?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study was to prepare and characterize the complexes between curcumin (CU) phosphatidylcholine (PC) and hydrogenated soya phosphatidylcholine (HSPC) and to evaluate their anticancer activity. These CU–PC and CU–HSPC complexes (CU–PC-C and CU–HSPC-C) were evaluated for various physical parameters like Fourier transform infrared spectroscopy, melting point, solubility, scanning electron microscopy and the in vitro drug release study. These data confirmed the formation of phospholipids complexes. The in vitro hemolysis study showed that the complex was non-hemolytic. The anti-cancer potential of the complexes was demonstrated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay in MCF-7 cell line. This increase may be due to the amphiphilic nature of the complexes, which significantly enhances the water and lipid solubility of the CU. Unlike the free CU (which showed a total of only 90% drug release at the end of 8 h), complex showed around 40–60% release at the end of 8 h in dissolution studies. It showed that (when given in equimolar doses) complexes have significantly decreased the amount of CU available for absorption as compared with CU-free drug. Both CU-PC-C and CU-HSPC-C were found to be non-toxic at the dose equivalent to 2000 mg/kg of body weight of CU in the toxicity study. Acute and subacute toxicity studies confirmed the oral safety of the formulation. A series of genotoxicity studies was conducted, which revealed the non-genotoxicity potential of the developed complexes. Thus, it can be concluded that the phospholipid complexes of CU may be a promising candidate in cancer therapy.
Introduction
Curcumin (CU) [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], a yellow natural polyphenol extracted from turmeric (Curcuma longa), has been focused to a multitude of investigations over the past few decades. These research demonstrated various health benefits ranging from anti-inflammatory, antioxidant (Balogun et al., Citation2003; Aggarwal et al., Citation2007) and anticarcinogenic properties (Duvoix et al., Citation2005; Reuter et al., Citation2008) of CU. Furthermore, antidiabetic (Peeyush et al., Citation2009) and anti-HIV (Mazumder et al., Citation1995) activities has also been demonstrated. The clinical studies indicate that CU has potential therapeutic value against most chronic diseases including neoplastic, neurological, cardiovascular, pulmonary, metabolic and psychological diseases (Aggarwal et al., Citation2003; Aggarwal & Sung, Citation2009; Fryer et al., Citation2009). Above all these clinical activities, the CU has been certified as a safe active compound by preclinical and clinical studies even at a very high dose (Anitha et al., Citation2011). Still after possessing number of activities and high safety profile, CU suffered with the poor aqueous solubility and low systemic bioavailability, which interns limit its therapeutic efficacy (Dhule et al., Citation2012).
Furthermore, for anticancer treatment, the drug molecule should present at the site of tumor for longer period of time to exert its therapeutic action (Byrne et al., Citation2008). Globally, many research groups are putting efforts in increasing the solubility of CU and also to improve the release/bioavailability. One possible way to increase its therapeutic utility is to develop a CU complex with phospholipids, which improves the solubility as well as controls the rate of drug release (Yadav et al., Citation2009).
Phospholipids play a major role in drug delivery technology. It is an important carrier for those drug molecules, which require controlled and sustained release in vivo due to faster elimination from the body. Soya lecithin/phosphatidylcholine (PC) and hydrogenated soya phosphatidylcholine (HSPC) are major constituents of cell membranes phospholipids. Soya lecithin and hydrogenated soya are freely attuned with other nutrients and when co-administered may enhance their absorption. Several studies have demonstrated that complexation of phospholipids with phyto-constituents increases their bioavailability (Conti et al., Citation1992; Morazzoni et al., Citation1993) and as a result, there is a raise in therapeutic effect also. Recent studies with quercetin and naringenin (rapid elimination) confirmed that complexation with phospholipids enhances the bioactivity of these phyto-molecules (Maiti et al., Citation2006, Citation2007).
In this investigation, CU-PC-C and CU-HSPC-C were prepared and characterized by melting point, solubility, drug content, Fourier transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). In vitro release of CU from the complexes and cytotoxicity studies against cancer cells were evaluated and compared with pure CU (Maiti et al., Citation2007).
Materials and methods
Materials
CU, PC, HSPC, sodium azide-flourescin isothiocynate, fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma Aldrich (St. Louis, MO). Dialysis bag (cut off mol. wt. 12 000 Dalton) were purchased from Himedia (Mumbai, India). HPLC grade acetonitrile and methanol were purchased from Spectrochem (Mumbai, India). All other chemicals used were of analytical grade. Milli Q water was used throughout the study was prepared by Milli-Q plus 185 purification system (Bedford, MA).
Preparation of CU–PC-C Complex
CU and PC in molar ratio of 1:1 were taken in a round bottom flask and dissolved in 20 mL of dichloromethane. This mixture was stirred for 2 h at room temperature on a magnetic stirrer. The solvent was evaporated under vacuum utilizing rotary evaporator (Buchi, Flawil, Switzerland) at 30 °C. The resultant CU–PC-C was washed with n-hexane, dried under vacuum and stored at room temperature (Gupta & Dixit, Citation2011).
Preparation of CU–HSPC-C complex
CU and HSPC in molar ratio of 1:1 were taken in a round bottom flask and dissolved in 20 mL of dichloromethane. This mixture was stirred for 2 h at room temperature on a magnetic stirrer. The solvent was evaporated under vacuum utilizing rotary evaporator (Buchi, Switzerland) at 30 °C. The resultant CU–HSPC-C was washed with n-hexane, dried under vacuum and stored at room temperature (Maiti et al., Citation2009).
Characterization
Solubility study
To determine the change in solubility due to complexation, solubility studies were performed by adding about 50 mg of the CU, CU–PC-C, CU–HSPC-C and physical mixture of CU and phospholipids in 10 mL of water or n-octanol in sealed glass container at room temperature (Maiti et al., Citation2007). The solutions were vortexed for 15 min, filtered and 1 mL of filtrate was mixed with 9 mL of methanol. Aliquot (20 µL) in triplicate of the resulting solution (after proper dilution) was subjected to RP-HPLC for CU content in complexes.
