?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Luteolin (LUT) is a promising molecule with potential anti-arthritic activity. This investigation presents formulation and evaluation of niosomal transgel for enhanced transdermal delivery of LUT. Different non-ionic surfactants and vesicle compositions were employed for preparation of niosomes. The vesicle size analysis showed that all vesicles were in the range from 534.58 to 810.22 nm which favoured efficient transdermal delivery. The entrapment of LUT in vesicle was found to be higher in all surfactant. The developed formulation was proved significantly superior in terms of amount of drug permeation with an enhancement ratio of 2.66 when compared to a control formulation. The in vivo bioactivity studies revealed that the prepared niotransgel formulation of LUT was able to provide good anti-arthritic activity and the results were comparable to standard (diclofenac gel for anti-arthritic and analgesic). Finally, the results were confirmed through radiological analysis which proved that the prepared niotransgel was effectively able to treat arthritis and results were comparable with the standard formulation.
Introduction
Luteolin (LUT) is chemically flavonoid (3,4,5,7-tetrahydroxy flavones) and important constituent of leaves of Vitex negundo L. (family: Verbenaceae), possessing high therapeutic activity. It is usually found in glycosylated forms in celery, green pepper, chamomile flower and honeysuckle flower (Rui et al., Citation2010). Flavonoids are such a group of polyphenolic compounds which are present in many plants, displaying a wide range of pharmacological properties, including anti-carcinogenic and anti-inflammatory activity (Middleton et al., Citation2000; Chahar et al., Citation2011). A wide range of pharmacological activities has been reported including anti-oxidant (Cotelle et al., Citation1996), cardiovascular (Shailajan & Yeragi, Citation2011), estrogenic (Hiremath et al., Citation2000), anti-bacterial (Tsou et al., Citation2001) and gasrtoprotective (Simoes et al., Citation1988). It can be useful in treatment of arthritis, in essence inhibition of necrosis factor (TNF-α), interleukin-1β (IL-1β) and suppression of activation of necrosis factor-кB (NF-кB). Khanna et al. (Citation2007) considered LUT as a gold mine for the treatment of arthritis. Drug delivery systems using colloidal particulate carriers such as liposomes (El-Nabarawi et al., Citation2013), niosomes or proniosomes (Mokhtar et al., Citation2008; Ammar et al., Citation2011; Imam et al., Citation2014) proved to have distinct advantages over conventional dosage with an increasingly important role in drug delivery. Proniosomes, hydrated by agitation in hot water for a short period of time, offer a versatile vesicle delivery concept with the potential for drug delivery via the dermal/transdermal route (El-Laithy et al., Citation2011). It can act as drug-containing reservoir, and modification of the particle composition or surface can adjust the drug release rate and/or the affinity for the target site (Hu & Rhodes, 1999). It can encapsulate both hydrophilic and hydrophobic substances in vesicles (Niemiec et al., Citation1995), thus, it is known that sparingly soluble drugs can be entrapped in vesicles (Arunothayanun et al., Citation2000). Consequently, non-ionic surfactant-based carbopol niotransgel is expected to offer a special advantage for LUT which is lipophilic with relative hydrophilicity. So, the aim of the present study is to design new transdermal formulation (niotransgel) for LUT characterized by safety and high therapeutic efficacy.
Materials and methods
Materials
LUT was kindly provided by Sami Labs Limited, Bengaluru, Karnataka, India. Span-40, Span-60, Tween-20, Tween-80 and Cholesterol were purchased from S.D. Fine Chemicals Limited, Mumbai, Maharashtra, India. Carbopol-934 was provided by S.D. Fine Chemicals Limited. High-performance liquid chromatography (HPLC) grade methanol, water and other chemicals of analytical grade were obtained from S.D. Fine Chemicals Limited. Complete Freund’s adjuvant (CFA) was purchased from MP Biomedicals Pvt Ltd, Mumbai, Maharashtra, India.
