Abstract
A major constraint in oral controlled release drug delivery is that not all the drug candidates are absorbed uniformly throughout the gastrointestinal tract (GIT). Drugs having “absorption window” are absorbed in a particular portion of GIT only or are absorbed to a different extent in various segments of the GIT. Thus, only the drug released in the region preceding and in close vicinity to the absorption window is available for absorption. The drug must be released from the dosage form in solution form; otherwise, it is generally not absorbed. Hence, much research has been dedicated to the development of gastroretentive drug delivery systems that may optimize the bioavailability and subsequent therapeutic efficacy of such drugs, as these systems have unique properties to bypass the gastric emptying process. These systems show excellent in vitro results but fail to give desirable in vivo performance. During the last 2–3 decades, researchers from the academia and industries are giving considerable importance in this field. Unfortunately, till date, few so-called gastroretentive dosage forms have been brought to the market in spite of numerous academic publications. The manuscript considers strategies that are commonly used in the development of gastroretentive drug delivery systems with a special attention on various parameters, which needs to be monitored during formulation development.
Introduction
Oral route is considered as most convenient and preferred route for drug administration due to high level of patient compliance (Lee & Mukherjee, Citation2006; Shahiwala, Citation2011). Oral route has variable and versatile physiological conditions in different parts, which enables development of formulations that can selectively release the medicament for optimal absorption and therapeutic benefit. Oral route offers multiple advantages like ease of administration and enormous surface area for passive diffusion of drugs (Hoffmann et al., Citation1983; Narayana et al., Citation2010). Because oral dosage forms do not need special attention for administration, avoid the emotional trauma and pain associated with injections, most drugs are designed for oral administration.
To maintain the drug concentration within the therapeutic range, it is often necessary to take the drug dose several times a day which may result in significant fluctuation in plasma drug concentration (Shen et al., Citation2003). This has led to the development of controlled release dosage forms, where the dosage form is designed to control the drug release such that its plasma profile is maintained within the therapeutic range for prolonged time. The term “controlled release” implies that the release of drugs from the delivery systems proceeds at a reproducible rate (Wilson & Crowley, Citation2011). The basic rationale for the development of controlled drug delivery is to control the drug concentration in the target tissue, reducing the number of administrations and to improve the efficacy of drugs by altering pharmacokinetics and pharmacodynamics of the drugs (Chien, Citation2009; Akala, Citation2010; Awasthi et al., Citation2010). However, a controlled release dosage form offers limited advantages for drugs that have an absorption window in the upper small intestine (Siegel & Rathbone, Citation2012). Despite the extensive absorption properties of the duodenum and jejunum, the extent of drug absorption from these sites is limited as the passage through this region is rapid (Davis, Citation2005). Once the dosage form is emptied from the stomach, the passage through this region is rapid, thus limiting the extent of absorption at this site. After crossing the absorption window, the released drug goes to waste with negligible or no absorption. This phenomenon drastically decreases the time available for drug absorption after it and limits the success of delivery system. In order to increase the bioavailability of such drugs, the residence time of the dosage form in the upper GIT needs to be prolonged that offer a new and better option for drug therapy (Streubel et al., Citation2006). This can be achieved by the development of gastroretentive systems that can withstand the contractions, grinding, crushing and peristaltic waves in the stomach and exhibit controlled release of drug in the gastric environment. The developed system should not have any effect on gastric motility or should not cause any gastric mucosal damage (Klausner et al., Citation2003a,Citationb). This is possible by developing gastroretentive drug delivery systems with physical properties like smaller size, high buoyancy with minimum lag time, along with the controlled release of drug in the gastric environment. The solute released in the stomach will empty along with the fluids and thus the whole surface area of the intestine will be available for absorption. An orally administered drug in gastroretentive delivery system must survive in the acidic environment of the stomach tract and should be absorbed. These systems are particularly useful for drugs that are primarily absorbed in the duodenum and upper jejunum segments (Sriamornsak et al., Citation2004; Jain & Gupta, Citation2009).
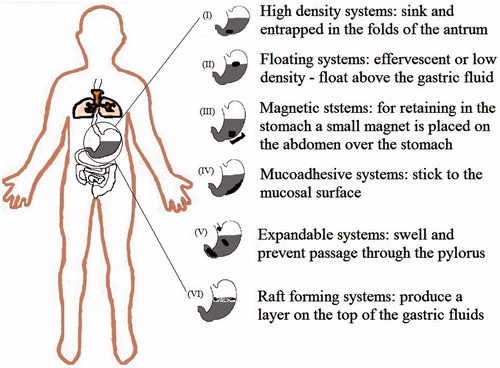
The first pioneering gastroretentive dosage form was suggested as far as back in 1957. Over the last 2–3 decades, numerous gastroretentive dosage forms such as high-density systems, floating systems, expandable systems, mucoadhesive or bioadhesive systems, magnetic systems, dual working systems and superporous systems have been designed to prolong the gastric residence time (Hwang et al., Citation1998; Pawar et al., Citation2011). After administration, these systems can remain in the stomach for a determined time period and thus, can maintain the drug concentration at the target site (Gangadharappa et al., Citation2007). Floating controlled release drug delivery system represents one of such approaches. There are numerous publications and patents about the development of controlled release dosage forms using gastroretentive approach.
Drug targeting to the stomach or upper small intestine can be attractive for several reasons such as drugs released over an extended period at a controlled rate and hence improve the patient compliance. A long-lasting local action on the gastrodudonal wall can be achieved, for example, drugs used in the treatment of gastric ulcer caused by H. pylori, such as amoxicillin. Certain drugs get benefited from gastroretentive devices such as drugs that are degraded in the colon or drugs, which primarily get absorbed in the stomach or act locally in the stomach. Weakly basic drugs with poor solubility in the basic environment can also benefit using gastroretentive delivery systems. The better therapeutic effect of short half-life drugs can be achieved by increasing the gastric retention (Talukder & Fassihi, Citation2004; Arora et al., Citation2005). Drugs administered as gastroretentive-controlled release dosage form have limitations such as (Singh & Kim, Citation2000; Waterman, Citation2007; Adebisi & Conway, Citation2011):
drugs which undergo significant first-pass metabolism may not be desirable candidates for floating drug delivery systems;
drugs which are well absorbed throughout the gastrointestinal tract (GIT) may not be desirable candidates for floating drug delivery systems;
the drugs that cause local irritation of the gastric mucosa may not be suitable for gastroretentive drug delivery systems.
The interest in the field remains high, over thousands papers on various aspects of gastroretentive drug delivery are published till now. Clearly, within the frame of a single paper, it is impossible to address all the logical relevant issues, but in this laconic review, we first describe the biological aspects of gastroretentive drug delivery systems, benefits and drawbacks associated with various gastroretention technologies and factors affecting gastroretention. Second, we discuss the formulation approaches used for the development of gastroretentive drug delivery systems. Finally, we outlined the basic challenges and essential processing parameters which need to be considered during the development of these systems for bringing attention to the main theme of the article.
Biological aspects of gastroretentive drug delivery systems
Gastrointestinal tract (GIT) is a 9 m long tube that runs through the middle of the body from mouth to anus. The major parts of the GIT are throat (pharynx), esophagus, stomach, small intestine (consisting of the duodenum, jejunum and ileum) and large intestine (consisting of the cecum, appendix, colon and rectum). The GIT has the same general structure from the esophagus to the anus, with some local variations in each region (; Waugh & Grant, Citation2001; Tortora & Derrickson, Citation2011). Gastrointestinal characteristics of healthy humans are given in .
Table 1. Salient features of upper gastrointestinal tract.
Table 2. Gastrointestinal characteristics of healthy humans.
Stomach
The stomach is a muscular sac situated in the left upper part of the abdominal cavity just below the diaphragm and liver. The volume of empty stomach is 20–25 ml, which can get expanded upto 1.5 l, on consumption of food. There are three major functions of stomach: physical digestion-churning action, chemical digestion and limited absorption (some water, alcohol, certain drugs). Stomach is anatomically divided into four segments namely, cardia, fundus, body (corpus) and pyloric antrum. The opening between stomach and small intestine is the pylorus. It is 12–13 mm wide in its open resting state, but the sphincter muscle can relax further to allow larger objects to pass. Muscle layers are well developed in the stomach, which help to break up food by churning action resulting in milky white liquid chyme. When the stomach digests the food as much as it can, the valve opens and the food travels into the small intestine (Wilson & Washington, Citation1989; Daniels & Allum, Citation2005; Marieb, Citation2007).
Gastrointestinal motility and gastric emptying of dosage forms
The emptying process of the stomach is caused by two mechanisms: tonic contraction of the stomach and peristaltic waves moving over the distal part of the gastric corpus. Two distinct patterns of gastrointestinal motility and secretion take the form of segmentation of mixing contractions and propulsive or peristaltic contractions.
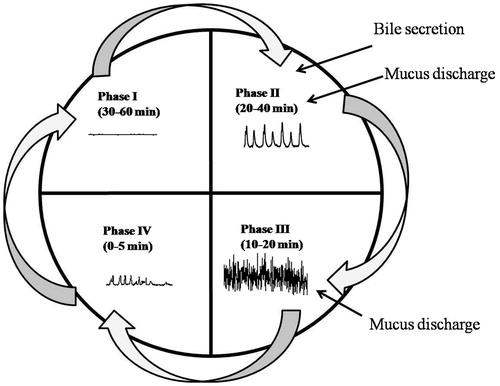
The bioavailability of orally administered drug depends on the fasted or fed state. In the fasted state, gastric emptying generally occurs within 2 h (Fell, Citation1996). This cyclical phenomenon is called the migrating motor complex (MMC). The process of MMC is divided into four consecutive phases: basal (Phase I), pre-burst (Phase II), burst (Phase III) and Phase IV intervals (; Vantrappen et al., Citation1979; Chawla et al., Citation2003; Takahashi, Citation2012). Plasma motilin level (stored in the duodenum) is highly associated with the appearance of gastric phase III of MMC (Sarna, Citation1985; Schemann & Ehrlein, Citation1986; Wilding et al. Citation2001; Romanski, Citation2009).
Phase I (basal phase) is a quiescent period with virtually no contractions, lasts for 40–60 min.
Phase II (pre-burst phase) lasts for 40–60 min with intermittent, irregular low amplitude contractions. The intensity and frequency also increase gradually as the phase progresses.
Phase III (burst phase) consists of short burst (4–6 min) of regular high amplitude contractions (house-keeper waves) due to which, all the undigested material is swept out of the stomach down to the small intestine.
Phase IV is a short transition period between phases III and I of two consecutive cycles and lasts for 0–5 min, with very little or no contractions.
Figure 1. Motility patterns of the gastrointestinal tract in the fasted state (Chawla et al., Citation2003).
The motor activity in the fed state is induced after 5–10 min of food ingestion and persists as long as the food remains in the stomach (Deshpande et al., Citation1996). The larger the amount of food ingested, the longer the period of feeding activity, with usual time spans of 2–6 h and phasic contractions similar to Phase II of MMC (Hasler, Citation1995). The effect of meal size and composition on gastric emptying of humans is presented in .
Table 3. Effect of meal size and composition on gastric emptying of humans (Dressman, Citation1986).
