?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Tuberculosis, MTB or tubercle bacillus (TB) is a lethal, infectious disease mainly caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. In this study, guar gum-based porous nanoaggregates were formulated by precipitation technique with two frontline antitubercular drugs, i.e. isoniazid and rifampicin. The formulations were optimized on the basis of various evaluation parameters such as morphology, density, entrapment efficiency and in vitro drug release. The optimized formulations were administered by inhalable route to Wistar rats for the evaluation of drugs in different organs (lungs, liver and kidneys). High drug encapsulation efficiency was achieved in guar gum porous nanoaggregates, ranging from 50% to 60%. A single pulmonary dose resulted in therapeutic drug concentrations of 30%–50% in the lungs and in other organs (less than 5%) for 24 h. From this study, we can conclude that delivering drugs through pulmonary route is advantageous for local action in lungs. Furthermore, the formulation showed sustained drug release pattern, which could be beneficial for reducing the drug dose or frequency of dosing, thus helpful in improving patient compliance.
Introduction
Tuberculosis (TB) is a global plague and the leading cause of mortality among infectious diseases affecting humans. TB came back in early 1990s with a great impact on human health due to increase in drug resistance and HIV/AIDS. One-third of the world's population is infected with Mycobacterium tuberculosis (causative agent of TB). The standard recommendation remains initial therapy with four drugs including isoniazid (INH), rifampin, pyrazinamide and ethambutol for the first two months, followed by four months of INH and rifampicin (RIF). The drawback associated with this conventional therapy is patient non-compliance and multiple drug resistance, leading to treatment failure (Shim & Jo, Citation2013). Global TB control policies include directly observed therapy short course (DOTS) for an effective control of TB. Although DOTS technique is effective, it is associated with relatively expensive monitoring and several operational difficulties, especially in developing countries (Baum & Lafair, Citation2003). So, highly effective treatment is urgently needed, which minimizes the duration of therapy and relapse rate. This derives the researchers to pursue novel drug delivery systems in order to develop therapeutically effective therapy for TB (Kaur et al., Citation2014a).
Various particulate drug carrier systems such as liposomes, niosomes, nanoparticles and microspheres have been investigated in recent years (Singh et al., Citation2014). Utility of these systems for pulmonary application is severely hindered because of their low inertia due to small dimensions and mass, which causes them to escape from the lungs (Garg et al., Citation2012). So to overcome this problem guar gum porous nanoaggregates particles (PNAPs) were formulated. Guar gum polymer shows sustained release action as well as free flowing properties and has great potential for anti-tubercular therapy because of its affinity toward macrophages due to the presence of mannan receptors (Gangotri et al., Citation2012). PNAPs have large particle size due to aggregates formation and low density due to porous structures. The large particle size and low density of nanoaggregates is specifically formulated to achieve high aerosolization efficiency and an effective lung deposition (Kaur et al., Citation2014b).
This study was planned to develop a guar gum base porous nanoaggregate carrier systems containing entrapped INH and RIF in combination, to be administered pulmonary. The deposition of drugs was evaluated in various organs of Wistar rats.
Materials and methods
Chemicals and drugs
Guar gum was purchased by Sigma Chemical Co. (St. Louis, MO). INH and RIF were procured from BV Patel Centre (Ahmadabad, India). Ethanol, acetone, chloroform, mannitol, leucine and Tween 80 were purchased by CDH Laboratory reagents (New Delhi, India) and methanol from HIMEDIA (Mumbai, India). All the materials used in this study were of analytical grade and obtained from standard companies.
Animals
Wistar rats of both sexes (180–220 g) obtained from ISF College of Pharmacy, Moga, India, were used in this study and were housed in an animal house facility under natural light conditions. The animals were fed a standard pellet diet and water as required. The project was approved by the Institute Animal Ethics Committee.
