?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To reduce the drug plasma concentration fluctuation without being destroyed by gastric fluid, novel Esomeprazole magnesium modified-release pellets (EMZ-MRPs) with suitable in vitro release profiles and good in vitro and in vivo correlation (IVIVC) were developed. Fluid-bed was used to obtain EMZ-loaded pellets by spraying drug suspension onto blank sugar pellets. The drug-loaded pellets were subsequently coated with Eudragit® RS30D/RL30D (ERS/ERL) aqueous dispersion to achieve sustained-release (SR) characteristics. Furthermore, the SR pellets were coated with Eudragit® L30D-55 (EL-55) aqueous dispersion to achieve enteric properties. Besides, isolated coating film was necessary between drug layer and SR layer, as well as SR and enteric-coated layer to protect from their possible reaction. The resulting pellets were filled into the hard gelatin capsules for in vitro release processing and single-dose pharmacokinetic study in rats. The optimal formulation achieved good SR feature both in vitro and in vivo with a relative bioavailability of 103.50%. A good IVIVC was characterized by a high coefficient of determination (r = 0.9945) by deconvolution method. Compared to those of EMZ enteric-coated pellets (EMZ-ECPs, trade name NEXIUM), the in vivo study make known that the EMZ-MRPs with decreased maximum plasma concentration (Cmax), prolonged peak concentration time (Tmax) and mean residence time (MRT), and similar values both area under concentration–time curve from 0 to t (AUC0–t) and 0 to infinity (AUC0–∞). Collectively, these results manifested EMZ-MRPs had a satisfactory sustained-release behavior, a desired pharmacokinetic property, improved in vivo retention and decreased plasma drug concentration fluctuation.
Introduction
Esomeprazole is a proton pump inhibitor (PPI) used for the treatment of gastric ulcers, gastroesophageal reflux disease (GERD), Zollinger Ellison syndrome, erosive esophagitis (Sean & Paul, Citation2002), long-term management of patients suffering from peptic ulcer and Helicobacter pyloriinfections alone or associated with other drugs such as non-steroidal anti-inflammatory drugs (NSAIDs, e.g. naproxen) (Goldstein et al., Citation2010), Aspirin (Chan et al., Citation2005), metronidazole and clarithromycin (Miehlke et al., Citation2003) or amoxicillin (Graham et al., Citation2006). As the PPI developed for the treatment of acid-related diseases (Andersson et al., Citation2001), esomeprazole, the S-isomer of omeprazole, owes higher bioavailability and so the stronger acid inhibitor effect. Moreover, esomeprazole has less individual differences and almost can be ignored side effect, compared to omeprazole. Esomeprazole is a BCS Class α drug with low solubility and high permeability. Esomeprazole magnesium (EMZ) is the salt form of Esomeprazole, it takes on well stability than esomeprazole, and its solubility and stability would increase with the solution pH increment. For the degration of EMZ in acid medium quickly, it was developed into enteric-coated oral preparation by AstraZeneca. However, for its short plasma half-life (t1/2) (∼1.3 h) like other PPIs, nocturnal acid breakthrough occurs frequently resulting in worse nighttime gastric acidity control than that of daytime. Tenatoprazole, a PPI with longer t1/2 has been proved to cause less nocturnal acid breakthrough (Galmiche et al., Citation2004). Moreover, EMZ counteracts the detrimental action of NSAIDs on ulcer repairing by acid-dependent and acid-independent effects. The latter action is related to reduction of tissue oxidation and apoptosis, and enhancement of nuclear factor-kB activation (Blandizzi et al., Citation2008; Pastoris et al., Citation2008; Colucci et al., Citation2009;), which mainly depends on drug plasma concentration. Based on the above two points, it is urgent to develop EMZ sustained preparation to maintain the drug plasma concentration and prolong the duration.
Pioneering studies (Biswas et al., Citation2008; Kabir et al., Citation2009) showed that EMZ matrix tablets have been prepared without enough consideration of the problems that the drug is prone to be destroyed by gastric juice, and comparing the relative bioavailability with the market products. As it is well known, compared to conventional single-unit dosage forms, the multiple unit formulation, e.g. pellets, has many remarkable advantages, including being more predictable for gastric transit time and drug absorption, lowering local irritation and reducing the risk of systemic toxicity originated from dose dumping. Even more, they are flexible for further modifications of the preparations to increase the bioavailability (Timmermans et al., Citation1998; Guthmann et al., Citation2007; Kim et al., Citation2007; Muschert et al., Citation2009). The fluid-bed technique, by which the coatings can be reproducibly performed with minimal product defects and highly efficient drying capacity, has become popular in the preparation of pellet-based dosage forms since it is easy to realize the industrial production (Heckötter et al., Citation2011). Moreover, considering the drug is prone to be destroyed by gastric juice, EMZ-MRPs, which could increase the drug's retention in vivo, reduce dosing frequency and increase patient compliance, were engineered by us through the fluid-bed coating method.
