Abstract
Topical route of administration is the most commonly used method for the treatment of ophthalmic diseases. However, presence of several layers of permeation barriers starting from the tear film till the inner layers of cornea make it difficult to achieve the therapeutic concentrations in the target tissue within the eye. In order to circumvent these barriers and to provide sustained and targeted drug delivery, tremendous advances have been made in developing efficient and safe drug delivery systems. Liposomes due to their unique structure prove to be extremely beneficial drug carriers as they can entrap both the hydrophilic and hydrophobic drugs. The conventional liposomes had several drawbacks particularly their tendency to aggregate, the instability and leakage of entrapped drug and susceptibility to phagocytosis. Due to this reason, for a long time, liposomes as drug delivery systems did not attract much attention of researchers and clinicians. However, over recent years development of new generation liposomes has opened up new approaches for targeted and sustained drug delivery using liposomes and has rejuvenated the interest of researchers in this field. In this review we present a summary of current literature to understand the anatomical and physiological limitation in achieving adequate ocular bioavailability of topically applied drugs and utility of liposomes in overcoming these limitations. The recent developments related to new generation liposomes are discussed.
Introduction
The topical application of drugs is the most common method of drug delivery for the treatment of ophthalmic ailments. However, achievement of adequate bioavailability of topically applied drugs is challenging due to unique anatomical and physiological barriers at the ocular surface that include tear barrier as well the tight barriers in the apical layers of cornea and conjunctiva. A comprehensive understanding of these barriers is essential for the development of efficient drug delivery systems that can provide therapeutic drug concentrations at the target tissue. In order to circumvent the permeation barriers for topically applied drugs, incredible advances have been made in the field of pharmaceutical development. Over the past few decades, the techniques to develop newer drug delivery systems have tried not only to address the major issue of prolonged and controlled drug delivery but also to achieve targeted drug delivery. These approaches are expected to provide maximum therapeutic benefits with minimal adverse effects. Ever increasing knowledge of pathological conditions is leading to discovery of new treatment strategies such as gene delivery, but such treatment approaches also pose challenges of devising efficient drug delivery systems. Among many novel drug delivery systems that have evolved over the past decades, liposomes occupy a prominent place.
The conventional liposomes are simple vesicles consisting of outer bilayer of lipids enclosing a central aqueous core. Because of this unique structure, they can entrap both the hydrophilic and hydrophobic drugs (Fielding, Citation1991). Besides this particular advantage, liposomes are also biocompatible, biodegradable and non-toxic (Schwendener, Citation2007). They are of sufficient flexibility to allow synthesis in various sizes and can be formulated as eye drops, gels and ointments for topical delivery. However, the conventional liposomes had the disadvantage of being unstable, getting aggregated and were susceptible to phagocytosis. In order to overcome these problems and to achieve prolonged and targeted drug delivery, a whole new generation of liposomes has evolved. As the area of new generation liposomes remains unexplored for topical drug delivery, in this paper we describe the characteristics of such liposomes along with an updated knowledge on the permeation barriers at ocular surface and conventional liposomes in topical delivery. This will help in opening up new area of investigations to understand and explore the possibilities of developing newer delivery systems for topical application.
In the first part of this review we present a summary of current literature to understand the barriers for topical drug absorption. Next, we present the characteristics of liposomes, their mechanisms of intracellular drug delivery and evolution of conventional liposomes into new generation liposomes. In the last part of this review we summarize the current status in the development of liposomal formulation of several classes of drugs for topical drug delivery. The literature search for this review was made using Pubmed search engine. Several keywords such as liposomes, topical, characteristics, size, charge, stability, new generation, conventions, etc., were used alone and in combinations. We have included a total of 154 papers published during 1965 to 2013.
Absorption barriers for topical drug delivery
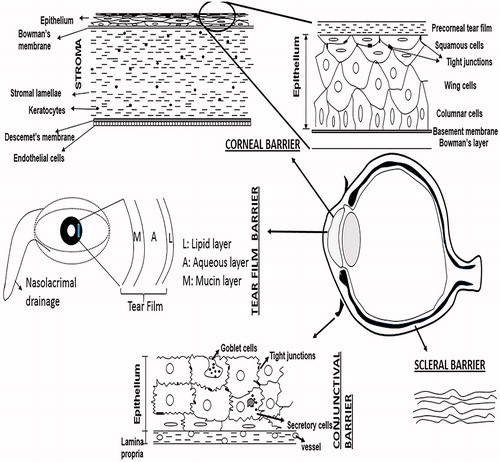
The ocular tissue absorption of topically administered drugs is estimated to be less than 5% (Urtti & Salminen, Citation1993). Majority of drug loss is attributed to overspill. The capacity of the conjunctival sac when lower lid is pulled away is approximately 25 µl and reduces to approximately 10 µl when eyelid returns to its normal position (Peters & Colby, Citation2006). The pathological conditions affecting conjunctiva may further limit the holding capacity of conjunctival sac. Hence, instillation of eye drops in a volume larger than 25 µl results in drug loss due to overspill. The amount of drug retained in the conjunctival sac mixes with the precorneal tear film before it comes in direct contact with ocular surface. It has been observed that even if the drug loss due to drainage is compensated by sustained drug delivery through a solid delivery system, the ocular bioavailability reaches only up to 10% indicating the importance of barriers on the ocular surface (Urtti et al., Citation1990). Not only the barrier functions of ocular surface tissue i.e. cornea, conjunctiva and sclera are now extensively studied, investigations have also revealed the importance of precorneal tear film as a significant barrier to drug absorption ().
Precorneal tear film
An intact tear film is essential for a healthy ocular surface, however, for the penetration of drugs applied topically to ocular surface, tear film is a significant barrier. The tear film is composed of three distinct layers: the outermost lipid layer (0.1 µm), the middle aqueous layer (8 µm) and innermost mucus layer (0.8 µm). The lipid layer prevents evaporation of tears, the aqueous layer allows spread of tears over the ocular surface and mucin layer adheres the tear film to ocular surface. In an unstimulated human eye at a normal blink rate of 15–20 blinks/min, the tear volume on the ocular surface is about 6–8 µl and the basal tear turnover rate is approximately 16%/min of the total tear volume (Norn, Citation1965). This tear turnover rate may increase significantly due to reflex tearing which may occur due to drug instillation resulting in accelerated washout of the drug. Tears are drained through the nasolacrimal duct into the nasal cavity. Blinking creates a pumping mechanism to facilitate flow of tears into the nasolacrimal duct (Peters & Colby, Citation2006). Drainage of the instilled drug into the nasolacrimal system along with tears is the main factor contributing to drug loss from precorneal tear film (Patton & Robinson, Citation1976) and reduction in the rate of tear drainage significantly increases the drugs’ ocular bioavailability (Linden & Alm, Citation1990).
Ocular surface barriers
The ocular surface comprises of the interpalpebral surface and the surface of both the conjunctival fornices. The cornea comprises of only the 5% of the total ocular surface while the rest is formed by the conjunctiva (Park & Karesh, Citation2006). The passage of substances through ocular surface takes place either transcellularly or paracellularly depending upon the lipophilicity or the hydrophilicity of the substance. Lipophilic drugs undergo transcellular absorption whereas hydrophilic and large molecules are transported through paracellular spaces (Liaw & Robinson, Citation1992). The conjunctiva has 230 times greater paracellular spaces compared to cornea due to 2 times larger pores and 16 times higher pore density (Hämäläinen et al., Citation1997b). Hence due to greater surface area and leakier epithelium compared to cornea, conjunctiva seems to be more favored as the route of absorption for hydrophilic and large molecules (Hämäläinen et al., Citation1997a; Prausnitz & Noonan, Citation1998; Geroski & Edelhauser, Citation2001). Despite these properties of conjunctiva, transcorneal route provides the greatest drug absorption because most of the clinically used drugs are small molecules that are fairly lipophilic and, moreover, rich network of capillaries and lymphatics in conjunctiva gives way to systemic rather than ocular absorption (Sasaki et al., Citation1995).
Transcorneal absorption
As stated above, the major route of absorption for topically applied drugs is transcorneal. Studies have shown that permeability of the corneal epithelium is 10−7–10−5 cm/s and the ocular drug bioavailability after topical ocular administration is less than 5% even for small lipophilic molecules (Urtti et al., Citation1990). The cornea comprises of three layers: outermost epithelium, middle stroma and innermost endothelium (). Each of these layers offers a different polarity and is a rate-limiting structure for drug permeation. The corneal epithelium is lipophilic, stroma is hydrophilic and endothelium is lipophilic. Corneal epithelium generally controls transcorneal transport, whereas corneal stroma and endothelium contribute significantly only to the barrier for small, lipophilic compounds (Prausnitz & Noonan, Citation1998). The corneal epithelium consists of a basal layer of columnar cells, 2–3 layers of wing cells and 1–2 outermost layers of squamous cells. The most superficial layers of epithelium function as greatest barriers to paracellular diffusion of molecules due to presence of the tight junctions () (Maurice & Mishima, Citation1984). Paracellular permeability depends upon the size of drug molecules and paracellular pore size, which is very small in corneal epithelium. Hämäläinen et al. (Citation1997a) showed that in the rabbit cornea the pore size is only 1.5–2.0 nm. As the pores of the corneal epithelium are negatively charged at physiological pH, negatively charged molecules permeate slower than positively charged and neutral molecules (Hämäläinen et al., 1997b). Hence most of the transcorneal drug absorption takes place transcellularly. Transcellular absorption of drugs is largely influenced by lipophilicity (i.e. partition coefficient) and in general, increased lipophilicity increases the permeability (Toropainen et al., Citation2003).
The stroma, which forms 90% of the corneal thickness, is hydrophilic in nature. It is relatively hypocellular and consists of large volume of tissue fluid. Because of the relatively open structure of stroma, molecules up to the size of 500 000 Dalton can pass through it (Maurice & Mishima, Citation1984). It is a rate-limiting barrier to small, highly lipophilic molecules due to its hydrophilic nature but allows easy passage to the hydrophilic molecules. Because of the large fluid volume, the stroma also acts as a reservoir for drugs that gain entry through the epithelium (Prausnitz & Noonan, Citation1998). The endothelium, which forms the innermost layer of corneal epithelium, is a single layer of hexagonal cells. It offers little resistance for the passage of drug molecules due to presence of the gap junctions and easily pumps out the tissue fluid from stroma into the aqueous humor.
Besides the anatomical characteristics of cornea that provide a significant barrier to drug absorption, presence of drug metabolizing enzyme is also considered a limiting factor. Activity of esterases, peptidases and proteases is present in corneal epithelium (Lee et al., Citation1986; Harris et al., Citation1992). Although these enzymatic activities are primarily viewed as a limitation in drug absorption, they may be facilitatory in the use of prodrugs such as dipivefrin (Anderson et al., Citation1980; Mindel et al., Citation1984).
Passive diffusion through the corneal epithelium is the most common method for drug absorption. The extent of passive diffusion depends upon the duration of contact between drug and cornea, drugs’ oil: water partition coefficient, molecular weight, charge and degree of ionization. Among all, lipophilicity is a major factor in determining the corneal drug penetration. While the corneal epithelium provides a significant barrier for the absorption of hydrophilic and large molecules, it provides easy passage for small lipophilic molecules and also acts as a depot for them (Sieg & Robinson, Citation1976). Huang et al. (Citation1983) showed that the optimal lipophilicity for passive diffusion through cornea corresponds to log D values of 2–3. For example, the permeability coefficient (Papp) of the lipophilic beta-blocker betaxolol (log D 1.59 (pH 7.4); Papp 2.7 × 10−5 cm/s) was 25 times higher than the permeability coefficient of the hydrophilic atenolol with similar molecular size (log D −1.77 (pH 7.4); Papp 1.1 × 10−6 cm/s) in the isolated rabbit cornea (Wang et al., Citation1991; Prausnitz & Noonan, Citation1998). Not only the transfer of hydrophilic molecules is limited through cornea, highly lipophilic molecules also have poor transcorneal absorption due to their restricted passage from lipophilic epithelium to largely hydrophilic stroma.