Multi-particulate systems avoid “all or none” gastric emptying process of single unit systems as these particles distribute evenly over the gastric intestinal tract which is independent of nutritional status (Rouge et al., Citation1997).
Factors affecting gastric retention (Kaus et al., Citation1984; O'Reilly et al., Citation1987; Sangekar et al., Citation1987; Hocking et al., Citation1988; Mazer et al., Citation1988; Mojaverian et al., Citation1988; Coupe et al., Citation1991, Citation1993; Clarke et al., Citation1993; Timmermans & Moes, Citation1994; Awasthi et al., Citation2010)
Age and gender - People over 70 years have a longer gastric retention time. Mean ambulatory gastric retention time in males (3.4 ± 0.6 h) is less compared with their age and race matched female counterparts (4.6 ± 1.2 h), regardless of the weight, height and body surface area.
Concomitant drug administration - Concomitant administration of anticholinergics, opiates and prokinetic agents can prolong gastric retention time.
Density - The buoyancy of gastroretentive dosage form depends on the density of the dosage form. It should be less than the gastric contents. However, sometimes the bulk density of a dosage form is not an appropriate parameter for describing the buoyancy due to the floating force kinetics of such dosage forms. This can be maintained by controlling the entry of water in the system.
Fed or unfed state - Under fasting conditions, the GI motility is characterized by MMC which sweeps undigested material from the stomach. The retention time of the dosage form is very short if the timing of administration of the formulation coincides with that of the MMC.
Frequency of feed - The gastric retention can increase by 6–7 h when successive meals are given compared with a single meal.
Nature of the meal - Indigestible polymers or fatty acid salts can decrease the gastric emptying rate. Gastric retention time can be increased by 4–10 h with a protein- and fat-rich meal.
Particle size - The dosage forms which are less than 10 mm in size can get emptied from the fed stomach.
Single or multiple unit formulations - It has been reported that multiparticulate systems avoid the “all or none” gastric emptying process of the single unit system. From the past literature, it can be concluded that multiple unit formulations have a more predictable release profile.
Formulation approaches for gastroretentive drug delivery systems
Formulation approaches used for the development of gastroretentive drug delivery systems are broadly classified into following seven categories based on formulation variables and mechanism of gastric retention:
High-density systems
Swelling and expandable systems
Mucoadhesive or bioadhesive systems
Superporus hydrogels
Magnetic systems
Floating systems
Dual working systems.
These systems have different principles of working and have their own merits and demerits. describes localization mechanisms of the different gastroretentive dosage forms. Benefits and drawbacks associated with gastroretention technology are described in .
Table 4. Benefits and drawbacks associated with various gastroretention technologies.
High-density systems
The density of gastric content is close to the density of water (∼1.004 g/cm3), whereas the density of these systems, is about 3 g/cm3. These systems are retained in the rugae of the stomach due to the high density (above a threshold density of 2.4–2.6 g/cm3) and are capable of withstanding its peristaltic movements. This phenomenon is confirmed by various clinical studies (Kaus et al., Citation1984; O'Reilly et al., Citation1987; Sangekar et al., Citation1987; Hocking et al., Citation1988; Mazer et al., Citation1988; Mojaverian et al., Citation1988; Coupe et al., Citation1991, Citation1993; Clarke et al., Citation1993, Citation1995; Timmermans & Moes, Citation1994; Tuleu et al., Citation1999; Hejazi & Amiji, Citation2002). The major drawback of high-density systems is that they are technically difficult to manufacture with a large amount of drug because the weight of matrix decreases progressively as the drug gets released (Bechgaard & Ladefoged, Citation1978; Davis et al., Citation1986; Rouge et al., Citation1998). High-density systems did not significantly extend the gastric residence time (Gupta & Robinson, Citation1995).
Swelling and expandable systems
The initial size of expandable dosage form should be minimum possible to facilitate swallowing and once the dosage form reaches to the stomach, the size of the dosage form should significantly increase rapidly and thus prevent premature passage through the pyloric sphincter. The size of the system needs to decrease after the complete drug release and enable the system to be evacuated from the stomach (Cargill et al., Citation1988; Fix et al., Citation1993; Kedzierewicz et al., Citation1999). For these systems, the stomach must be filled with fluids as swelling is due to the fluid absorption. The super porous hydrogels can reduce this problem to a certain limit as they have high swelling capacity. The expansion can be achieved by swelling due to the osmosis or unfolding of polymeric chains. Extensive study of unfolding gastroretentive devices has been carried out by Caldwell et al. (Citation1988a,Citationb,Citationc).
An expandable system based on unfolding mechanism has been developed for veterinary use (Laby, Citation1974). In terms of safety, the expandable systems should not interfere with gastric motility, must be biodegradable, and must not cause any local damage to the gastric mucosa on prolonged retention. Since permanent retention of rigid, large single-unit may cause bowel obstruction, intestinal adhesion and gastropathy, the system should be designed in such a way that it gets eliminated from the body after completion of drug release (Hou et al., Citation2003).
Mucoadhesive or bioadhesive systems
Adhesion of the dosage forms to the mucosal membrane of the stomach is an attractive approach to increase the retention of dosage form in the stomach or upper small intestine (Ponchel & Irache, Citation1998). These formulations utilize bioadhesive materials, namely, polyacrylic acids (Carbopol® 974P and 971P), Chitosan, cholestyramine, sodium alginate, hydroxypropyl methylcellulose (HPMC), sucralfate, tragacanth, dextrin, polyethylene glycol (PEG), gliadin etc., which enables the device to adhere to the gastric mucosal wall (Longer et al., Citation1985; Castellanos et al., Citation1993; Akiyama & Nagahara, Citation1999; Harding et al., Citation1999; He et al., Citation1999). The bioadhesive material should form a strong non-covalent bond with the mucin-epithelial cell surface of the GIT. The gastric mucoadhesion of the dosage form may follow any of the reported mechanisms of mucoadhesion such as wetting, diffusion, absorption or electron transfer. It has been reported that the anionic polymers have better mucoadhesion property than neutral or cationic polymers (Lehr, Citation1994; Huang et al., Citation2000). The bioadhesion of polymers to the mucus membrane is achieved by the formation of electrostatic and hydrogen bonding at the mucus-polymer boundary. Based on the past clinical and pre-clinical trials, it seems that the mucoadhesive polymers are unable to effectively/significantly control gastrointestinal transit of the dosage form (Chickering et al., Citation1995). It is very difficult to maintain effective mucoadhesion, due to continuous renewal of mucus on the walls of the stomach, resulting in unpredictable adherence (Lee et al., Citation2000; Chun et al., Citation2005). Mucoadhesive delivery systems can cause local side effects due to the intimate contact of system with gastric mucosa for prolonged periods of time.
However, mucoadhesion is regarded as one of the best approach to achieve gastroretention, but, in most clinical trials, there was little or no benefit of mucoadhesion approach was observed in gastroretention. In a study, similar time for 50% of particles to pass the pyloric sphincter was observed when cholestyramine powder was evaluated for the in vivo performance in humans using γ-scintigraphy. The comparison of mucoadhesion was done using carbopol® 934P, a pH-dependent polymer and sucralfate as a non-adhesive control (Jackson et al., Citation2001). In a study, erratic results were observed for gastroretention when microcrystalline Chitosan granules were evaluated by γ scintigraphy for determination of mucoadhesion (Sakkinen et al., Citation2004).
Superporous hydrogels
Hydrogels have been used in various pharmaceutical formulations due to their biodegradability and biocompatibility (Kamath & Park, Citation1993). Hydrogels are cross-linked network of hydrophilic polymers that are insoluble in water. Hydrogels have the ability to swell by absorbing water or gastric fluid (Park et al., Citation1993). The rate of swelling of conventional hydrogels is very slow, and hence, there are chances of premature evacuation of the dosage form through the pyloric sphincter (Chen et al., Citation2000). Therefore, these conventional hydrogels are commonly not used in gastroretentive drug delivery systems. Superporous hydrogels (pore size >100 µm) swell very fast due to rapid water uptake by capillary action, and hence, these hydrogels are important for in the development of gastroretentive delivery systems. These hydrogels can retain their mechanical strength due to their water insoluble nature (Mayur et al., Citation2013). Examples of superporous hydrogels are poly (acrylamide-co-acrylic acid)/polyethyleneimine polymer networks, polymerized vinyl monomers, or acrylate derivatives, sucrose hydrogels, Ac-Di-Sol® (croscarmellose sodium; Qiu & Park, Citation2003; Omidian, Citation2005).
Magnetic systems
Magnetic systems contain a small internal magnet (iron powder) and an extracorporal magnet placed on the abdomen over the position of the stomach, which control or guide the gastrointestinal transit of the dosage form (Fujimori et al., Citation1994). Various studies based on human trials have been reported by many researchers (Groning & Berntgen, Citation1996). The images are taken by very sensitive bio-magnetic measurement equipment. The major drawback of these systems is that the effectiveness of the therapy depends on the position of the external magnet, which might compromise patient compliance. The prolonged residence time of such delivery systems in the stomach of human volunteers has been proved by magnetic resonance imaging of the dosage forms (Groning et al., Citation1998).
Floating drug delivery systems
Floating systems are low-density systems which float over the gastric fluids and thus increase the retention time of the dosage form at the site of drug absorption, particularly in the stomach (Hwang et al., Citation1998; Yang et al., Citation1999). The first floating system was described by Sheth & Tossounian in 1975. These delivery systems are formulated by the incorporation of carbonate or bicarbonate salts in the swellable polymer matrix. The floatation is achieved by the entrapment of carbon dioxide gas within the polymer matrix (Mouzam et al., Citation2011), which decreases the density of the dosage form. These systems release the drug in a controlled manner while the system floats over the gastric fluid, which results in increased bioavailability of the drug with reduced fluctuation in plasma concentration (Reddy & Murthy, Citation2002). This study reports that the bulk density of a dosage form is not a appropriate parameter for describing buoyancy capability. Further, the report suggests that the optimization of floating force can be done by either slowing water penetration inside the formulation or by improving the swelling properties of the dosage form. represents companies investing in the development of gastroretentive drug delivery systems and represent different floating dosage forms formulated either single or multiple systems. The effectiveness of the buoyancy process is dependent on physiological conditions and the characteristics of dosage form.
Table 5. Companies investing in the development of gastroretentive drug delivery systems (Pies, Citation1982; Washington et al., Citation1986; Chouza et al., Citation1987; Erni & Held, Citation1987; Ceballos-Baumann et al., Citation1990; Degtiareva et al., Citation1994; Fabregas et al., Citation1994).
Table 6. List of drugs explored for various floatations based gastroretentive dosage forms.
Classification of floating drug delivery systems
Floating systems can be classified as effervescent, non-effervescent, low density and raft forming systems, depending on the formulation variables. The first category of floating systems is effervescent systems which are obtained by the incorporation of bicarbonate salt, which is responsible for gas generation or by volatilization of an organic solvent which make hollow cavity. The other category is non-effervescent systems which are formulated using the gel-forming, highly swellable polymers (Hascicek et al., Citation2011).