Preparation of niosomes
Niosomes-containing LUT were prepared by thin-film hydration technique (Balakrishnan et al., Citation2009). The compositions of niosomes prepared with different non-ionic surfactants have been given in . Various surfactant, phospholipids and cholesterol were dissolved in chloroform: methanol mixture (1:1) followed by 50 mg of LUT was dissolved in a round-bottom flask. The organic phase was evaporated using a rotary vacuum evaporator (HS-2005 V-N; Hahnshin Scientific Co., Bucheon, South Korea) at 40 °C to form thin film on the wall of the flask. The flask was kept in a desiccator under vacuum for 24 h to ensure complete removal of trace organic solvents. The hydration of deposited film was carried out using 10 mL of phosphate-buffered saline (PBS) at 55 °C, which is above the gel–liquid transition temperature (Tc) of sorbitan monoesters and polyoxyethylene alkyl ether surfactants (Kibbe, Citation2000). Then, the vesicle suspension was sonicated (titanium probe, Ultrasonicator, Model-UP100H; Hielscher Ultrasonics GmbH, Berlin, Germany) in three cycles of 1-min “on” and 1-min “off” leading to the formation of multilamellar niosomes. The niosomal suspension was left to mature overnight at 4 °C and stored at refrigerator temperature for further studies.
Table 1. Composition of different niosomal formulations.
Entrapment efficiency
Entrapment efficiency was calculated in terms of LUT content. The LUT-containing niosomes were separated from untrapped drug by centrifuging at 14 000 rpm at 4 °C for 1 h. The supernatant was collected, diluted with appropriate medium and assayed spectrophotometrically at 289 nm (UV-1601; Shimadzu Corporation, Kyoto, Japan). The entrapment percentage of the LUT was calculated by the equation given below (El-Laithy et al., Citation2011):
where, Ct is concentration of total LUT and Cr is concentration of free LUT.
Vesicles size and morphology
The size and of formulation was measured by dynamic light scattering (DLS) technique using Malvern Instruments (HAS-3000, Malvern, UK). Niosomal suspensions were mixed with the appropriate medium and measurements were taken in triplicate. The polydispersity index (PDI) was determined as a measure of homogeneity. Small values of PDI < 0.1 indicate a homogeneous population, while PDI > 0.3) indicate high heterogeneity (El-Laithy et al., Citation2011). The morphology of the niosome was determined by transmission electron microscopy (TEM Morgagni 268-D; FEI Company, Eindhoven, Netherlands). A drop of the dispersion was applied to a carbon-coated copper grid and left for 1 min to allow niosomes to adhere to the carbon substrate. The remaining dispersion was removed by absorbing the drop with piece of filter paper. Subsequently, a drop of 1% phosphotungstic acid was placed on the grid and sample was air dried and scanned. The image was visualized using Soft imaging viewer software (Olympus Soft Imaging Solutions GmbH, Munster, Germany).
Ex vivo transport study
Ex vivo transport studies were performed on rat skin using Franz diffusion cell with 1 cm2 area and 10 mL volume of receiver cell. Full-thickness skin was mounted after removal of hair and fat on diffusion cells with water jacket to assess skin permeability. The stratum corneum side was facing upward into the donor compartment and dermal side was facing downward into receptor compartment. The donor cell was filled with 2 mL niosomal dispersion and receptor medium was filled with permeation media at 37 °C with 600 rpm. One millilitre of aliquot was collected from the receiver cell at definite time intervals for 24 h and an equal volume of fresh receptor medium was replenished immediately. The collected samples were diluted and were filtered using 0.45 µm membrane filter and analysed using UV spectrophotometer. Similar experiments were performed for control formulation of LUT to compare the permeation enhancement. To determine the extent of permeation enhancement, enhancement ratio (ER) was calculated as follows:
In vitro release studies
The optimized niosomes formulation was converted into gel on the basis of highest entrapment efficiency and ex vivo transport data. Niotransgel was prepared by adding Carbopol®-934 (1%, w/w) and kept overnight for complete humectation of polymer chains. Other ingredients like 15% (w/v) PEG-400 and triethanolamine (TEA) (0.5%, w/v) were added to get homogeneous dispersion of gel (Chaudhary et al., Citation2013; Ahad et al., Citation2014) and were taken for assessment of analgesic and anti-arthritic activity. The control gel formulation (hydroethanolic solution 7:3) was also converted in gel as above procedure. The in vitro release experiments were performed for niotransgel formulations by paddle method. The release was performed using phosphate buffer (pH 5.5) and temperature was adjusted at 32 ± 0.5 °C to simulate both, human skin pH and temperature. Both the formulation (containing 10 mg of LUT) were accurately weighed and placed in dialysis bag closed from both sides. The assembly was placed at the bottom of the USP dissolution tester (VDA-8DR; Veego Scientific, Mumbai, Maharashtra, India). The vessel contained 500 mL buffer solution and speed was adjusted to 50 rpm (Siewert et al., Citation2003). Aliquots of 5 mL were withdrawn from the release medium at different time intervals and replaced by equivalent volume of release media. The amount of drug released from the gel was determined spectrophotometrically at 289 nm. The data obtained from the release studies were kinetically analysed and order of drug release from both formulations were determined.