The role of active transport may be significant for the passage of hydrophilic molecules through the corneal epithelium. The extent of active transport across cornea depends upon the expression of corresponding transporters in the apical cell membranes, the affinity of drug for the transporter and the drug’s concentration (Mannermaa et al., Citation2006). Several transport systems such as amino acid, oligopeptide, monocarboxylate, nucleoside and organic anion transporters as well as efflux transporters have been identified in corneal epithelium (Mannermaa et al., Citation2006).
Although, it has been suggested that transporters in cornea may provide important avenue for enhanced drug delivery, their role and clinical significance remains unclear.
Transconjunctival-scleral absorption
The cells in the superficial layer of conjunctival epithelium have tight junctions (), however, as stated earlier these tight junctions are leaky. Therefore, conjunctival permeability to hydrophilic drugs is higher than that of corneal permeability and molecules up to the size of 20 000–40 000 Dalton can pass through the conjunctiva (Huang et al., Citation1989; Prausnitz & Noonan, Citation1998; Geroski & Edelhauser, Citation2001). Mannitol has 55 times higher conjunctival permeability as compared to corneal permeability (Huang et al., Citation1989). Conjunctival permeability coefficients for many compounds, like β-blockers (Ahmed et al., Citation1987; Wang et al, Citation1991) and timolol prodrugs (Chien et al., Citation1991), are higher than their corneal permeabilities. The ocular availability of peptides through conjunctiva is expected to be limited not only due to large molecular size but also due to degradation by enzymes secreted by conjunctiva. Presence of carrier-mediated mechanisms in the conjunctival epithelium has also been suggested to play an important role in transferring drug molecules to the interior of the eye (Ueda et al., Citation2000). Since conjunctiva is richly supplied with blood vessels and lymphatics and has a large surface area, it is also a site of significant loss of topically applied drugs due to systemic absorption (Urtti et al., Citation1985). Because of systemic drug absorption following conjunctival uptake even substantial prolongations of the residence times of the vehicle in the conjunctival sac may not always result in significant improvements in ocular drug absorption (Gurny et al., Citation1987). For example, nearly all timolol that is released from a controlled release insert to the lacrimal fluid in humans is absorbed systemically (Urtti et al., Citation1994).
Sclera mainly consists of collagen fibers and proteoglycans embedded in an extracellular matrix. Scleral permeability is comparable to that of the corneal stroma and is inversely proportional to the molecular radius. Hence, globular proteins permeate more easily compared to dextrans with linear molecular structure (Geroski & Edelhauser, Citation2001). The charge of the drug molecule also affects its transscleral permeability. Positively charged molecules permeate poorly possibly due to their binding to the negatively charged proteoglycan matrix. For example, transscleral iontophoresis with the negatively charged form of ciprofloxacin resulted in higher ciprofloxacin concentrations in the vitreous body than with the positively charged form (Yoshizumi et al., Citation1991). Scleral permeability was found to be higher than the corneal permeability for some β-blockers, sucrose and inulin (Ahmed et al., Citation1987).
Approaches to enhance ocular drug permeation
Considering the challenges in ocular drug delivery, efforts have been made to formulate ophthalmic formulation in a way that can minimize the drug loss and maximize the drugs’ ocular bioavailability (Lee & Robinson, Citation1986; Pal Kaur & Kanwar, Citation2002). The characteristics of an ideal topical drug delivery system can be summarized as follows:
Should be able to resist precorneal clearance and provide prolonged corneal contact time.
Should be delivered in a dosage form that provides adequate transcorneal absorption (paracellular or transcellular).
Should be of suitable viscosity that provides good corneal contact time but avoids reflex blinking, tearing and blurred vision.
Should have a suitable pH that favors the absorbable form of drug molecule (non-ionized) but is non-irritant to ocular surface.
Should cause minimal adverse effects.
Should be easy to administer.
Should require sufficiently low frequency of administration to ensure patient compliance.
In order to achieve the above-mentioned characteristics of a topical drug delivery system, several approaches have been adapted. Viscosity enhancing polymers such as polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), methylcellulose, hydroxyethyl cellulose, hydroxypropyl methylcellulose (HPMC), and hydroxypropyl cellulose are added to topical formulation to reduce precorneal drug clearance and enhance the corneal contact time. Penetration enhancers that modify the integrity of corneal epithelium and increase drug permeability have also been used. Some of the examples of penetration enhancers include cetylpyridinium chloride, lasalocid, benzalkonium chloride, parabens, Tween 20, saponins, bile salts and bile acids. These substances have the disadvantage of causing corneal toxicity. A prodrug approach has also been used to achieve suitable polarity of drug molecules that favors transcellular uptake. Some of the examples include dipivefrin (prodrug for adrenaline) and acyclovir (prodrug for ganciclovir).
Over the past few decades, colloidal drug delivery systems have become important entities for successful delivery of loaded drugs. They act as vectors that entrap, retain, transport and finally deliver the loaded drugs in the vicinity of the target site. This approach helps to achieve therapeutic benefits at much smaller doses and produces less toxic effects. Colloidal drugs delivery systems may be particulate or vesicular dosage form in nanometer size range such as microemulsion, liposomes, niosomes and nanoparticles. In this review we focus on the liposomal formulations for topical drug delivery.
Liposomes
Liposomes are artificial vesicles consisting of outer covering of lipids that encloses the inner core. In the early twentieth century, liposomes were considered as artificial cells due to their lipid bilayer covering. In the mid-1960s, Alec Bangham and his colleagues observed that smears of egg lecithin react with water and form quite intricate structures. Their observations revealed that these intricate structures are vesicles with unique property of encapsulating solvent into their interior when thin lipid films are agitated in water (Bangham et al., Citation1965). These lipid vesicles were found to be more or less homogenous under electron microscope and were later named as liposomes (Sessa & Weissmann, Citation1968; Bangham, Citation1972). In the subsequent years, liposomes were mainly used as biomembrane models to study the transport functions, mechanisms and absorption kinetics of various substances. Soon after, liposomes were recognized as important drug delivery systems and their potential uses in cancer chemotherapy were investigated (Gregoriadis et al., Citation1974). Since then, liposomes have undergone extensive investigation to develop them for targeted and sustained drug delivery.
The liposomal vesicles vary in size from 10 nm to 1 μm or greater. Structurally liposomes are classified into unilamellar vesicles (ULVs) and multilamellar vesicles (MLVs). Based on the size of vesicles, ULVs are further classified into small unilamellar vesicles (SUVs), giant unilamellar vesicles (GUVs), and large unilamellar vesicles (LUVs) (). In ULVs, single lipid bilayer consisting of lecithin or phosphatidylglycerol encloses the aqueous core. MLVs consist of more than one lipid bilayer, each separated by an aqueous compartment. Structure of liposomal vesicle allows them to serve as carrier for hydrophilic drugs that can be encapsulated into the aqueous core as well as hydrophobic and amphiphilic drugs that can be embedded in the lipid bilayer. Since the liposomes with multiple compartments have greater aqueous space, their capability to entrap hydrophilic drugs is higher than those with single compartments.
Figure 2. Schematic representation of the structure of liposomes. Small squares indicate entrapped drug in liposome.
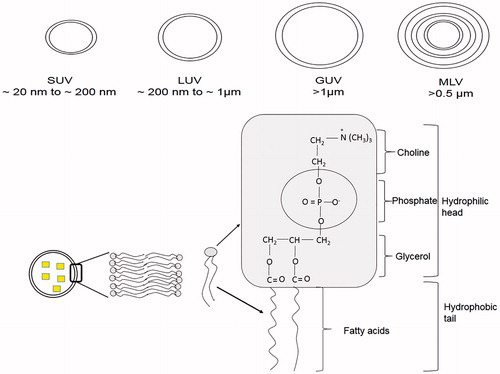
Lipids commonly used for the preparation of liposomes are phospholipids, which can be synthetic or naturally occurring (). The phospholipids that are commonly used include egg phosphatidylcholine, brain and synthetic phosphatidylserine, sphingomyelin, synthetic dipalmitoyl-dl-α-phosphatidylcholine, phosphatidylinositol and ovolecithin. A nonionic or zwitterionic lipid is generally used as the basic lipid and to introduce surface charge other lipids are included such as stearylamine for positive charge and diacetylphosphate, phosphatidyl glycerol or phosphatidyl serine for negative charge. Incorporation of charged lipids leads to a greater overall volume for aqueous entrapment and the likelihood of aggregation reduces (Goldbach et al., Citation1995). However, stearylamine containing cationic liposomes have been shown to cause toxicity in rabbit (Yoshihara & Nakae, Citation1986). Incorporation of cholesterol increases the stability, enhances the fluidity or microviscosity of the lipid bilayer and reduces the leakage of water soluble molecules (Senior & Gregoriadis, Citation1982; Weiner et al., Citation1989).
Mechanisms of permeation of liposomes through ocular surface
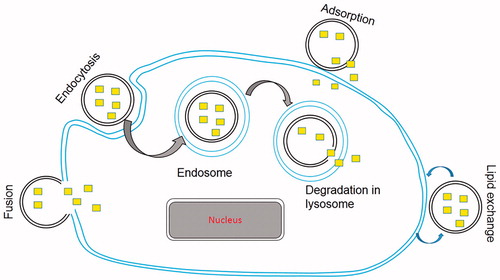
The mechanisms of interaction of liposomes with cell membranes that result into intracellular drug delivery have been studied extensively but are poorly understood. Due to highly complex nature of this interaction, the interpretation of experimental data is often difficult. The initial liposome-cell membrane interaction is the key process that leads to intracellular drug delivery. This liposome-cell membrane interaction may involve different receptors on different cell types or more than one receptor on a particular cell and is greatly affected by the lipid composition of liposomes (Duzgunes & Nir, Citation1999). Largely, four mechanisms of intracellular drug delivery by liposomes are widely accepted and are as follows ().
Adsorption: Adsorption of liposomes to cell membrane is one of the important mechanisms of intracellular drug delivery. The adsorbed liposomes, in the presence of cell surface proteins, become leaky and release their contents in the vicinity of cell membrane. This results in a higher concentration of drug close to cell membrane and facilitates cellular uptake of drug by passive diffusion or transport (Allen et al., Citation1981).
Endocytosis: Adsorption of liposomes on the surface of cell membrane is followed by their engulfment and internalization into endosomes. Endosomes transport liposomes to lysosomes. Subsequently, lysosomal enzymes degrade the lipids and release the entrapped drug into the cytoplasm (Lipowsky, Citation1995).
Fusion: Fusion of lipid bilayer of liposomes with lipoidal cell membrane by intermixing and lateral diffusion of lipids results in direct delivery of liposomal contents into the cytoplasm (Knoll et al., Citation1988).
Lipid exchange: Due to the similarity of liposomal membrane lipids with the cell membrane phospholipids, lipid transfer proteins in the cell membrane recognize liposomes and consequently cause lipid exchange. This results in the destabilization of liposomal membranes and intracellular release of drug molecules (Wojewodzka et al., Citation2005).
