Abstract
Among all cancers, lung cancer is the major cause of deaths. Lung cancer can be categorized into two classes for prognostic and treatment purposes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Both categories of cancer are resistant to certain drugs. Various mechanisms behind drug resistance are over-expression of superficial membrane proteins [glycoprotein (P-gp)], lung resistance-associated proteins, aberration of the intracellular enzyme system, enhancement of the cell repair system and deregulation of cell apoptosis. Structure–performance relationships and chemical compatibility are consequently major fundamentals in surfactant-based formulations, with the intention that a great deal investigation is committed to this region. With the purpose to understand the potential of P-gp in transportation of anti-tumor drugs to cancer cells with much effectiveness and specificity, several surfactant-based delivery systems have been developed which may include microspheres, nanosized drug carriers (nanoparticles, nanoemulsions, stealth liposomes, nanogels, polymer–drug conjugates), novel powders, hydrogels and mixed micellar systems intended for systemic and/or localized delivery.
Introduction
Among all cancers, lung cancer is the major cause of deaths. In lung cancer, survival rate is very low comparative to all other cancers. In 2008 survey, lung cancer was considered as the most commonly diagnosed cancer. Cancer-related deaths in males are mainly due to lung cancer and in females, due to breast cancer, which is another cause for cancer-related mortality (Jemal et al., Citation2011; Nurwidya et al., Citation2012). In 2012, estimation of mortality rate due to lung cancer is 26% in females and 29% in males (Siegel et al., Citation2012). Lung cancer is mainly due to tobacco usage in both males and females. The tumor is asymptomatic for a long-time duration due to poor diagnosis of lung cancer (Saintigny & Burger, Citation2012). Patients are diagnosed at advanced stage where surgery is no longer an option. Two major problems that lead to difficulty in treatment are metastasis and drug resistance. Lung cancer can be categorized into two classes for prognostic and treatment purposes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Both categories of cancer are resistant to certain drugs. NSCLC cells are resistant to some anticancer drugs and SCLC cells are resistant with persistent administration of drug.
Small cell lung cancer is a sort of lung cancer that involves characters like short cell doubling time, hasty, destructive and headway occurrence of blood-borne and lymph metastasis (Chen et al., Citation2012). It initiates from pulmonary neuroendocrine cells and recent studies suggest that a patient suffers ∼15–20% of other different types of lung cancers (Rodriguez & Lilenbaum, Citation2010). Among different types of lung cancer, patients suffering from SCLC express highest malignancy. When compared with NSCLC, SCLC is different in its clinical and biological characteristics. Malignancy of SCLC is higher when compared to other lung cancers due to destructive deeds, hasty growth and premature extend to different sites. Primarily, SCLC retort well to chemotherapy and relapse therapy (RT), but after the primary treatment, relapse is a well-known trouble. In general, ∼2% of SCLC patients continued existence after the initiation of disease. Moreover, drug resistance, mainly multi-drug resistance (MDR), has become major cause for decline in SCLC patients (Chen et al., Citation2012). Researchers concluded that mutually single factors and multiple factor cause multi-drug resistance (MDR) when both factors are in combination and neutralize the positive, preliminary possessions of chemotherapy. Patients having NSCLC treated with chemotherapeutic agents have survival of ∼8–10 months (Ohe et al., Citation2007). Histologically, both SCLC and NSCLC differ from each other due to dissimilar responses of their treatment therapies. NSCLC can be cured with surgery and radiotherapy, whereas SCLC does not respond to surgery or radiotherapy, whereas median survival rate can be increased from 7 weeks to 1 year by combination therapies, i.e. by combining chemotherapy or chemotherapy and radiotherapy. Consequent relapse is familiar ∼10% with 2-year survival (Vogelsang et al., Citation1985). Relapse arises during therapy due to huge extent of cells having drug resistance and leads to unsuccessful chemotherapy. Researchers show different mechanisms behind SCLC drug resistance that are as follows:
Over-expression of superficial membrane proteins
Lung resistance-associated proteins
Aberration of the intracellular enzyme system
Enhancement of the cell repair system
Dysregulation of cell apoptosis (Chen et al., Citation2012)
P-glycoprotein (P-gp) is a membrane of ATP-binding cassette (ABC) family which involves in over-expression of superficial membrane proteins; enzymes involving an aberration on intracellular enzyme system are topoisomerase (Top) and gamma-glutamyl transpeptidase (γ-GGT); and over-expression of anti-apoptotic genes involves in dysregulation of cell apoptosis which are Bcl-2 and c-myc. Moreover, alteration of key genes also leads to expansion of drug resistance in the body (Roberti et al., Citation2006). In SCLC patients, alteration in genes includes stimulation of proto-oncogenes and no or less stimulation of tumor suppressor genes (Fong et al., Citation1999). Fong et al. (Citation1999) categorized SCLC into two types: classic and variant. Classic type shows large proliferation and susceptibility to chemotherapy and variant type show lesser proliferation, rapid progression rate and responds less to chemotherapy. C-myc expression level is high in variant type, whereas down-regulation of c-myc in classic type.