Melting point
Melting point of CU, PC, CU-PC-C and CU-HSPC-C complexes were determined by digital melting point apparatus.
Drug content
Drug content was determined and estimated by using the HPLC method, which was developed.
Quantitative estimation of drug with HPLC and validation
Equipment
The HPLC system was equipped with LC 10 ATVP binary isocratic pumps (Shimadzu), A Rheodyne (Cotati, CA) model 7125 injector with a 20 µl loop and SPD 10 AVP UV-VIS detector (Shimadzu, Japan). HPLC separation was achieved on a LiChrospher LichroCART RP–C18 (5 µm) column. Column effluent was monitored at 251 and 425 nm. Data were acquired and processed using Shimadzu LC Solution Software.
Preparation of stock and working standard solution
Stock solution of CU was prepared by dissolving 10 mg of drug in a 10 mL of volumetric flask. Volume was made up to 10 mL with methanol. Working solutions were prepared from stock solution in the range of 200 ng/mL to 4 µg/mL by serial dilution method. Sample (20 µl) was injected into the column. Chromatographic conditions used in the analysis are depicted below.
Preparation of mobile phase
The mobile phase was a mixture of acetonitrile:triple distilled water:glacial acetic acid (650:340:10) v/v. The solution were filtered and degassed before use via Bath Sonicator. Chromatography was performed at 30 °C at a flow rate of 1.00 mL/min.
Calibration curve of CU
The HPLC of the CU sample showed retention time of 3.97 min. Accuracy was tested by percentage DFA of mean of three determinations of CU at different concentrations. The precision was accessed by testing the repeatability of three different standard solutions three times in same day (intra-day) and by the intermediate precision analyzing the same three standard solutions three times on different days (inter-day), and results are listed in . The HPLC of the drug sample showed retention time of 3.97 min. The correlation coefficients were found to be 0.9994. The slope and intercept on Y-axis were 174.54 and 1780.4, respectively.
Table 1. Inter and intra assay variations of CU.
Solubility study
To determine the CU content in the complexes, complexes equivalent to about 10 mg of CU were weighed and dissolved in 100 mL of methanol individually. The volumetric flasks were stirred continuously for 24 h on a magnetic stirrer. Dilutions were made suitably and drug content was measured by HPLC method described as above. The experiments were carried out in triplicate.
Fourier transforms infrared spectroscopy
The prepared CU complexes were confirmed by FT-IR spectrum, which has been recorded on FT-IR multiscope spectrophotometer (Perkin-Elmer, Seer Green, Beaconsfield, and Buckinghamshire, UK) equipped with spectrum v3.02 software. The complexes were grinded with purified potassium bromide and then pressed with mechanical press to form a translucent pellet. This pellet was used for spectrophotometer.
Scanning electron microscope
For morphological characterization of prepared complexes, SEM (Leo Electron Microscope Ltd., Cambridge, UK) studies were performed. For the SEM analysis, the samples were coated with gold and palladium. These coated samples were placed over a copper grid and were subjected to SEM analysis.
In vitro dissolution study
In vitro dissolution studies for plain CU, CU-PC-C and CU-HSPC-C were carried out in triplicate in a USP dissolution test apparatus (Apparatus II, 100 rpm, 37 ± 0.5 °C) with slight modification (Li et al., Citation2008; Mohanty & Sahoo, Citation2010). Tween 80 (1%, v/v) was added to the dissolution medium to maintain sink conditions. The treated dialysis bag containing accurately weighed amount of the complexes were first immersed in the dissolution medium. Samples (3 mL each) of dissolution fluid were withdrawn at different intervals and replaced with the dissolution medium. Withdrawn samples were filtered (through a 0.4-µm membrane filter), and the filtrates were analyzed by HPLC.
Hemolytic toxicity study
Hemolytic toxicity study was performed by the reported method (Torne et al., Citation2012). Whole human blood was isolated from heparinized vials. The RBCs were separated by centrifugation (2800 rpm for 5 min) and re-suspended in normal saline solution (pH 7.4). After centrifugation and discarding of the supernatant, the washing steps were repeated three times and finally re-suspended in phosphate-buffered saline, and volume was made up to 10 mL. Solutions of different concentrations (50 µL) of CU, CU-PC-C and CU-HSPC-C were added in 1 mL of RBC suspension and incubated at 37 °C for 90 min. Samples were then centrifuged for 5 min at 3000 rpm, and absorbance of the supernatant was taken in ELISA plate reader at 545 nm. Phosphate buffer saline (pH 7.4) was used as the negative control with 0% hemolysis, the positive control comprised of 50 µL Triton-X-100 (4%) with 100% hemolysis. Each concentration was evaluated in triplicate. The percent hemolysis was calculated using following equation:
where, ABS100 and ABS0 are the absorbances of the solution at 100 and 0% hemolysis, respectively.
Cytotoxicity studies by MTT assay
The cytotoxicity of CU-PC-C and CU-HSPC-C against MCF-7 cancer cells were measured by MTT assay. MTT is a colorimetric assay that measures the reduction of yellow colored 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) into an insoluble dark purple colored formazan product by mitochondrial succinic dehydrogenase. MCF-7 cells were normally maintained in Dulbecco’s Modified Eagle Medium (Sigma Aldrich) supplemented with 10% FBS (Merck) and 1% antibiotic antimycotic solution (Merck and Co. Inc., NJ, USA) at 37 °C in a humidified incubator with 5% CO2. All stock solution of complexes were prepared in cell culture grade dimethyl sulfoxide (DMSO) and stored in −20 °C. Complexes were diluted in culture media prior to use in experiments. MCF-7 cancer cells were seeded onto 96-well plates at a density of 1 × 104 cells/well for 24 h were subjected to CU-PC-C and CU-HSPC-C at a concentration 1.35 µM, 2.72 µM, 13.5 µM and 27.1 µM equivalent of CU with plain CU and control group. These were incubated for 24 h under humidified atmosphere of 5% CO2 at 37 °C. Cytotoxicity was estimated by staining live cell by 0.5 mg/mL MTT (formazan crystal formation), after incubation for 4 h at 37 °C. DMSO (150 μl) was added to each well with gentle shaking, the optical density values were determined at 540 nm using ELISA plate reader. Triplicates of each sample were analyzed. The data obtained were mean values from three different experiments.