Preparation of guar gum nanoporous aggregates
Guar gum nanoporous aggregates (NPs) were prepared by precipitation technique by using ethanol as anti-solvent. Guar gum solution was prepared in different concentrations from 0.1 to 0.7%, by dissolving guar gum in cold distilled water with proper stirring for 15 min. Surfactant (Tween 80, 0.4%) solution was added to the above solution. Then different ratio were optimized for ethanol to water, which was added drop wise to the above solution and then spray-dried adding varying concentrations of the mannitol and leucine. Drugs (RIF and INH) loaded NPs were prepared by adding drugs in different amounts in ethanol to water mixture, which was added drop wise to the guar gum solution (Soumya et al., Citation2010). The process yield of spray-dried powder can be calculated as follows:
Characterization of NPs
Morphological studies of NPs
The size and shape of porous nanoaggregates were determined with the help of particle size analyzer (Beckman Coulter Pvt. Ltd., Delsa Nano C). For the measurement of size and zeta potential, 2 ml of NP suspension was placed into cuvette and zeta potential cell of Beckman coulter (Mumbai, India), respectively. Scanning electron microscopy (SEM) was employed for visualization of shape and size of the prepared NPs (Goyal et al., Citation2013). SEM analysis was done in National Institute of Pharmaceutical Education & Research, Mohali, India. From SEM images, mean aerodynamic diameter was calculated.
Entrapment efficiency of NPs
The RIF and INH-loaded NPs were separated from the NPs suspension by centrifugation at 25 000 rpm for 1 h at controlled temperature of 4 °C. Then 1 ml supernatant was taken and diluted up to 100 ml with water in case of INH, whereas methanolic phosphate buffer solution (PBS) was used as a solvent for RIF. Finally, drug content was analyzed at 261.90 nm for INH and at 473 nm for RIF, respectively, using UV-spectrophotometer (Perkin Elmer, Tokyo, Japan) (Parnami et al., Citation2013). Entrapment efficiency (EE) was calculated using following formula:
Spray drying of optimized formulations
The suspension of optimized formulations was subjected to spray drying after addition of different concentrations of mannitol and leucine.
Density profile analysis. The spray-dried formulations were taken, and the bulk volume was noted. The formulations were then tapped 100 times and the tapped volume was noted. Furthermore, the Carr's index was calculated by using the formula C = 100 ((Vb − Vt)/Vb) and Hausner's ratio by using formula H = 100/(100 − C) (Misra et al., Citation2011). Where C = Carr's index, Vb = bulk volume, Vt = tapped volume and H = Hausner's ratio.
Angle of repose analysis. It was determined by fixed funnel method. The pile of the powders was carefully built up by dropping the powder material through a funnel tip from a height of 2 cm. The angle of repose was calculated as follows:
where, θ is angle of repose, h is height of the particles pile and r is distance from the center of the pile to the edge (Duret et al., Citation2012).
Moisture content
The moisture content of the dry powders was performed by the Karl Fisher volumetric titration method. This method is designed to determine the water content in substances. The moisture content in a sample is determined by measuring the quantity of electricity, which is required for the electrolysis (i.e. for the production of iodine), based on the quantitative reaction of the generated iodine with water. The water content was measured in triplicate on approximately (Hirota et al., Citation2010).
In vitro drug release studies
The in vitro release of antitubercular drugs (ATDs) from the optimized formulations was determined by dialysis tubing technique. In brief, accurately weighed ATDs-loaded guar gum porous nanoaggregates was transferred into pre-soaked dialysis membrane and suspended in a conical flask (100 ml) containing 25 ml of methanolic PBS for simultaneous estimation of both the ATDs. The whole set was placed in shaking incubator (constant speed 100 rpm) (Daihan Labtech Co. Ltd., Kyonggi-Do, Korea) maintained at a temperature of 37 °C. Samples were collected at different time intervals of 48 h for guar gum NPs and assayed spectrophotometrically at 261.90 nm for INH and at 473 nm for RIF (Doan & Olivier, Citation2009).
In vivo studies
Wistar rats of either sex (180–220 gm) were used for organ distribution and pharmacokinetic studies of optimized formulations. Experiments were conducted as per Committee for Prevention, Control and Supervision of Experimental animals, approval no. ISF/CPCSEA/IEAC/2013/100 guidelines. Organ distribution and pharmacokinetic parameters were obtained after pulmonary administration of guar gum nanoaggregates formulations. First, all standard curves were plotted in different organs such as lungs, liver and kidney by using UV spectrophotometer. For organ distribution studies, various organs like lungs, liver and kidney were removed at fixed intervals of 1, 2, 4, 8 and 24 h after blood sampling. Organ homogenates were prepared in phosphate buffer using organ homogenizer. Then centrifugation was done at 6000 rpm for 30 min. Then 0.2 ml of the supernatant from different organs was taken followed by acetonitrile for the purpose of deproteinization. Vortexing was done for 5 min, and then centrifugation was done for 15 min at 3000 rpm. After that, 1 ml of the supernatant was removed from the centrifuged samples and is diluted with 1 ml of methanolic PBS and assayed spectrophotometrically (Islam & Gladki, Citation2008; Garg et al., Citation2013).