Coating of pharmaceutical solid dosage forms can achieve the modified-release characteristics. Organic and aqueous-based film coating techniques have been popular in industrial production in the pellet-based dosage forms. As the growing concerns of the environment, safety and cost, pharmaceutical manufacturing has been moving from organic forms to aqueous coating systems, e.g. aqueous polymer dispersions were developed and marketed-drived to substitute organic coating solutions (Nollenberger and Albers, Citation2013). Eudragit®, including a series Poly(meth)acrylates, which is well known and widely used in the pharmaceutical industry allows the active ingredients of solid dosage form to perform during the passage of the human body. The desired drug release profile at the right place and the right time can be achieved by the flexibility of combination of different polymers (Joshi, Citation2013). ERS and ERL are the aqueous dispersions, the varying of their ratio and coating weight enables the expected drug release profile. EL-55 is a prior choice of coating polymers to protect the active ingredient from the gastric fluid and to improve drug effectiveness. In addition, isolated coating film is necessary between drug layer and SR film, as well as SR film and enteric-coated film to avoid the possible reaction between them (Alhnan & Basit, Citation2011).
The Food and Drug Administration defines IVIVC as “A predictive mathematical model describing the relationship between an in vitro property of an extended release dosage form (usually the rate or extent of drug dissolution or release) and a relevant in vivo response, e.g. the plasma drug concentration or the amount of drug absorbed”. Four levels of correlation (Level A, B, C and multiple level C) have been defined according to the concept (Food and Drug Administration, Citation1997). Among of them level A of correlation, which represents a point-to-point relationship between the in vitro dissolution rate and the in vivo input rate of the drug from the dosage form, is the highest category of correlation. Convolution and deconvolution methods are mostly used for IVIVC. The deconvolution technology is to compare the in vivo dissolution profile obtained from the plasma drug levels, which requires elaborate mathematical and computing expertise (Madden et al., Citation1996; Gaynor et al., Citation2008), with the in vitro dissolution profile of formulation. Numerical deconvolution requires data obtained after both oral and unit impulse response (UIR) administration in the same subject, while the latter is basically derived from intravenous administration.
In conclusion, this study concerns the preparation of EMZ-MRPs, in detail, the drug solution was spraying onto blank pellets cores, the sustained-release coating was carried out and the enteric coating were performed. An isolated film was necessary between drug layer and sustained layer, as well as SR layer and enteric-coated layer. The prepared EMZ-MRPs were characterized by scanning electron microscope (SEM) for their internal and external morphology, as well as the research of the IVIVC of level A correlation, pharmacokinetics and the relative bioavailability in rats compared to EMZ-ECPs.
Materials and methods
Materials
Esomeprazole magnesium (EMZ) and EMZ standard were purchased from Shandong Chengchuang Pharmaceutical R&D Co. Ltd (Jinan, China) and the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), respectively. EMZ-ECPs capsules (trade name Nexium) were the product of Astra Zeneca Pharmaceutical Co Ltd (Wilmington, DE). Tinidazole (99.9%) was provided by Hubei Hongyun long biological technology Co. Ltd. (Wuhan, China). Sugar spheres (30–35 meshes) were from JRS Pharma (Rosenberg, Germany). Hydroxy-propyl cellulose (HPC) and hydroxy-propyl methyl cellulose (HPMC) were supplied by Colorcon Coating Tech., Ltd. (Shanghai, China). Eudragit® RS30D (ERS), RL30D (ERL) and L30D-55 (EL-55) were kindly donated from Evonik Industries (Darmstadt, Germany). Triethyl citrate (TEC) was purchased from Shanghai Chemical Agent Company, Ltd. (Shanghai, China). Both glycerol monostearate (GMS) and hydrochloric acid were obtained from Nanjing Chemical Reagent Co. Ltd (Nanijing, China). Tween-80 was the product of Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Talc (1200 mesh) was delivered from Merck-Schuchardt (Hohenbrunn, Germany). Sodium phosphate dibasic was purchased from Shanghai Lingfeng Chemical Reagent Co. Ltd (Shanghai, China). All other reagents used were of analytical grade, but the mobile phase was chromatographic grade and purchased from Chemical Reagent Co., Ltd. (Nanjing, China).
Preparation of EMZ-MRPs
Preparation of drug-loaded pellets
Esomeprazole magnesium-loaded pellets were prepared by spraying the drug-binder dispersion onto the blank sugar cores using a fluid-bed (JHQ-100; Shenyang, China). After several earlier trials, it was found that the binder composition was HPC-EF and HPC-LF at a weight ratio of 1:1 with concentration of 3.5% aqueous solution. EMZ concentration of 10% showed it was suitable to deposit drug onto sugar pellets with a high yield.