In vivo studies
The animal experiments have been carried out by following experimental protocol guidelines of Council for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India. Experiments were conducted after ethical clearance by the Institutional Animal Ethics Committee of the institute (IAEC approval no. 2012/41/Pharmaceutics/IAEC). Healthy adult Wistar rats (2–3 weeks old) with the average body weight (BW) of 150 ± 25 g were employed for acute and sub-acute toxicity studies, whereas 6–8 week old, healthy Balb/c mice with the average BW of 25 ± 5 g were employed for the micronucleus (MN) assay as well as chromosome aberration (CA) assay. The animals were maintained at the controlled temperature of 23 ± 1 °C, humidity of 55 ± 5% in a 14 h light/10 h dark cycle. Throughout the study, the animals were provided with soy-free and filtered drinking water. For the acute and sub-acute-toxicity study, the CU complexes equivalent to 2000 mg/kg BW of CU and 100 mg/kg BW of CU, respectively, were administered (Reddy et al., Citation2005; Camacho-Barquero et al., Citation2007).
Acute-toxicity study of complexes
The animals, 10 males, were assigned to each of the following test groups namely Group I (vehicle control; distilled water), Group II (CU–PC-C to 2000 mg/kg BW of CU in distilled water), Group III (CU–HSPC-C equivalent to 2000 mg/kg BW of CU in distilled water), and the respective doses suspended in 5 mL of the vehicle were given in divided doses at the time interval of 2 h for 15 d followed by monitoring of the animals for any occurrence of signs and symptoms of toxicity or mortality (OECD guidelines for the testing of chemicals, Citation1995; Development of ECa, Citation1996). On conclusion of the treatment, the animals were sacrificed by cervical dislocation and necropsied to facilitate gross pathological examination of organs.
Sub-acute toxicity
The animals, six males and six females, were assigned to the following four test groups namely Group I (vehicle control; distilled water), Group II (CU–PC-C equivalent to 100 mg/kg BW of CU in distilled water), Group III (CU–HSPC-C equivalent to 100 mg/kg BW of CU in distilled water) (Development of ECa, Citation1996). The animals were gavaged the relevant doses suspended in 0.5 mL of the vehicle, once daily, for a period of 28 d. During the dosing, the animals were examined for any clinical signs of morbidity, mortality, changes in BW and changes in food consumption. At the end of the treatment, the animals were bled from the orbital sinus for clinical pathology assessments, which included analysis of various hematology parameters like hemoglobin [Hgb (g/dl)], total red cell count (RBC), total white cell count, packed cell volume % and blood biochemistry parameters like total protein (mg/dl), albumin (mg/dl), cholesterol (mg/dl), glucose (mg/dl), creatinine (mg/dl), urea (mg/dl), uric acid (mg/dl), triglycerides (mg/dl), calcium (mg/dl) and phosphorous (mg/dl). The animals were sacrificed by cervical dislocation and were necropsied for the gross evaluation of the various vital organs like liver, spleen, kidneys and intestine. Finally, the dissected tissues were fixed in 10% neutral buffered formalin, processed and embedded in paraffin wax. Sections (5 µm) of these tissues taken on glass slides were stained using a combination of hematoxylin-eosin before observing under a microscope for histopathological evaluations.
Genotoxicity testing
The genotoxic potential of complexes of CU-PC-C and CU-HSPC-C were evaluated in a sequence of short-term assays, which measured aneugenicity and clastogenicity. Experiments have been carried out according to the reported procedures (More et al., Citation2009). The animals used for all the genotoxicity studies were assigned to the following five test groups, namely Group I (vehicle control; distilled water), Group II (CU–PC-C equivalent to 100 mg/kg BW of CU in distilled water), Group III (CU–HSPC-C equivalent to 100 mg/kg BW of CU in distilled water) and Group IV (cyclophosphamide equivalent to 40 mg/kg BW in distilled water as positive control) (Rao et al., Citation2004; Putman et al., Citation2006).
MN assay
Healthy Balb/c mice, n = 4 were gavaged the respective doses suspended in 0.25 mL of the vehicle, once daily, over a period of two days. At the end of the treatment, the animals were sacrificed by cervical dislocation, and the bone marrow cells were collected from both femurs of the mice into a small amount (5 mL) of FBS with the help of a 22G needle and mixed homogeneously to facilitate the cell collection. The resulting cell suspension was centrifuged at 2000 rpm for 10 min, the supernatant was disposed off and the pellet was then suspended and mixed in the residue FBS (about 100 µL) to provide a indiscriminate distribution of cells. A single layer of adequately spread cells was obtained by smearing the pellet on clean glass slides. The smears were fixed in methanol for 5 min and were stained using a 0.25% solution of May-Grunwald (Himedia) stain in methanol for 2 min and in Giemsa (Sigma) for 10 min, finally mounted with cover glass and DPX. Slides were observed under a microscope (Leica, Wetzlar, Germany) with 100× magnification. At least 1000 polychromatic erythrocytes (PCEs) were scored for the presence of micronuclei for assessing the Genotoxicity. The normochromatic erythrocytes were also determined as the indicator of bone marrow toxicity.