Results and discussion
Preparation and characterization of guar gum NPs
Guar gum NPs were successfully prepared by precipitation method and were optimized on the basis of various parameters like guar gum concentration (), solvent ratio () and drug entrapment ( and ).
Table 1. Optimization of guar gum concentration.
Table 2. Optimization of solvent (ethanol:water) ratio.
Table 3. Isoniazid entrapment optimization in optimized guar gum batch (S-GGN 1).
Table 4. Rifampicin entrapment optimization in optimized guar gum batch (S-GGN 1).
Primarily, the concentration of guar gum was optimized under constant volume of precipitant. Results indicated that there is a proportionate increment in the particle size and polydispersity index (PDI) with respect to the concentration of guar gum. An abrupt increment in PDI could be associated to uncontrolled precipitates of guar gum at its higher concentration. Furthermore, it could be inferred from the results that polarity acquired by 1:1 (ethanol:water) is sufficient to induce the homogeneous molecular aggregation with a PDI value of 0.291. However, at a concentration beyond 0.3% w/v of guar gum, there was no sufficient water left in the medium to maintain a homogeneous dispersion of guar gum. Furthermore, alcohol being a precipitant with low surface tension, easily precipitate into the molecular space by replacement of water, resulting in an uncontrolled precipitate of guar gum. There was a significant difference observed in terms of particle size and size distribution with 0.1% w/v of guar gum. However a higher particle density with 0.2% w/v of similar morphology to that of 0.1% w/v play a key role in determining the drug pay load and particle yield.
Once the concentration of guar gum was optimized under a fixed polarity of precipitant, the precipitant concentration was optimized taking the optimized polymer concentration, i.e. 0.3% w/v as a fixed concentration of polymer. Results show precipitant ratio consisting of 1:1 (ethanol:water) develop a contusive environment for controlled precipitation of guar gum. As the ethanol concentration was increased, there was an abrupt increment of PDI and size. This could be clearly indicated that ethanol at higher concentration replace more of the water from the molecular space, resulting an increase terminal hydrophobicity of polymer leads to uncontrolled precipitation of guar gum.
Furthermore, the individual drug concentrations were optimized with respect to the above-mentioned optimized concentration of polymer and precipitant to achieve desire particle morphology for pulmonary administration. In case of INH, results indicated that there is a proportionate increment in the size with increase concentration of INH with a maximum % entrapment observed at drug:polymer ratio (2:3). As the concentration of INH was increased further, a proportionate decrease in the % entrapment was observed. This could be clearly indicated that at 2:3 ratio of INH:polymer, a saturated state of drug, was developed under the specified polymer and precipitant combination. Furthermore, increase in INH concentration induces a super saturation state with the separation of INH from the polymer network, resulting decrease in % entrapment. However in case of RIF, an abrupt increase in particle size was observed above the ratio of 2:3 (RIF:polymer). This could be associated to nonpolar nature of RIF where RIF, together with alcohol, synergizes the precipitation of guar gum, indicated by the change in PDI from 0.41 to 0.64. Moreover 2:3 ratio of RIF to polymer exhibit % entrapment of 50.4% followed by a significant decrease in % entrapment with increase concentration of RIF. This could be clearly indicated that guar gum being a hydrocolloid entity possesses the maximum drug holding capacity at 2:3 ratio of (RIF:polymer). Above that concentration, RIF remains outside of the polymer aggregate and facilitate the precipitation of guar gum.
Characterization of optimized formulations after spray drying
To improve the yield and flow properties of powders, mannitol and leucine were used as excipients in spray-drying process. Mannitol was added to improve the yield and the flow properties of experimental formulation. Leucine was used because of its dispersibility enhancing properties, which improved the aerosolization characteristic of powders. represents some characterization parameters of optimized formulations after spray drying.
Table 5. Characterization of optimized formulations after spray drying.