Different coating processes and conditions were optimized and validated before the final coating. More specifically, the fluid-bed was loaded with 5 g of 30–35 meshes sugar pellets for each run. Firstly, sugar pellets were fluidized by opening the inlet air flap till the outlet temperature reached 35 °C. The drug solution under continuous stirring was then bottom-sprayed onto sugar pellets from a nozzle (1.2 mm) attached to a peristaltic pump (HL-2, Shanghai, China). The air rate was 80–140 ml/min with an atomizing air pressure of 1.1–1.2 bar and coating temperature of 35–36 °C. The resulting pellets were further fluidized for 30 min to dry.
Preparation of isolated coating solution, SR coating dispersion and EC coating dispersion
The optimal isolated coating aqueous solution was composed of HPC-EF and HPMC-E5 at a weight ratio of 3:7 with the concentration at 4%, and an addition of 2% talcum as antisticking agent.
The SR coating dispersion contained ERS and ERL, in addition 20% (weigh to dry polymer) of TEC as the plasticizer, 5% (weigh to dry polymer) of GMS as the antisticking agent, and 0.063% (weigh to dry polymer) of Tween-80 as the emulsifier. In total the solid content of the dispersion was 20%. The blend solution was sieved with a 60 meshes prior to the film coating procedure.
EL-55, 10% TEC (weigh to dry polymer, serving as plasticizer), 5% GMS (weigh to dry polymer, serving as antisticking agent), and 1.8% Tween-80 (weigh to dry polymer, serving as emulsifier) formed the enteric coating dispersion with the solid content of 20.6%. Before the film coating process, the blend aqueous dispersion was sieved through a 60 meshes.
Preparation of EMZ-MRPs
The drug-loaded pellets were fluidized to reach the predetermined temperature. After gaining 20% weight by spraying isolate layer, the membrane-controlled suspension under continuous stirring was bottom-sprayed onto cores by a nozzle (1.2 mm) attached to a peristaltic pump (HL-2, Shanghai, China), and the air rate was 100–160 ml/min with a atomizing air pressure of 1.1–1.2 bar and a coating temperature of 37–38 °C. After coating, the pellets were blended with talc prior to the curing step in oven for different time (2, 4, 6, 8, 10 and 12 h) for film curing. Several ratios of ERS and ERL (5:5, 6:4, 7:3, 8:2, 9:1 and 10:0, w/w) and coating weights (5%, 6%, 8%, 10%, 12% and 15%, w/w) were investigated.
The SRPs were once more coated by isolate solution as described above with the identical weight gain ∼20%. The enteric coating dispersion under continuous stirring was bottom-sprayed onto the SRPs by a nozzle (1.2 mm) equipped with a peristaltic pump with the weight gain of 30%. The process parameters were set as follows: 1.1–1.2 bar atomization pressure, 0.6 bar fluidization pressure, 120–180 ml/min air rate and 31–32 °C coating temperature. Finally, after being fluidized in the chamber at 30 °C for 30 min, the coated pellets were obtained.
All operations were protected from light. The coating weight percentage was calculated with the following equation: , where, Wa and Wb were pellets weight before and after coating, respectively.
In vitro release study
Quantitative analysis of EMZ
The concentrations of EMZ were determined by UV-visible spectroscopy (spectrophotometer WFZ UV-2000, Nanjing, China) at 305 nm wavelengths. The linearity of the method was studied in which the drug concentration is in the range of 2.5–20 μg/ml (r = 0.9997). The RSD of the intraday and interday precision for EMZ were < 2%. The recovery rates for EMZ were in the range of 98–102%.
In vitro drug release
The USP 34 XXIII, apparatus II (ZRS-8G; Tianjin, China; basket method) was employed to assess the dissolution behavior of the EMZ-MRPs. Pellets (equivalent to 20 mg of EMZ) were accurately weighed and filled into a size 5 hard gelatin capsule. Each capsule was put into a separate metal basket in a vessel containing 300 ml of 0.1 M HCl. The speed of the paddle was 75 rpm and the temperature of the solution was 37 ± 0.5 °C. For gastric resistance of enteric-coated pellets, the pellets were collected on a filter and the residual drug was determined according to the section titled “Quantitative analysis of EMZ”; for the MRPs, after 2 h, 600 ml of 0.1 M Na2HPO4 pre-equilibrated to 37 ± 0.5 °C was added to each vessel. The pH of the solution was adjusted to pH 6.8 ± 0.05 or 7.4 ± 0.05. At predetermined time points, 5 ml samples were withdrawn. The medium was kept at a constant volume by refilling it with fresh buffer solution. The withdrawn samples subsequently were filtered through a 0.45-μm millipore filter and assayed for the dissolved EMZ concentration by UV/Vis spectrophotometric at 305 nm as described above. The test lasted 10 h in total with all tests being performed with six capsules and the mean values are reported.
Analysis of release data
Difference in the release profiles was evaluated using the similarity factor (f2), a criterion for the different release profiles comparison recommended by the United States Food and Drug Administration. The f2 was calculated by the following equation:
(1)
(1)
where n is the number of time points, Rt and Tt are the reference and test dissolution at the predetermined time points, respectively. The two formulations release profiles are considered to be similar when the value of f2 is 50 or above.