Effervescent systems
Effervescent systems are prepared by using swellable polymers such as methylcellulose and a gas forming agent like carbonate or bicarbonate salt with or without tartaric acid/citric acid (Rubinstein & Friend, Citation1994). Floatation can also achieve by the volatilization of an organic solvent (e.g. dichloromethane, ether, cyclopentane etc.). The most common approach for preparing these systems involves resin beads loaded with bicarbonate and coated with ethylcellulose. The insoluble but permeable coating allows water to permeate through it. Thus, carbon dioxide is released, causing the beads to float in the stomach. Other approaches and materials that have been reported are mixtures of hydroxypropyl methylcellulose, hydroxypropyl cellulose, ethyl cellulose or Carbopol® together with sodium bicarbonate (Xiaoqiang et al., Citation2006), light mineral oils (Bera et al., Citation2009), polypropylene foam powder (Streubel et al., Citation2003a), a mixture of alginate and bicarbonate that generate carbon dioxide when ingested (Stockwell et al., Citation1986), floating minicapsules with a core of sodium bicarbonate (Shaha et al., Citation2009) and floating systems based on ion-exchange resin technology (Atyabi et al., Citation1996). From the formulations containing carbonate salt, carbon dioxide gas is generated upon contact with acidic gastric fluid. The generated gas remains entrapped in the hydrated polymer matrix to make the dosage form remain buoyant for a prolong time period. The working of these systems depends on the net resultant force acting on the system. Buoyancy and sinking of system is balances by the upward and downward forces. The upward forces are caused by the generation of CO2 gas and increased density of system caused by continuous penetration of gastric fluid, respectively.
Strubing et al. (Citation2008b) investigated the drug release behavior of poly(vinyl acetate)-based membrane controlled floating tablets. The results of benchtop MRI study of selected samples suggested that the drug release was sustained for a period of 24 h. A multi-layer coated floating system based on gas formation using acrylic polymers (Eudragit RL 30D, RS 30D, NE 30D) and ethylcellulose was developed by Sungthongjeen et al. (Citation2008). The results of in vitro floatation and in vitro drug release studies showed that the floatation time was not affected when the amount of the gas forming agent was increased, but the drug release from the system was increased with the increase in concentration of gas forming agent. Optimization of floating tablets containing hydroxypropyl methylcellulose, ethyl cellulose and sodium bicarbonate was done using a simplex lattice design. The optimized formulation remained buoyant for more than 12 h and showed zero-order release profile (Patel et al., Citation2007a).
The work of Shishu et al. (Citation2007b) presents a floating system of 5-fluorouracil using hydrocolloids, such as, hydroxypropyl methylcellulose (HPMC) and Carbopol® 934P, gas forming agents like sodium bicarbonate and citric acid. The results of the in vitro study demonstrated that the formulation remained buoyant for 16 h and the drug release was sustained for a period of 24 h. Nakagawa et al. (Citation2006) developed double-compressed floating drug delivery system by pulsed plasma-irradiation of 5-Fluorouracil with an outer layer of a 68/17/15 weight ratio of Povidone (PVP), Eudragit RL (E-RL) and sodium bicarbonate. The formulation showed sustained drug release due to plasma-induced cross-linking reaction on the outer layer of tablet. X-ray imaging method was used to evaluate the buoyancy behavior of captopril bilayer-floating tablet in human subjects (Rahman et al., Citation2006). The X-ray images showed that the tablets remained in the stomach for about 6.4 h. An attempt was made to improve the dissolution profile of gliclazide by developing floating alginate beads by ionotropic gelatin method using various biodegradable polymers like gelatin, pectin and hydroxy propyl methylcellulose. The mechanism of drug release was Fickian diffusion with swelling. The in vivo sub-acute hypoglycemic study in high fat diet induced diabetic C57BL/6J mice demonstrated significant (p < 0.05) hypoglycemic effect over a period of 12 h and 24 h, respectively, with HPMC and pectin beads. A significant (p < 0.05) reduction in fasting and non-fasting blood glucose levels, reduction in fasting plasma insulin level and a significant improvement in glucose tolerance was observed in animals treated with formulations (Awasthi & Kulkarni, Citation2012).
Floating tablets of dextromethorphan HBr have been evaluated in healthy humans for the determination of pharmacokinetic parameters (Hu et al., Citation2011). There was no significant difference the pharmacokinetic values for the test and reference formulations, but the Tmax of floating tablets was significantly delayed compared with the conventional tablets. Effervescent tablets of ciprofloxacin HCl were evaluated for pharmacokinetic parameters after administration to the human subjects (Mostafavi et al., Citation2011). The study reported the Cmax and Tmax were 0.945 µg/mL and 6.0 h, respectively. Cmax and Tmax for conventional product were estimated to be 2.1 ± 0.46 µg/ml and 1.42 ± 0.59 h, respectively. The effect of metolose SH 4000 SR on drug release from floating matrix tablets of captropril has been reported (Martinez et al., Citation2010). The study concluded that the higher level of gas forming agent caused hindrance on drug release, as carbon dioxide bubbles obstructed the diffusion path and decreased the matrix coherence. The developed formulations remained buoyant for a period of more than 8 h. The addition of polymer decreased drug release rate due to an increasing tortuosity and length of the diffusion path through the matrix. Optimization studies on floating tablets containing nimodipine solid dispersion has been reported by Barmpalexis et al. (Citation2011). PXRD diffractograms of formulation indicated the existence of nimodipine in crystalline form. The floatation duration varied from 1 to 20 h with a lag time less than 3 min. The in vitro release profile followed both the Korsmeyer–Peppas and zero-order kinetic models.
A comparative study was done for in vivo evaluation of coated bicarbonate loaded resin beads against uncoated, bicarbonate-loaded ion exchange resins using γ-scintigraphy. Half-life for gastric emptying of coated test beads was about 3 h in fed human volunteers whereas uncoated control beads showed only about a 2 h time for half emptying (Atyabi et al., Citation1996). In vivo performance of the verapamil containing coated carbonate minitablets was determined based on pharmacokinetic study against an immediate release control formulation in healthy human volunteers. An increase in AUC was observed in test minitablet against the control. The study could not conclude the gastric retention because of the indirect link between pharmacokinetics and the gastrointestinal position (Sawicki, Citation2002).
Non-effervescent systems
These delivery systems are developed using a high level of one or more gel-forming, highly swellable polymers. Hydroxypropyl methylcellulose (HPMC) is the most commonly used excipient for the development of non-effervescent floating systems, although agar, carrageenans, hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC) and sodium carboxymethycellulose (NaCMC) are also used. These gel forming polymers hydrate and form a gel barrier that controls the fluid penetration into the device and the consequent drug release from the device. The buoyancy of the dosage form depends on the density of swollen polymeric matrix, which can be reduced due to the entrapped air within the matrix (Nakamichi et al., Citation2001).
Hydrodynamically balanced systems
Hydrodynamically balanced systems (HBS) are useful for drugs having a better solubility in gastric environment and useful for drugs that are primarily absorbed in the stomach. These systems are able to remain in the stomach for a longer period by maintaining their low apparent density less than the gastric fluid, while the polymer hydrates and forms a gelled barrier at the outer surface (Seth & Tossounian, Citation1984; Bardonnet et al., Citation2006). Nama et al. (Citation2008) have reported an HBS of clarithromycin for the eradication of H. pylori. The results of in vivo radiographic study in healthy male volunteers suggested that the gastric residence time of tablet was increased, which leads to the effective localized action of the clarithromycin. The drug release from the floating tablets was through the anomalous diffusion process and followed zero-order kinetics. An optimized single unit HBS of metformin was formulated and evaluated by Ali et al. (Citation2007). The in vivo buoyancy and pharmacokinetic parameters were assessed by gamma scintigraphy in rabbits. The developed system remained buoyant during 5 h of the study. An increase in AUC was observed when the animals were treated with an optimized formulation. Artificial neural networks (ANNs) as modeling tools for prediction of drug release from HBS composed with Metholose 90SH (hydroxy propyl methylcellulose) has been reported by Mendyk et al. (Citation2006). It was found that ANNs were capable to accurately predict release patterns of different drugs from HBS based on the description of the formulation as well as chemical structure of the drug.
Low-density systems
Gas generating systems have a lag time before floating on the stomach contents, during which, the dosage form may undergo premature evacuation through the pyloric sphincter. Low-density systems (<1 g/cm3) with immediate buoyancy do not have this kind of problem. They are made of low-density materials, entrapping oil or air. Most of them are multiple unit systems, such as microspheres and are also called as “microballoons or hollow microspheres” because of the low-density core. They are characteristically free flowing powder with a size less than 200 µm. Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout the particle matrix have the potential for controlled release of drugs. In low-density approach, the globular shells with a density lower than that of gastric fluid are used as a carrier (Streubel et al., Citation2003b). A buoyant dosage form can also be obtained using a fluid-filled system that floats on the stomach. These are further coated with a drug–polymer mixture. The polymer of choice can be either ethylcellulose or hydroxyl propylcellulose depending on the type of release desired. Finally, the product floats on the gastric fluid while releasing the drug gradually over a prolonged duration (Sharma & Pawar, Citation2006; Goole et al., Citation2007; Garg & Gupta, Citation2008). For easy administration and accurate dose, these systems can be compressed into fast disintegrating tablets (Streubel et al., Citation2003a). Recently, sublimation material based floating tablets were investigated by Oh et al. (2013).
An attempt was made to improve the release profile of gliclazide by developing hollow alginate beads by the ionotropic gelation method in combination with low methoxyl pectin and hydroxypropylmethylcellulose. The beads remained buoyant for more than 12 h. The drug release from beads followed Fickian diffusion with swelling (Awasthi & Kulkarni, Citation2014). An in vivo study of a floating depot system of metformin HCl in healthy male albino rats was conducted by Choudhury et al. (Citation2008). The developed ethylcellulose microspheres had good encapsulation efficiency (73–93%). It was found that the drug release and plasma sugar levels were controlled more efficiently from the prepared microspheres. Strubing et al. (Citation2008a) developed floating Kollidon® SR matrix tablets of Propranolol. They studied the floatation behavior of tablets and the drug release profiles. Results showed that the tablets remained buoyant for 24 h with a very short lag time. The floatation was found to depend on the level of Kollidon® SR.
Wakode & Bajaj (Citation2008) optimized pramipexole-loaded floating microspheres of ethyl cellulose and hydroxyl propyl methylcellulose on the basis of 23 level factorial design. The particle size and morphology of formulations was characterized by image analyzer and scanning electron microscopy, respectively. The results of in vitro drug release kinetics showed that the drug release from microspheres was diffusion controlled. Shishu et al. (Citation2007a) developed floating alginate beads of 5-fluorouracil. The prepared beads were evaluated for percent drug loading, buoyancy, surface topography and in vitro drug release. The in vivo antitumor study was done using an optimized formulation to check the therapeutic efficacy of the floating dosage form against benzto(a)pyrene induced stomach tumors in albino female mice. The developed system was found to reduce the tumor incidence in mice by 74%, while the conventional tablet reduced tumor by only 25%. Melt granulation technique was used for the development of floating granules of ranitidine HCl by Patel et al. (Citation2007b). Gelucire 50/13 and Gelucire 43/01 were used as lipid carrier. The optimization was done by 32 full factorial design. The drug release was controlled by a moderate amount of polymer. A similar formulation approach was used by Shimpi et al. (Citation2004) for the preparation of floating granules of diltiazem HCl using Gelucire 43/01. The results of in vivo γ-scintigraphy study showed that the granules were retained in stomach of healthy human volunteers for 6 h and 65–80% of drug was released over 6 h with an initial fast release from the surface.