Among all the cancers, non-small cell lung cancer is the major cause for death (Haura, Citation2001). NSCLC can be classified by their histological bases which are squamous cell carcinoma, large cell carcinoma and adeno carcinoma (Pore et al., Citation2013). Classic therapy for NSCLC is platinum-planted double-drug combination therapy (Pfister et al., Citation2004). But, this therapy is not preferable (Greco et al., Citation2002; Scagliotti et al., Citation2002; Schiller et al., Citation2002; Smit et al., Citation2003) and endurance issue is poor (Mean endurance rate, 8–10 months; 1-year endurance rate 35–40%) (Ettinger, Citation2002). Consequently, novel, acceptable therapies can be necessarily required for better endurance in NSCLC. Novel NSCLC therapies gave indicative comfort and discrete growth in survival rate (Group, Citation1995); little change in response like development of progression time from 3 to 5 months. Development of platinum-based drug regimen by second-line therapy with docetaxel decreases mortality rate due to NSCLC (Fossella et al., Citation2000; Shepherd et al., Citation2000). Therefore, at present, there is no distinct need for third-line therapy. An ineffectiveness third line therapy was established (Massarelli et al., Citation2003), which examines increase in only 2% response and median survival of 4 months. Shepherd et al. (Citation2000) demonstrated to facilitate patients treated with docetaxel following the unsuccessful double or more therapy regimens; endurance was similar to those patients with sympathetic concern. Epidermal growth factor receptor (EGFR) is a fraction of composite signal-induction system that is fundamental to many significant cellular movements. EGFR is frequently seen in NSCLC cells (Rusch et al., Citation1997; Brabender et al., Citation2001). Proliferation in NSCLC is not fast when compared with SCLC; thus patients who are detected at former phase are possibly healable, although NSCLC may frequently degenerate on supplementary metastatic point. Moreover, when compared with SCLC, NSCLC is not more reactive to chemotherapy, hence so as to yet by surgical incision at former diagnosis, ∼50% of patients suffers from relapse of NSCLC (Kelsey et al., Citation2006). Although, after surgery, yet ∼40–75% cannot survive for >5 years having Stage I to Stage IIIA of tumor growth which is stimulated by vascular endothelial growth factor (VEGF) (Folkman et al., Citation1971; Ferrara, Citation2002; Bergers & Benjamin, Citation2003; Ferrara et al., Citation2003) and rise in VEGF is familiar in case of NSCLC which leads to undesirable clinical results. A humanized monoclonal antibody having activity in decrease in VEGF, Bevacizumab (Ferrara et al., Citation2004) has better results in therapy of first line non-squamous NSCLC (Hurwitz et al., Citation2004; Hainsworth et al., Citation2005; Miller et al., Citation2005; Sandler et al., Citation2006; Giantonio et al., Citation2007).
Multiple drug resistance occurs not with single anticancer drug, but it is due to resistance of variety of anticancer moieties having distinct structures and cellular target. When drugs like doxorubicin, VP-16 and cisplatin used alone cause drug resistance but when used in combination with others anticancer drugs produce an increase in response rate in formerly untreated patients while less efficient in relapsed patients. P-gp is a major part of multidrug resistance in many malignancies which is due to P-glycoprotein drug efflux pump that is produced by the MDR gene but, there is no convincing fact about P-glycoprotein which is broadly concerned in the inherent resistance of NSCLC tumors or the acquired resistance of SCLC tumors. Mostly, anticancer drugs that cause MDR are hydrophobic in nature, amphipathic natural products, such as the taxanes (paclitaxel and docetaxel), vinca alkaloids (vinorelbine, vincristine and vinblastine), anthracyclines (doxorubicin, daunorubicin and epirubicin), epipodophyllotoxins (etoposide and teniposide), antimetabolites (methorexate, fluorouracil, cytosar, 5-azacytosine, 6-mercaptopurine and gemcitabine) topotecan, dactinomycin and mitomycin C.
Treatment strategies
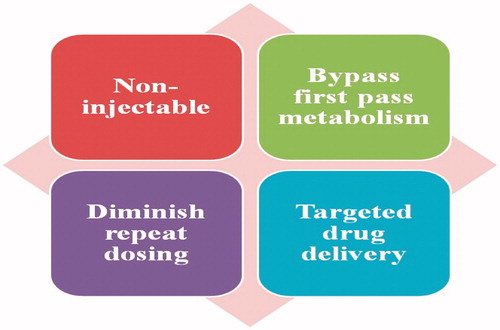
Inhalation therapy is beneficial for pulmonary disease management rather than other routes like parenteral and oral (). Formulations used for lung inflammation having extremely hydrophobic API, surfactants and co-solvents frequently exploit to prepare stable formulations utilized for pulmonary route (Mehnert & Mäder, Citation2001). Incorporation of hydrophobic ingredient in nanoparticulate system will give stable formulation through defending the main compound from deterioration and releases the incorporated drug in controlled mode for continued duration (Yang et al., Citation2008). Target to lung is interesting because of non-injectable through intake of aerosols, by-pass first pass metabolism, release directly at target site for management of pulmonary disorders, and accessibility of massive superficial site for drug action and penetration of drug. Colloidal carriers (i.e. nanocarrier systems) in pulmonary drug delivery offer many advantages such as the potential to achieve relatively uniform distribution of drug dose among the alveoli, achievement of improved solubility of the drug from its own aqueous solubility, a sustained drug release which consequently reduces dosing frequency, improves patient compliance, decreases incidence of side effects and the potential of drug internalization by cells (Patton & Byron, Citation2007; Sung et al., Citation2007). Nanotechnology-based formulations in respiratory disorders are beneficial in many ways which involves:
Probably to attain consistent delivery of drug dose along with the alveolus
Acquisition of improved solubility of the active ingredient over its own water solubility