Morphology of film-coated pellets
The shape, surface and cross-section morphology of the EMZ-MRPs were visualized using a SEM (S-3000N, Hitachi, Japan). All samples were coated with a golden layer using an ion-sputter device (E1030; Hitachi, Tokyo, Japan)
In vivo studies
Experimental design
All care and handling of animals were approved by the University Ethics Committee for the use of experimental animals and carried out in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. A random, crossover and single-dose pharmacokinetic study was conducted on a total of six healthy male rats weighing 220 ± 20 g. The rats were randomly divided into three groups: (A) intravenous injection group; (B) EMZ-ECPs group and (C) EMZ-MRPs group. The detail of the test run was listed in . Prior to drug administration, all rats were kept for overnight fasting but allowed water ad libitum. The intravenous injection group was employed to obtain the weigh parameters at a dose equivalent to 4 mg/kg of EMZ. The intravenous injection was prepared in the 0.9% normal saline with EMZ and filtered through the 0.45 μm membrane. Both EMZ-ECPs and EMZ-MRPs at a dose equivalent to 4 mg/kg of EMZ were filled into a size 5 gelatin capsules and orally administered to the rats (n = 6), respectively. Rats were anesthetized by ether, and blood samples (1 ml) were collected from retro-orbit sinus in heparinized tubes immediately just before (time zero) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14 and 24 h after dosing. The plasma was obtained by centrifugation at 10 000 rpm for 10 min and then stored at −20 °C for subsequent analysis.
Table 1. Studies schedule in rats for different formulations.
Determination of EMZ in rat plasma
All the operations were protected from light. Frozen rats plasma samples were firstly thawed at ambient temperature. About 200 μl plasma sample was mixed with 30 μl internal standard solution (2 μg/ml tinidazole in methanol solution). About 0.45 ml acetonitrile was added to the mixture to precipitate plasma proteins. The mixture was vortexed for 1 min, followed by centrifuging at 10 000 rpm for 10 min, and then the organic layer was collected and evaporated under a nitrogen stream at 40 °C. The residue was reconstituted in 100 μl methanol, drawing 20 μl of the supernatant injected into the HPLC system for analysis. The standard linear calibration curve was in the concentration ranges between 0.02 and 10 μg/ml (r = 0.9949).
Pharmacokinetic data analysis and statistics
Pharmacokinetic parameters, involving Cmax, Tmax, MRT, AUC0–t and AUC0–∞ were calculated by non-compartmental analyses using the software program PKSolver (Version 1.0) (Zhang et al., Citation2010). All results were expressed as mean ± SD (n = 6). Two-tailed Student's t-test (SPSS, version 10.0) was used for statistically significant differences with p < 0.05 or p < 0.01. In order to compare the relative bioavailability between the test and the reference (EMZ-ECPs), the relative bioavailability (Frel%) was calculated (from AUC0–∞) as the following equation: F = AUCtest/AUCreference × 100%.
In vitro–in vivo correlation (IVIVC) by deconvolution approach
The deconvolution procedure (Gaynor et al., Citation2008) was adopted to obtain the in vivo input profiles of EMZ from the EMZ-MRPs dosage forms. This approach is based on the convolution relation between the response function R(t), input function I(t), and the weigh function W(t). The relation is described as the following formula (2):
(2)
(2)
Here, R(t) is the transient plasma drug concentration at time t of the modified release dosage form; I is the in vivo release rate and W represents the concentration time profile resulting from an UIR which is typically from bolus intravenous injection or reference oral solution data (here obtained by intravenous injection); θ is the variable of integration. Both response and weigh functions were achieved by experimental measurements. Therefore, the input function could be determined by the deconvolution as formula (3):
(3)
(3)
Then, the in vivo cumulative release can be obtained by formula (4):
(4)
(4)
MATLAB software (Version 7.0, 3 Apple Hill Drive Natick, MA) was used for performing the numerical deconvolution process. In this method, the time course of the drug input is estimated by using a mathematical model based on convolution integral. A plot of the percent of in vivo input versus the percent of in vitro dissolution at various time points was constructed to illustrate whether an IVIVC model could be developed.
Results and discussion
Preparation of EMZ-MRPs
Film formulation and mechanism of EMZ release
Poly(meth)acrylate coating formulations can contain not only the polymer and the dispersing but also several additives such as surfactants, plasticizers, glidants and pigments. Understanding the mechanisms behind the film formation is critical for selecting the appropriate coating excipients. Minimum film forming temperature (MFT) and glass transition temperature (Tg) are key parameters for the efficient film formation from aqueous dispersions (Nollenberger & Albers, Citation2013). In the work, TEC was used as plasticizers to lower MFT. GMS, as antisticking agents, which was dispersed into water with an emulsifier of polysorbate 80, were employed to prevent tackiness and agglomeration during coating, drying or storage, as these would otherwise cause film damage. The amount and the process have been verified previously (data not shown).