Ishak et al. (Citation2007) used ionotropic gelation method for the development of Chitosan-treated floating alginate beads of metronidazloe for the eradication of H. pylori infection. The results of histopathologic study showed that metronidazole loaded floating beads gave a better effect than the corresponding suspension. Gohel & Sarvaiya (Citation2007) developed novel gastroretentive tablets of rifampicin and isoniazid by wet granulation to minimize their degradation in acidic medium using hydroxypropyl methylcellulose, calcium carbonate and polyethylene glycol 4000. The degradation of rifampicin was arrested because of the minimization of physical contact between the two drugs and controlled release of rifampicin in acidic medium. Badve et al. (Citation2007) developed hollow calcium pectinate beads for floating-pulsatile release of diclofenac sodium. The developed floating beads had Ft50% of 14–24 h due to the porous (34% porosity) structure with bulk density less than 1. In vivo gamma scintigraphy results showed that the beads remained buoyant for 5 h in rabbit stomach. Ravala et al. (Citation2007) investigated the effects of formulation and processing parameters on floating matrix controlled drug delivery system. Poly (styrene-divinyl benzene), a highly porous co-polymer, was used for the development of low-density system. Excellent in vitro floating behavior of the tablets was obtained at a concentration of 15% (w/w).
Tanwar et al. (Citation2008) developed floating microspheres of verapamil HCl using cellulose acetate, acrycoat S100 and eudragit S100. Radiographic images of dog stomach revealed that cellulose acetate microspheres loaded with barium sulfate floated on the gastric fluid for about 3.2 h and in vitro release study demonstrated non-Fickian diffusion of the drug. Pharmacokinetic studies of hollow microspheres of piroxicam were reported in male albino rabbits by Joseph et al. (Citation2002). As compared to the free drug, bioavailability of the drug from the microspheres was about 1.4 times, whereas it was about 4.8 times when the microspheres were administered with a loading dose. The elimination half-life was increased by about three times for the microsphere preparation alone and nearly about six times for the dosage form consisting of microspheres and a loading dose in comparison to the free drug. Jain et al. (Citation2006a) evaluated floating microspheres of repaglinide by gamma scintigraphy study in albino rabbits. The gastric residence time was found to be around 6 h. The relative bioavailability of drug loaded floating microspheres was 3.17 times higher than the marketed tablet.
Jain et al. (Citation2006b) have reported gamma scintigraphic and pharmacokinetic studies in albino rabbits for a comparative study of Orlistat loaded floating microspheres and marketed Xenical capsule. Gamma scintigraphic images showed gastric residence time of 6 h. In contrast, non-floating marketed formulation showed gastroretention of less than 2 h. The results showed that the time to reach peak plasma concentration (Tmax) and area under the curve (AUC) were increased from 4 to 8 h and 69.0–113.3 ng h/ml, respectively, for floating microspheres. Anti-H. pylori activity of floating microspheres containing acetohydroxamic acid was carried out by Umamaheshwari et al. (Citation2006) in Mongolian gerbils. The microspheres exhibited greater anti-H. pylori activity due to the prolonged residence time. Stithit et al. (Citation1998) prepared buoyant theophylline microspheres by emulsion solvent evaporation method using cellulose acetate butyrate and Eudragit RL 100. The microspheres remained buoyant for 24 h, and the drug release from microspheres followed nearly zero-order kinetics.
In a study, Lee et al. (Citation2001) determined the effect of solvent composition and non-volatile oil on floatation and release profile of drug from microspheres. Model drugs used were cyclosporine A, ketoprofen, piroxicam, tacrine HCl and tenoxicam. The best formulation was obtained when the ratio of dichloromethane: ethanol: isopropanol was maintained at 5:6:4. The formulations containing oil had less dense and more porous channels. The drug release was found to increase with the increase in pH of the dissolution media. A novel solvent diffusion evaporation method was reported by Soppimath et al. (Citation2001) using nifedipine, nicardipine HCl, verapamil HCl and dipyridamole as model drugs. The drug release was controlled for 8–10 h following different transport mechanisms.
Low-density foam powder was used for the preparation of floating matrix tablets by Streubel et al. (Citation2002). The study reports that the floatation and release of drug could be modified by varying the matrix-forming polymer/foam powder ratio. Porous calcium silicate based floating microspheres of repaglinide were developed by emulsion solvent diffusion method (Jain et al., Citation2005). The optimized formulation demonstrated good buoyancy (84 ± 6.0%), high encapsulation (75 ± 3.0%) and a sustained in vitro drug release in pH 2.0, 6.8 and 7.4. The drug release was found to decrease with increase in concentration of calcium silicate. A similar method was used Muthusamy et al. (Citation2005) for the development of floating micropellets of lansoprazole. In this study, it was observed that the drug loaded micropellets floated on the simulated gastric fluid for more than 12 h with sustained drug release over a period of 12 h.
The solvent evaporation method was used for the preparation of citrimide microspheres by Srivastava et al. (Citation2005). They demonstrated that the prepared microspheres exhibited prolonged drug release and remained buoyant for more than 10 h. Nepal et al. (Citation2007) prepared hollow microspheres of josamin by solvent diffusion and evaporation technique using Eudragit E100. The loading efficiency of the drug in the microspheres was 64.7%. In a period of 45 min, the drug was released completely in the simulated gastric fluid of rainbow trout (pH 2.7). Kale & Tyade (Citation2007) developed floating microspheres of piroxicam by an emulsification solvent evaporation method using Eudragit S100. The microspheres remained buoyant for a period of 10 h. DSC and X-ray diffraction studies showed that drug incorporated in the outer shell of the polymer was in amorphous form. SEM images indicated that the developed microspheres were spherical with a hollow internal cavity. The drug release at intestinal pH was faster and continuous as compared to the gastric pH. Varshosaz et al. (Citation2007) reported diffusion solvent evaporation technique for the development of floating microballons to increase the solubility and bioavailability of cinnarizine. During the development of formulations, the effect of process variables such as eudragit type, stirring rate, time of stirring on the yield, particle size, loading, release and floating behavior of microspheres was evaluated using factorial design.
Soppimath et al. (Citation2006) studied the effect of co-excipients on drug release profile and floation behavior of the hollow microspheres of nifedipine. The results of in vitro buoyancy study showed that the microspheres floated for more than 12 h and their buoyancy followed the rank order of: blank (no-coexcipient) > dibutylpathalate > polyethyleneglycol > poly(ε-caprolactone) and the drug was released in a controlled manner. Chauhan et al. (Citation2004) have studied the release characteristics of risedronate sodium and Gelucire® 39/01 floating matrices using melt solidification technique. A change in the crystal structure of Gelucire® was observed due to the ageing of the product, which was responsible for an increase in drug release.
A 32 full factorial design was used by Dave et al. (Citation2004) for the optimization of ranitidine HCl floating tablets. The study was conducted to demonstrate the effect of stearic acid and citric acid on drug release. It was suggested that low amount of citric acid and high amount of stearic acid favors sustained drug release from the prepared formulations. Sato et al. (Citation2004) have examined pharmacokinetic data of riboflavin containing microballoons by urinary excretion method on healthy human subjects. It was noticed that the larger microballoons (particle size 500–1000 µm) showed better buoyancy in comparison to smaller particles (particle size < 500 µm). Talukder & Fassihi (Citation2004) reported floating hollow beads developed either using calcium and methoxylated pectin or calcium, methoxylated pectin and sodium alginate. The results showed that calcium-pectinate-alginate beads released their contents at faster rates than calcium-pectinate beads.
The floating mucoadhesive microspheres of melatonin were prepared using ionic interaction of chitosan and sodium dioctyl sulfosuccinate by El-Gibaly et al. (Citation2002). The microcapsules exhibited zero-order release kinetics in simulated gastric fluid. The formulations remained buoyant for more than 12 h.
Solid dispersion of furosemide in polyvinylpyrrolidone was used in floating multiple unit system by Iannuccelli et al. (Citation2000). The results of X-ray diffraction study showed a decrease in crystallinity of furosemide solid dispersion, which lead to the improved solubility and thus improved dissolution of the drug. Durig & Fassihi (Citation2000) developed swellable hydrophilic floating matrix tablets of verapamil HCl using a guar gum matrix. The study was aimed to compare the dissolution profiles using USP dissolution apparatus type I and type II. The study concluded that a double mesh device may provide an alternative to current compendial dissolution methods when the release kinetics of floating and sticking delivery system is required. A comparative gamma scintigraphic study of floating and non-floating beads was done by Whitehead et al. (Citation1998) in healthy human volunteers. The study demonstrated that the floating beads remained buoyant for 5–6 h. The gastric residence time for non-floating beads was only 1 h. Slight increase in gastric residence time for flavine mononucleotide floating particles versus dense particles as control was observed based on pharmacokinetics (Lippold & Gunther Citation1991).
A gastroretentive floating system of amoxicillin was developed and optimized for the efficient treatment of peptic ulcer induced by H. pylori infection. Floating microballoons were developed using central composite design (CCD), and optimization was done by employing response surface methodology. The in vitro MIC results showed a sustained drug effect from the microballoons. The study results conclude that CCD is a valuable second-degree design to develop and optimize GFS of amoxicillin which in turn provides a basis to localize the drug release in the gastric region for effective treatment of H. pylori-mediated infection (Awasthi et al., Citation2012; Awasthi & Kulkarni, Citation2013).
Raft forming systems
Raft forming gastroretentive system is a boat-like structure that floats over the gastric fluid and allow a constant drug release. Here, gel forming solution (e.g. sodium alginate solution containing carbonate or bicarbonate) swells and forms a viscous cohesive gel containing entrapped CO2 bubbles on contact with gastric fluid. These formulations generally contain antacids such as aluminum hydroxide or calcium carbonate to reduce gastric acidity. Since the raft forming systems produce a layer on the top of gastric fluid, they are also used for treatment of gastroesophageal reflux (Rajinikanth et al., Citation2007). Rajinikanth & Mishra (Citation2008) prepared floating in situ gelling system of clarithromycin to eradicate H. pylori using gellan as gelling polymer and calcium carbonate as floating agent. They studied H. pylori clearance efficiency of prepared system and clarithromycin suspension following oral administration to H. pylori infected Mongolian gerbils by polymerase chain reaction technique and by a microbial culture method. Floating in situ gelling system showed a significant anti-H. pylori effect than that of clarithromycin suspension.
Dual working systems
Systems based on the combination of bioadhesion and floation principles have more potential to increase to improve the in vivo performance of the drug. Furthermore, the combination of mucoadhesion and flotation technology can meliorate drawbacks associated with floating technology like floating lag time and requirement of fluid in the stomach for proper floatation.