By adding leucine, particle size of spray-dried powders decreased significantly and there was increase in the process yield because of its anti-adherent properties. The optimized ratio of mannitol and leucine was 1% and 0.2% for guar gum nanoaggregates on the basis of desired flow properties. The inlet temperature was maintained at 80 °C, outlet temperature at 40–50 °C and vacuum at 100–120 of water column (WC). Results of the micrometrics study of spray-dried formulations indicated that all the parameters were found within the specification require for pulmonary administration. Particularly, aerodynamic diameter below 5 µm is considered to be essential for formulation to access the infection site. In this study, both the formulations exhibit an aerodynamic diameter of 3.53 µm and 3.11 µm for INH and RIF, respectively.
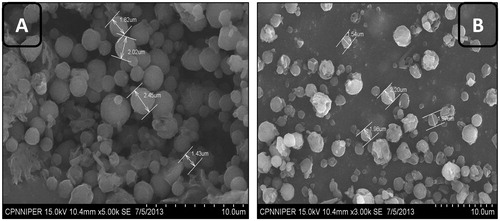
SEM images of guar gum nanoaggregates
Optimized formulations were visualized under SEM for surface morphology. SEM photographs revealed that spray-dried nanoaggregates were of spherical shape. SEM images of optimized formulations are shown in . SEM images further revealed that the particles were spherical, homogeneous and uniform in nature. Moreover, the optimized compositions provide a contusive atmosphere for controlled evaporation of solvent during the specified spray-dried conditions, resulting in the homogenous distribution of aggregates.
In vitro release studies
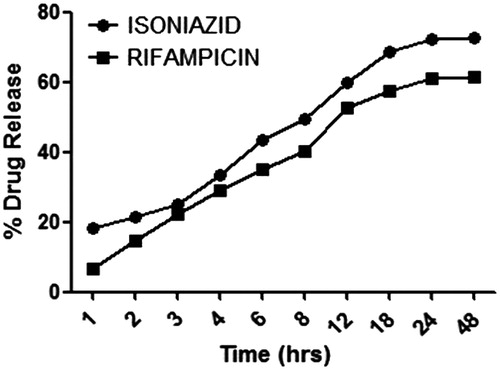
In vitro drug release profile of optimized formulations using dialysis bag in methanolic PBS (pH 7.4) is shown in . Drug release study was performed in methanolic PBS. There is no significant difference observed in the release profile of INH and RIF in methanolic buffer. However, an initial burst release of 20% in INH and 5% in RIF was observed, this could be associated to drug solubility in the selected medium. Furthermore, a relatively slow release of RIF could be attributed to hydrophobic interaction between RIF and hydrophobic site of guar gum, which is expected during precipitation process.
In vivo studies
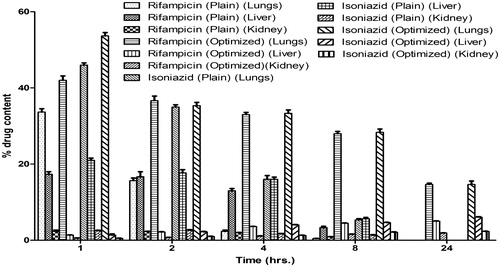
Wistar rats of either sex (180–220 g) were used to study pharmacokinetics and organ distribution of optimized formulations. The amount of drug distributed in the different organs (lungs, liver and kidney) is shown in . These results showed that maximum amount of the drug reaches the lungs as the dose was administered through pulmonary route. With time, the drug concentration decreases in the lungs and increases in different vital organs.
Organ distribution studies showed that experimental formulations shows greater accumulation in the lungs compared to free drug. Furthermore, in case of plain drugs, no drug was detected in the lungs after 24 h. However, approximately 15.21% of the drug was estimated after 24 h of administration in case of experimental formulations. This could be attributed to the mucoadhesive nature of guar gum and controlled release of drugs from gel core structure of guar gum. A major concern of using INH and RIF in TB therapy is their hepatotoxicity. Results of organ distribution clearly indicated that the prepared formulation shows a significant low level of drug distribution in the vital organs as compared to plain drug. This could be due to a controlled release of the drugs from the developed formulation. The observed values further suggested that the pulmonary administration of prepared formulation using guar gum is not only effective in rapid attainment of high drug concentrations in alveolar macrophages (lungs) but could also maintain the concentration over a prolonged period of time when compared to plain drug. This establishes the significance of the targeting potential of the developed systems.