ERS (slightly permeable) and ERL (highly permeable) are poly(meth)acrylates, which can be mixed at any ratio in aqueous form to adjust permeability and obtain specific release patterns, as a result, it is commonly used for SR film coatings. After contacting with gastrointestinal fluids, the film coatings swell, independent of pH and release the active by a diffusion-controlled mechanism (Nollenberger & Albers, Citation2013).
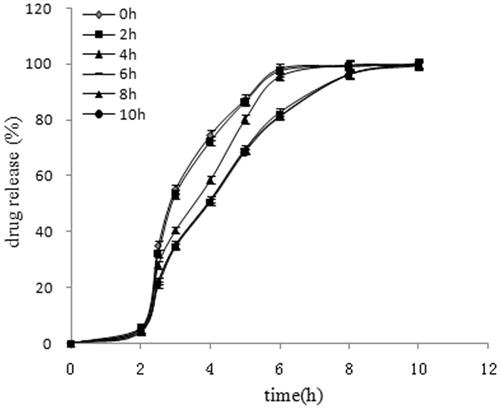
Effect of curing time on EMZ release
Esomeprazole magnesium release curves of EMZ-MRPs with different curing time were given in . As illustrated, the release behaviors of pellets largely depended on the curing time. In comparison with curing time of 6 h, the f2 about 37.00, 39.11, 52.75, 96.56 and 92.09, respectively, at 0, 2, 4, 8 and 10 h. It indicated that the drug release speed changes adversely according to the curing time, till 6 h becomes stable. The slower release rates with increased curing time were attributed to greater polymer particles coalescence (Bhattacharjya & Wurster, Citation2008). When films are placed at the temperature above Tg, aging or further coalescence can occur and the free volume in the polymer film will be reduced. The dissolution properties varied due to lower permeability of the film. To complete the film-forming process and to achieve storage-stable films, special post-processing (curing) is required for coating. Curing at an excessively low temperature is time-consuming and can cause the incomplete film formation. Higher curing temperature and longer curing time can help enhance the film formation (Qiao et al., Citation2010), whereas it could lead to an irreversible agglomeration of pellets and damage to the coating upon separation of the beads, which resulted in faster release than uncured beads. Concomitantly, the stability of EMZ depends on temperature (Karim et al., Citation2013). Considering the above, the curing temperature was set at 40 °C. Blending the pellets with talc just prior to the curing step can eliminate the agglomeration and, therefore, film damage (Wesseling et al., Citation1999)
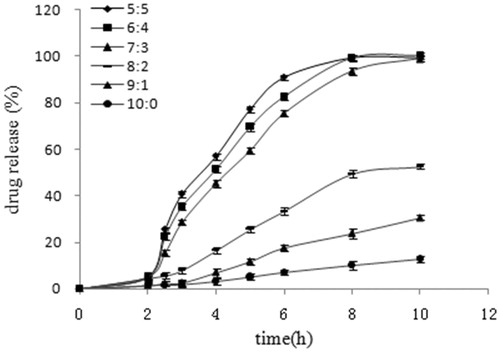
Effect of ERS/ERL ratio on EMZ release
Fixing the coating weight gain at 5%, the effect of copolymer ratio of ERS and ERL in coating formulation on the drug release profile is shown in . As learned from , an increase the weight ratio of ERS results in a significant decrease in the drug release rate. ERS and ERL ratio of 7:3 as controls, 5:5, 6:4, 8:2, 9:1 and 10:0 groups release curve of f2 were 46.39, 58.35, 24.49, 16.49 and 13.74. It revealed that different ratio of ERS and ERL in vitro release profiles were in significant difference. Owing to the two polymers containing different amounts of quaternary ammonium groups, they interact with water and drug molecules in different ways, thus water and drug within the polymeric networks move differently (Jambhekar et al., Citation1987; Samir AlKhatib & Sakr, Citation2003). Incorporation of more hydrophilic quaternary ammonium groups helped ERL take up more water compared to ERS. The polymer chain mobility increases with the increasing ERL content, resulting in increased drug mobility within the polymeric network. A wide range of drug release behaviors could be achieved by varying the ratios of ERS/ERL in the formulation (Amighi & Moes, Citation1995). Too low ERL content would exhibit the insufficient release at the predetermined time, while large amount of ERL content needs more coating thickness to obtain the desired SR characteristic which consumes more time and cost. In this regard, ERS/ERL at 7:3 was employed.