Varshosaz et al. (Citation2006) prepared floating-bioadhesive tablets of ciprofloxacin using sodium carboxymethylcellulose, polyacrylic acid, citric acid and sodium bicarbonate. All the tablets floated for more than 24 h. It was observed that an increase in sodium carboxymethylcellulose amount caused higher mucoadhesion than polyacrylic acid. Chavanpatil et al. (Citation2006) reported swellable and bioadhesive system of ofloxacin using psyllium husk, hydroxyl propylmethylcellulose and crosspovidone. The results showed that the formulation containing crosspovidone had a good swelling property and swelling was increased with increasing concentration of crosspovidone. The bioadhesive property of the developed formulation was found to be significantly increased in combination as compared to hydroxypropyl methylcellulose and psyllium husk alone. Umamaheshwari et al. (Citation2002, Citation2003) developed floating-bioadhesive particulate systems of acetohydroxamic acid and cholestyramine containing sodium bicarbonate as gas forming agent. The cellulose acetate butyrate coated microcapsules showed better buoyancy than uncoated resin particles. In vitro growth inhibition studies were performed in an isolated H. pylori culture. The microspheres showed a better inhibition rate than plain acetohydroxamic acid. Floating-bioadhesive bilayer tablets of rosiglitazone maleate have been developed by Sonara et al. (Citation2007). The formulations showed a unique combination of floatation and bioadhesion to prolong the gastric residence. Gamma scintigraphy images showed that the tablets were buoyant for 8 h in the human stomach. The drug release followed first-order kinetics.
In the past few years, many studies related to the animal experimentation on floating mucoadhesive microparticulate gastroretentive drug delivery systems have been reported. Pharmacokinetic study of clarithromycin loaded floating-bioadhesive microparticles was carried out by Zheng et al. (Citation2006) in male Sprague–Dawley rats. The microparticles were developed by emulsification/evaporation and internal/ion gelation methods. The results showed that the after 4 h about 61% of the microparticles remained in the stomach and the concentration of clarithromycinin in gastric mucosa was greater than that of the solution, and the difference at 2 h was statistically significant (p < 0.05). The effect of food intake on the performance of various types of gastroretentive drug delivery systems based on in vivo studies is given in .
Table 7. The effect of food intake on the performance of various types of gastroretentive drug delivery systems in human volunteers.
Advancements in designing of floating drug delivery
Intragastric floating gastrointestinal drug delivery systems
Intragastric floating drug delivery system composed of a drug reservoir encapsulated in a microporous compartment with apertures along its top and bottom surfaces. The peripheral walls of the drug reservoir compartment are properly sealed to prevent any direct physical contact of the undissolved drug with the stomach mucosal surface. The intragastric floating can be achieved using low-density additives (e.g. fatty acids and fatty alcohols) and gas-generating agent. These systems can be prepared by simple ionotropic gelation method (Harrigan, Citation1977).
Inflatable gastrointestinal drug delivery devices
These devices composed of an inflatable chamber containing a volatile liquid such as ether which gasified at body temperature to cause the chamber to inflate in the stomach. The inflatable chamber also contained a biodegradable polymer filament. These systems contain a copolymer of polyvinyl alcohol and polyethylene that gradually dissolved in the gastric fluid. Dissolution of this copolymer is responsible for the release of gas from the system after an extended period of time to permit the spontaneous ejection of the system from the stomach (Michaels, Citation1974).
Intragastric osmotically controlled floating drug delivery devices
These devices comprised of a hollow deformable polymeric capsule shell. The capsule is divided into two compartments separated by a semipermeable membrane. The inner compartment is a drug reservoir, which is covered by an outer osmotically active compartment. The osmoticlally active compartment contains a volatile liquid such as cyclopentane or ether that vaporizes at the body temperature. Vaporization of liquid increases the size of unit to inflate. The device contained a bioerodible plug that allows the vapors to escape from the device and return it to the original collapsed position after an extended period of time for easy removal from the body (Michaels et al., Citation1975).
Evaluation of floating drug delivery systems
In vitro evaluation
In vitro parameters that need to be evaluated in gastroretentive drug delivery systems includes differential scanning calorimetry to examine the thermal behavior of drug in formulations, X-ray diffraction studies to examine the physical state (amorphous or crystalline) of drug in formulations, infrared spectroscopy for investigation of possible interaction between drug and excipients, specific gravity, flow properties, particle size analysis, yield, size and shape, in vitro buoyancy behavior (buoyancy lag time and buoyancy duration), content uniformity and in vitro drug release profiles. The performance of such systems is depending on the density of the system, thus it is an important to evaluate density of such system. For a system to float on the gastric fluid, the system should have a density lower than that of the gastric fluid (∼1.004 g/cm3). The true density can be determined using the photographic counting method or the liquid displacement method. The percentage buoyancy of floating gastroretentive systems can be determined by taking a predetermined amount of dosage form in 100 ml of suitable medium such as 0.1 N hydrochloric acid (pH 1.2). In case of particulate systems, the dosage form that floated and those settled are collected after a specified time period. The fractions of dosage units are weighed and buoyancy can be determined by the following formula:
where Wf and Ws are the weights of floating and settled dosage units, respectively.
The test for in vitro drug release is generally performed in simulated gastric fluids at 37°C. Investigation of drug release profiles at slight higher pH, such as phosphate buffer pH 5.8 or 6.8, is also recommended, due to the variation in gastric pH based on fasting or fed conditions. Dissolution tests generally performed using USP II dissolution apparatus. USP 28 states “the dosage unit is allowed to sink to the bottom of the vessel before rotation of the blade is started”. A small, loose piece of non-reactive material with not more than a few turns of a wire helix may be attached to the dosage units that would otherwise float. It is reported that the drug release from the delivery system is reduced by the use of helical wire. To overcome this limitation, a method has been developed in which the floating drug delivery system was fully submerged under a ring or mesh assembly (Soppimath et al., Citation2001). Recently, Eberle et al. (Citation2014) developed a custom-built stomach model to, simultaneously, analyze buoyancy behavior and drug release profiles. In silico dissolution and floatation profiles of the floating tablet were simulated using a three-dimensional cellular automata-based model. In this study, the floating tablets showed instant floatation in simulated gastric fluid.
In the case of particulate gastroretentive delivery systems, the percentage encapsulation is determined by taking 10 mg of the microparticles. Powdered microparticles are suspended in 25 ml of suitable medium. After 24 h shaking, the filtrate is analyzed for the drug content by UV-spectrophotometer after suitable dilution. The percentage encapsulation can be calculated as follows:
where Da is the actual amount of drug present in the microparticles and Dt is the theoretical amount of drug added in the preparation of microparticles.
The surface and internal morphology are observed by scanning electron microscopy. During sample coating for scanning electron microscopy analysis, it is generally exposed to high vacuum, to make the sample conductive.
In vivo evaluation
Pharmacokinetics
This technique uses collection and analysis of blood samples at predetermined time intervals. Various pharmacokinetic parameters, such as, maximum plasma concentration of drug (Cmax), time to reach maximum plasma concentration (Tmax) and area under the curve (AUC) are determined for the in vivo performance measurement of the dosage form.
γ-Scintigraphy
This technique is used to evaluate in vivo buoyancy behavior of different type of gastroretentive systems. This technique is based on the incorporation of a radioisotope like 111In within the system. The radioisotope labeled formulation is administered to the human volunteers. Ionization radiations, limited topographic information, low resolution are the major drawbacks of γ-Scintigraphy technique. This technique is complicated and expensive (Wilding et al., Citation2001; Goole et al., Citation2008).
Radiology
Radiology is a simple technique used for estimation of gastroretention. However, this technique has not gained popularity due to the exposure to X-rays. Radiographs are taken at various periodic time intervals after administration of the dosage form (Iannuccelli et al., Citation1998; Baumgartner et al., Citation2000).
Gastroscopy
Gastroscopy involves visual observation of dosage form in the stomach using optic-fibers and a video camera. Retained blood or food in the stomach may lead to poor study results (Klausner et al., Citation2003a,Citationb).
Ultrasonography
Ultrasonic waves are used to produce images of body structures. The waves travel through tissues and are reflected back where density differs. The reflected echoes are received by an electronic apparatus that measures their intensity level and the position of the tissue reflecting them. The results can be displayed as images or as a moving picture of the inside of the body (Hendee, Citation1994).
Magnetic resonance imaging
This is a non-invasive technology which uses a magnetic field, radio frequency pulses, and a computer to produce a detailed image. The advantage of this technique over γ-scintigraphy is that it does not use ionizing radiation such as X-rays. Harmless paramagnetic and supra-magnetic imaging contrast agents are applied to obtain better study results (Dorozynski et al., Citation2007).
Future recommendations
Based on various in vitro and in vivo data, the evidence for successful gastroretentive dosage forms remains limited. The expected gastric retention, especially with low calories or fasted conditions is discombobulating. The benefits of gastric retention for drug delivery can be attained with the current technologies using particulate systems given in the fed state. Current industrial applications of gastroretentive delivery systems are based on their physical properties (size, buoyancy, drug content and drug release behavior) as they are specified during their preparation. To provide evidence that a gastroretentive technology actually works, the development and in vivo testing of the system should carry out by considering following parameters:
As the performance of the system is based on the buoyancy, so the analysis of the position of dosage form should be done using an imaging technique (such as γ-scintigraphy) rather than pharmacokinetics.
The caloric content of the meal should be carefully controlled. The break between two successive meals should be for at least 6 h.
The optimization of buoyancy behavior should be done by either controlling water penetration inside the formulation or by improving the swelling properties of the dosage form.
The size, shape and surface of dosage form should be controlled. The control should not break into parts during the testing period and should release the drug at the same rate as the test to rule out any motility effects of the drug.
Conclusion
Gastroretentive drug delivery is not a brand new concept. Along with the years, various drugs have been investigated to modify their properties more surely for better absorption. In addition, new technologies such as intragastric floating systems, inflatable devices, intragastric osmotically controlled floating devices, have been setting standards for years, but they could not hide their own technological and practical limitations. Development of dosage forms based on gastroretention technology for prolonged and controlled drug release needs to conceptualization to proof of concept, technology transfer for global regulatory filings and commercialization of the products. Developing a gastroretentive-controlled release drug delivery formulation is very challenging, as continuous entry of the medium in the dosage form leads to alter the density of the system. A dual working buoyant and mucoadhesive system might be a promising formulation. Floating devices administered in a single unit form such as HBS are unreliable in prolonging the gastric retention time owing their “all or none” emptying process. Thus, they may cause high variability in bioavailability and local irritation due to large amount of drug delivered at particular sites of the GIT. In contrast, multiple unit particulate doses form such as microspheres and beads have the advantages that they pass uniformly through the GIT to avoid the vagaries of gastric emptying and provide an adjustable release, thereby, reducing the inter-subject variability in absorption and risk of local irritation. The special consideration has been given to the microparticulate systems such as microspheres or beads. At present, floating microparticulate systems are considered as one of the most promising buoyant systems as they combine the advantages of multiple systems with good floating properties with high drug loading and controlled release profile.
Declaration of interest
The authors report no conflicts of interest.