Continuous-release lowers the dose repetition of drug
Appropriate release of macromolecules
Decline in occurrence of adverse effects
Better patient compatibility and
Probable of drug internalization through cells (Bailey & Berkland, Citation2009)
Initial new drug treatment to create a statistically endurance advantage above regular treatment is use of combination therapy of Vinorelbine or Vindesine and Cisplatin (Wozniak et al., Citation1998). Effectiveness of combination of Vinorelbine and cisplatin was proved for metastatic NSCLC (Hitzman et al., Citation2006). When cisplatin alone was used for 1 year, endurance was 20%; but, when used in combination, endurance was 36%. Thus, combination of Vinorelbine and cisplatin becomes novel treatment strategy. Along with some pulmonary therapies, nanoparticulate systems have established numerous benefits in conditions of defending the drug from deterioration and continuous release of drug at extended duration of time. For pulmonary delivery, nanoparticles and liposomes are used to carry anticancer moieties but having some disadvantages mainly the major limitations of these systems are alteration during nebulization, biodegradability, drug outflow and drug-related unfavorable conditions (Bonomi et al., Citation2000; Chougule et al., Citation2006). Paclitaxel is one more new drug for treatment in NSCLC which reports 20–25% rate in metastatic patients (Hande, Citation1998). Combination of paclitaxel with cisplatin recorded extended endurance in phase II trials leads to a phase III trial (Bunn & Kelly, Citation1998). Low-dose paclitaxel with cisplatin was reported as novel classic therapy for management of NSCLC. Etoposide is another anticancer drug which causes breakdown of DNA and then cell damage because of development of ternary compound through topoisomerase II and DNA (Smit et al., Citation1989) which becomes part of the first line treatment strategy for small cell lung carcinoma (Novello et al., Citation2007). Intended and modified strategies have been used based on the symptoms of cancer patients; thus, generally, endurance rates are unsuccessful to express the probable general endurance (Subramanian et al., Citation2011; García Sar et al., Citation2012). Additionally, resistance to anticancer drugs has been noticed mostly due to apoptotic mechanism in NSCLC cell lines (Hosomi et al., Citation2011). In SCLC, 5-year endurance noticed <10%, although different drug combinations have been utilized (Shepherd et al., Citation2007; Hunter et al., Citation2011). Besides, acquired resistance has been reported in SCLC (Schiller et al., Citation2002). Thus, new treatment strategies are largely required. For efficacious therapy, active ingredient must be reached in solid tumors and, so far drug concentration at the tumor location has been reached in short concentration behind systemic chemotherapy (De Vore & Johnson David, Citation2000; Minchinton & Tannock, Citation2006). In past studies from 1980s, basis for treatment of lung cancer is combination therapy that gives large progress in overall endurance prospects (Eberhardt & Korfee, Citation2003). Though, when cisplatin was used in cancer management strategy, endurance time period was 7–11 months, and in clinical trials reported shortens progress (Eberhardt & Korfee, Citation2003). Syndrome restricted toward chest, called limited-disease SCLC (LD-SCLC), can be treated by chemotherapy alone, and it results in endurance time of 10–14 months (Group, Citation1995; Eberhardt & Korfee, Citation2003). Though, when optimizing organization manage (exterior the brain) through the combination chemotherapy, management become critical.
Drugs inducing MDR
Curcumin
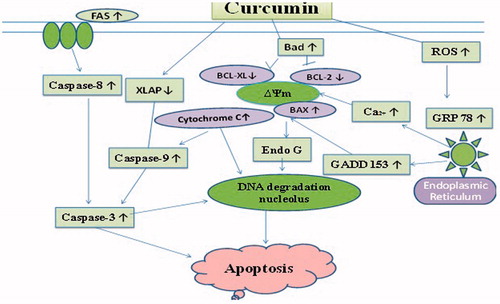
Curcumin is a phenolic complex extracted from the plant Curcuma longa. Curcumin shows cytotoxic activity in different types of cancer cell line in vitro, accompanied by hematologic malignancies, head and neck, genitourinary, gastrointestinal, breast, ovarian and neurologic cancer, melanoma and sarcoma (Lin, Citation2007; Anand et al., Citation2008). These anticancer behaviors are partially recognized to its property of various molecular targets concerned with cell cycle, apoptosis, alteration, proliferation, survival, invasion, angiogenesis and metastasis of cancer cells (Wu et al., Citation2010). Though, the mechanisms behind curcumin-inhibited cell growth and curcumin-induced apoptosis in NSCLC are still not clear. For assurance of its anticancer property, NCI-H460 (NSCLC cells) was treated with curcumin. Various concentrations of curcumin were treated with NCI-H460 cells at different time intervals that results in succeeding alterations in the cell structure, viability, cell cycle, mRNA and protein expressions (). Apoptotic structural alterations in NSCLC cells due to curcumin were dependent upon the concentration of drug used. Then, results were concluded as over-regulation of BAX and BAD, and less regulation of BCL-2, BCL-XL and XIAP. This study leads to elevation of reactive oxygen species (ROS), intracellular Ca2+ and endoplasmic reticulum (ER) stress in NCI-H460 cells treated with curcumin. Apoptosis occurs due to too much enhancement of curcumin through the FAS/caspase-8 which is a exterior path and ER stress proteins, growth arrest- and DNA damage-inducible gene 153 (GADD153) and glucose-regulated protein 78 (GRP78) was stimulated in the NCI-H460 cells. Apoptosis which occurs due to curcumin was inverted considerably by pretreatment with ROS scavenger or caspase-8 inhibitor. Moreover, the NCI-H460 cells tended to be detained at the G2/M cell cycle stage following curcumin action and involvement of decrease in regulation of cyclin-dependent kinase 1 (CDK1). In evaluation, curcumin shows its anti-cytotoxic property on lung cancer NCI-H460 cells by apoptosis (Wu et al., Citation2010).