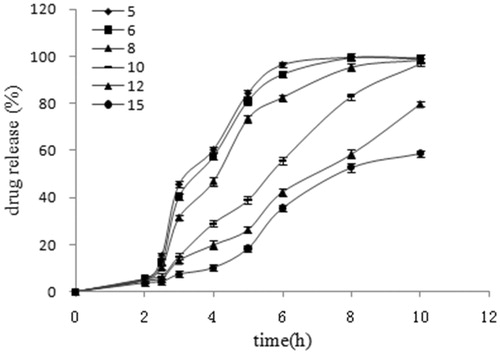
Effect of coating thickness on EMZ release
As depicted in , the effect of coating level on the drug release profile was investigated with an ERS/ERL ratio of 7:3. The coated pellets with a coating level of 12% and 15% showed much slower drug release than the ones with lower coating levels (5%, 6%, 8% and 10%). As the film-controlled drug release, whether or not the crack occurs in the polymeric membranes were determined by the mechanical stability of the film coatings and the hydrostatic pressure (Schultz & Kleinebudde, Citation1997; Lecomte et al., Citation2003). When the formulation contacts with the aqueous media, water diffuses into the pellets core and a monotonically increased hydrostatic pressure is induced inside the pellets. If the mechanical stability of the film coating could not withstand this hydrostatic pressure at a given time point, a crack formation in the film is generated. The hydrostatic pressure built up inside the pellets core with lower coating levels (5%, 6% and 8%) exceeds the film coating mechanical stability, and results in the premature formation of cracks during the dissolution test. Once the cracks formed, the drug release was primarily controlled via diffusion through water-filled channels instead of polymeric film. This former manner leads to much higher release rates than that through the intact polymeric film networks (10%, 12% and 15% coating level) (Qiao et al., Citation2010). So too low coating levels caused dose dumping, however, too high coating levels were time consuming and lead to incomplete release. In the current study, 10% coating weight was applied.
Gastric resistance of enteric-coated pellets
When enteric-coated pellets was released in 0.1 M HCl medium for 2 h, the percentage of residual drug was 95% above, this showed a good acid-resisting property (data not shown).
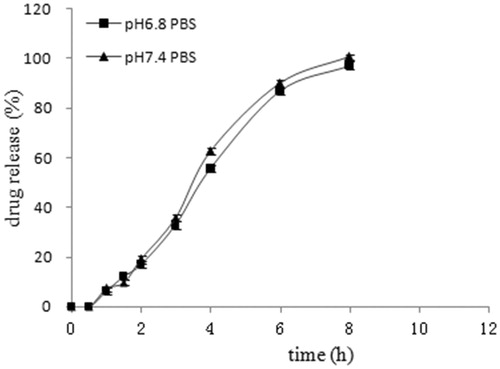
The effect of different pH release medium on EMZ release
Esomeprazole magnesium modified-release pellets release profiles in medium of pH 6.8 PBS and pH 7.4 PBS was recorded in . It was shown that the drug release was independent of pH, which was consistent with the property of the selected sustained-release material Eudragit® RS and RL.
EMZ-MRPs and EMZ-ECPs release profiles and mathematical modeling fitting
The release profiles of EMZ-ECPs and EMZ-MRPs were presented in . The result suggested that drug released from EMZ-ECPs fast and reached 90% above within 45 min after acid-resisting stage and the release from EMZ-MRPs at a lower steady rate within 8 h after acid-resisting phase. Different dissolution kinetic models were adopted to investigate the drug release from EMZ-MRPs, it proved that EMZ-MRPs were the best fitted to Korsmeyer–Peppas model, r = 0.9894. Seen in , the value of the Korsmeyer–Peppas release exponent is 0.8, which was between 0.43 and 0.85, indicating an anomalous transport mechanism (non-Fickian diffusion).
Figure 5. In vitro release profiles of EMZ-MRPs and EMZ-ECPs. Each point represents the mean ± SD (n = 3).
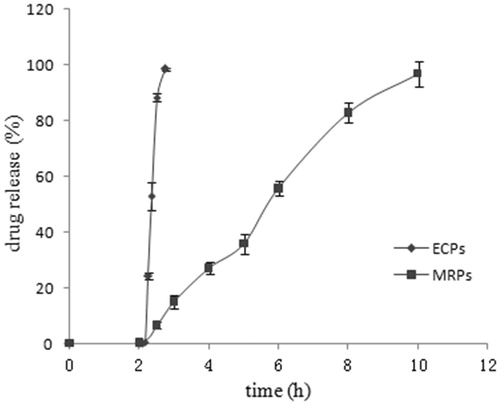
Table 2. Mathematical modeling and drug release kinetics from EMZ-MRPs.
Visualization by SEM
showed the SEM images of outer surface and cross-section of EMZ-MRPs loaded pellets. Pellets displayed a fine sphericity () with a clearly smooth outer surface (). As illustrated, EMZ-MRPs from inner to outer were composed of five layers: sugar starter cores were used as substrate to load innermost EMZ layer; the second layer using HPC-EF and HPMC-E5 as an isolated coating film (20% coating weight) was applied to prevent from the possible reaction between drug and polymer; the third layer was ERS/ERL polymer blends, which could adjust the drug release flexibly by varying blend ratios and coating weight; the fourth layer was HPC-EF and HPMC-E5 as an isolated coating film (20% coating weight) which was employed against the interaction between the sustained polymer and the enteric polymer; and the outermost layer was enteric polymer blends, which could prevent drug from being destroyed by gastric fluid. For each layer in the cut pellets, the texture of the cross-section was homogenous and compact, indicating a uniform polymer formation.