Figure 3. Schematic representation of various mechanism of intracellular drug delivery by liposomes. Small squares indicate entrapped drug in liposome.
An understanding of the mechanisms of intracellular drug delivery by liposomes provides the basis for bringing about manipulations in the characteristics of liposomes to enhance their favorable interaction with cell membranes and hence the drug delivery.
Conventional liposomes: modifications to meet the challenges
Liposomes have the advantage of delivering both the lipophilic drugs that they can entrap in their lipid covering and hydrophilic drugs that are incorporated into their aqueous core. Studies have shown that liposomes are also efficient carriers of amphiphilic drugs (Schaeffer & Krohn, Citation1982). Liposomes enhance the corneal permeability of lipophilic, hydrophilic as well as amphiphilic drugs due to their ability to come in close contact with cornea and conjunctiva (Schaeffer & Krohn, Citation1982; Dharma et al., Citation1986) and increase the extent of corneal uptake by prolonging the corneal contact time (Rathod & Deshpande, Citation2010). Additionally, liposomes are completely biodegradable and relatively nontoxic. Despite these advantages, some limitations of the liposome-based formulations have restricted their therapeutic uses in the past. One of the major limitations is their stability. Liposomes may become chemically unstable due to hydrolysis or oxidation of their constituent unsaturated lipids. They may also become physically unstable due to leakage of entrapped drug. Liposomes may aggregate to form larger particles that interfere with ocular absorption (Inokuchi et al., Citation2010) and also make them susceptible to phagocytosis by phagocytic cells. Instability may also be due to partitioning of lipophilic drug from the lipid bilayer to solvent. Conventional liposomes do not differentiate the target cells from others and manipulation of the structure of liposomes in order to achieve targeted drug delivery is another important issue. Additionally, the requirement of prolonged sustained drug delivery also warrants manipulations of the liposome characteristics. Efficacy of liposomes as a drug carrier depends upon various factors such as charge, size, lipid composition of the liposomal membrane, stability and corneal residence time (Lee et al., Citation1985).
To circumvent the limitations of liposomes summarized above and to achieve the characteristics of an ideal drug carrier, several modifications particularly to the surface characteristics and lipid composition of the liposomes have been explored.
Surface charge and size of liposomes
In general, charged liposomes resist aggregation and fusion better compared to uncharged liposomes and positively charged liposomes provide greater duration of action and higher drug delivery compared to negatively charged liposomes (Schaeffer & Krohn, Citation1982; Weissmann et al., Citation1985; Rathod & Deshpande, Citation2010). This is because positively charged liposomes intimately interact with negatively charged cornea leading to prolonged residence time (Law et al., Citation2000). It has also been suggested that cationic vehicle slows down the drug drainage with lacrimal fluid by increasing the viscosity and interaction with negative charges of the mucus (Felt et al., Citation1999a,Citationb). The effect of surface charge of liposomes on ocular irritation has also been evaluated. Positively charged liposomes significantly increase the rabbit eye blinking rate compared to neutral liposomes, however, the mean total score on Draize test remains below “practically non-irritating level” and no corneal histological changes appeared (Taniguchi et al., Citation1988). The size of the liposomes also affects their efficiency of drug delivery. Schaeffer & Krohn (Citation1982) showed that MLV have prolonged retention compared to SUV of same lipid composition and the interaction of liposomes with cornea decreases in the order of MLV+ > SUV+ > MLV− > SUV− > MLV. However, in another study done by Lee & Carson (Citation1986), it was observed that inulin-loaded neutral MLV, despite lesser affinity for cornea, provide 100-times greater and sustained inulin concentration in the anterior segment of eye compared to positively charged MLV. This effect was attributed to a two-fold faster disappearance rate of positively charged MLV from the tear pool. Monem et al. (Citation2000) also showed that MLV demonstrate prolonged drug delivery. Fitzgerald et al. (Citation1987) showed that increase in vesicle size restricts drainage from the inner canthal region, hence providing prolonged residence time.
Lipid composition
Lipid composition of the liposomal membrane determines its capacity to resist leakage of the entrapped drug. Barber & Shek (Citation1986, Citation1990) showed that tear-induced leakage of entrapped drug can be reduced by incorporating increasing amounts of cholesterol in the vesicle bilayers. The lipid composition of the liposomes needs to be optimized depending on the drug to be loaded. While preparing the liposomes loaded with pilocarpine nitrate, Rathod & Deshpande (Citation2010) observed that phosphatidylcholine and cholesterol in a ratio of 10:4 provided the maximum entrapment efficiency. Similarly, drug to phospholipid ratio also affects the entrapment efficiency of the liposomes. The type of lipid used also determines the surface charge of liposomes.
Stability and corneal residence time
Some of the stability problems may be overcome by lyophilization (Rathod & Deshpande, Citation2010). Use of bioadhesive polymers to prolong the residence time has been explored widely. Chitosan, a biodegradable and nontoxic mucoadhesive substance, has been used to coat liposomes. Mehanna et al. (Citation2010) showed that coating liposomes with high molecular weight chitosan inhibits their aggregation, increases the viscosity and hence the corneal residence time. The chitosan coating slowed the rate of drug release and provided 1.74-fold higher corneal permeation due to absorption enhancing nature of chitosan. Furthermore, chitosan-coated ciprofloxacin liposomes showed higher efficacy in a rabbit model of Pseudomonas aeruginosa conjunctivitis compared to the marketed preparation, ciloxan. Earlier studies have suggested that water-soluble low molecular weight chitosan as potential biopolymer as it has greater advantages in preventing liposome aggregation, providing slow drug release and better corneal permeability (Li et al., Citation2009). Similarly, compared to uncoated liposomes, N-trimethyl chitosan chloride coated coenzyme Q10 loaded liposomes were demonstrated to have high stability, 4.8-fold higher corneal residence time and higher anticataract efficacy in Sprague–Dawley rats (Zhang & Wang, Citation2009). Yerushalmi & Margalit (Citation1994) used collagen-coated liposomes and Kaufman et al. (Citation1994) coupled liposomes with collagen matrix. In both cases, prolonged drug delivery was achieved. Bochot et al. (Citation1998) used poloxamer 407, which has unique thermal gelling properties. It can be administered as drop and forms gel as it comes in contact with ocular surface due to change in temperature. The preparation provided better controlled release compared to simple gel. Davies et al. (Citation1992) used carbopol 934P and carbopol 1342 as a mucoadhesive polymer to coat the liposomes and assessed the effects of coating on corneal bioavailability of drugs. It was observed that initial precorneal rapid drainage was significantly reduced and carbopol 1342 was most effective.
Fujisawa et al. (Citation2012) used surface modified diclofenac-loaded liposomes to enhance their stability and achieve sustained drug delivery. The surface modification of liposomes was done using polyvinyl alcohol (PVA) and polyvinyl alcohol derivatives bearing a hydrophobic anchor (C16H33 – S –) at the terminal of the molecule (PVR). It was observed that PVR liposomes had higher physical stability and showed lesser particle aggregation. Due to higher chain flexibility and good dispersion properties, PVR-coated liposomes demonstrated higher mucoadhesion. The drug delivery of diclofenac to retina was significantly higher with both the PVA- and PVR-coated liposomes compared to non-liposomal formulation and it was observed that high retinal delivery was achieved via a non-corneal pathway. Li et al. (Citation2009) also prepared the diclofenac-loaded liposomes for topical application. However, in this study liposomes were coated with an 8 kDa low molecular weight chitosan, which changed the liposome surface charge and slightly increased the particle size without affecting the encapsulation. The chitosan-coated liposomes displayed a prolonged in-vitro drug release profile, improved physicochemical stability and significantly prolonged retention compared to non-coated liposome or drug solution. The chitosan coating also provided penetration enhancing effect without increasing the corneal toxicity. Schaeffer et al. (Citation1982) prepared phosphatidylcholine liposomes that also incorporated mixed brain gangliosides. Rabbit corneas were treated with these liposomes after pretreatment with wheat germ agglutinin, a plant lectin that binds strongly to both human and rabbit corneal epithelium. Ganglioside-containing liposomes showed a 2.5-fold increase in their binding to rabbit corneas in vitro as the ganglioside acts as receptors for lectin. The study indicated that ligands can be successfully used for site-specific binding of liposomes and targeted drug delivery.
Liposomes: evolution from conventional to new generation
Development of liposome-based formulations has undergone tremendous changes since the introduction of conventional liposomes in therapeutics. Modifications in the structure of liposomes have been made in order to achieve high stability, prolonged duration of drug release and targeted drug delivery. In this section, we summarize the new generation of liposomes. gives a summary of conventional and new generation liposomes and schematically shows the evolution of conventional liposomes into multifunctional new generation liposomes.
Figure 4. Schematic representation of the conventional and new generation liposomes. (1) Conventional liposomes with outer bilayer lipid covering and inner aqueous core; (2) stealth liposomes protected by polymer chains attached on the outer surface; (3) immunoliposomes equipped with antibodies for targeted drug delivery; (4) stealth immunoliposomes bearing antibodies either on the surface of liposomes or attached to the end of the polymer chains; (5) A representation of multifunctional liposomes with protective polymer to provide stealth properties; antibodies for targeted drug delivery; pH sensitive lipids to enhance intracellular drug delivery (•); incorporation of cationic lipids for gene delivery (+); attachment of cell penetrating peptides to enhance cell penetration (P). A: Aqueous soluble drug; L: Lipid soluble drug.
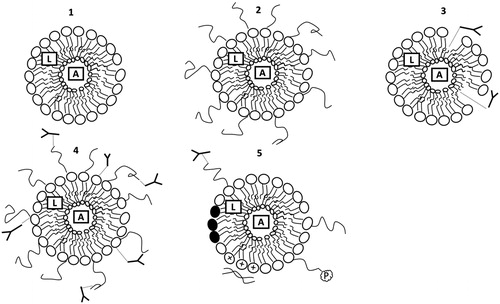
Stealth liposomes
Loss of liposomes due to engulfment by phagocytic cells is another issue to be addressed. Coating with inert, biocompatible polymers, such as polyethylene glycol (PEG), which form a protective layer over the liposome surface, provide stearic hindrance to recognition by opsonins and, hence, reduce their clearance (Klibanov et al., Citation1990; Blume & Cevc, Citation1993). These are known as “stealth liposomes”. Stealth properties of liposomes are particularly important when topical drug delivery targets posterior segment through noncorneal routes. Current methods attach PEG in a removable fashion on the surface of liposomes to facilitate their capture by target cells. After PEG-liposomes accumulate at the target site, the PEG coating is detached due to local pathological conditions such as reduced pH at the site of inflammation (Maeda et al., Citation2001). Yamashita et al. (Citation2007) have described the development of PEG coated liposomes. In this study when rabbit corneal cells were exposed to plasmids containing green fluorescent protein (GFP) cDNA followed by PEG-liposomes, gene transfer efficacy was significantly higher than conventional liposome group. PEG offers several advantages as it is nonionic, highly soluble both in water and organic solvents and has excellent biocompatibility. However, PEGylation requires excess of cholesterol to prevent PEG chain–chain interaction and aggregation (Lehtonen & Kinnunen, Citation1995). PEGylated liposomes can also initiate immunogenic reaction through complement activation (Szebeni et al., Citation2002). Although, PEG still remains the gold standard, other substitutes such as polyvinyl pyrrolidone (PVP), polyglutamic acid (PGA), poly(hydroxyethyl-l-asparagine) (PHEA) and poly(hydroxyethyl-l-glutamine) (PHEG) water-soluble polyoxazolines, zwitterionic lipopolymers have been used to overcome the drawbacks of PEG (Nag & Awasthi, Citation2013).