Erlotinib
Erlotinib is usually used on patients with EGFR alterations, however, normally does not effectively used in management of lung cancer lacking malignant cells relapse. Even though various mechanisms for drug resistance are observed, these mechanisms do not successfully explain the description in support of each and every case in drug resistance. Though, investigation gave the hopeful possibility in targeting numerous tumorigenic pathways concurrently. Erlotinib in combination with other anticancer drugs has already established achievement in several lung cancer cell lines. Antitumor activity has formerly been showed in numerous studies. In recent studies, novel drugs are examined that develop chemotherapy and target on identifying prospective aspects for beneficial management of cancer (Leighl, Citation2012). Further research on these pathways may progress our information of the anticancer efficiency and durability, which will confidently progress in management of lung cancer.
Surfactant-based delivery systems
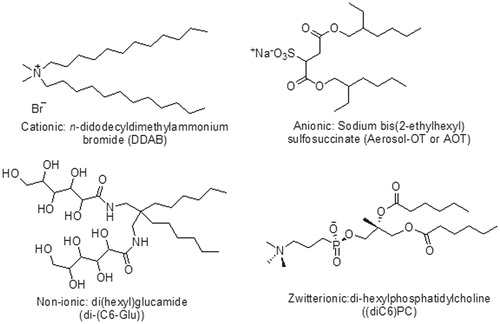
Surfactants as wetting agents are widely used in pharmaceutical formulations to develop dissolution and absorption of poorly soluble drugs. shows various examples of surfactants along with their nature. For this, rational and low molecular weight ionic surfactants like sodium lauryl sulfate are used in that concentration which are not harmful to the intestinal mucosa. Ionic surfactants have been observed in previous studies which are even more harmful to biological membrane than non-ionic surfactants (Davis et al., Citation1970). Furthermore, non-ionic surfactants which are lipophilic in nature have better capability to dissolve poorly soluble moieties. Thus, non-ionic surfactants are more effective than ionic surfactants in the dissolution of poorly soluble drugs. Non-ionic surfactants are extremely proficient emulsifiers which are to be used in self-emulsifying drug delivery systems. Non-ionic surfactants may effect on various factors of particles such as size, shape and stability of the particles. These factors can be determined by various analytical techniques such as dynamic light scattering (DLS), scanning electron microscopy (SEM), size-exclusion high-performance liquid chromatography (SEHPLC) and circular dichroism (CD). The most common non-ionic surfactants that can affect various factors of particles are Tween 80, Tween 20 and Brij 97. In earlier studies, it is concluded that the molecular configuration of surfactants can alter the self-assembly prototype of nanostructures (Tummala & Striolo, Citation2009; Suttipong et al., Citation2011). Various alterations can probably occur in the configuration of both the head and tail part of surfactants. The head part can be charged or neutral, small and compact in size, or a polymeric chain. The tail part is generally a single or double, straight or branched hydrocarbon chain, but may also be a fluorocarbon, or a siloxane, or contain aromatic group(s) (Parnami et al., Citation2013; Kataria et al., Citation2014). Chemical structures of typical double-chain surfactants are shown in . Since the hydrophilic part generally attains its solubility due to ionic interactions or hydrogen bonding, the simplest classification is on the basis surfactant head group type, through further sub-groups on the basis of the nature of the lyophobic moiety (Holmberg, Citation2003; Singh et al., Citation2014a). Fundamental classes consequently appear as listed in .
Table 1. Classification of surfactants long with suitable examples.
Table 2. Commercially available hydrophobic and hydrophilic surfactants (Griffin, Citation1955).
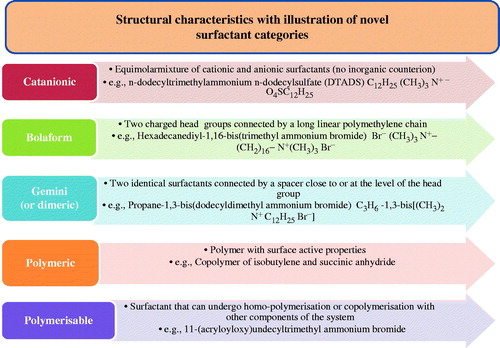
Surfactants having low HLB value provide a w/o emulsion, whereas surfactants having high HLB range give an o/w emulsion. NLC is resulting from an o/w emulsion method, thus the surfactant available here were of a high HLB range which are preferably dissolved in an aqueous external phase of the emulsion. Through the regular investigation for improving surfactant properties, novel structures have latterly emerged that display affecting synergistic relations or improved surface and aggregation effects (Singh et al., Citation2014b). These new surfactants have great significance and consist of the catanionics, bolaforms, gemini (or dimeric) surfactants, polymeric and polymerizable surfactants (Dickinson, Citation1992; Solans & Kunieda, Citation1996). Characteristics and typical examples are shown in . An additional significant motivation for this investigate is required for improved surfactant biodegradability. In exacting for special care products and household detergents, regulations require high biodegradability and constituents which are not harmful are required in the formulation.
Surfactant uses and development
Surfactants can exist from natural or synthetic sources. Naturally existing amphiphiles are primary standard surfactant which include lipids that are glycerol-based surfactants and are essential factor of the cell membrane (Solans & Kunieda, Citation1996). When animal and vegetable oils were combined with alkaline salts, soap-like matter was produced which may be beneficial for management of skin-related disease and also used for washing (Garg et al., Citation2013). Currently, synthetic surfactants are fundamental ingredients in various manufacturing processes and formulations (Dickinson, Citation1992). Depending on the specific chemical character of the product, the effects like emulsification, detergency and foaming may be displayed in changeable extent. The numeric and arrangement factors of the hydrocarbon groups collectively among the character and position of the hydrophilic groups merge to find out the surface-active effects of the molecule (Kaur et al., Citation2014a). As C12 to C20 is usually regarded as the vary covering optimal detergency, even though wetting and foaming are achieved greatest through shorter chain lengths. Structure–performance relationships and chemical compatibility are consequently major fundamentals in surfactant-based formulations, with the intention that a great deal investigation is committed to this region (Kaur et al., Citation2014b). discusses some common uses of surfactant with their special features.