In vivo pharmacokinetic study
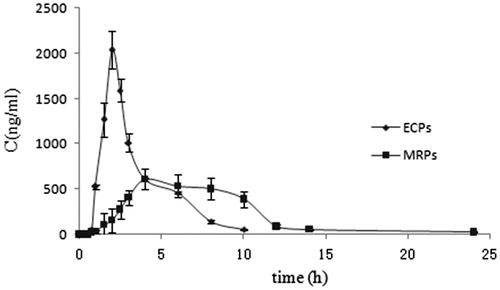
The mean EMZ plasma concentration and time curves after single-dose administration in model animals were presented in . The obtained pharmacokinetics parameters were summarized in . As learned from , it is obvious demonstrated that less plasma drug concentration fluctuation for MRPs than that of ECPs formulation. The MRT was significantly longer in EMZ-MRPs than that of in EMZ-ECPs (p < 0.05), respectively, was 7.36 ± 0.391 h and 2.73 ± 0.119 h. Prolonged Tmax significantly was achieved in MRPs than ECPs (p < 0.05), respectively, was 6.00 ± 0.526 h and 1.45 ± 0.735 h. No significant difference (p > 0.05) in AUC0–t and AUC0–∞ values were found between the two groups, and the Frel was 103.50%.
Table 3. The pharmacokinetic parameters of EMZ for EMZ-ECPs and EMZ-MRPs after single oral administration in model rats (n = 6).
In vitro–in vivo correlation
From the linear regression analysis between the mean in vitro percentage releases and in vivo dissolution rates values, the IVIVC of EMZ-MRPs was obtained. A correlation coefficient of 0.9945 manifested a good level A correlation between the percent in vitro release and in vivo input. The high correlations suggested that the drug dissolution characteristic in the physiological conditions could be illustrated by the in vitro release test under the current conditions.
Conclusions
In this study, we successfully prepared EMZ-MRPs using fluidized-bed technology. ERS and ERL were used as SR membrane materials, and EL-55 was employed as enteric material. In contrast to the ECPs, the in vitro characterization revealed that EMZ-MRPs had desired release profiles, and the in vivo results provided evidence for a lower Cmax, prolonged Tmax and MRT, and similar AUC0–t and AUC0–∞ of MRPs. In the percent of in vitro release and in vivo input, IVIVC model exhibited a good linear regression relationship. Based on the present results, it is evident that EMZ-MRPs might were a more suitable formulation in treating acid related disease without nocturnal acid breakthrough, and a potential dosage form counteracting the detrimental action like NSAIDs for less plasma drug fluctuation and longer MRT. Furthermore, Eudragit series are alternative materials for preparing EMZ-MRPs.
Acknowledgements
Thanks to FMC, Meggle GmbH, ISP and Evonik Industries for providing the excipients and coating materials.
Declaration of interest
This article is submitted to be considered for publication as an “Original Article” in your journal. Neither the entire article nor any part of its content has been published or accepted elsewhere. This study is financially supported by the major project of National College Students Innovation Project for the R&D of Novel Drugs (No. J1030830).
References
- Alhnan MA, Basit AW. (2011). Engineering polymer blend microparticles: an investigation into the influence of polymer blend distribution and interaction. Eur J Pharm Sci 42:30–6
- Amighi K, Moes A. (1995). Evaluation of thermal and film forming properties of acrylic aqueous polymer dispersion blends: application to the formulation of sustained-release film coated theophylline pellets. Drug Dev Ind Pharm 21: 2355–69
- Andersson T, Hassan-Alin M, Hasselgren G, et al. (2001). Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet 40:411–26
- Bhattacharjya S, Wurster DE. (2008). Investigation of the drug release and surface morphological properties of film-coated pellets, and physical, thermal and mechanical properties of free films as a function of various curing conditions. AAPS PharmSciTech 9:449–57
- Biswas BK, Islam MS, Begum F, et al. (2008). In vitro release kinetic study of esomeprazole magnesium from methocel K15M and methocel K100 LVCR matrix tablets. Dhaka Univ J Pharm Sci 7:39–45
- Blandizzi C, Tuccori M, Colucci R, et al. (2008). Clinical efficacy of esomeprazole in the prevention and healing of gastrointestinal toxicity associated with NSAIDs in elderly patients. Drugs Aging 25:197–208
- Chan FK, Ching JY, Hung LC, et al. (2005). Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 352: 238–44
- Colucci R, Fornai M, Antonioli L, et al. (2009). Characterization of mechanisms underlying the effects of esomeprazole on the impairment of gastric ulcer healing with addition of NSAID treatment. Digest Liver Dis 41:395–405