Targeted liposomes
PEGylated liposomes do not provide a targeted drug delivery. However, if one or more functional groups or ligands are inserted on the surface of the liposomes, the drug delivery to target cells and even the cell organelles can be achieved. Immunoliposomes are an example of targeted liposomes. They have the antibodies attached to their surface, which recognizes specific proteins on target cells. Immunoglobulins (Ig) of the IgG class and their fragments are commonly used. These ligands are attached to liposome surface without affecting their integrity. Tan et al. (Citation2003) generated immunoliposomes to target non-viral vectors to cell surface receptors on endothelium. The antibodies used were against both transferrin receptor and E-selectin. The immunoliposomes in this study were shown to augment transfection efficiency of liposomes to cells expressing the appropriate antigens including those of cornea. Norley et al. (Citation1987) had earlier shown that anti-IgD immunoliposomes loaded with acyclovir proved far more effective at inhibiting viral replication in the cornea than free drug or drug delivered in untargeted liposomes.
Besides antibodies, the ligands may also be proteins and peptides targeting specific cellular proteins. Cell penetrating peptides such as TAT-peptide, poly-arginine penetratin, etc., may also be incorporated on the liposome surface. These peptides facilitate rapid cell internalization of liposomes without targeting specific cells. However the mechanism of the internalization process remains unclear. Since these peptides are susceptible to enzymatic degradation, PEGylation is required to provide stearic protection (Laufer & Restle, Citation2008).
The targeted liposomes may also be prepared using PEGylated liposomes with ligands attached either to liposome surface or at the distal end of PEG chains. Presence of ligand on the surface of liposomes may be hidden due to stearic hindrance by PEG chains. Therefore, presentation of ligand at the distal end of PEG chains provides better ligand recognition and multivalent binding due to flexibility of PEG chains (Blume et al., Citation1993; Gabizon et al., Citation1999; Loomis et al., Citation2010).
pH sensitive liposomes
Liposomes deliver their contents to endosomes (pH 6.5–6) and then to lysosomes (pH <5) after endocytosis. The enzymatic degradation by lysosomal enzymes may result in loss of drug. To overcome this challenge, fusogenic lipids or peptides are incorporated which destabilize membranes after conformational activation at low pH of endosomes and deliver the drugs into the cytoplasm and escape degradation. Hence, pH sensitive liposomes help in achieving high cytoplasmic drug delivery by pH-sensitive release of liposome content. Efforts have also been made to confer the property of pH sensitivity to stealth and targeted liposomes (Torchilin, Citation2005; Bonacucina et al., Citation2009). This area of research is still evolving and many pH sensitive liposomes are being investigated (Perche & Torchilin, Citation2013).
Cationic liposomes
Cationic liposomes have been investigated particularly for the delivery of genetic material. They consist of positively charged lipids that interact with and neutralize the negatively charged DNA and hence, condense the DNA into a more compact structure. Such lipid complexes provide protection to entrapped genetic material and enhance its intracellular delivery. Cationic liposomes also incorporate helper lipid such as dioleoyl phosphatidylethanolamine and dioleoyl phosphatidylcholine, which stabilize the liposome complex. Li et al. (Citation2013) have described the synthesis of zwitterionic polycarboxybetaine (PCB) based distearoyl phosphoethanolamine-polycarboxybetaine (DSPE-PCB) lipid containing cationic liposome as siRNA delivery system. Kuesters & Campbell (Citation2010) showed that intracellular uptake of bevacizumab-modified PEGylated cationic liposomes by VEGF-secreting cells is enhanced, hence providing a targeted delivery. Cationic liposomal formulations are the youngest members among the new generation liposomes and hold promising future particularly for gene delivery.
Therapeutic applications of liposomal formulations in topical ocular drug delivery: current status
Antiviral drugs
Herpes Simplex virus (HSV) keratitis is a common cause of cornea related blindness worldwide. Global incidence of HSV keratitis is roughly 1.5 million, including 40 000 new cases of severe monocular visual impairment or blindness each year (Farooq & Shukla, Citation2012). Several antiviral drugs have been used in the treatment of HSV keratitis.
Acyclovir, a nucleoside analog, has been shown to be effective against HSV. However, because of its limited water solubility, poor lipophilicity and low bioavailability following topical application, it has not shown effectiveness in the treatment of HSV keratitis. Kitagawa et al. (Citation1989) compared the bioavailability of topically administered acyclovir with that of subconjunctival administration. The corneal levels of acyclovir were 2.5 times lower and aqueous levels were 5 times lower with topical administration compared to subconjunctival. Systemic acyclovir has been shown to be more effective than topically applied ointment in terms of recurrence rate, functional improvement and graft survival in patients after keratoplasty (Ghosh et al., Citation2008). Prodrug strategy using more stable amino acid esters of acyclovir has been shown to have enhanced bioavailability (Katragadda et al., Citation2008). Use of liposomal formulations to enhance bioavailability of topically applied acyclovir has also been evaluated.
In one of the studies, in vitro corneal penetration and in vivo corneal absorption of acyclovir containing liposomes was evaluated using rabbits. Liposomes were prepared by drug-lipid hydration method. Phosphatidylcholine and cholesterol were used as lipids, stearylamine as cationic charge-inducing agent and dicetylphosphate as anionic charge-inducing agent. The loading concentration of acyclovir in the liposome dispersion was 1.24 mg/ml. In vitro studies showed that the acyclovir in solution had fastest permeation through cornea while the corneal permeability of negatively charged liposomes was two times lower and that of positively charged 3.6 times lower than solution. Because of the slower permeation, negatively charged liposomes provided longer permeation time and the same was even longer with positively charged liposomes. In vivo studies also showed that positively charged liposomes penetrate cornea slower than negatively charged liposomes and solution, however, due to prolonged corneal contact time, the extent of corneal penetration is highest with positively charged liposomes (Law et al., Citation2000). Fresta et al. (Citation1999) studied acyclovir delivery by topical application using liposome. 1, 2-Dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC) and cholesterol were used as the lipids. Negative charge was introduced by adding 1, 2-dipalmitoyl-sn-glycerol-3-phosphatidic acid (DPPA) and positive charge by stearylamine or dimethyl-dioctadecyl glycerole bromide (DDAB). Liposomes were prepared following four different techniques; thin-layer evaporation, reverse phase evaporation, frozen and thawed, and dehydrated-rehydrated. Positively charged oligolamellar liposomes were shown to have most suitable bioadhesivity towards corneal epithelium. In vivo studies exhibited 42.3-folds increase in the acyclovir concentration in aqueous humor by acyclovir-loaded liposomes compared to free drug.
In another study, pharmacokinetic profile of acyclovir in aqueous humor was studied for positively charged acyclovir-containing liposomes in comparison with acyclovir ointment. Liposomal dispersion of acyclovir was found to produce significantly higher concentration profile as compared to ointment with same concentration. Furthermore, much higher dose of 1.5 mg versus 0.18 mg with full-strength 3.0% ointment produced an area under curve (AUC) that was only 1.6 times greater than that resulting from corresponding liposomal vehicle. Additionally, the aqueous humor acyclovir concentration achieved with liposomes at the plateau was in the upper range of the ID(50) reported for HSV (Chetoni et al., Citation2004). Stephen et al. (Citation1987) showed that acyclovir containing immunoliposomes that incorporated monoclonal antibody to HSV glycoprotein D were superior in preventing viral replication in infected corneas of mice as compared to untargeted acyclovir containing liposomes or acyclovir in solution.
Ganciclovir is another antiviral drug that finds application in the treatment of ocular herpes and cytomegalovirus infections. Due to poor lipid solubility, its ocular bioavailability is poor after topical application. Alternatively, systemic administration is done to achieve higher ocular tissue levels of ganciclovir. Intravitreal implants have also been developed for prolonged delivery of ganciclovir in the posterior segment of eye; however, this method involves surgical procedure, which is associated with cost and complications. Liposomal drug delivery system has been evaluated for effective delivery of ganciclovir to ocular tissue.
Shen & Tu (Citation2007) prepared negatively charged ganciclovir liposomes of an average size of about 200 nm by reverse phase evaporation using a mixture of phosphatidylcholine, cholesterol and sodium deoxycholate mixture. Final liposomal solution contained ganciclovir 1.0 mg/ml. In vitro studies showed a 1.7 times higher ocular bioavailability in rabbits treated with ganciclovir liposomes compared to commercially available ganciclovir solution. Liposome-treated group showed 3.5 times higher corneal permeation of ganciclovir compared to solution-treated group. This is in contrast to earlier description stating slower permeation of acyclovir in negatively charged liposomes compared to acyclovir solution (Law et al., Citation2000). This difference might be attributed to larger particle size of acyclovir. In vivo studies using ganciclovir liposomes showed 2–10 times higher drug concentration in all ocular tissues compared to commercially available ganciclovir solution. Highest ganciclovir levels were observed in sclera and cornea and these levels were higher than IC50 for cytomegalovirus. However, similar levels could not be achieved in retina and vitreous.
Idoxuridine (IDU), another antiviral drug is known to have poor corneal permeability. The liposomal preparation of IDU has been studied for the extent of corneal permeation in rabbits. Dharma et al. (Citation1986) formulated IDU liposomes using phosphatidic acid, phosphatidyl choline, and alpha-tocopherol by the reverse phase evaporation method. As compared to 0.1% solution of I125-labelled aqueous IDU, the liposomal preparation of I125-labelled IDU showed significantly higher corneal permeation up to 6 h post-instillation.
Nucleoside analogs such as stavudine that act as reverse transcriptase inhibitor (NARTI) are of therapeutic importance in the treatment of HIV-related ocular complications. Stavudine has been formulated in neutral, positively charged and negatively charged liposomes by the reverse-phase evaporation method. Transcorneal and transconjunctival permeation of these liposomes was studied in vitro using rabbit cornea. The study demonstrated the cornea–conjunctiva drug uptake ratios of 1.60, 2.79, 3.72, and 0.78 for free drug, neutral, positive, and negative liposomes, respectively. It was concluded that any of the liposomal formulations, although, did not enhance stavudine transport but the formulations were useful in preferentially localizing drug in either cornea or conjunctiva. Neutral and positively charged liposomes were found to localize stavudine to cornea, while negatively charged provided conjunctival localization (Kompella et al., Citation1998).
Antibacterial drugs
Several antibacterial agents have been formulated in liposomal forms to enhance the ocular bioavailability of these agents in the treatment of bacterial ocular infections. Tetracyclines, a group of broad spectrum antibiotics, are known to have several non-antimicrobial effects. Owing to the wide range of properties, tetracyclines find therapeutic application not only in ophthalmic infections but also in other ophthalmic conditions such as dry eye syndrome (Federici, Citation2011). Tetracycline in aqueous or oily solution applied topically fails to provide sufficient aqueous humor levels; however, ointment formulation succeeds in achieving aqueous humor levels at bacteriostatic concentration (Massey et al., Citation1976). Systemic administration of tetracycline in rabbits was also shown to provide relatively low aqueous humor levels compared to vascular structures in eye. Furthermore, the vitreous levels were half that of aqueous humor (Salminen, Citation1977). Keeping in view the difficulties is achieving ocular permeation, liposomal preparations of tetracycline for topical application have been studied.