Table 3. Common uses of surfactant categories with their features (Dickinson, Citation1992; Solans & Kunieda, Citation1996).
Pharmaceutical excipients as P-gp inhibitors
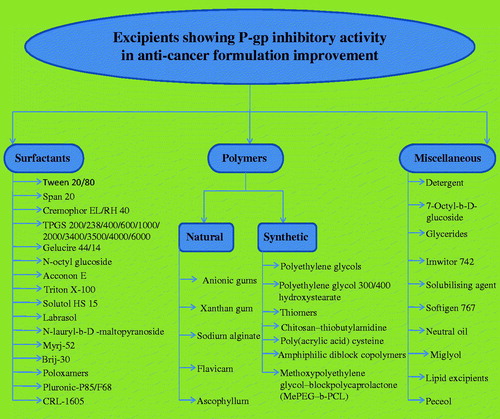
A variety of pharmaceutical products produced from either synthetic or natural origin, associated with the classes of co-solvents, surfactants, polymers and lipid ingredients have been publicized to have P-gp inhibition (). These workings enhance the absorptive transportation of P-gp substrates by inhibiting discharge intended for transport (Kaur et al., Citation2014c). The mechanism changes with the type of excipients that inhibit P-gp inhibition which is presently under research (Lo, Citation2003). Though some theories have been anticipated ().
Figure 6. Mechanism undertaken for P-glycoprotein-mediated efflux for excipients (Bansal et al., Citation2009).
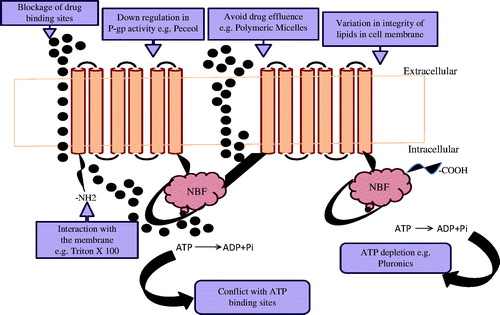
Solvents and surfactants act together by the polar head of the lipid bilayers which may change hydrogen bonding and ionic forces and this polar head may embed between the lipophillic tails of the bilayers (Gagandeep et al., Citation2014). These membrane disturbances have been exposed to alter P-gp activity leads to fluidization of the lipid layers (Cornaire et al., Citation2004). Batrakova & Kabanov (Citation2008) showed that pluronics can excite P-gp which causes prohibition of ATPase activity leading to depletion in ATP, whereas peceol and Gelucire 44/14 downregulate MDR1 gene expression and P-gp protein expression in Caco-2 cells (Batrakova & Kabanov, Citation2008). It is concluded from studies that several ingredients too influence direct binding to the P-gp, reduce Protein kinase C action, decrease phosphorylation of P-gp and alter P-gp-mediated efflux. Verapamil is familiar with P-gp inhibition by which mixed micelles have been noted to circumvent P-gp drug efflux, while the drug augmentation does not change (Garg, Citation2014).
Surfactants used in P-gp efflux
The initial surfactant which expressed as chemosensitizing agent was polysorbate 80, which was used with daunomycin (Riehm & Biedler, Citation1972). Then research was preceded by Woodcock et al. (Citation1992) which then conclude some other non-ionic surfactants, e.g. Tweens, Spans, Cremophors (EL and RH40), Pluronics and vitamin E TPGS having P-gp inhibition action (Collnot et al., Citation2007). Cremophor EL was used as a component of marketable formulations of paclitaxel (Taxol), however this formulation was found to be toxic. Usually, non-ionic surfactants can improve dissolution in water insoluble moieties because of being highly hydrophobic and comparatively lesser toxicity to biological membranes (Rege et al., Citation2002). Further research reveals the capability of Tweens to inhibit efflux pumps. Lo (Citation2003) established that Tween 20, Tween 80, Myrj 52 and Brij 30 enhance the epirubicin transport and decrease in efflux of diffusion chambers with excised rat intestinal mucosa. P-gp inhibition action is based on two essential parameters, i.e. surfactant concentration and hydrophilic–lipophilic balance (HLB). Surfactant concentrations which are non-hazardous to the intestinal mucosa may be usually preferred for the inhibition of P-gp. Greater than the crucial micelle concentration (CMC) surfactants become much beneficial in the solubilization of lipophilic substrates, while they would gave double achievement of solubilizing lipophilic drugs and inhibiting efflux. Though, the prototype of P-gp inhibition alters on the basis of kind of excipients used. On the basis of research, P-gp inhibitory activity raises when CMC is achieved and after CMC there is failure of the inhibitory activity lead to drug (P-gp substrate) entrapment in the micelles (Garg & Goyal, Citation2012). In a further development, inhibitory action raises yet beyond CMC and this could be accredited to the information that substrate entrapped in micelles avoid P-gp-mediated efflux. The most advantageous HLB range of surfactant systems among appropriate hydrocarbon chains and polar part is a significant issue in designing potential drug formulations (Garg & Goyal, Citation2014b). The most favorable development on the intracellular aggregation of epirubicin was attributing among intermediary HLB ranges ranging from 10 to 17 (Lo, Citation2003). Pluronics may possibly increase Caco-2 cell aggregation of rhodamine at concentrations less than the CMC while they demonstrate larger permeability by such concentrations (Batrakova et al., Citation1998). Pluronics (poloxomers) are extremely effective, non-hazardous, realistic and near-market utilize pharmaceutical ingredients. The biological effect of Pluronics is accredited toward their capability in the direction of incorporation into membranes followed via consequent translocation into the cells along with disturbing different cellular functions, such as mitochondrial respiration, ATP production, action of drug efflux transporters, apoptotic signal transduction and gene expression (Goyal et al., Citation2013a; Garg & Goyal, Citation2014a). Consequently, Pluronics cause extreme sensitization of MDR tumors to different anti-cancer drugs, improve drug transportation towards the blood brain and intestinal barriers, with causes transcriptional stimulation of gene expression both in vitro and in vivo (Batrakova & Kabanov, Citation2008; Garg et al., Citation2014).