- Food and Drug Administration. (1997). Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. : Food and Drug Administration
- Galmiche J, Bruley Des Varannes S, Ducrotte P, et al. (2004). Tenatoprazole, a novel proton pump inhibitor with a prolonged plasma half-life: effects on intragastric pH and comparison with esomeprazole in healthy volunteers. Aliment Pharmacol Ther 19:655–62
- Gaynor C, Dunne A, Davis J. (2008). A comparison of the prediction accuracy of two IVIVC modelling techniques. J Pharm Sci 97:3422–32
- Goldstein J, Hochberg M, Fort J, et al. (2010). Clinical trial: the incidence of NSAID-associated endoscopic gastric ulcers in patients treated with PN 400 (naproxen plus esomeprazole magnesium) vs. enteric-coated naproxen alone. Aliment Pharmacol Ther 32: 401–13
- Graham D, Abudayyeh S, El-Zimaity H, et al. (2006). Sequential therapy using high-dose esomeprazole-amoxicillin followed by gatifloxacin for Helicobacter pylori infection. Aliment Pharmacol Ther 24:845–50
- Guthmann C, Lipp R, Wagner T, et al. (2007). Development of a Multiple Unit Pellet Formulation for a Weakly Basic Drug. Drug Dev Ind Pharm 33:341–9
- Heckötter UM, Larsson A, Sriamornsak P, et al. (2011). Effect of annealing time and addition of lactose on release of a model substance from Eudragit® RS coated pellets produced by a fluidized bed coater. Chem Eng Res Des 89:697–705
- Jambhekar SS, Breen PJ, Rojanasakul Y. (1987). Influence of formulation and other factors on the release of chlorpheniramine mateate from polymer coated beads. Drug Dev Int Pharm 13:2789–810
- Joshi M. (2013). Role of Eudragit in targeted drug delivery. Int J Curr Pharm Res 5:58–62
- Kabir AKL, Jesmeen T, Talukder MMU, et al. (2009). In vitro release kinetics study of different brands of esomeprazole sustained release tablets available in Bangladesh. S J Pharm Sci 2:27–31
- Karim S, Baie SH, Hay YK, et al. (2013). Stability study of paracetamol and omeprazole pellets formulated through sieving-spheronisation. Lat Am J Pharm 32:431–6
- Kim MS, Kim JS, You YH, et al. (2007). Development and optimization of a novel oral controlled delivery system for tamsulosin hydrochloride using response surface methodology. Int J Pharm 341:97–104
- Lecomte F, Siepmann J, Walther M, et al. (2003). Blends of enteric and GIT-insoluble polymers used for film coating: physicochemical characterization and drug release patterns. J Control Release 89:457–71
- Madden FN, Godfrey KR, Chappell MJ, et al. (1996). A comparison of six deconvolution techniques. J Pharmacokinet Biophys 24:283–99
- Miehlke S, Schneider-Brachert W, Bästlein E, et al. (2003). Esomeprazole-based one-week triple therapy with clarithromycin and metronidazole is effective in eradicating Helicobacter pylori in the absence of antimicrobial resistance. Aliment Pharmacol Ther 18:799–804
- Muschert S, Siepmann F, Leclercq B, et al. (2009). Prediction of drug release from ethylcellulose coated pellets. J Control Release 135:71–9
- Nollenberger K, Albers J. (2013). Poly (meth) acrylate-based coatings. Int J Pharm 457:461–9
- Pastoris O, Verri M, Boschi F, et al. (2008). Effects of esomeprazole on glutathione levels and mitochondrial oxidative phosphorylation in the gastric mucosa of rats treated with indomethacin. Naunyn-Schmiedeberg's Arch Pharmacol 378:421–9
- Qiao M, Luo Y, Zhang L, et al. (2010). Sustained release coating of tablets with Eudragit® RS/RL using a novel electrostatic dry powder coating process. Int J Pharm 399:37–43
- Samir AlKhatib H, Sakr A. (2003). Optimization of methacrylic acid ester copolymers blends as controlled release coatings using response surface methodology. Pharm Dev Technol 8:87–96
- Schultz P, Kleinebudde P. (1997). A new multiparticulate delayed release system: part I: dissolution properties and release mechanism. J Control Release 47:181–9
- Sean CS, Paul B. (2002). Martindale: the complete drug reference. : Pharmaceutical Press, 219–599
- Timmermans J, Amighi K, Puigdevall J, et al. (1998). Peroral sustained-release film-coated pellets as a means to overcome physicochemical and biological drug-related problems. II. Bioavailability and tolerance assessment in dogs. Drug Dev Ind Pharm 24:517–25
- Wesseling M, Kuppler F, Bodmeier R. (1999). Tackiness of acrylic and cellulosic polymer films used in the coating of solid dosage forms. Eur J Pharm Biopharm 47:73–8
- Zhang Y, Huo MR, Zhou JP, et al. (2010). PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–14