In vitro studies have shown that for minimum inhibitory concentration, the anti-Chlamydia trachomatis activity increases by 2–6 folds with liposomal doxycycline and 4–10 fold with liposomal tetracycline compared to corresponding free drug solutions (Sangare et al., Citation1999) indicating possibility of higher effectiveness of liposomal formulations in the treatment of trachoma (Shafaa et al., Citation2011). In another study, liposomal formulation of tetracycline was prepared using drug-lipid hydration method. The efficacy of the formulation in dry eye syndrome was evaluated in rabbits. The liposome-treated group was shown to provide greater clinical and histopathological improvement compared to animals treated with empty liposomes or aqueous solution (El-Shazly et al., Citation2009).
Aminoglycoside group of antibiotics have wide therapeutic application in ophthalmology as they are effective against wide range of bacteria, have post-antibiotic effect and provide rapid concentration-dependent bactericidal effect. Moreover, the incidence of resistance against aminoglycosides is low. However, systemic administration is associated with nephrotoxicity as well as toxicity to audio-vestibular system and, therefore, drug delivery systems that can provide targeted delivery of aminoglycosides and reduce the toxicities are of great interest.
Gentamicin injected subconjunctivally provides higher drug corneal levels, however, the drug level in cornea falls rapidly reaching the non-detectable levels by 12–24 h (Barza, Citation1980). Topical instillation of gentamicin also provides comparable drug levels in cornea (Baum & Barza, Citation1983). In both cases, repeated administration is required and, therefore, liposomes as a drug delivery system to achieve sustained drug delivery have been investigated.
Gentamicin containing negatively charged liposomes were prepared by reverse-phase evaporation method using phosphatidic acid, phosphatidylcholine, and α-tocopherol. Corneal levels of gentamicin were compared among rabbits injected subconjunctivally with 5 mg of gentamicin given as liposome-encapsulated, with “empty” liposomes, or gentamicin in solution. The corneal gentamicin levels were markedly higher with the liposome-encapsulated drug than with the other two preparations. At 24 h, the differences were 5- to 20-fold higher in the cornea and were statistically significant for temporal cornea (Barza et al., Citation1984).
Efficacy of liposome-encapsulated tobramycin in rabbit model of pseudomonas keratitis was studied by Assil et al. (Citation1991). The efficacy of single subconjunctival injection of liposomes was comparable to hourly instilled topical fortified tobramycin and both the treatment groups showed higher efficacy compared to another group treated with subconjunctival injection of free tobramycin. In another study, using rabbits with Pseudomonas aeruginosa keratitis, the efficacy of large multivesicular liposomes enmeshed in a fibrin sealant applied once topically was comparable with hourly topical application of fortified tobramycin and was higher compared to liposomes without fibrin sealant (Frucht-Perry et al., Citation1992). These studies indicate prolongation of drugs’ half-life by encapsulation in liposome. However, the rate of clearance of liposomes is related to their size and lipid composition. Prolongation of half-life is greater with larger vesicles compared to small vesicles and with the cholesterol containing vesicles, as they are more stable. Furthermore, clearance of liposomes is faster in infected eyes compared to normal eyes (Barza et al., Citation1987; Zeng et al., 1993).
Ciprofloxacin, a fluoroquinolone antibiotic, is widely used in the treatment of ophthalmic infections; however, the efficacy is often limited due to poor ocular bioavailability of aqueous solutions. Budai et al. (Citation2007) prepared multilamellar liposomes containing ciprofloxacin by thin film hydration method using lecithin and α-l-dipalmithoylphosphatidylcholine (DPPC). Release profile of the liposomes was then studied in vitro. DPPC liposomes were found to significantly prolong the release half-time especially when 0.1% polymethacrylic acid was incorporated in the formulation as vehicle. Ciprofloxacin-loaded liposomes coated on soft contact lenses have also investigated for efficient drug delivery. The contact lenses with ciprofloxacin in liposomes inhibited both the Staphylococcus aureus and Pseudomonas aeruginosa on an agar plate and showed an enhanced antibacterial effect as determined by minimal inhibitory concentrations. The system was found to be nontoxic in in vitro experiment (Jain & Shastri, Citation2011). Abdelbary (Citation2011) studied in vitro and in vivo distribution of ciprofloxacin hydrochloride using liposomes coated with chitosan complex. Liposomes were prepared by thin film hydration method. It was observed that positively charged liposomes entrapped high level of ciprofloxacin. However, when chitosan complex coating was added, even higher entrapment was seen with negatively charged liposomes. In vivo studies showed that negatively-charged chitosan-coated liposomes provide significantly higher level of ciprofloxacin in external eye tissues with prolonged duration of up to 8 h compared to non-coated positively-charged liposomes (4 h) and commercial eye drop (2 h). In a recent study by Taha et al. (Citation2013), it was reported that ciprofloxacin-loaded liposomes provide higher ocular bioavailability compared to commercially available drug.
Norfloxacin is another fluoroquinolone with limited utility in ophthalmology due to poor ocular bioavailability. Lin et al. (Citation1996) prepared the norfloxacin-loaded liposomes using different phospholipids and studied their release profile and corneal penetration in vitro. It was observed that norfloxacin-loaded passed through the cornea at a slower rate compared to free drug. These liposomes accumulated in cornea and their drainage out from the cornea was slower than the free drug. Thus the liposomes provided corneal drug loading that reached the maximum in hours. The drug corneal retention was affected by the pH of the environment and was maximum with the usage of distearoyl-l-alpha-phosphatidylcholine. The study suggested that the norfloxacin-loaded liposomes are absorbed by the cornea via endocytosis. Other studies have also shown that encapsulation in liposomes prolongs the duration of drug release and the release pattern depends on the lipid composition of liposomes (Hosny, Citation2010).
Chloramphenicol is a broad spectrum antibacterial agent that is commonly used in ophthalmic infections. Mahmoud et al, (Citation2008) studied dimyristoylphosphatidylcholine (DMPC) liposomes containing chloramphenicol. The liposomes were prepared using different ways and the efficacy against Staphylococcus aureus infection was measured. When the aqueous phase to hydrate lipid film was prepared by dissolving chloramphenicol in buffer, entrapment efficiency as well as efficacy against Staphylococcus aureus was highest. Wutzler et al. (Citation2000) have also reported higher chlamydicidal activity of povidone-iodine loaded liposomes against Chlamydia trachomatis compared to solution.
Antifungal agents
Ocular fungal infections are often difficult to treat. Although, several antifungal agents are available for systemic use their ocular bioavailability after topical or systemic administration is poor and intraocular administration is associated with toxic effects.
Amphotericin B is an important antifungal drug for the treatment of mycotic infections in eye, however, topical use of the drug causes irritation and ocular permeation is poor. Therefore, use of liposome encapsulated amphotericin B has been investigated. Application of one drop of amphotericin containing unilamellar liposomes to rabbit eyes provided very stable corneal amphotericin B level and had the benefit of lowered ocular toxicity (Pleyer et al., Citation1995).
In a study involving 11 patients with culture positive Candida keratitis, the treatment was given as topical liposomal fluconazole (2 mg/ml) 3 times daily over a period of 30 d. Among all, eight patients showed complete improvement, one patient showed partial improvement while two patients had no improvement. Thus the fluconazole in liposomal form was found to be highly effective in treating Candida keratitis (Abdel-Rhaman et al., Citation2012). Although topical fluconazole in liposomal form was found to be superior to solution form in the rabbit model of Candida keratitis, in Candida endophthalmitis model the intravitreal liposomal fluconazole showed lower efficacy than fluconazole in solution (Gupta et al., Citation2000; Habib et al., Citation2010).
Anti-inflammatory and immunomodulatory agents
Several anti-inflammatory and immunomodulatory agents are widely used in the treatment of ocular inflammatory and immunological diseases. To enhance the ocular bioavailability and reduce the toxic effects following topical or intravitreal adminsitration, liposomal forms of many of these drugs have been evaluated.
Diclofenac, 2(2,6-dichloroanilino) phenyl acetic acid, is a commonly used anti-inflammatory agent in several ophthalmic conditions associated with pain and inflammation such as corneal epithelial defects, non-infectious inflammation, allergy and surgical of accidental trauma. Diclofenac is used as 0.1% aqueous solution of its sodium salt. At physiological pH of tears it largely remains in ionized form and therefore, is absorbed poorly. Liposomal formulation of diclofenac has been investigated for better corneal absorption. Sun et al. (Citation2006) prepared the cationic liposomes containing diclofenac by reverse-phase evaporation method and studied the ocular pharmacokinetics of topically applied liposomes in rabbits. Compared to solution, precorneal clearance of liposomes was slower. The maximum drug concentration (Cmax) in cornea and aqueous humor was higher with liposomes compared to solution and the half-life of diclofenac was prolonged in liposome formulation. However, the time to reach the Cmax (Tmax) in cornea and aqueous humor was found to be the same for liposomes and aqueous solution. The ocular bioavailability of liposome in aqueous humor was 211% compared to that of solution. Fujisawa et al. (Citation2012) showed higher drug delivery of diclofenac to retina in PVA- and PVR-coated liposomes compared to free drug.
Cyclosporin is an anti-inflammatory and immunomodulatory drug and is used for the treatment of ophthalmic conditions like dry eye syndrome and in patients undergoing corneal transplant. Milani et al. (Citation1993) studied the efficacy of cyclosporine encapsulated in liposomes in preventing the corneal graft rejection in rats that received allogenic graft. When compared with cyclosporine in olive oil or empty liposomes, the group treated with cyclosporine-loaded liposomes showed increased mean survival time. The graft survival rate was 77% in liposome treated group compared to 45% in cyclosporine in olive oil or 36% in empty liposomes treated group. In another study cyclosporine loaded liposomes or olive oil containing equivalent amount of cyclosporine was applied topically to rabbit eyes at 15-min intervals within the first hour and then one hourly over a 6-h period. Additionally, collagen shields soaked for 30 min in the liposome preparation of cyclosporine were also tested in vitro and in vivo. It was observed that cyclosporine in liposomes applied topically or in collagen shields delivered significantly higher drug levels to the cornea and sclera at 1 and 3 h compared to cyclosporine in olive oil. Similarly, the levels of cyclosporine in aqueous and vitreous humor were significantly higher in animals treated with liposomes topically or in collagen shields compared to cyclosporine in olive oil (Pleyer et al., Citation1994). The aqueous humor concentration of cyclosporine was also found to be higher after subconjunctival injection of cyclosporine containing liposomes in rabbits compared to subconjunctival injection of the same quantity of free cyclosporine (Alghadyan et al., Citation1988). One of the recent studies in rabbits has demonstrated that topically applied fusogenic liposomes are safe and in some eyes may produce mild conjunctival injection (Mosallaei et al., Citation2012). Tacrolimus, another immunomodulatory agent, when applied topically in liposomal formulation delivered significantly higher drug concentrations to all ocular tissues and particularly aqueous and vitreous humor up to 120 min post-instillation compared to treatment with tacrolimus solution in oil. The study demonstrated that liposomal formulation of tacrolimus may be a useful system for drug delivery to ocular tissue (Pleyer et al., Citation1993). Whitcup et al. (Citation1998) studied the anti-inflammatory efficacy of tacrolimus in rat model of endotoxin-induced uveitis using liposomes that contained 20-fold lower concentrations of drug compared to its solution in oil. It was observed that the efficacy of liposomes was similar to that of oily drug solution despite low drug concentration. In a recent study, Dai et al. (Citation2013) prepared tacrolimus loaded liposome to which bile salts were added to improve the corneal penetration. Three different bile salts were used; sodium glycocholate, sodium deoxycholate, and sodium taurocholate. It was observed that all three bile salts increased the cellular uptake of drug and corneal permeability increased by 3–4-fold compared to conventional liposomes. However, liposomal formulation containing sodium deoxycholate caused toxicity both in vitro and in vivo. 5-Fluorouracil, a synthetic pyrimidine is used in the treatment of proliferative vitreoretinopathy and to prevent the closure of conjunctival filtering blebs. Repeated subconjunctival and intravitreal injections are required as the half-life of 5-fluorouracil is short. Moreover, because of high drug concentrations achieved in several ocular tissues, toxicities are observed. However, the subconjunctival administration of liposomal 5-fluorouracil provides a lower peak concentration in aqueous humor and in all ocular tissues examined except for the conjunctiva overlying the liposomal injection site up to 96 h. This is of clinical benefit as the preparation provides high drug concentration at target site with lower toxicity to other ocular tissues (Simmons et al., Citation1988).