Formulation advances on the basis of P-gp modulation
With the purpose to understand the prospective of P-gp in transportation of anti-tumor drugs to cancer cells along with much effectiveness as well as specificity, numerous delivery systems have been developed which may include microspheres, nanosized drug carriers (nanoparticles, nanoemulsions, stealth liposomes, nanogels, polymer–drug conjugates), novel powders, hydrogels, mixed micellar systems intended for systemic and/or localized delivery (Garg et al., Citation2012; Goyal et al., Citation2013b) ().
Table 4. Advanced formulations on the basis of P-gp modulation.
Conclusion
Surfactant-based delivery system has infinite potential with novel applications continuously being developed for use in cancer diagnosis, detection, imaging and treatment. These systems are previously assisting to address key matters with traditional anticancer agents such as non-specific targeting, low therapeutic efficiencies, untoward side effects and drug resistance as well as greater their forerunners with the skill to sense early metastasis. The ability of above systems to be personalized for an adapted medicine strategy makes them ideal vehicles for the treatment of lung cancer. In general, above systems allow design flexibility in drug delivery of poorly water-soluble molecules as well as imparting the ability to overcome biological barriers and selectively target desired sites within the body.
Acknowledgements
Authors Dr. Amit K. Goyal thankful to Indian Council of Medical Research, New Delhi, INDIA for providing financial assistance to carry out research.
Declaration of interest
The authors report no conflict of interest.
References
- Anand P, Sundaram C, Jhurani S, et al. (2008). Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267:133–64
- Bailey MM, Berkland CJ. (2009). Nanoparticle formulations in pulmonary drug delivery. Med Res Rev 29:196–212
- Bansal T, Jaggi M, Khar R, Talegaonkar S. (2009). Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci 12:46–78
- Batrakova EV, Han H-Y, Alakhov VY, et al. (1998). Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res 15:850–5
- Batrakova EV, Kabanov AV. (2008). Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Rel 130:98–106
- Bergers G, Benjamin LE. (2003). Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–10
- Bonomi P, Kim K, Fairclough D, et al. (2000). Comparison of survival and quality of life in advanced non–small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol 18:623–31
- Brabender J, Danenberg KD, Metzger R, et al. (2001). Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res 7:1850–5
- Bunn P, Kelly, K. (1998). New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res 4:1087–100
- Chavanpatil MD, Patil Y, Panyam J. (2006). Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. Int J Pharm 320:150–6
- Chen Y-T, Feng B, Chen L-B. (2012). Update of research on drug resistance in small cell lung cancer chemotherapy. Asian Pac J Cancer Prev 13:3577–81
- Chougule MB, Padhi BK, Misra, A. (2006). Nano-liposomal dry powder inhaler of amiloride hydrochloride. J Nanosci Nanotechnol 6:9–10
- Collnot E-M, Baldes C, Wempe MF, et al. (2007). Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm 4:465–74
- Cornaire G, Woodley J, Hermann P, et al. (2004). Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharma 278:119–31
- Dabholkar RD, Sawant RM, Mongayt DA, et al. (2006). Polyethylene glycol–phosphatidylethanolamine conjugate (PEG–PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int J Pharm 315:148–57
- Davis W, Pfeiffer R, Quay J. (1970). Normal and promoted gastrointestinal absorption of water‐soluble substances I: induced rapidly reversible hyperabsorptive state in the canine fundic stomach pouch. J Pharm Sci 59:960–3
- de Vore R, Johnson David, H. (2000). Chemotherapy for small cell lung cancer. lung cancer: principles and practice. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins
- Dickinson, E. (1992). Introduction to food colloids. Oxford, UK: Oxford University Press
- Du J, Lu W-L, Ying X, et al. (2009). Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood−brain barrier and survival of brain tumor-bearing animals. Mol Pharm 6:905–17
- Eberhardt W, Korfee, S. (2003). New approaches for small-cell lung cancer: local treatments. Cancer Control 10:289–96
- Elkharraz K, Faisant N, Guse C, et al. (2006). Paclitaxel-loaded microparticles and implants for the treatment of brain cancer: preparation and physicochemical characterization. Int J Pharm 314:127–36
- Emilienne Soma C, Dubernet C, Bentolila D, et al. (2000). Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials 21:1–7
- Ettinger DS. (2002). Is there a preferred combination chemotherapy regimen for metastastic non-small cell lung cancer? Oncologist 7:226–33
- Ferrara, N. (2002). Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 10:10–14
- Ferrara N, Gerber H-P, Lecouter, J. (2003). The biology of VEGF and its receptors. Nat Med 9:669–76
- Ferrara N, Hillan KJ, Gerber H-P, Novotny, W. (2004). Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3:391–400
- Föger F, Schmitz T, Bernkop-Schnürch, A. (2006). In vivo evaluation of an oral delivery system for P-gp substrates based on thiolated chitosan. Biomaterials 27:4250–5
- Folkman J, Merler E, Abernathy C, Williams, G. (1971). Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133:275–88
- Fong KM, Sekido Y, Minna JD. (1999). Molecular pathogenesis of lung cancer. J Thorac Cardiovasc Surg 118:1136–52