References
- Adebisi A, Conway BR. (2011). Gastroretentive microparticles for drug delivery applications. J Microencapsul 28:689–708
- Akala EO. (2010). Oral controlled release solid dosage forms. In: Jasti BR, Ghosh TK, eds. Theory and practice of contemporary pharmaceutics. 2nd ed. Florida: CRC Press, 333–66
- Akiyama Y, Nagahara N. (1999). Novel formulation approaches to oral mucoadhesive drug delivery systems. In: Mathiowitz E, Chickering DE III, Lehr CM, eds. Bioadhesive drug delivery systems – fundamentals, novel approaches and development. New York: Dekker, 477–505
- Ali J, Arora S, Ahuja A, et al. (2007). Formulation and development of hydrodynamically balanced system for metformin: in vitro and in vivo evaluation. Eur J Pharm Biopharm 67:196–201
- Arora S, Ali J, Ahuja A, et al. (2005). Floating drug delivery system: a review. AAPS PharmSciTech 6:E372–E399. Article-47
- Atyabi F, Sharma HL, Mohammad AH, Fell JT. (1996). In vivo evaluation of a novel gastric retentive formulation based on ion exchange resins. J Control Release 42:105–13
- Awasthi R, Kumar P, Pawar VK. (2010). Chronotherapy: science and technology of drug scheduling on the basis of biological rhythm. J Chronother Drug Deliv 1:1–8
- Awasthi R, Kulkarni GT. (2012). Development of novel gastroretentive floating particulate drug delivery system of gliclazide. Curr Drug Deliv 9:437–51
- Awasthi R, Kulkarni GT. (2014). Development of novel gastroretentive drug delivery system of gliclazide: hollow beads. Drug Dev Ind Pharm 40:398–408
- Awasthi R, Kulkarni GT, Pawar VK, Garg G. (2012). Optimization studies on gastroretentive floating system using response surface methodology. AAPS PharmSciTech 13:85–93
- Awasthi R, Kulkarni GT. (2013). Development and characterization of amoxicillin loaded floating microballoons for the treatment of Helicobacter pylori induced gastric ulcer. Asian J Pharm Sci 8:174–80
- Awasthi R, Pawar V, Kulkarni GT. (2010). Floating microparticulate systems: an approach to increase gastric retention. Indian J Pharm 1:17–26
- Babu VBM, Khar RK. (1990). In vitro and in vivo studies of sustained-release floating dosage forms containing salbutamol sulfate. Pharmazie 45:268–70
- Badve SS, Sher P, Korde A, Pawar AP. (2007). Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur J Pharm Biopharm 65:85–93
- Bardonnet PL, Faivre V, Pugh WJ, et al. (2006). Gastroretentive dosage forms: overview and special case of Helicibacter pylori. J Control Release 111:1–18
- Barmpalexis P, Kachrimanis K, Georgarakis E. (2011). Solid ispersions in the development of a nimodipine floating tablet formulation and optimization by artificial neural networks and genetic programming. Eur J Pharm Biopharm 77:122–31
- Baumgartner S, Krist J, Vreer F, et al. (2000). Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int J Pharm 195:125–35
- Bechgaard H, Ladefoged K. (1978). Distribution of pellets in the gastrointestinal tract: the influence on transit time exerted by density or diameter of pellets. J Pharm Pharmacol 30:690–2
- Bera R, Mandal B, Bhowmik M, et al. (2009). Formulation and in vitro evaluation of sunflower oil entrapped within buoyant beads of furosemide. Sci Pharm 77:669–78
- Caldwell LJ, Gardner CR, Cargill RC. (1988a). Drug delivery device which can be retained in the stomach for a controlled period of time. US Patent US 4,735,804
- Caldwell LJ, Gardner CR, Cargill RC, Higuchi T. (1988b). Drug delivery device which can be retained in the stomach for a controlled period of time. US Patent US 4,758,436
- Caldwell LJ, Gardner CR, Cargill RC. (1988c). Drug delivery device which can be retained in the stomach for a controlled period of time. US Patent US 4,767,627
- Cargill R, Caldwell LJ, Engle K, et al. (1988). Controlled gastric emptying. 1. Effects of physical properties on gastric residence times of nondisintegrating geometric shapes in beagle dogs. Pharm Res 5:533–6
- Castellanos NRJ, Zia H, Rhodes CT. (1993). Mucoadhesive drug delivery systems. Drug Dev Ind Pharm 19:143–94
- Ceballos-Baumann AO, Kummer R, Eckert W, Weicker H. (1990). Controlled-release levodopa/benserazide (Madopar HBS): clinical observations and levodopa and dopamine plasma concentrations in fluctuating Parkinsonian patients. J Neurol 237:24–8
- Chauhan B, Shimpi S, Mahadik KR, Paradkar A. (2004). Preparation and evaluation of floating risedronate sodium Gelucire® 39/01 matrices. Acta Pharm 54:205–14
- Chavanpatil MD, Jain P, Chaudhari S, et al. (2005). Development of sustained release gastroretentive drug delivery system for ofloxain: in vitro and in vivo evaluation. Int J Pharm 304:178–84
- Chavanpatil MD, Jain P, Chaudhari S, et al. (2006). Novel sustained release swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm 316:86–92
- Chawla G, Gupta P, Koradia V, Bansal AK. (2003). Gastroretention a means to address regional variability in intestinal drug absorption. Pharm Technol 6:50–68
- Chen J, Blevins WE, Park H, Park K. (2000). Gastric retention properties of superporous hydrogel composites. J Control Release 64:39–51
- Chickering DE, Jacob JS, Mathowitz E. (1995). Bioadhesive microspheres II: characterisation and evaluation of bioadhesion involving hard, bioerodible polymers and soft tissue. React Polym 25:189–206
- Chien YW. (2009). Novel drug delivery systems. USA: Informa, Healthcare Inc
- Choudhury PK, Kar M, Chauhan CS. (2008). Cellulose acetate microspheres as floating depot system to increase gastric retention of antidiabetic drug: formulation, characteristics and in vitro-in vivo evaluation. Drug Dev Ind Pharm 34:349–54
- Chouza C, Romero S, de Medina O, et al. (1987). Substitution of standard madopar by madopar HBS in parkinsonians with fluctuations. Eur Neurol 27:59–67
- Chun MK, Sah H, Choi HK. (2005). Preparation of mucoadhesive microspheres containing antimicrobial agents for eradication of H. pylori. Int J Pharm 297:172–9
- Clarke GM, Newton JM, Short MD. (1993). Gastrointestinal transit of pellets of differing size and density. Int J Pharm 100:81–92
- Clarke GM, Newton JM, Short MB. (1995). Comparative gastrointestinal transit of pellet systems of varying density. Int J Pharm 114:1–11
- Coupe AJ, Davis SS, Evans DF, Wilding IR. (1991). Correlation of the gastric emptying of nondisintegrating tablets with gastrointestinal motility. Pharm Res 8:1281–5
- Coupe AJ, Davis SS, Evans DF, Wilding IR. (1993). Do pellet formulations empty from the stomach with food? Int J Pharm 92:167–75
- Daniels IR, Allum WH. (2005). The anatomy and physiology of the stomach. In: Fielding JWL, Hallissey MT, eds. Upper gastrointestinal surgery. The Netherlands: Springer, 17–37
- Dave BS, Amin AF, Patel MM. (2004). Gastroretentive drug delivery system of ranitidine hydrochloride: formulation and in vitro evaluation. AAPS PharmSciTech 5:77–82. Article 34
- Davis SS. (2005). Formulation strategies for absorption windows. Drug Discov Today 10:249–57
- Davis SS, Stockwell AF, Taylor MJ. (1986). The effect of density on the gastric emptying of single and multiple unit dosage forms. Pharm Res 3:208–13
- Degtiareva II, Bogdanov A, Khatib Z, et al. (1994). The use of 3rd-generation antacid preparations for the treatment of patients with nonulcerous dyspepsia and peptic ulcer complicated by reflux esophagitis. Lik Sprava 5:119–22
- Deshpande AA, Rhodes CT, Shah NH, Malick AW. (1996). Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm 22:531–9
- Dorozynski P, Kulinowski P, Jachowicz R, Jasinski A. (2007). Development of a system for simultaneous dissolution studies and magnetic resonance imaging of water transport in hydrodynamically balanced systems: a technical note. AAPS PharmSciTech 8:E1–4
- Dressman JB. (1986). Comparison of canine and human gastrointestinal physiology. Pharm Res 3:123–31
- Durig T, Fassihi R. (2000). Evaluation of floating and sticking extended release delivery systems: an unconventional dissolution test. J Control Release 67:37–44
- Eberle VA, Schoelkopf J, Gane PAC, et al. (2014). Floating gastroretentive drug delivery systems: comparison of experimental and simulated dissolution profiles and floatation behavior. Eur J Pharm Sci 58:34–43
- El-Gibaly I. (2002). Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non8floating chitosan microspheres. Int J Pharm 249:7–21
- Erni W, Held K. (1987). The hydrodynamically balanced system: a novel principle of controlled drug release. Eur Neurol 27:21–7
- Fabregas JL, Claramunt J, Cucala J, et al. (1994). In-vitro testing of an antacid formulation with prolonged gastric residence time (Almagate Flot-Coat). Drug Dev Ind Pharm 20:1199–212
- Fell JT. (1996). Targeting of drugs and delivery systems to specific sites in the gastrointestinal tract. J Anat 189:517–9
- Fix JA, Cargill R, Engle K. (1993). Controlled gastric emptying. III. Gastric residence time of a nondisintegrating geometric shape in human volunteers. Pharm Res 10:1087–9
- Fujimori J, Machida Y, Nagai T. (1994). Preparation of a magnetically-responsive tablet and configuration of its gastric residence in beagle dogs. STP Pharma Sci 4:425–30
- Gangadharappa HV, Kumar P, Kumar S. (2007). Gastric floating drug delivery systems: a review. Ind J Pharm Educ Res 41:295–305
- Garg S, Gupta GD. (2008). Progress in controlled gastroretentive delivery systems. Trop J Pharm Res 7:1055–66
- Goole J, Gansbeke BV, Pilcer G, et al. (2008). Pharmacoscintigraphic and pharmacokinetic evaluation on healthy human volunteers of sustained-release floating minitablets containing levodopa and carbidopa. Int J Pharm 364:54–63
- Goole J, Vanderbist F, Aruighi K. (2007). Development and evaluation of new multiple-unit levodopa sustained-release floating dosage forms. Int J Pharm 334:35–41
- Gohel MC, Sarvaiya KG. (2007). A novel solid dosage form of rifampicin and isoniazid with improved functionality. AAPS PharmSciTech 8:E133–E139. Article 68
- Groning R, Berntgen M. (1996). Estimation of the gastric residence time of magnetic dosage forms using the heidelberg capsule. Pharmazie 51:328–31
- Groning R, Berntgen M, Georgarakis M. (1998). Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur J Pharm Biopharm 46:285–91
- Gupta PK, Robinson JR. (1995). Effect of volume and viscosity of coadministered fluid on gastrointestinal distribution of small particles. Int J Pharm 125:185–93
- Gusler G, Berner B. (2000). Metformin (GRTM) gastric retentive tablets: GI transit and pharmacokinetics in healthy volunteers. Millenial World Congress of Pharm Sci.: San Francisco, 2–5019