Antiglaucoma agents
Several antiglaucoma agents are used topically to lower intraocular pressure for the treatment of glaucoma. However, topical use is associated with adverse effects and the reduction in intraocular pressure is often suboptimal. Use of these drugs in liposome form may enhance their efficacy by increasing the ocular bioavailability and on the other hand may reduce the adverse effect by slow drug release over prolonged period.
Pilocarpine, a non-selective muscarinic receptor agonist, has been used in reducing intraocular pressure (IOP) for over a century (Rosin, Citation1991). Benita et al. (Citation1984) first compared the IOP reduction in response to liposomes loaded with pilocarpine 0.2% with that produced by pilocarpine solution 1% after topical application. It was observed that the satisfactory therapeutic levels were not reached with liposomes at these smaller doses. Durrani et al. (Citation1992) later used carbopol 1342 coated liposome containing pilocarpine to increase the precorneal retention time and using these liposomes they demonstrated sustained and prolonged IOP lowering and miotic activity of pilocarpine compared to conventional liposome and free drug. In this study pilocarpine concentration entrapped in carbopol 1342 coated liposome was 20–23%, compared to 3.8% in the study by Benita et al. (Citation1984). Monem et al. (Citation2000) studied effects of pilocarpine hydrochloride (HCl)-loaded liposomes in ocular normotensive and hypertensive rabbits. They used 0.1 mg/ml pilocarpine HCl and the entrapment efficiency of liposome was 96%. These pilocarpine HCl-loaded liposomes lowered the IOP for up to 4–5 h in normotensive eyes and up to 7 h in hypertensive eyes compared to free drug (1–2 h). Rathod & Deshpande (Citation2010), prepared liposomal formulation containing pilocarpine nitrate using thin lipid film hydration method to produce multilamellar vesicles. Positively charged liposome showed greater duration of action and higher IOP reduction.
Latanoprost, a prostaglandin analog, is one of the most effective drugs in lowering IOP. It is a lipophilic substance and is available as oil/water emulsion. Studies have been done to encapsulate latanoprost in liposomes and achieve long-term delivery for better patient compliance. In one of the studies, latanoprost-loaded large unilamellar liposomes were given subconjunctivally in rabbits as a single injection and reduction in intraocular pressure was monitored. A comparison was made with daily topical application of latanoprost in solution. It was observed that the liposomal formulation provided a greater sustained IOP lowering effect compared with daily administration of topical latanoprost beyond 90 d and no signs of inflammation were evident in the eyes (Natarajan et al., Citation2012). Liposome entrapped brimonidine tartrate and diltiazem hydrochloride also exhibit greater IOP lowering effects compared to corresponding free drug solutions (Prabhu et al., Citation2010; Ibrahim et al., Citation2013).
Acetazolamide, a carbonic anhydrase inhibitor, is used systemically for lowering IOP. El-Gazayerly & Hikal (Citation1997) studied effect of liposome containing acetazolamide (2%) in normotensive rabbit eyes. Positively charged liposomes containing acetazolamide had the highest entrapment efficiency and the most significant IOP reduction lasting for 4-h post instillation. Hathout et al. (Citation2007) used 1% acetazolamide in different type of liposomal formulation and studied the IOP lowering effect in normotensive rabbits. Results showed that positively charged multilamellar vesicles of liposomes containing acetazolamide provide maximum IOP reduction after 3 h of application and duration of action lasted for 8 h.
Afouna et al. (Citation2005) used demeclocycline in liposomal formulation and compared its IOP lowering effect with commercially available pilocarpine solution. Demeclocycline reduces IOP by blocking desmopressin, positively charged liposome containing demeclocycline exhibited earlier onset of action when administered three times a day for three days compared to pilocarpine.
Other potential uses
Use of liposomes for gene therapy is another potential application that has recently been investigated. Masuda et al. (Citation1996) and Matsuo et al. (Citation1996) studied the gene transfer using liposomes. Both of these studies showed that N-(alpha-trimethylammonioacetyl)-didodecyl-d-glutamate (TMAG) liposomes can effectively transfer plasmid DNA to retinal ganglionic cells.
Antioxidants are finding application in several disease processes. Edavarone, a radical scavenger, was used in liposomal formulation to study its effect against light-induced retinal damage. The liposomal formulation of edavarone was shown to reduce the photic damage as well as apoptotic cell death of the retinal ganglion cells much more effectively compared to free drug formulation (Hironaka et al., Citation2011; Shimazaki et al., Citation2011).
Experimental study of Ito et al. (Citation2000) suggested that liposome formulations are effective for transcorneal drug delivery of anticataract agents such as disulfiram. Another study (Lee & Tsai, Citation2010) demonstrated that liposomal CoQ10 is a promising candidate for the topical application of CoQ10. In our experimental studies we demonstrated that liposomal formulations of magnesium taurate and tocotrienol delay the onset and progression of galactose-induced cataract in rats. (Abdul Nasir et al., Citation2013; Iezhitsa et al., Citation2013; Saad et al., Citation2013; Zakaria et al., Citation2013).
Conclusions
Topical route of administration is the most commonly used method for the treatment of ophthalmic diseases. However, presence of several layers of permeation barriers starting from the tear film till the inner layers of cornea make it difficult to achieve the therapeutic concentrations in the target tissue within eye. In order to circumvent these barriers and to provide sustained and targeted drug delivery tremendous advances have been made in developing drug carriers. Liposomal drug delivery systems due to their unique structure prove to be extremely beneficial as they can entrap both the hydrophilic and hydrophobic drugs. The conventional liposomes had several drawbacks particularly their tendency to aggregate, instability and leakage of entrapped drug and susceptibility to phagocytosis. The modification in the surface characteristics of liposomes has helped in overcoming some of these limitations. However, over recent years development of new generation liposomes has opened up entirely new approaches for targeted and sustained drug delivery using liposomes. Such new generation liposomes have been extensively investigated for cancer chemotherapy and many are under clinical trial. Despite these developments, the therapeutic application of liposomal drug delivery systems in topical ophthalmic drug delivery remains limited. Currently, some of the liposomal formulations incorporating vitamins, isoflavonoids or hyaluronic acid are available for the treatment of dry eye (Craig et al., Citation2010; Pult et al., Citation2012). However, investigations into the topical use of liposomes for most of the other ocular diseases still remain in preclinical stage and none has progressed to clinical trials. Identification of appropriate target molecules on the corneal epithelial cells or perhaps the tight junctions will provide a lead in preparing targeted liposomes. Furthermore, immunoliposomes and cationic liposomes are likely to find much wider application in topical ophthalmic drug delivery.
Declaration of interest
This research received grants from Ministry of Higher education, Government of Malaysia under grant number 600-RMI/RAGS 5/3 (105/2013) and Universiti Teknologi MARA under grant number 600-RMI/DANA 5/3/RIF (17/2012) and 600-RMI/DANA 5/3/RIF (491/2012). The authors declare that no competing interests exist.
References
- Abdelbary G. (2011). Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm Dev Technol 16:44–56
- Abdel-Rhaman MS, Soliman W, Habib F, Fathalla D. (2012). A new long-acting liposomal topical antifungal formula: human clinical study. Cornea 31:126–9
- Abdul Nasir NA, Agarwal R, Tripathy M, et al. (2013). Tocotrienol delays onset and progression of galactose-induced cataract in rat. Abstracts of the 12th Meeting of the Asia Pacific Federation of Pharmacologists. Shanghai, China, July 9–13, 2013. Acta Pharmacol Sin 34:147
- Afouna MI, Khattab IS, Reddy IK. (2005). Preparation and characterization of demeclocycline liposomal formulations and assessment of their intraocular pressure-lowering effects. Cutan Ocul Toxicol 24:111–24
- Ahmed I, Gokhale RD, Shah MV, Patton TF. (1987). Physicochemical determinants of drug diffusion across the conjunctiva, sclera and cornea. J Pharm Sci 76:583–6
- Alghadyan AA, Peyman GA, Khoobehi B, et al. (1988). Liposome-bound cyclosporine: aqueous and vitreous level after subconjunctival injection. Int Ophthalmol 12:101–4
- Allen TM, Mc Allister L, Mausolf S, Gyorffy E. (1981). Liposome-cell interactions. A study of the interactions of liposomes containing entrapped anti-cancer drugs with the EMT6, S49 and AE1 (transport-deficient) cell lines. Biochimica et Biophysica Acta 643:346–62
- Anderson JA, Davis WL, Wei CP. (1980). Site of ocular hydrolysis of a prodrug, dipivefrin, and a comparison of its ocular metabolism with that of the parent compound, epinephrine. Invest Ophthalmol Vis Sci 19:817–23
- Assil KK, Frucht-Perry J, Ziegler E, et al. (1991). Tobramycin liposomes. Single subconjunctival therapy of pseudomonal keratitis. Invest Ophthalmol Vis Sci 32:3216–20
- Bangham AD. (1972). Lipid bilayers and biomembranes. Ann Rev Biochem 41:753–76
- Bangham AD, Standish MM, Watkins JC. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–52
- Barber RF, Shek PN. (1986). Liposomes and tear fluid. I. Release of vesicle-entrapped carboxyfluorescein. Biochim Biophys Acta 879:157–63
- Barber RF, Shek PN. (1990). Tear induced release of liposome entrapped agents. Int J Pharm 60:219–27
- Barza M. (1980). Treatment of bacterial infections of the eye. In: Remington JS, Swartz MN, eds. Current Clinical Topics in Infectious Diseases. Vol. 1. New York: McGraw-Hill Book Company, 158–94
- Barza M, Baum J, Szoka Jr F. (1984). Pharmacokinetics of subconjunctival liposome-encapsulated gentamicin in normal rabbit eyes. Invest Ophthalmol Vis Sci 25:486–90
- Barza M, Stuart M, Szoka Jr F. (1987). Effect of size and lipid composition on the pharmacokinetics of intravitreal liposomes. Invest Ophthalmol Vis Sci 28:893–900