- Fossella FV, Devore R, Kerr RN, et al. (2000). Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non–small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol 18:2354–62
- Gagandeep G, Garg T, Malik B, et al. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–16
- García Sar D, Aguado L, Montes Bayón M, et al. (2012). Relationships between cisplatin-induced adducts and DNA strand-breaks, mutation and recombination in vivo in somatic cells of Drosophila melanogaster, under different conditions of nucleotide excision repair. Mutat Res Genet Toxicol Environ Mutagen 741:81–8
- Garg, T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol 20:1–8
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Rath G, Goyal AK. (2014). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. [Epub ahead of print]
- Garg T, Singh O, Arora S, Murthy, R. (2012). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. (2007). Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–44
- Goyal AK, Rath G, Garg T. (2013a). Nanotechnological Approaches for Genetic Immunization. DNA RNA Nanobiotechnol Med Diagn Treat Dis 67–120
- Goyal G, Garg T, Malik B, et al. (2013b). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv
- Greco FA, Gray JR, Thompson DS, et al. (2002). Prospective randomized study of four novel chemotherapy regimens in patients with advanced nonsmall cell lung carcinoma. Cancer 95:1279–85
- Griffin WC. (1955). Calculation of HLB values of non-ionic surfactants. Am Perfumer Essent Oil Rev 65:26–9
- Group, N-SCLCC. (1995). Chemotherapy in non-small cell lung cancer. A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 311:899–909
- Guillemard V, Saragovi HU. (2004). Prodrug chemotherapeutics bypass p-glycoprotein resistance and kill tumors in vivo with high efficacy and target-dependent selectivity. Oncogene 23:3613–21
- Hainsworth JD, Sosman JA, Spigel DR, et al. (2005). Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol 23:7889–96
- Hande, K. (1998). Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34:1514–21
- Haura EB. (2001). Treatment of advanced non-small-cell lung cancer: a review of current randomized clinical trials and an examination of emerging therapies. Cancer Control 8:326–36
- Hitzman CJ, Elmquist WF, Wattenberg LW, Wiedmann TS. (2006). Development of a respirable, sustained release microcarrier for 5‐fluorouracil I: in vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J Pharm Sci 95:1114–26
- Ho EA, Soo PL, Allen C, Piquette-Miller, M. (2007). Impact of intraperitoneal, sustained delivery of paclitaxel on the expression of P-glycoprotein in ovarian tumors. J Control Rel 117:20–7
- Holmberg, K. (2003). Novel surfactants: preparation applications and biodegradability, revised and expanded. Florida, USA: CRC Press
- Hosomi Y, Shibuya M, Niho S, et al. (2011). Phase II study of topotecan with cisplatin in Japanese patients with small cell lung cancer. Anticancer Res 31:3449–56
- Hunter TB, Manimala NJ, Luddy KA, et al. (2011). Paclitaxel and TRAIL synergize to kill paclitaxel-resistant small cell lung cancer cells through a caspase-independent mechanism mediated through AIF. Anticancer Res 31:3193–204
- Hurwitz H, Fehrenbacher L, Novotny W, et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med 350:2335–42
- Iffert T, Soldan M, Moeller A, Maser, E. (2000). Modulation of daunorubicin toxicity by liposomal encapsulation and use of specific inhibitors in vitro. Toxicology 144:189–95
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. Cancer J Clin 61:69–90
- Kataria K, Sharma A, Garg T, et al. (2014). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Kaur M, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014b). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv
- Kaur R, Garg T, Malik B, et al. (2014c). Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv 1–6 . [Epub ahead of print]
- Kelsey CR, Clough RW, Marks LB. (2006). Local recurrence following initial resection of NSCLC: salvage is possible with radiation therapy. Cancer J 12:283–8
- Koziara JM, Whisman TR, Tseng MT, Mumper RJ. (2006). In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Rel 112:312–19
- Lamprecht A, Benoit J-P. (2006). Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Rel 112:208–13
- Leighl, N. (2012). Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 19:S52-8
- Lin J-K. (2007). Molecular targets of curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. New York, USA: Springer
- Lo Y-L. (2003). Relationships between the hydrophilic–lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Rel 90:37–48
- Massarelli E, Andre F, Liu D, et al. (2003). A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer 39:55–61
- Mehnert W, Mäder, K. (2001). Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Miller K, Wang M, Gralow J, et al. (2005). A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: a trial coordinated by the Eastern Cooperative Oncology Group (E2100). Breast Cancer Research and Treatment. New York, NY: Springer 233 Spring Street, S6–6
- Minchinton AI, Tannock IF. (2006). Drug penetration in solid tumours. Nat Rev Cancer 6:583–92
- Novello S, Longo M, Levra MG. (2007). Toward therapies tailored to patient characteristics. J Thorac Oncol 2:S38–41
- Nurwidya F, Takahashi F, Murakami A, Takahashi, K. (2012). Epithelial mesenchymal transition in drug resistance and metastasis of lung cancer. Cancer Res Treat 44:151–6
- Ohe Y, Ohashi Y, Kubota K, et al. (2007). Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-arm cooperative study in Japan. Annals Oncol 18:317–23
- Parnami N, Garg T, Rath G, Goyal AK. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol
- Patel NR, Rathi A, Mongayt D, Torchilin VP. (2011). Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int J Pharm 416:296–9
- Patil Y, Sadhukha T, Ma L, Panyam, J. (2009). Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Rel 136:21–9
- Patton JS, Byron PR. (2007). Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 6:67–74
- Pfister DG, Johnson DH, Azzoli CG, et al. (2004). American Society of Clinical Oncology treatment of unresectable non–small-cell lung cancer guideline: update 2003. J Clin Oncol 22:330–53
- Pore MM, Hiltermann TJN, Kruyt FA. (2013). Targeting apoptosis pathways in lung cancer. Cancer Lett 332:359–68
- Rege BD, Kao JP, Polli JE. (2002). Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci 16:237–46
- Riehm H, Biedler JL. (1972). Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res 32:1195–200
- Roberti A, Sala DL, Cinti, C. (2006). Multiple genetic and epigenetic interacting mechanisms contribute to clonally selection of drug‐resistant tumors: current views and new therapeutic prospective. J Cell Physiol 207:571–81
- Rodriguez E, Lilenbaum RC. (2010). Small cell lung cancer: past, present, and future. Curr Oncol Rep 12:327–34
- Rusch V, Klimstra D, Venkatraman E, et al. (1997). Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 3:515–22
- Saintigny P, Burger JA. (2012). Recent advances in non-small cell lung cancer biology and clinical management. Discov Med 13:287–97
- Sandler A, Gray R, Perry MC, et al. (2006). Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 355:2542–50
- Scagliotti G, de Marinis F, Rinaldi M, et al. (2002). Phase III randomized trial comparing three platinum-based doublets in advanced non–small-cell lung cancer. J Clin Oncol 20:4285–91
- Schiller JH, Harrington D, Belani CP, et al. (2002). Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med 346:92–8
- Shepherd FA, Crowley J, van Houtte P, et al. (2007). The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2:1067–77
- Shepherd FA, Dancey J, Ramlau R, et al. (2000). Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–103
- Siegel R, Naishadham D, Jemal, A. (2012). Cancer statistics, 2012. Cancer J Clin 62:10–29
- Singh H, Sharma R, Joshi M, et al. (2014a). Transmucosal delivery of docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol
- Singh O, Garg T, Rath G, Goyal AK. (2014b). Microbicides for the treatment of sexually transmitted hiv infections. J Pharm 1–18
- Smit E, Carney D, Harford P, et al. (1989). A phase II study of oral etoposide in elderly patients with small cell lung cancer. Thorax 44:631–3
- Smit EF, van Meerbeeck JP, Lianes P, et al. (2003). Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group – EORTC 08975. J Clin Oncol 21:3909–17
- Solans C, Kunieda, H. (1996). Industrial applications of microemulsions. New York, USA: Marcel Dekker
- Song XR, Cai Z, Zheng Y, et al. (2009). Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci 37:300–5
- Št’astný M, Plocová D, Etrych T, et al. (2002). HPMA-hydrogels containing cytostatic drugs: kinetics of the drug release and in vivo efficacy. J Control Rel 81:101–11
- Št’astný M, Strohalm J, Plocova D, et al. (1999). A possibility to overcome P-glycoprotein (PGP)-mediated multidrug resistance by antibody-targeted drugs conjugated to N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer carrier. Eur J Cancer 35:459–66
- Subramanian J, Waqar SN, Govindan, R. (2011). Targeted therapy in lung cancer: lessons learned from past experiences. J Thorac Oncol 6:S1786–8
- Sung JC, Pulliam BL, Edwards DA. (2007). Nanoparticles for drug delivery to the lungs. Trends Biotechnol 25:563–70
- Suttipong M, Tummala NR, Kitiyanan B, Striolo, A. (2011). Role of surfactant molecular structure on self-assembly: aqueous SDBS on carbon nanotubes. J Phys Chem C 115:17286–96
- Tummala NR, Striolo, A. (2009). SDS surfactants on carbon nanotubes: aggregate morphology. ACS Nano 3:595–602
- Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. (2005). Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Control Rel 107:143–57
- Vogelsang GB, Abeloff MD, Ettinger DS, Booker SV. (1985). Long-term survivors of small cell carcinoma of the lung. Am J Med 79:49–56
- Wong HL, Bendayan R, Rauth AM, Wu XY. (2006). Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J Control Rel 116:275–84
- Woodcock D, Linsenmeyer M, Chojnowski G, et al. (1992). Reversal of multidrug resistance by surfactants. Br J Cancer 66:62–8
- Wozniak AJ, Crowley JJ, Balcerzak SP, et al. (1998). Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 16:2459–65
- Wu J, Lu Y, Lee A, et al. (2007). Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci 10:350–7
- Wu S-H, Hang L-W, Yang J-S, et al. (2010). Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade-and mitochondria-dependent pathways. Anticancer Res 30:2125–33
- Xiao Yan L, McTan T, Lee H. (2005). In vitro cytotoxicity of Stealth liposomes co-encapsulating doxorubicin and verapamil on doxorubicin-resistant tumor cells. Biol Pharm Bull 28:822–8
- Yang S, Gursoy RN, Lambert G, Benita, S. (2004). Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm Res 21:261–70
- Yang W, Peters JI, Williams RO III. (2008). Inhaled nanoparticles – a current review. Int J Pharm 356:239–47
- Zhang Z, Feng S-S. (2006a). The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly (lactide)–tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials 27:4025–33
- Zhang Z, Feng S-S. (2006b). Self-assembled nanoparticles of poly (lactide)–vitamin E TPGS copolymers for oral chemotherapy. Int J Pharm 324:191–8