CA assay
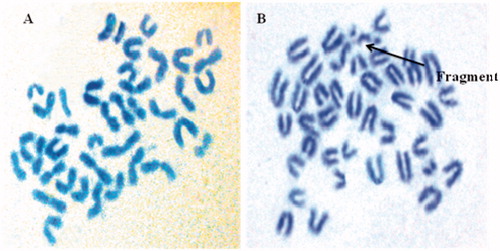
Healthy Balb/c mice, n = 4 were gavaged the respective doses suspended in 0.5 mL of the vehicle, once daily, over a period of two days. At the end of the treatment, the animals were sacrificed by cervical dislocation, 2 h before which they were treated with 4 mg/kg BW of colchicine, administered as intraperitoneal injection. Bone marrow cells were collected from both the femurs of the mice into 5 mL of 1% sodium citrate with the help of a 22G needle, mixed homogeneously, incubated at 37 °C for 10 min. The resulting pellet is treated with 3 mL of hypotonic solution (0.075 M KCl), mixed gently and incubated at 37 °C for 20 min. The resulting cell suspension was centrifuged at 2000 rpm for 10 min to obtain a pellet. The cell pellet was fixed with 3 mL cold Carnoy’s fixative (glacial acetic acid/methanol, 1:3 v/v) and mixed gently. The pellet was centrifuged and resuspended in the same for additional three times to separate the cells from the garbage; 200 µL of fresh Carnoy’s fixative was added to the suspension, and resulting suspension was then dropped onto chilled glass slides, dried on a hot plate at 45 °C for 5 min and stained for 5 min with a freshly prepared, 10% (v/v) Giemsa’s solution (Sigma) in Sorenson buffer and mounted with cover glass and DPX. Finally, dried slides were observed under a microscope (Leica) with 100× magnification. The frequency of CA determined by scoring 100 well-spread metaphase cells, from each animal, was observed as a marker of genotoxicity. Various chromatid and chromosome-type of aberrations included gaps, breaks, fragments and rings. Though gaps and aneuploid cells were recorded, they were not considered for the percent CA calculations.
Statistical analysis
All data are expressed as mean ± standard deviation. Statistical analysis was done with one-way analysis of variance (ANOVA) followed by the Turkey–Kramer multiple comparison test, using GraphPad Instat software (Graph Pad Software Inc., San Diego, CA). p < 0.01 was considered to be statistically significant. Genotoxicity data were statistically analyzed by one-way ANOVA followed by the Newman–Keüls post analysis test using GraphPad prism version 5.00.
Results
Characterization of complexes
Solubility study
As listed in , the CU–PC-C and CU-HSPC has different solubility profile than plain CU. The CU–PC-C and CU–HSPC-C has enhanced aqueous solubility, i.e. 8.23 ± 0.22 µg/mL and 4.09 ± 0.26 mg/mL, respectively, and n-octanol solubility, i.e. 25.87 ± 1.28 µg/mL and 45.79 ± 2.34 mg/mL, respectively. The higher solubilization indicated the formation of complex with CU.
Table 2. Solubility of CU, PC, HSPC, CU–PC-C, CU–HSPC-C and physical mixture in water and n-octanol at 25 °C.
Melting point
Melting point of CU, PC, HSPC, CU-PC-C and CU-HSPC-C was found to be 176–178 °C, 108–112 °C, 45–48 °C, 74–76 °C and 75–77 °C, respectively.
Drug content
Drug content (percent loading of CU) in the CU-PC-C and CU-HSPC-C was estimated by HPLC and were found to be 99.21 ± 0.054 % (w/w) and 98.11 ± 0.032 % (w/w), respectively.
Fourier transform infrared spectroscopy
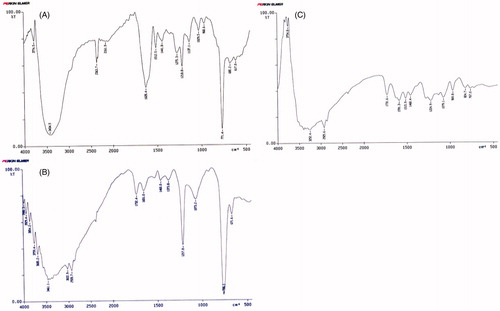
The FT-IR spectrums for CU, CU-PC-C and CU-HSPC-C are shown in . The spectrum of CU () shows characteristic absorption bands at 3434 cm−1, which represent phenolic OH at 1628 cm−1 C=O stretching peak of conjugated ketone at 1512 cm−1 and 1441 cm−1 represents aromatic and aliphatic C=C stretching, respectively, and at 1029 cm−1 C–O–C stretching peak of ether (Pan et al., Citation2006). The CU-PC-C shows its IR (KBr) peaks at 3461, 1732, 3029, 1217 and 1651 cm−1. The CU-HSPC-C shows its IR peaks at 3252, 1731, 1224 and 1079 cm−1. The IR spectra of CU-PC-C and CU-HSPC-C showed a broad peak for hydroxyl group appears at 3461 cm−1 and 3252 cm−1, respectively, which indicated some interaction at hydroxyl (–OH) group (Rao et al., Citation2004).
Scanning electron microscope (SEM)
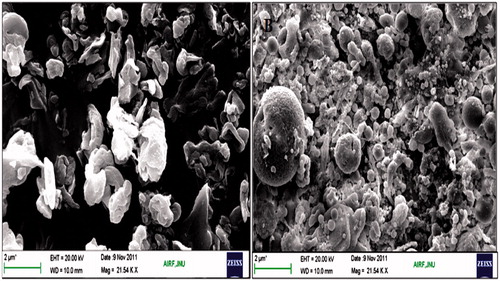
Scanning electron micrographs of the complexes are shown in . The CU-HSPC-C was found to be of arch shaped structure and sometime needle-shaped structure, and CU-PC-C was irregular shaped with rough surface morphology. Complexes were found to be as free sinuous units.