Pharmacokinetic parameters
Peak plasma concentration (Cmax) and time taken to reach Cmax (Tmax), elimination half-life (t½), area under plasma drug concentration over time curve (AUCtot) and mean residence time were calculated using Kinetic 5.0 software. represents various pharmacokinetic parameters of optimized formulations.
Table 6. Pharmacokinetic data of optimized formulations.
Various pharmacokinetics parameters such as AUC 0–24 (µg h/ml), Cmax (μg/ml), Tmax (h), and t1/2 (h) of the developed formulation was determined and compared with the plain drug. It was observed that peak plasma concentrations of the drug were achieved quickly in case plain drug compared to the developed formulation. However, the mean residence time of the drug in plasma remained for longer period with developed systems. This may be attributed to the controlled release of the drug from the prepared formulation. Furthermore, the biological half-life (t1/2) of prepared formulation was found to be significantly higher than the plain drugs accounted to be 9.11 ± 1.1 h and 23.5 ± 1.54 h for INH and RIF, respectively, against plain drugs, which was 2.91 ± 1.20 h and 3.7 ± 0.5 h, respectively. The results further supported the sustained release behavior of the developed formulation. The AUC of prepared formulations were 174.6 ± 10.25 μg/ml/h and 324.5 ± 15.27 μg/ml/h for INH and RIF, respectively, which was higher, compared to plain drug, which could be due to maintenance of concentration of drug within the pharmacologically effective range for longer period of time from the developed formulations. It could be concluded from the pharmacokinetic studies that the anti-tubercular drug-loaded guar gum micro-particle would be available at the infection site above minimum inhibitory concentration (MIC) for a longer period to produce desired pharmacological effect.
Conclusion
INH- and RIF-loaded porous nanoaggregates were successfully prepared using guar gum as polymers. The optimized inhalable formulations have demonstrated excellent flow properties. In vitro release studies showed sustained release of drugs up to 48 h from guar gum based formulations. Furthermore, alveolar deliveries of developed nanoparticulate formulations in animal have shown predominant deposition of drugs within the lungs. Studies revealed that delivering drugs through pulmonary route is advantageous for local action as the maximum amount of drug concentration was observed in the lungs. Furthermore, the optimized formulations showed sustained drug release pattern, which could be beneficial for reducing the drug dose or frequency of dosing, thus helpful in improving patient compliance.
Notice of Correction:
In the version of this article published on 28 May 2014, the fourth author's name was incorrectly written as ‘Umesh Das Gupta’. This has been corrected in this version, published on 4 July 2014.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Baum GL, Lafair J. (2003). DOTS-S: directly observed therapy short course-with smiles. Int J Tuberc Lung Dis 7:200–1
- Doan TV, Olivier JC. (2009). Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int J Pharm 382:61–6
- Duret C, Wauthoz N, Merlos R, et al. (2012). In vitro and in vivo evaluation of a dry powder endotracheal insufflator device for use in dose-dependent preclinical studies in mice. Eur J Pharm Biopharm 81:627–34
- Gangotri W, Jain-Raina R, Babbar SB. (2012). Evaluation of guar gum derivatives as gelling agents for microbial culture media. World J Microbiol Biotechnol 28:2279–85
- Garg T, Singh O, Arora S, Murthy R. (2012). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Goyal G, Garg T, Malik B, et al. (2013). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug delivery
- Hirota K, Hasegawa T, Nakajima T, et al. (2010). Delivery of rifampicin-PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J Control Release 142:339–46
- Islam N, Gladki E. (2008). Dry powder inhalers (DPIs) – a review of device reliability and innovation. Int J Pharm 360:1–11
- Kaur M, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014b). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug delivery
- Misra A, Hickey AJ, Rossi C, et al. (2011). Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb) 91:71–81
- Parnami N, Garg T, Rath G, Goyal AK. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol
- Shim TS, Jo KW. (2013). Medical treatment of pulmonary multidrug-resistant tuberculosis. Infect Chemother 45:367–74
- Singh H, Sharma R, Joshi M, et al. (2014). Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol. [Epub ahead of print]
- Soumya RS, Ghosh S, Abraham ET. (2010). Preparation and characterization of guar gum nanoparticles. Int J Biol Macromol 46:267–9