (2008). Differential activation of ERK and Rac mediates the proliferative and anti-proliferative effects of hyaluronan and CD44. J Biol Chem 283:31823–9
- Lee H, Mok H, Lee S, et al. (2007). Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Contr Rel 119:245–52
- Morra M. (2005). Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules 6:1205–23
- Peer D, Margalit R. (2004). Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer 108:780–9
- Sleeman J, Rudy W, Hofmann M, et al. (1996). Regulated clustering of variant CD44 proteins increases their hyaluronate binding capacity. J Cell Biol 135:1139–50
- Slevin M, Krupinski J, Gaffney J, et al. (2007). Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 26:58–68
- Stern R, Asari AA, Sugahara KN. (2006). Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85:699–715
- Toole BP. (2004). Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4:528–39
- Yang C, Cao M, Liu H, et al. (2012). The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem 287:43094–107
- Yu H, Mu H, Xiu L, et al. (2013). A novel ketal-based chitosan as nano-vehicles for potential ph-sensitive nanomedicine delivery. Nanosci Nanotechnol Lett 5:1007–11