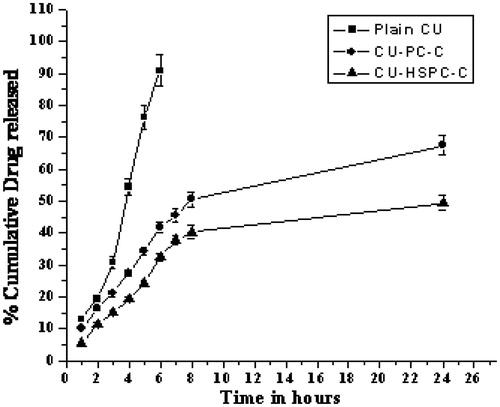
In vitro dissolution study
The complexes were evaluated by in vitro dissolution study. From the in vitro release study, it was evident that the release of CU from complexes was sustained and endured for a longer period of time as compared to plain CU (). Release of CU was almost completed within 6 h, whereas it was evident that both complexes (CU-PC-C and CU-HSPC-C) exhibited faster dissolution rates (67.45% and 49.43%, respectively) up to 24 h. The enhancement of dissolution rate obtained with inclusion complexes could also be due to CU wettability and formation of readily soluble complexes in the dissolution medium (Yadav et al., Citation2009).
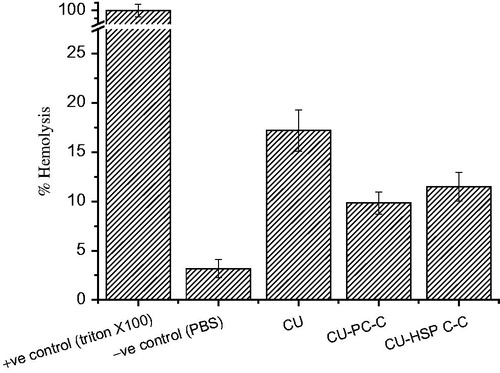
Hemolytic toxicity study
Hemolysis causes impairment to red blood cells via the release of iron-containing protein hemoglobin into plasma. The CU complexes must first be checked for hemolytic compatibility, which is mandatory requirement for parental administration. The CU–PC-C and CU–HSPC-C had shown only 1–12% of the hemolytic activity on RBC (). It was clear from this study that complexes significantly suppressed the hemolytic toxicity of CU up to the tested concentration of 1 mg/mL.
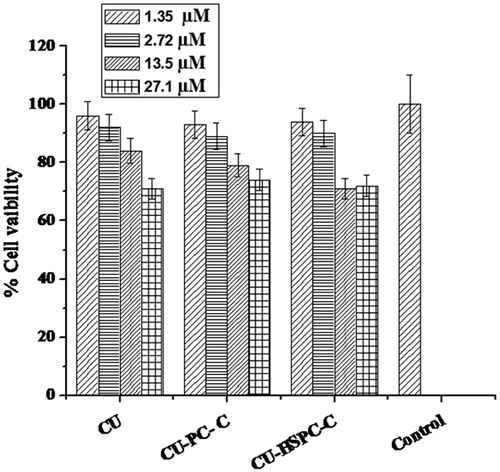
Cytotoxicity studies by MTT assay
Cytotoxicity studies were studied by using MTT assay. MCF-7 cells were exposed for 24 h to a series of equivalent concentrations of CU, CU-PC-C and CU-HSPC-C. A dose-dependent decrease in the cell viability was noticed in all the cases ().
In vivo studies
Acute-toxicity study
The animals in different treatment with CU complexes as well as the control group did not exhibit any mortality and any treatment-related abnormal behavior during the observation period. Gross pathological inspection of the vital organs did not reveal any evidence of toxicity during the animal necropsies. Thus CU-PC-C and CU-HSPC-C were found to be orally safe at the single-limit dose equivalent to 2000 mg/kg of CU.
Sub-acute toxicity
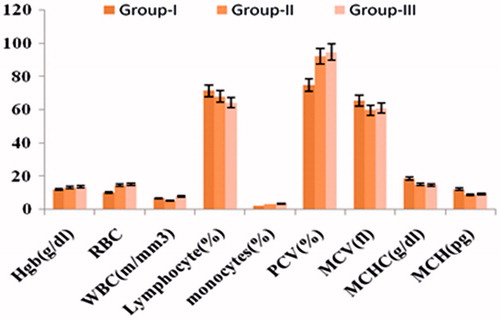
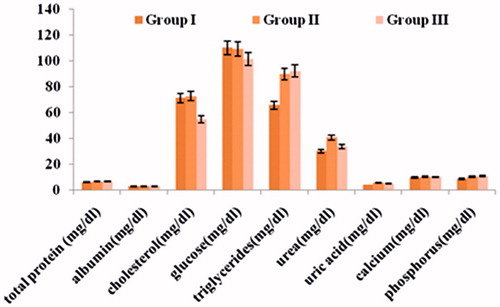
In the sub-acute toxicity studies, no animal mortalities were noticed in any of the study groups throughout the study period. The animals did not exhibit any treatment-related abnormal behavioral character. The increase in the weight of the animals in all groups was analogous to the weight increased in the control group. The observations indicated that long-term administration of the CU-PC-C and CU-HSPC-C had no adverse effects on the general health of the animals. The hematological parameters of the animals treated with CU-PC-C and CU-HSPC-C did not vary considerably from that of the control group (), thus indicating the oral safety of the CU-PC-C and CU-HSPC-C to the various tissue systems.
During the serum biochemistry investigations, the various biochemical parameters of the treatment with CU-PC-C and CU-HSPC-C did not vary significantly from that of the control group animals. These results have been depicted in . Similarly, the histopathology of various organs like heart, liver, spleen, kidneys and intestine () post necropsy indicated that the natural style of the various organs remained unaffected. No dose associated toxicity lesions were observed. Thus, the acute and sub-acute toxicity studies clearly indicated the oral safety of the developed CU complexes.