- Baum J, Barza M. (1983). Topical vs. subconjunctival treatment of bacterial corneal ulcers. Ophthalmology 90:162–8
- Benita S, Plenecassagne JD, Cave G, et al. (1984). Pilocarpine hydrochloride liposomes: characterization in vitro and preliminary evaluation in vivo in rabbit eye. J Microencapsul 1:203–16
- Blume G, Cevc G. (1993). Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta 1146:157–68
- Blume G, Cevc G, Crommelin MD, et al. (1993). Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim Biophys Acta 1149:180–4
- Bochot A, Fattal E, Gulik A, et al. (1998). Liposomes dispersed within a thermosensitive gel: a new dosage form for ocular delivery of oligonucleotides. Pharm Res 15:1364–9
- Bonacucina G, Cespi M, Misici-Falzi M, Palmieri GF. (2009). Colloidal soft matter as drug delivery system. J Pharmaceut Sci 98:1–42
- Budai L, Hajdú M., Budai M, et al. (2007). Gels and liposomes in optimized ocular drug delivery: studies on ciprofloxacin formulations. Int J Pharm 343:34–40
- Chetoni P, Rossi S, Burgalassi S, et al. (2004). Comparison of liposome-encapsulated acyclovir with acyclovir ointment: ocular pharmacokinetics in rabbits. J Ocul Pharmacol Ther 20:169–77
- Chien D-S, Sasaki H, Bundgaard H, et al. (1991). Role of enzymatic lability in the cornea and conjunctival penetration of timolol ester prodrugs in the pigmented rabbit. Pharm Res 8:728–33
- Craig JP, Purslow C, Murphy PJ, Wolffsohn JS. (2010). Effect of a liposomal spray on the pre-ocular tear film. Cont Lens Anterior Eye 33:83–7
- Dai Y, Zhou R, Liu L, et al. (2013). Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine 8:1921–33
- Davies NM, Farr SJ, Hadgraft J, Kellaway IW. (1992). Evaluation of mucoadhesive polymers in ocular drug delivery. II. Polymer-coated vesicles. Pharm Res 9:1137–44
- Dharma SK, Fishman PH, Peyman GA. (1986). A preliminary study of corneal penetration of 125l-labelled idoxuridine liposome. Acta Ophthalmol (Copenh.) 64:298–301
- Durrani AM, Davies NM, Thomas M, Kellaway IW. (1992). Pilocarpine bioavailability from a mucoadhesive liposomal ophthalmic drug delivery system. Int J Pharm 88:409–15
- Duzgunes N, Nir S. (1999). Mechanisms and kinetics of liposome–cell interactions. Adv Drug Deliv Rev 40:3–18
- El-Shazly AH, El-Shazly LH, El-Gohary AA. (2009). The potential effect of liposome encapsulated tetracycline topical application for the management of experimental dry eye model in albino rabbits. Med J Cairo Univ 77:149–57
- El-Gazayerly ON, Hikal AH. (1997). Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int J Pharm 158:121–7
- Farooq AV, Shukla D. (2012). Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 57:448–62
- Federici TJ. (2011). The non-antibiotic properties of tetracyclines: clinical potential in ophthalmic disease. Pharmacol Res 64:614–23
- Felt O, Baeyens V, Zignani M, et al. (1999b). Mucosal drug delivery. In: Mathiowitz E, ed. The encyclopedia of controlled drug delivery. New York: Wiley, 605–26
- Felt O, Furrer P, Mayer JM, et al. (1999a). Topical use of chitosan in ophthalmology: tolerance assessment and evaluation of precorneal retention. Int J Pharm 180:185–93
- Fielding RM. (1991). Liposomal drug delivery. Advantages and limitations from a clinical pharmacokinetic and therapeutic perspective. Clin Pharmacokinet 21:155–64
- Fitzgerald P, Hadgraft J, Wilson CG. (1987). A gamma scintigraphic evaluation of the precorneal residence of liposomal formulations in the rabbit. J Pharm Pharmacol 39:487–90
- Fresta M, Panico A, Bucolo C, et al. (1999). Characterization and in-vivo ocular absorption of liposome-encapsulated acyclovir. J Pharm Pharmacol 51:565–76
- Frucht-Perry J, Assil KK, Ziegler E, et al. (1992). Fibrin-enmeshed tobramycin liposomes: single application topical therapy of Pseudomonas keratitis. Cornea 11:393–7
- Fujisawa T, Miyai H, Hironaka K, et al. (2012). Liposomal diclofenac eye drop formulations targeting the retina: formulation stability improvement using surface modification of liposomes. Int J Pharm 436:564–7
- Gabizon A, Horowitz AT, Goren D, et al. (1999). Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: in vitro studies. Bioconjug Chem 10:289–98
- Geroski DH, Edelhauser HF. (2001). Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev 52:37–48
- Ghosh S, Jhanji V, Lamoureux E, et al. (2008). Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol 145:198–202
- Goldbach P, Herve B, Pascal W, Andre S. (1995). Sterile filtration of liposomes: retention of encapsulated carboxyfluorescein. Int J Pharm 117:225–30
- Gregoriadis G, Wills EJ, Swain C, Tavill AS. (1974). Drug-carrier potential of liposomes in cancer chemotherapy. Lancet 1:1313–6
- Gupta SK, Dhingra N, Velpandian T, Jaiswal J. (2000). Efficacy of fluconazole and liposome entrapped fluconazole for C. albicans induced experimental mycotic endophthalmitis in rabbit eyes. Acta Ophthalmol Scand 78:448–50
- Gurny R, Ibrahim H, Aebi A, et al. (1987). Design and evaluation of controlled release systems for the eye. J Control Release 6:367–73
- Habib FS, Fouad EA, Abdel-Rhaman MS, Fathalla D. (2010). Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta Ophthalmol 88:901–4
- Hämäläinen KM, Kontturi K, Murtomäki L, et al. (1997a). Estimation of pore size and porosity of biomembranes from permeability measurements of polyethylene glycols using an effusion-like approach. J Control Release 49:97–104
- Hämäläinen KM, Kananen K, Auriola S, et al. (1997b). Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 38:627–34
- Harris D, Liaw JH, Robinson JR. (1992). Routes of delivery: case studies: ocular delivery of peptide and protein drugs. Adv Drug Deliv Rev 8:331–9
- Hathout RM, Mansour S, Mortada ND, Guinedi AS. (2007). Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharmscitech 8:E1–12
- Hironaka K, Inokuchi Y, Fujisawa T, et al. (2011). Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur J Pharm Biopharm 79:119–25
- Hosny KM. (2010). Ciprofloxacin as ocular liposomal hydrogel. AAPS PharmSciTech 11:241–6
- Huang AJ, Tseng SC, Kenyon KR. (1989). Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 30:684–9
- Huang HS, Schoenwald RD, Lach JL. (1983). Corneal penetration behavior of beta-blocking agents: II. Assessment of barrier contributions. J Pharm Sci 72:1272–9
- Mokhtar Ibrahim M, Tawfique SAH, Mahdy MM. (2014). Liposomal diltiazem HCl as ocular drug delivery system for glaucoma. Drug Dev Ind Pharm 40:765–73
- Iezhitsa I, Agarwal R, Awaludin NA, et al. (2013). Evaluation of the anticataract effects of magnesium taurate in vitro. Abstracts of the 12th Meeting of the Asia Pacific Federation of Pharmacologists. Shanghai, China, July 9–13, 2013. Acta Pharmacol Sin 34:24
- Inokuchi Y, Hironaka K, Fujisawa T, et al. (2010). Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eye drop administration. Invest Ophthalmol Vis Sci 51:3162–70
- Ito Y, Cai H, Koizumi Y, et al. (2000). Effects of lipid composition on the transcorneal penetration of liposomes containing disulfiram, a potential anti-cataract agent, in the rabbit. Biol Pharm Bull 23:327–33
- Jain RL, Shastri JP. (2011). Study of ocular drug delivery system using drug-loaded liposomes. Int J Pharm Investig 1:35–41
- Katragadda S, Gunda S, Hariharan S, Mitra AK. (2008). Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm 359:15–24
- Kaufman HE, Steinemann TL, Lehman E, et al. (1994). Collagen-based drug delivery and artificial tears. J Ocul Pharmacol 10:17–27
- Kitagawa K, Fukuda M, Sasaki K. (1989). Intraocular penetration of topically administered acyclovir. Lens Eye Toxic Res 6:365–73
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. (1990). Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268:235–7
- Knoll G, Burger KNJ, Bron R, et al. (1988). Fusion of liposomes with the plasma membrane of epithelial cells: fate of incorporated lipids as followed by freeze fracture and autoradiography of plastic sections. J Cell Biol 107:2511–21
- Kompella UB, Katragadda AK, Aukunuru JV, Betageri GV. (1998). Effect of liposomal charge on stavudine transport across rabbit cornea and conjunctiva. Pharm Pharmacol Commun 4:339–43
- Kuesters GM, Campbell RB. (2010). Conjugation of bevacizumab to cationic liposomes enhances their tumor-targeting potential. Nanomedicine (Lond.) 5:181–92
- Laufer SD, Restle T. (2008). Peptide-mediated cellular delivery of oligonucleotide-based therapeutics in vitro: quantitative evaluation of overall efficacy employing easy to handle reporter systems. Curr Pharm Des 14:3637–55
- Law SL, Huang KJ, Chiang CH. (2000). Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and absorption. J Control Release 63:135–40
- Lee VH, Carson LW. (1986). Ocular disposition of inulin from single & multiple doses of positively charged multilamellar liposomes: evidence for alterations in tear dynamics and ocular surface characteristics. J Ocul Pharmacol 2:353–64
- Lee VH, Urrea PT, Smith RE, Schanzlin DJ. (1985). Ocular drug bioavailability from topically applied liposomes. Surv Ophthalmol 29:335–48
- Lee VHL, Carson LW, Kashi SD, Stratford RE. (1986). Metabolic and permeation barriers to the ocular absorption of topically applied encephalins in albino rabbits. J Ocul Pharmacol 2:345–52
- Lee VH, Robinson JR. (1986). Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol Ther 2:67–108
- Lee WC, Tsai TH. (2010). Preparation and characterization of liposomal coenzyme Q10 for in vivo topical application. Int J Pharm 395:78–83
- Lehtonen JY, Kinnunen PK. (1995). Poly(ethylene glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophys J 68:525–35
- Li N, Zhuang C, Wang M, et al. (2009). Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm 379:131–8
- Li Y, Cheng Q, Jiang Q, et al. (2013). Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J Control Release. Available from: ISSN 0168-3659, http://dx.doi.org/10.1016/j.jconrel.2013.12.007 [last accessed 21 Dec 2013]
- Liaw J, Robinson JR. (1992). The effect of poly ethylene glycol molecular weight on corneal transport and the related influence of penetration enhancers. Int J Pharm 88:125–40
- Lin HH, Ko SM, Hsu LR, Tsai YH. (1996). The preparation of norfloxacin-loaded liposomes and their in-vitro evaluation in pig’s eye. J Pharm Pharmacol 48:801–5
- Lindén C, Alm A. (1990). The effect of reduced tear drainage on corneal and aqueous concentrations of topically applied fluorescein. Acta Ophthalmol (Copenh.) 68:633–8
- Lipowsky R. (1995). Generic interactions of flexible membranes. In: Lipowsky R, Sackmann E, eds. Handbook of biological physics. Chapter 11. Vol 1. The Netherlands: B.V. Elsevier Science, 521–602