- Gusler G, Gorsline J, Levy G, et al. (2001). Pharmacokinetics of metformin gastric-retentive tablets in healthy volunteers. J Clin Pharmacol 41:655–61
- Harding SE, Davis SS, Deacon MP, Fiebring I. (1999). Biopolymer mucoadhesives. Biotechnol Genet Eng Rev 16:41–86
- Harrigan RM. (1977). Drug delivery device for preventing contact of undissolved drug with the stomach lining. US Patent US 4,055,178
- Hascicek C, Tilkan GY, Turkmen B, Ozdemir N. (2011). Effect of formulation parameters on the drug release and floating properties of gastric floating two-layer tablets with acetylsalicylic acid. Acta Pharm 61:303–12
- Hasler LH. (1995). Motility of the small intestine. In: Yamada T, ed. Textbook of gastroenterology II. Vol. 1. Philadelphia: J.B. Lippincott, 181–225
- He P, Davis SS, Illum L. (1999). Chitosan microspheres prepared by spay drying. Int J Pharm 187:53–65
- Hejazi R, Amiji M. (2002). Stomach-specific anti H. pylori therapy I: preparation and characterization of tetracycline of a floating multiple-unit, capsule, a high-density loaded chitosan microcapsules. Int J Pharm 235:87–94
- Hendee WR. (1994). Fundamentals of diagnostic imaging: characteristics of the radio-graphic image. In: Putman CE, Ravin CE, eds. Textbook of diagnostic imaging, Vol. 1. Philadelphia: WB Saunders Co., 9–10
- Hilton AK, Deasy PB. (1992). In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxicillin trihydrate. Int J Pharm 86:79–88
- Hocking MP, Brunson ME, Vogel SB. (1988). Effect of various prokinetic agents on post Roux-en-Y gastric emptying. Digest Dis Sci 33:1282–7
- Hoffmann AF, Pressman JH, Code CF, et al. (1983). Controlled entry of orally administered drugs: physiological considerations. Drug Dev Ind Pharm 9:1077–109
- Hou SY, Cowles VE, Berner B. (2003). Gastric retentive dosage forms: a review. Crit Rev Ther Drug Carrier Syst 20:459–97
- Hu L, Li L, Yang X, et al. (2011). Floating matrix dosage form for dextromethorphan hydrobromide based on gas forming technique: in vitro and in vivo evaluation in healthy volunteers. Eur J Pharm Sci 42:99–105
- Huang Y, Leobandung W, Foss A, Peppas NA. (2000). Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J Control Release 65:63–71
- Hwang SJ, Park H, Park K. (1998). Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst 15:243–84
- Iannuccelli V, Coppi G, Leo E, et al. (2000). PVP solid dispersion for the controlled release of furosemide from a floating multiple unit system. Drug Dev Ind Pharm 26:595–603
- Iannuccelli V, Coppi G, Sansone R, Ferolla G. (1998). Air compartment multiple-unit for prolonged gastric residence part II. In vivo evaluation. Int J Pharm 174:55–62
- Ingani HM, Timmermans J, Moes A. (1987). Conception and in vivo investigation of peroral sustained release floating dosage forms with enhanced gastrointestinal transit. Int J Pharm 35:157–64
- Ishak RAH, Awad GAS, Mortada ND, Nour SAK. (2007). Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J Control Release 119:207–14
- Jackson SJ, Bush D, Perkins AC. (2001). Comparative scintigraphic assessment of the intragastric distribution and residence of cholestyramine, Carbopol 934P and sucralfate. Int J Pharm 212:55–62
- Jain SK, Agrawal GP, Jain NK. (2006a). A novel calcium silicate based microspheres of repaglinide: in vivo investigations. J Control Release 113:111–6
- Jain SK, Agrawal GP, Jain NK. (2006b). Evaluation of porous carrier-based floating orlistat microspheres for gastric delivery. AAPS PharmSciTech 7:E54–E62. Article 90
- Jain SK, Awasthi AM, Jain NK, Agrawal GP. (2005). Calcium silicate based microspheres of repaglinide for gastroreteneive floating drug delivery: preparation and in-vitro characterization. J Control Release 107:300–9
- Jain SK, Gupta A. (2009). Development of gelucire 43/01 beads of metformin hydrochloride for floating delivery. AAPS PharmSciTech 10:1128–36
- Joseph NJ, Lakshmi S, Jayakrishnan A. (2002). A floating type oral dosage form for piroxicam based on hollow polycarbonate microspheres: in-vitro and in-vivo evaluation in rabbits. J Control Release 79:71–9
- Kale RD, Tyade PT. (2007). A multiple unit floating drug delivery system of piroxicam using eudragit polymer. Ind J Pharm Sci 1:120–4
- Kamath K, Park K. (1993). Biodegradable hydrogels in drug delivery. Adv Drug Deliv Rev 11:59–84
- Kaus LC, Fell JT, Sharma H, Taylor DC. (1984). Gastric emptying and intestinal transit of non-disintegrating capsules the influence of metoclopramide. Int J Pharm 22:99–103
- Kedzierewicz F, Thouvenot P, Lemut J, et al. (1999). Evaluation of peroral silicone dosage forms in humans by gamma-scintigraphy. J Control Release 58:195–205
- Khosla R, Davis SS. (1987). The effect of polycarbophil on the gastric emptying of pellets. J Pharm Phamacol 39:47–9
- Klausner EA, Lavy E, Friedman M, Hoffman A. (2003a). Expandable gastroretentive dosage forms. J Control Release 90:143–62
- Klausner EA, Lavy E, Barta M, et al. (2003b). Novel gastroretentive dosage forms: evaluation of gastroretentivity and its effect on levodopa absorption in humans. Pharm Res 20:1466–73
- Laby RH. (1974). Device for administration to ruminants. US Patent US 3,844,285
- Lee JW, Park JH, Robinson JR. (2000). Bioadhesive-based dosage forms: the next generation. J Pharm Sci 89:850–66
- Lee VHL, Mukherjee SK. (2006). Drug delivery: oral colon8specific. In: Swarbrick J, ed. Encyclopedia of pharmaceutical technology. London: Informa Healthcare, 1228–41
- Lee JH, Park TG, Lee YB, et al. (2001). Effect of adding non-volatile oil as a core material for the floating microspheres prepared by emulsion solvent diffusion method. J Microencapsul 18:65–75
- Lehr CM. (1994). Bioadhesion technologies for the delivery of peptide and protein drugs to the gastrointestinal tract. Crit Rev Ther Drug Carrier Syst 11:119–60
- Lippold BC, Gunther J. (1991). In-vivo Prufung einer multipartikularen Retard-Schwimmarzneiform. Eur J Pharm Biopharm 37:254–61
- Longer MA, Ching HS, Robinson JR. (1985). Bioadhesive polymers as platform for oral control drug delivery III-oral delivery of chlorthiazide using bioadhesive polymer. J Pharm Sci 74:406–10
- Marieb EN. (2007). Essentials of human anatomy and physiology. 1st ed: Delhi: Pearsons Education
- Martinez IJ, Ramirez AMD, Robles LV. (2010). Effect of antioxidants on captopril floating matrices. Pharm Dev Technol 15:230–40
- Mayur C, Senthilkumaran K, Hemant G. (2013). Super porous hydrogels: a recent advancement in gastroretentive drug delivery system. Indonesian J Pharm 24:1–13
- Mazer N, Abisch E, Gfeller JC, et al. (1988). Intragastric behavior and absorption kinetics of a normal and “floating” modified-release capsule of isradipine under fasted and fed conditions. J Pharm Sci 77:647–57
- Mendyk A, Jachowicz R, Dorozynski P. (2006). Artificial neural networks in the modeling of drugs release profiles from hydrodynamically balanced systems. Acta Poloniae Pharm Drug Res 63:75–80
- Michaels AS. (1974). Drug delivery device with self-actuated mechanism for retaining device in selected area. US Patent US 3,786,813
- Michaels AS, Bashwa JD, Zaffaroni A. (1975). Integrated device for administering beneficial drug at programmed rate. US Patent US 3,901,232
- Mojaverian P, Vlasses PH, Kellner PE, Rocci ML. (1988). Effects of gender, posture, and age on gastric residence time of an indigestible solid: pharmaceutical considerations. Pharm Res 10:639–44
- Mostafavi A, Emami J, Varshosaz J, et al. (2011). Development of a prolonged-release gastroretentive tablet formulation of ciprofloxacin hydrochloride: pharmacokinetic characterization in healthy human volunteers. Int J Pharm 409:128–36
- Mouzam MI, Dehghan MHG, Asif S, et al. (2011). Preparation of a novel floating ring capsule-type dosage form for stomach specific delivery. Saudi Pharm J 19:85–93
- Muthusamy K, Govindarazan G, Ravi TK. (2005). Preparation and evaluation of Lansoprazole floating micropellets. Indian J Pharm Sci 67:75–9
- Nakagawa T, Kondo SI, Sasai Y, Kuzuya M. (2006). Preparation of floating drug delivery system by plasma technique. Chem Pharm Bull 54:514–8
- Nakamichi K, Yasuura H, Fukui H, et al. (2001). Evaluation of a floating dosage form of nicardipine hydrochloride and hydroxypropylmethylcellulose acetate succinate prepared using a twin-screw extruder. Int J Pharm 218:103–12
- Nama M, Gonugunta CSR, Veerareddy PR. (2008). Formulation and evaluation of gastroretentive dosage forms of clarithromycin. AAPS PharmSciTech 9:231–7
- Narayana CR, Basavaraj BV, Madhavan V. (2010). Microballoons of famotidine: a non-effervescent gastroretentive controlled drug delivery system using Eudragit L-100. Der Pharm Lett 2:176–89
- Nepal PR, Chun MK, Choi HK. (2007). Preparation of floating microspheres for fish farming. Int J Pharm 341:85–90
- Oh TO, Kim JY, Ha JM, et al. (2013). Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur J Pharm Biopharm 83:460–7
- Omidian H, Rocca JG, Park K. (2005). Advances in superporous hydrogels. J Control Release 102:3–1012
- O'Reilly S, Wilson CG, Hardy JG. (1987). The influence of food on the gastric emptying of multiparticulate dosage forms. Int J Pharm 34:213–6
- Park H, Park K, Shalaby WSW. (1993). Biodegradable hydrogels for drug delivery. PA, USA: Technomic Publishing Co., Inc
- Patel DM, Patel NM, Pandya NN, Jogani PD. (2007a). Gastroretentive drug delivery system of carbamazepine: formulation optimization using simplex lattice design: a technical note. AAPS Pharma Sci Tech 8:E82–E86. Article 11
- Patel DM, Patel NM, Patel VF, Bhatt DA. (2007b). Floating granules of ranitidine hydrochloride-gelucire 43/01: formulation optimization using factorial design. AAPS PharmSciTech 8:E25–E31. Article 30
- Pawar VK, Kansal S, Garg G, et al. (2011). Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug Deliv 18:97–110
- Phuapradit W, Bolton S. (1991). The influence of tablet density on the human oral absorption of sustained release acetaminophen tablets. Drug Dev Ind Pharm 17:1097–107
- Pies R. (1982). Value of once daily dosing of diazepam questioned-and defended. J Clin Psychopharmacol 2:355–6
- Ponchel G, Irache JM. (1998). Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev 34:191–219
- Qiu Y, Park K. (2003). Superporous IPN hydrogels having enhanced mechanical properties. AAPS PharmSciTech 4:406–412. Article 51
- Rahman Z, Ali M, Khar RK. (2006). Design and evaluation of bilayer floating tablets of captopril. Acta Pharm 56:49–57
- Rajinikanth PS, Balasubramanium J, Mishra B. (2007). Development and evaluation a novel floating in situ gelling system of amoxicillin for eradicating of Helicobacter pylori. Int J Pharm 335:114–22