Figure 8. Representative photomicrographs of (A) liver (Group I), (B) liver (Group III), (C) kidney(Group I), (D) kidney (Group III), (E) intestine (Group I), (F) intestine (Group III), (G) spleen (Group I), (H) spleen (Group III), [Cv: central vein; Lf: lymphoid follicle; Gm: glomerulus; Tb: tubule; M: mucosa; and Sm: sub-mucosa]. The various organ sections were stained with hematoxylin and eosin (10×).
![Figure 8. Representative photomicrographs of (A) liver (Group I), (B) liver (Group III), (C) kidney(Group I), (D) kidney (Group III), (E) intestine (Group I), (F) intestine (Group III), (G) spleen (Group I), (H) spleen (Group III), [Cv: central vein; Lf: lymphoid follicle; Gm: glomerulus; Tb: tubule; M: mucosa; and Sm: sub-mucosa]. The various organ sections were stained with hematoxylin and eosin (10×).](/cms/asset/d8b60572-c39c-4a04-a6c5-3669a02f9dc2/idrd_a_936988_f0008_c.jpg)
Genotoxicity testing
MN assay
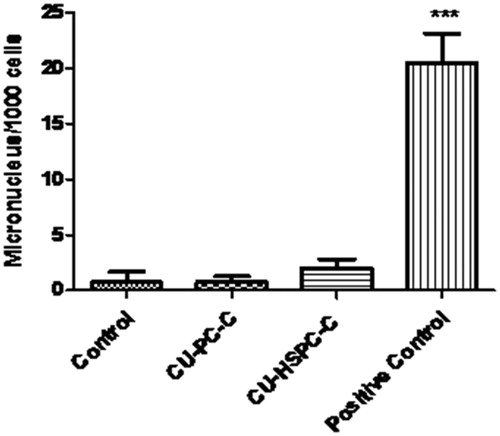
The administration of CU-PC-C and CU-HSPC-C did not result in any significant increase in the rate of micronucleated cells in the animals, when compared with the cyclophosphamide-treated mice. However, a significant increase in micronucleated cells (p < 0.001) were observed in the animals treated with cyclophosphamide, the animals treated with CU-PC-C and CU-HSPC-C were comparable with the control, which were non-clastogenic ( and ).
CA assay
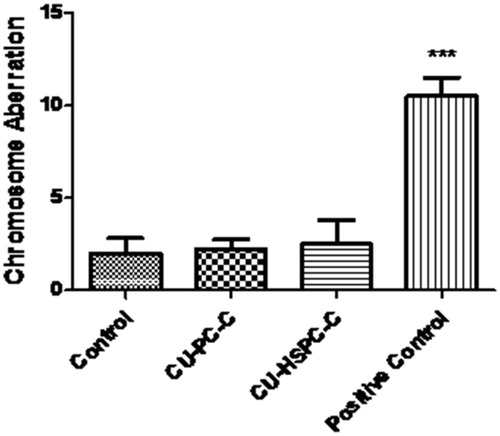
The administration of CU-PC-C and CU-HSPC-C did not induce aberrations and were comparable to control. Cyclophosphamide-treated mice, however, produced a significant number (p < 0.001) of aberrations. The results clearly indicate that CU-PC-C and CU-HSPC-C are non-clastogenic in nature ( and ).
Figure 10. Results of the chromosome aberration assay conducted in bone marrow cells of mice. ***p < 0.0001.
Discussion
Phyto-medicines prepared from plants had been used for vigor protection since ancient times, but many of them have limited use due to their poor absorption after oral administration. The recent developments were to improve the therapeutic performance of the conventional drug by formulating them as new drug delivery system, which could maximize the properties of these molecules (Yanyu et al., Citation2006; Sharma et al., Citation2010). Phyto-medicines complexes are chemical mixtures, prepared from herbal drugs, showed a high percentage of drug loading, which makes the delivery of drug clinically feasible (Semalty et al., Citation2010).
Molecules like CU having enormous therapeutic potentials as a drug can play a major role in the management of cancer (Aggarwal et al., Citation2003; Duvoix et al., Citation2005). This study dealt with the simple and reproducible preparation and evaluation of novel phospholipid complexes of CU, which increases its therapeutic efficacy. The physicochemical investigations showed that CU formed a complex with phospholipids. The CU-PC-C and CU-HSPC-C had enhanced aqueous and n-octanol solubility may be due to higher cross-linking character of complexes. The main purpose of increasing solubilization efficiency over plain CU had been served by synthesizing the complexes (Maiti et al., Citation2007). Increased solubility of CU with these complexes had been reported to the fact that during chemical treatment there is exchange to amorphous mixture of isomeric derivatives (Baglole et al., Citation2005). Drug contents of CU in the CU-PC-C and CU-HSPC-C, as estimated by HPLC, were found to be 99.21 ± 0.054 % (w/w) and 98.11 ± 0.032% (w/w), respectively. The formation of the complex was confirmed by the FT-IR spectroscopy comparing the spectrum of the complex with the spectrum of the individual components. FT-IR spectra showed modification of peaks of phenolic –OH of CU, which indicates that the phospholipid has interacted with CU through these phenolic –OH groups in the process of complexation.