- Loomis K, Smith B, Feng Y, et al. (2010). Specific targeting to B cells by lipid-based nanoparticles conjugated with a novel CD22-ScFv. Exp Mol Pathol 88:238–49
- Maeda H, Sawa T, Konno T. (2001). Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 74:47–61
- Mahmoud SS, Gehman JD, Azzopardi K, et al. (2008). Liposomal phospholipid preparations of chloramphenicol for ophthalmic applications. J Pharm Sci 97:2691–701
- Mannermaa E, Vellonen KS, Urtti A. (2006). Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev 58:1136–63
- Massey JY, Hanna C, Goodart R, Wallace T. (1976). Effect of drug vehicle on human ocular retention of topically applied tetracycline. Am J Ophthalmol 81:151–6
- Masuda I, Matsuo T, Yasuda T, Matsuo N. (1996). Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci 37:1914–20
- Matsuo T, Masuda I, Yasuda T, Matsuo N. (1996). Gene transfer to the retina of rat by liposome eye drops. Biochem Biophys Res Commun 219:947–50
- Maurice DM, Mishima S. (1984). Ocular pharmacokinetics. In: Sears MC, ed. Handbook of experimental pharmacology, pharmacology of the eye. Vol. 69. Berlin, Heidelberg: Springer-Verlag, 16–119
- Mehanna MM, Elmaradny HA, Samaha MW. (2010). Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm 36:108–18
- Milani JK, Pleyer U, Dukes A, et al. (1993). Prolongation of corneal allograft survival with liposome-encapsulated cyclosporine in the rat eye. Ophthalmology 100:890–6
- Mindel JS, Cohen G, Barker LA, Lewis DE. (1984). Enzymatic and nonenzymatic hydrolysis of d, l-dipivefrin. Arch Ophthalmol 102:457–60
- Monem AS, Ali FM, Ismail MW. (2000). Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharmaceutics 198:29–38
- Mosallaei N, Banaee T, Farzadnia M, et al. (2012). Safety evaluation of nanoliposomes containing cyclosporine a after ocular administration. Curr Eye Res 37:453–6
- Nag O, Awasthi V. (2013). Surface engineering of liposomes for stealth behavior. Pharmaceutics 5:542–69
- Natarajan JV, Ang M, Darwitan A, et al. (2012). Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomedicine 7:123–31
- Norley SG, Sendele D, Huang L, Rouse BT. (1987). Inhibition of herpes simplex virus replication in the mouse cornea by drug containing immunoliposomes. Invest Ophthalmol Vis Sci 28:591–5
- Norn MS. (1965). Tear secretion in normal eyes. Estimated by a new method: the lacrimal streak dilution test. Acta Ophthalmologica 43:567–73
- Pal Kaur I, Kanwar M. (2002). Ocular preparations: the formulation approach. Drug Dev Ind Pharm 28:473–93
- Park DJJ, Karesh JW. (2006). Topographic anatomy of eye. In: Tasman W, Jaeger EA, eds. Duane’s foundations of clinical ophthalmology on CD Rom. Vol. 1. Chapter 1. Philadelphia: Lippincott Williams & Wilkins
- Patton TF, Robinson JR. (1976). Quantitative precorneal disposition of topically applied pilocarpine nitrate in rabbit eyes. J Pharm Sci 65:1295–301
- Perche F, Torchilin VP. (2013). Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Deliv 2013 (2013), Article ID 705265, 32 pages. doi:10.1155/2013/705265
- Peters E, Colby K. (2006). The tear film. In: Tasman W, Jaeger EA, eds. Duane’s foundations of clinical ophthalmology on CD Rom. Vol. 2. Chapter 3. Philadelphia: Lippincott Williams & Wilkins
- Pleyer U, Elkins B, Rückert D, et al. (1994). Ocular absorption of cyclosporine A from liposomes incorporated into collagen shields. Curr Eye Res 13:177–81
- Pleyer U, Grammer J, Pleyer JH, et al. (1995). Amphotericin B–bioavailability in the cornea. Studies with local administration of liposome incorporated amphotericin B. Ophthalmologe 92:469–75
- Pleyer U, Lutz S, Jusko WJ, et al. (1993). Ocular absorption of topically applied FK506 from liposomal and oil formulations in the rabbit eye. Invest Ophthalmol Vis Sci 34:2737–42
- Prabhu P, Nitish KR, Koland M, et al. (2010). Preparation and evaluation of nano-vesicles of brimonidine tartrate as an ocular drug delivery system. J Young Pharm 2:356–61
- Prausnitz MR, Noonan JS. (1998). Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci 87:1479–88
- Pult H, Gill F, Riede-Pult BH. (2012). Effect of three different liposomal eye sprays on ocular comfort and tear film. Cont Lens Anterior Eye 35:203–7
- Rathod S, Deshpande SG. (2010). Design and evaluation of liposomal formulation of pilocarpine nitrate. Indian J Pharma Sci 72:155–60
- Rosin A. (1991). Pilocarpine. A miotic of choice in the treatment of glaucoma has passed 110 years of use. Oftalmologia (Romania) 35:53–5
- Saad SD, Zakaria FK, Azizan IL, et al. (2013). Calcium and magnesium level in cataractous lenses of galactosemic rats topically treated with liposomal formulation of magnesium taurate. Abstracts of the 12th Meeting of the Asia Pacific Federation of Pharmacologists. Shanghai, China, July 9–13, 2013. Acta Pharmacol Sin 34:147
- Salminen L. (1977). Penetration of ocular compartments by tetracyclines. Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie 204:189–99
- Sangare L, Morisset R, Ravaoarinoro M. (1999). In-vitro anti-chlamydial activities of free and liposomal tetracycline and doxycycline. J Med Microbiol 48:689–93
- Sasaki H, Igarashi Y, Nagano T, et al. (1995). Penetration of β-blockers through ocular membranes in albino rabbits. J Pharm Pharmacol 47:17–21
- Schaeffer HE, Breitfeller JM, Krohn DL. (1982). Lectin-mediated attachment of liposomes to cornea: influence on transcorneal drug flux. Invest. Ophthalmol Vis Sci 23:530–3
- Schaeffer HE, Krohn DL. (1982). Liposomes in topical drug delivery. Invest Ophthalmol Vis Sci 22:220–7
- Schwendener RA. (2007). Liposomes in biology and medicine. Adv Exp Med Biol 620:117–28
- Senior J, Gregoriadis G. (1982). Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol com-ponents. Life Sci 30:2123–36
- Sessa G, Weissmann G. (1968). Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res 9:310–8
- Shafaa MW, El Shazly LH, El Shazly AH, et al. (2011). Efficacy of topically applied liposome-bound tetracycline in the treatment of dry eye model. Vet Ophthalmol 14:18–25
- Shen Y, Tu J. (2007). Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J 9:E371–7
- Shimazaki H, Hironaka K, Fujisawa T, et al. (2011). Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Invest Ophthalmol Vis Sci 52:7289–97
- Sieg JW, Robinson JR. (1976). Mechanistic studies on transcorneal permeation of pilocarpine. J Pharm Sci 65:1816–22
- Simmons ST, Sherwood MB, Nichols DA, et al. (1988). Pharmacokinetics of a 5-fluorouracil liposomal delivery system. Br J Ophthalmol 72:688–91
- Stephen GN, Deborah S, Leaf H, Barry TR. (1987). Inhibition of herpes simplex virus replication in the mouse cornea by drug containing immunoliposomes. Invest Ophthalmol Vis Sci 28:591–5
- Sun KX, Wang AP, Huang LJ, et al. (2006). Preparation of diclofenac sodium liposomes and its ocular pharmacokinetics. Yao Xue Xue Bao 41:1094–8
- Szebeni J, Baranyi L, Savay S, et al. (2002). Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res 12:165–72
- Taha EL, El-Anazi MH, El-Bagory IM, Bayomi MA. (2014). Design of liposomal colloidal systems for ocular delivery of ciprofloxacin. Saudi Pharmaceutical Journal, 22:231–9 http://dx.doi.org/10.1016/j.jsps.2013.07.003
- Tan PH, Manunta M, Ardjomand N, et al. (2003). Antibody targeted gene transfer to endothelium. J Gene Med 5:311–23
- Taniguchi K, Yamamoto Y, Itakura K, et al. (1988). Assessment of ocular irritability of liposome preparations. J Pharmacobiodyn 11:607–11
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Disc 4:145–60
- Toropainen E, Ranta VP, Vellonen KS, et al. (2003). Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur J Pharm Sci 20:99–106
- Ueda H, Horibe Y, Kim KJ, Lee VH. (2000). Functional characterization of organic cation drug transport in the pigmented rabbit conjunctiva. Invest Ophthalmol Vis Sci 41:870–6
- Urtti A, Pippin JD, Rork GS, et al. (1990). Controlled drug delivery devices for experimental ocular studies with timolol. 2. Ocular and systemic absorption in rabbits. Int J Pharm 61:241–9
- Urtti A, Salminen L. (1993). Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol 37:435–56
- Urtti A, Salminen L, Miinalaincn O. (1985). Systemic absorption of ocular pilocarpine is modified by polymer matrices. Int J Pharm 23:147–61
- Urtti A, Rouhiainen H, Kaila T, Saano V. (1994). Controlled ocular timolol delivery: systemic absorption and intraocular pressure effects in humans. Pharm Res 11:1278–82
- Wang W, Sasaki H, Chien DS, Lee VH. (1991). Lipophilicity influence on conjunctival drug penetration in the pigmented rabbit: a comparison with corneal penetration. Curr Eye Res 10:571–9
- Weiner N, Martin F, Riox M. (1989). Liposomes as drug delivery system. Drug Dev Ind Pharm 15:1523–54
- Weissmann G, Sessa G, Weissmann S. (1985). Effect of steroids and ‘Triton X-100’ on glucose-filled phospholipid/cholesterol structures. Nature 208:649–51
- Whitcup SM, Pleyer U, Lai JC, et al. (1998). Topical liposome-encapsulated FK506 for the treatment of endotoxin-induced uveitis. Ocul Immunol Inflamm 6:51–6
- Wojewodzka J, Pazdzior G, Langne M. (2005). A method to evaluate the effect of liposome lipid composition on its interaction with the erythrocyte plasma membrane. Chem Phys Lipids 135:181–7
- Wutzler P, Sauerbrei A, Clocking R, et al. (2000). Virucidal and chlamydicidal activities of eye drops with povidone-iodine liposome complex. Ophthalmic Res 32:118–25
- Yamashita T, Sonoda S, Suzuki R, et al. (2007). A novel bubble liposome and ultrasound-mediated gene transfer to ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp Eye Res 85:741–8
- Yerushalmi N, Margalit R. (1994). Bioadhesive, collagen-modified liposomes: molecular and cellular level studies on the kinetics of drug release and on binding to cell monolayers. Biochim Biophys Acta 1189:13–20
- Yoshihara E, Nakae T. (1986). Cytolytic activity of liposomes containing stearylamine. Biochim Biophys Acta 854:530–46
- Yoshizumi MO, Cohen D, Verbukh I, et al. (1991). Experimental transscleral iontophoresis of ciprofloxacin. J Ocul Pharmacol 7:163–7
- Zakaria FK, Saad SD, Azizan IL, et al. (2013). Effects of magnesium taurate in liposomal formulation on the onset and progression of cataract in galactose-fed rats. Abstracts of the 12th Meeting of the Asia Pacific Federation of Pharmacologists. Shanghai, China, July 9–13, 2013. Acta Pharmacol Sin 34:159
- Zeng S, Hu C, Wei H, et al. (1993). Intravitreal pharmacokinetics of liposome-encapsulated amikacin in a rabbit model. Ophthalmology 100:1640–4
- Zhang J, Wang S. (2009). Topical use of coenzyme Q10-loaded liposomes coated with trimethyl chitosan: tolerance, precorneal retention and anti-cataract effect. Int J Pharm 372:66–75