- Rajinikanth PS, Mishra B. (2008). Floating in situ gelling system for stomach site-specific delivery of clarithromycin to eradicate H. pylori. J Control Release 125:33–41
- Ravala JA, Patel JK, Li N, et al. (2007). Ranitidin hydrochloride floating matrix tablets based on low density powder: effects of formulation and prossing parameters on drug release. Asian J Pharm Sci 2:130–42
- Reddy LH, Murthy RS. (2002). Floating dosage forms in drug delivery. Crit Rev Ther Drug Carrier Syst 19:553–85
- Romanski KW. (2009). Migrating motor complex in biological sciences: characterization, animal models and disturbances. Indian J Exp Biol 47:229–44
- Rouge N, Leroux JC, Cole ET, et al. (1997). Prevention of sticking tendency of floating mini tablets filled into hard gelatin capsules. Eur J Pharm Biopharm 43:165–71
- Rouge N, Allemann E, Gex-Fabry M, et al. (1998). Comparative pharmacokinetic study of a floating multiple-unit capsule, a high density multiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helbetiae 73:81–7
- Rubinstein A, Friend DR. (1994). Specific delivery to the gastrointestinal tract. In: Domb AJ, ed. Polymeric site-specific pharmacotherapy. Chichester: Wiley, 282–3
- Sakkinen M, Tuononen T, Jurjenson H, et al. (2003). Evaluation of microcrystalline chitosans for gastro-retentive drug delivery. Eur J Pharm Sci 19:345–53
- Sakkinen M, Marvola J, Kanerva H, et al. (2004). Gamma scintigraphic evaluation of the fate of microcrystalline chitosan granules in human stomach. Eur J Pharm Biopharm 57:133–43
- Sangekar S, Vadino WA, Chaudry I, et al. (1987). Evaluation of the effect of food and specific gravity of tablets on gastric retention time. Int J Pharm 35:187–91
- Sarna SK. (1985). Cyclic motor activity; migrating motor complex. Gastroenterology. 89:894–913
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. (2004). In- vitro and in-vivo evaluation of riboflavin-containing microballoons for a controlled drug delivery system in healthy humans. Int J Pharm 275:97–107
- Sawicki W. (2002). Pharmacokinetics of verapamil and norverapamil from controlled release floating pellets in humans. Eur J Pharm Biopharm 53:29–35
- Schemann M, Ehrlein HJ. (1986). Mechanical characteristics of phase II and phase III of the interdigestive migrating motor complex in dogs. Gastroenterology 91:117–23
- Seth PR, Tossounian J. (1984). The hydrodynamically balanced system, a novel drug delivery system for oral use. Drug Dev Ind Pharm 10:313–39
- Shaha SH, Patel JK, Pundarikakshu Dua K, Patel NV. (2009). An overview of a gastro-retentive floating drug delivery system. Asian J Pharm Sci 4:65–80
- Shahiwala A. (2011). Formulation approaches in enhancement of patient compliance to poral drug therapy. Expert Opin Drug Deliv 8:1521–9
- Sharma S, Pawar A. (2006). Low density multiparticulate system for pulsatile release of meloxicam. Int J Pharm 313:150–8
- Shen SI, Jasti BR, Li X. (2003). Design of controlled-release drug delivery systems. In: Myer K, ed. Standard handbook of biomedical engineering and design. New York: McGraw-Hill, 5.1–5.14
- Shimpi S, Chauhan B, Mahadik RK, Paradkar A. (2004). Preparation and evaluation of diltiazem hydrochloride-glucire 43/01 floating granules prepared by melt granulation. AAPS PharmSciTech 5:51–66. Article 43
- Shishu Gupta N, Aggarwal N. (2007a). Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS PharmSciTech 8:E143–E149. Article 48
- Shishu Gupta N, Aggarwal N. (2007b). A gastro-retentive floating delivery system for 5-fluorouracil. Asian J Pharm Sci 2:143–9
- Siegel RA, Rathbone MJ. (2012). Overview of controlled release mechanisms. In: Siepmann J, ed. Fundamentals and applications of controlled release drug delivery, advances in delivery science and technology. Controlled Release Society. Netherlands: Springer, 19–43
- Singh BN, Kim KH. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 63:235–59
- Sonara GS, Jaina DK, Moreb DM. (2007). Preparation and in vitro evaluation of bilayer and floating-bioadhesive tablets of rosiglitazone maleate. Asian J Pharm Sci 2:161–9
- Soppimath KS, Aminabhavi TM, Agnihotri SA, et al. (2006). Effect of coexipients on nifedipine hollow microspheres: a novel gastroretentive drug delivery system. J Appl Polym Sci 100:486–94
- Soppimath KS, Kulkarni AR, Aminabhavi TM. (2001). Development of hollow microspheres as floating controlled-release system for cardiovascular drugs: preparation and release characteristics. Drug Dev Ind Pharm 27:507–15
- Srivastava AK, Ridhurkar DN, Wadhwa S. (2005). Floating microsphere of cimetidine: formulation, characterization and in-vivo evaluation. Acta Pharm 55:277–85
- Sriamornsak P, Thirawong N, Puttipipatkhachorn S. (2004). Morphology and buoyancy of oil-entrapped calcium pectinate gel beads. AAPS J 6:65–71. Article 24
- Stithit S, Chen W, Price JC. (1998). Development and characterization of buoyant theophylline microspheres with near zero order release kinetics. J Microencapsul 15:725–37
- Stockwell AF, Davis SS, Walker SE. (1986). In vitro evaluation of alginate gel system as sustained release drug delivery system. J Control Release 3:167–75
- Streubel A, Siepmann J, Bodmeier R. (2006). Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol 6:501–8
- Streubel A, Siepmann J, Bodmeier R. (2002). Floating microparticles based on low density foam powder. Int J Pharm 241:279–92
- Streubel A, Siepmann J, Bodmeier R. (2003a). Floating matrix tablets based on low density foam powder: effects of formulation and processing parameters on drug release. Eur J Pharm Sci 18:37–45
- Streubel A, Siepmann J, Bodmeier R. (2003b). Multiple unit gastroretentive drug delivery systems: a new preparation method for low density microparticles. J Microencapsul 20:329–47
- Strubing S, Abboud T, Contri RV, et al. (2008b). New insights on poly(vinyl acetate)-based coated floating tablets: characterisation of hydration and CO2 generation by benchtop MRI and its relation to drug release and floating strength. Eur J Pharm Biopharm 69:708–717
- Strubing S, Metz H, Mader K. (2008a). Characterization of poly (vinyl acetate) based floating matrix tablets. J Control Release 126:149–55
- Sugihara H, Matsui Y, Takeuchi H, et al. (2014). Development of a gastric retentive system as a sustained-release formulation of pranlukast hydrate and its subsequent in vivo verification in human studies. Eur J Pharm Sci 53:62–8
- Sungthongjeen S, Sriamornsak P, Puttipipatkhachorn S. (2008). Design and evaluation of floating multi-layer coated tablets based on gas formation. Eur J Pharm Biopharm 69:255–63
- Takahashi T. (2012). Mechanism of interdigestive migrating motor complex. J Neurogastroenterol Motil 18:246–57
- Talukder R, Fassihi R. (2004). Gastroretentive delivery systems: hollow beads. Drug Dev Ind Pharm 30:405–12
- Tanwar YS, Naruka PS, Ojha GR. (2008). Development and evaluation of floating microspheres of verapamil hydrochloride. Brazil J Pharm Sci 43:1–6
- Timmermans J, Moes AJ. (1994). Factors controlling the buoyancy and gastric retention capabilities of floating matrix capsules: new data for reconsidering the controversy. J Pharm Sci 83:18–24
- Tortora GJ, Derrickson BH. (2011). Principles of anatomy and physiology, 11th ed. Hoboken, NJ: John Wiley and Sons
- Tuleu C, Andrieux C, Boy P, Chaumeil JC. (1999). Gastrointestinal transit of pellets in rats: effect of size and density. Int J Pharm 180:123–31
- Umamaheshwari RB, Jain S, Bhadra D, Jain NK. (2006). Floating microspheres bearing acetohydroxamic acid for the treatment of Helicobacter pylori. J Pharm Pharmacol 55:1607–13
- Umamaheshwari RB, Jain S, Tripathi PK, et al. (2002). Floating bioadhesive microspheres containing acetohydroxamic acid for clearance of Helicobacter pylori. Drug Deliv 9:223–31
- Umamaheshwari RB, Jain S, Jain NK. (2003). A new approach in gasreoretentive drug delivery system using cholestyramine. Drug Deliv 10:151–60
- Vantrappen GR, Peeters TL, Janssens J. (1979). The secretory component of interdigestive migratory motor complex in man. Scand J Gastroenterol 14:663–7
- Varshosaz J, Tavakoli N, Roozbahani F. (2006). Formulation and in vitro characterization of ciprofloxacin floating and bioadhesive extended release tablets. Drug Deliv 13:277–85
- Varshosaz J, Tabbakhian M, Zahrooni M. (2007). Development and characterization of floating microballoons for oral delivery of cinnarizine by a factorial design. J Microencapsul 24:253–62
- Wakode RR, Bajaj AN. (2008). Formulation and characterization of pramipexole loaded microspheres
- Washington N, Washington C, Wilson CG, Davis SS. (1986). What is “Liquid Gaviscon”? A comparison of four international formulations. Int J Pharm 34:105–9
- Washington N, Wilson CG, Greaves JL, Danneskiold-Samsoe P. (1988). An investigation into the floating behaviour of a pectin-containing anti-reflux formulation (FF5005) by means of gamma scintigraphy. Scand J Gastroenterol 23:920–4
- Waterman KC. (2007). A critical review of gastric retentive controlled drug delivery. Pharm Dev Technol 12:1–10
- Waugh A, Grant A. (2001). Anatomy and physiology in health and illness. 9th ed. Churchill Livingstone: Elsevier
- Whitehead L, Fell JT, Collett JH, et al. (1998). Floating dosage forms: an in-vivo study demonstrating prolonged gastric retention. J Control Release 55:3–12
- Wilding IR, Coupe AJ, Davis SS. (2001). The role of gamma-scintigraphy in oral drug delivery. Adv Drug Deliv Rev 46:103–24
- Wilson CG, Crowley PJ, eds. (2011). Controlled release in oral drug delivery series. Advances in delivery science and technology. Controlled Release Society. Netherlands: Springer
- Wilson CG, Washington N. (1989). The stomach: its role in oral drug delivery. In: Rubinstein MH, ed. Physiological pharmaceutical: biological barriers to drug absorption. UK: Ellis Horwood, 47–70
- Xiaoqiang X, Minjie S, Feng Z, Yiqiao H. (2006). Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: in vitro and in vivo evaluation in healthy volunteers. Int J Pharm 310:139–45
- Xu WL, Tu XD, Lu ZD. (1991). Development of gentamicin sulfate sustained-release tablet floating in stomach. Yaoxue Xuebao 26:541–5
- Yang L, Eshraghi J, Fassihi R. (1999). A new intragastric delivery system for the treatment of Helicobacter pylori associated gastric ulcer: In vitro evaluation. J Control Release 57:215–22
- Zheng J, Liu C, Bao D, et al. (2006). Preparation and evaluation of floating bioadhesive microparticles containing clarithromcin for the eradication of Helicobacter pylori. J Appl Polym Sci 102:2226–32