The in vitro release profiles of CU obtained from the different formulations is shown in . The CU-HSPC-C clearly showed the almost one-fold lower release rate compared with that of the CU-PC-C. Similar findings were noted previously by Chen et al. (Citation2012), who showed that the release rate of CU encapsulated in SPC liposomes was two-fold faster than that of CU encapsulated in HSPC liposomes. These results might be the significance of a lower phase-transition temperature for SPC (below 0 °C) compared to that of HSPC (about 50 °C). The phase-transition temperature of a bilayer lipid membrane directly determines its liquidity, which in turn affects the release of CU from liposomes. Under the experimental temperature conditions, the lower film liquidity of the bilayer lipid membrane in CU-HSPC-C was less than in CU-SPC-C, which slowed down the release of CU from the CU-HSPC-C. Hemolysis is a type of acute toxicity assay used to evaluate the hemocompatibility of the complexes and to detect hemolyzation of red blood cells (Koziara et al., Citation2005; Ciochina et al., Citation2009; Mayer et al., Citation2009; Shelma & Sharma, Citation2011). To evaluate the in vivo utility of a CU-PC-C and CU-HSPC-C as a carrier for CU, the hemolytic potential in human blood needs to be tested (Fischer & Chan, Citation2007; Grainger, Citation2009). Therefore, we evaluated a direct complexes–erythrocyte membrane interaction in which the extent of disruption of the erythrocyte membrane was a direct measure of complexes toxicity (). Red blood cells exposed to plain CU, CU-PC-C and CU-HSPC-C as well as negative and positive hemolysis-inducing agents were evaluated for significant variations providing ideal evidence of hemolysis. However, a significant change, i.e. loss of biconcavity, was observed when red blood cells were treated with the plain CU, suggesting an increase in ghost cells and heavy hemolysis may be occurred. The CU-PC-C and CU-HSPC-C have lower protein binding, with no signs of hemolysis, demonstrating their suitability for therapeutic application. In vivo studies of CU complexes have shown improved bioavailability and therefore are more effective systems for delivering CU into tumors (Maiti et al., Citation2007; Yadav et al., Citation2009). Phospholipids, the major components of the complexes that have good biocompatibility, could promote the delivery through the cell membrane and enhance drug concentration in the cells, further enhancing the anti-cell effect of the drugs. In addition, at the cell-culture temperature condition (37 °C), the different concentrations of CU, CU-HSPC-C formulation had higher cell-inhibition ratios than CU-PC-C, the greater liquidity and hydrophilic form of CU-HSPC-C increased the ability of the complexes to interact with the cell membrane.
The design of complexes of CU-PC-C and CU-HSPC-C and its effect on regulatory policies for the advantageous complexes was foiled by the incomplete information about the health and the environmental safety of the complexes. Ascertaining the entire toxicological profile was thus essential for proving the human safety of complexes, which was one of the key ideas of the current investigation. In acute toxicity test study, the probability of the complexes to cause toxicity following single revelation for a short duration is usually less than 24 h. In this investigation, the CU-PC-C and CU-HSPC-C were tested at the limit dose since CU had been stated to be safe for oral administration at very high doses (Reddy et al., Citation2005).
Sub-acute toxicity tests analyze the possibility of complexes of CU to yield collective and potential biochemical, hematological and histopathological changes following multiple administrations. There were no clinical signs of toxicity detected during the experimental observation, and hence the complexes of CU were judged to be having non toxicological significance. Increase in the weight was observed, but it was comparable to the control group, so it was considered of non toxicological importance. The food consumption was normal. The toxicological difference in the hematological parameters has not been observed, which is the direct signal of the absence of tissue injury. The histopathological examination of different organs was studied, and their images were examined for any change in their anatomy. No significant change in the pictography was detected. The results indicate the safety of the complexes of CU without causing any significant difference in the parameters related to toxicity (Araujo & Leon, Citation2001; Keller & Banks, Citation2006).
Genotoxicity studies were carried out to evaluate the potential effects CU-PC-C and CU-HSPC-C on DNA/chromosomes, which may further direct to oncogene establishment or functional loss of tumor suppressor genes and hence carcinogenicity. Furthermore, these studies shows the possibility of complexes to change the genetic material of the germ cells heading to mutagenicity (Rao et al., Citation2004; Putman et al., Citation2006). For all the genotoxicity assays, cyclophosphamide, a known clastogenic agent, was applied as the positive control to confirm that a response was obtained under the realized test conditions (Putman et al., Citation2006). The MN assays investigate the complexes of micronuclei in anucleated PCE of the bone marrow of animals, which is used to calculate the toxicity in chromosomes. Visualization of micronuclei was assisted by the differential staining procedure, wherein the micronuclei were stained by the Giemsa stain as purple dots in the PCEs, which successively can be separated on their relevant violet or red color imparted by the May-Grunwald stain (). An increased occurrence of micronucleated cells indicating the chromosomal damage or a decreased PCE to NCE ratio pinpointing of suppressed bone marrow proliferation were regarded as the end point valuations of this attempt (Putman et al., Citation2006; More et al., Citation2009).
CA assay involves examination of mitotically arrested metaphase cells for the presence of structural chromosomal alterations induced by the complexes. Before sacrificing the animals, colchicine was administered to arrest the cells at the first mitosis of the cell cycle. At this stage, the metaphase chromosomes exhibit a contracted morphology, which facilitates the best visualization of the induced aberrations and the most accurate estimation of CA frequency (Putman et al., Citation2006). Chromosome-type aberrations including dicentric chromosomes, centric ring chromosomes, acentric fragments and ring chromosomes. The various types CAs have been represented in . During the study, macerated chromosome/s and cells, strictly damaged cells, fragments were recorded but were not considered for the percent CA determination.
Conclusion
In this study, CU complex has been prepared by a simple and reproducible method with the phospholipids. The complexes of CU have been found to produce better therapeutic efficacy in in vitro cells lines, which demonstrates the potential of CU–phospholipids complex as a drug delivery system. The various toxicological evaluations proved the oral safety of the CU complex for short-term as well as prolonged administration in animal models. The end-point evaluations of the various genotoxicity studies demonstrated no evidence of chromosome or DNA damage. Thus, a safe toxicological profile of the developed CU–PC-C and CU-HSPC indicates their potential as a capable anticancer against.
Supplementary material available online.
Supplemental Material.pdf
Download PDF (45.9 KB)Acknowledgements
The authors are particularly grateful to SAIF, CDRI, Lucknow, India, for spectral analysis and JNU, New Delhi, India, for SEM analysis. The Communication Number is 8717.
Declaration of interest
The authors report no conflicts of interest.
The authors are grateful to ICMR, New Delhi, India, for their financial support.
References
- Aggarwal BB, Kumar A, Bharti AC. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–98
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. (2007). Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75
- Aggarwal BB, Sung B. (2009). Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 30:85–94
- Anitha A, Maya S, Deepa N, et al. (2012). Curcumin-loaded N,O-carboxymethyl chitosan nanoparticles for cancer drug delivery. J Biomater Sci Polym Ed 23:1381–400
- Araujo CC, Leon LL. (2001). Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz 96:723–8
- Baglole KN, Boland PG, Wagner BD. (2005). Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J Photochem Photobiol A 173:230–7
- Balogun E, Hoque M, Gong P, et al. (2003). Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371:887–95
- Byrne JD, Betancourt T, Brannon-Peppas L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–26
- Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, et al. (2007). Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol 7:333–42
- Chen Y, Wu Q, Zhang Z, et al. (2012). Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 17:5972–87
- Ciochina AD, Bredeţean O, Dimitriu DC, Iacob G. (2009). [Considerations on in vitro and in vivo magnetic nanoparticles hemocompatibility testing]. Rev Med Chir Soc Med Nat Iasi 113:279–85
- Conti M, Malandrino S, Magistretti MJ. (1992). Protective activity of silipide on liver damage in rodents. Jpn J Pharmacol 60:315–21
- Development of ECa. (1996). OECD guidelines for the testing of chemicals, Test guideline 423, Acute oral toxicity–acute toxic class method. Paris: OECD
- Dhule SS, Penfornis P, Frazier T, et al. (2012). Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine 8:440–51
- Duvoix A, Blasius R, Delhalle S, et al. (2005). Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223:181–90
- Fischer HC, Chan WCW. (2007). Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol 18:565–71
- Fryer RA, Galustian C, Dalgleish AG. (2009). Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol 4:102–12
- Grainger DW. (2009). Nanotoxicity assessment: all small talk? Adv Drug Deliv Rev 61:419–21
- Gupta NK, Dixit VK. (2011). Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J Pharm Sci 100:1987–95
- Keller KA, Banks C. (2006). Toxicological testing handbook principles, applications, and data interpretation. In: Jacobson-Kram D, Keller KA, eds. M.g.t.s. New York: Informa Healthcare USA, 499
- Koziara JM, Oh JJ, Akers WS, et al. (2005). Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm Res 22:1821–8
- Li Y, Yang DJ, Chen SL, et al. (2008). Comparative physicochemical characterization of phospholipids complex of puerarin formulated by conventional and supercritical methods. Pharm Res 25:563–77
- Maiti K, Mukherjee K, Gantait A, et al. (2007). Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330:155–63
- Maiti K, Mukherjee K, Gantait A, et al. (2006). Enhanced therapeutic potential of naringenin-phospholipid complex in rats. J Pharm Pharmacol 58:1227–33
- Maiti K, Mukherjee K, Murugan V, et al. (2009). Exploring the effect of Hesperetin-HSPC complex – a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech 10:943–50
- Mayer A, Vadon M, Rinner B, et al. (2009). The role of nanoparticle size in hemocompatibility. Toxicology 258:139–47
- Mazumder A, Raghavan K, Weinstein J, et al. (1995). Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol 49:1165–70
- Mohanty C, Sahoo SK. (2010). The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 31:6597–611
- Morazzoni P, Montalbetti A, Malandrino S, Pifferi G. (1993). Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmacokinet 18:289–97
- More AB, Chilgunde SN, Kamble JC, et al. (2009). Polyethylene sebacate: genotoxicity, mutagenicity evaluation and application in periodontal drug delivery system. J Pharm Sci 98:4781–95
- OECD guidelines for the testing of chemicals, T.g. (1995). Repeated dose 28-day oral toxicity study in rodent. Paris: OECD – Organization for Economic Cooperation and Development
- Pan CJ, Tang JJ, Weng YJ, et al. (2006). Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J Control Release 116:42–9
- Peeyush KT, Gireesh G, Jobin M, Paulose CS. (2009). Neuroprotective role of curcumin in the cerebellum of streptozotocin-induced diabetic rats. Life Sci 85:704–10
- Putman DL, Clarke JJ, Escobar P, et al. (2006). Toxicological testing handbook principles, applications, and data interpretation. In: Jacobson-Kram D, Keller KA, eds. Genetic toxicology. New York: Informa Healthcare USA, Inc, 207–15
- Rao KS, Xu Y, Shaw E, Parton JW. (2004). Mutagenicity testing applied for regulation of developing products. Curr Separations 20:141–4
- Reddy RC, Vatsala PG, Keshamouni VG, et al. (2005). Curcumin for malaria therapy. Biochem Biophys Res Commun 326:472–4
- Reuter S, Eifes S, Dicato M, et al. (2008). Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol 76:1340–51
- Semalty A, Semalty M, Singh D, et al. (2010). Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J Incl Phenom Macrocycl Chem 67:253–60
- Sharma A, Gupta NK, Dixit VK. (2010). Complexation with phosphatidyl choline as a strategy for absorption enhancement of boswellic acid. Drug Deliv 17:587–95
- Shelma R, Sharma CP. (2011). Development of lauroyl sulfated chitosan for enhancing hemocompatibility of chitosan. Colloids Surf B Biointerfaces 84:561–70
- Torne S, Darandale S, Vavia P, et al. (2012). Cyclodextrin-based nanosponges: effective nanocarrier for Tamoxifen delivery. Pharm Dev Technol 18:619–25
- Yadav VR, Suresh S, Devi K, Yadav S. (2009). Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 10:752–62
- Yanyu X, Yunmei S, Zhipeng C, Qineng P. (2006). The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm 307:77–82


![Figure 11. Representative bone marrow cells stained with May Grunwald–Giemsa and exhibiting the presence of micronuclei [micronucleated normochromatic erythrocyte (MNPCE)].](/cms/asset/6a595358-0040-4310-b2ea-ca393c05a4b0/idrd_a_936988_f0011_c.jpg)