Biological evaluation
Study design
Albino Wistar rats (6–8 weeks/100–125 g) were supplied by Central Animal House of Hamdard University, New Delhi, India, and kept under standard laboratory conditions in 12-h light/dark cycle at 25 ± 2 °C. The animals were received for study was duly approved by the University Animal Ethics Committee, (Jamia Hamdard, approval number 902) and CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India). The in vivo study was carried out to assess the analgesic activity and anti-arthritic activity of LUT-loaded carbopol niotransgel and control gel formulation. The rats were divided into four groups (n = 6). Group A was taken as negative control, Group B was applied marketed formulation, Group C received LUT-loaded carbopol niotransgel and Group D was applied with control gel formulation, respectively.
In vitro anti-arthritic activity
In vitro anti-arthritic activity of niotransgel and marketed gel (Omnigel, 1%; Cipla Ltd, Baddi, Himachal Pradesh, India) formulation were assessed by protein denaturation method. The mechanism of denaturation probably involves alteration in electrostatic hydrogen and disulphide bonding (Deshpande et al., Citation2009; Singh et al., Citation2011). The reaction mixture (0.5 mL) consisted of 0.45 mL of bovine serum albumin (5% aqueous solution) and 0.5 g each of both formulations. The pH was adjusted at 6.3 using a small amount of 1 N HCl. The samples were incubated at 37 °C for 20 min and heated at 57 °C for 30 min. The sample was cooled and 2.5 mL of PBS (pH 6.3) was added in each sample. Similarly, control test of was performed and turbidity were measured spectrophotometrically at 660 nm. The percentage inhibition of protein denaturation was calculated as follows:
Analgesic activity
Analgesic activity was performed by tail-flick hot water immersion method. The treatment was given by gently applying the formulations of each group on the dorsal surface of paw with the index finger. Five-centimetre portion of the tail of animals was immersed into hot water at 55 ± 5 °C after 15-min treatment of gel. Due to thermal stimulus, rats withdrew their tail within few seconds. Cut-off time of 50 s was taken to avoid damage to tail tissue. Time at which the animals withdrew their tail was noted.
CFA-induced anti-arthritis
The study was carried out for 21 days. On Day 0, all the groups except Group1 were inoculated with CFA. About 0.1 mL of the suspension was injected in sub-plantar region of left hind paw of all groups. CFA principally contains 1 mg/mL of heat-killed Mycobacterium tuberculosis suspended in mineral oil. It produces inflammatory responses within 24 h (Ekambaram & Perumal, Citation2010). The paw volume and haematological parameters were taken as parameters to assess the anti-arthritic activity. Radiological analysis was also done to confirm the activity. The image were taken and compared.
Results and discussion
Entrapment efficiency
It depends on the type and amount of surfactant forming the bilayers, amount of cholesterol and phospholipid. The maximum entrapment efficiency (EE) (%) was found in Formulation 4 (F4) (89.12 ± 3.12) and the lowest was found for F2 (67.83 ± 2.29) (). The vesicle prepared using Spans as surfactant showed more EE (%) than those prepared from Tweens. These results indicated that chemical structure of the surfactant which is related to length of alkyl chain was also governing the EE. Surfactants of longer saturated alkyl chains showed higher EE (Guinedi et al., Citation2005). Another important factor that commonly helps to explain the vesicles entrapment patterns is the gel-to-liquid phase transition temperature (Tc) of the surfactant forming the niosomal membrane which is directly proportional to the surfactant alkyl hydrocarbon chain length. Surfactants of higher Tc are more likely in the ordered gel form forming less leaky bilayers, thus, having higher EE, while surfactants of lower Tc are more likely in the less ordered liquid form (Mohammed et al., Citation2004).
Table 2. Characterization of niosomal formulation.
Vesicle size and morphology
The size of all formulae were of small vesicle size ranging from 534.58 to 810.22 nm with low PDI (<0.3) and unimodal size distribution (), which favours transdermal delivery of the drug (Mohammed et al., Citation2004). Direct proportionality did exist between the vesicle size and both chain length and degree of hydrophilicity of the surfactants forming the vesicle bilayer (Balakrishnan et al., 2009). The differences in the mean vesicle size could be explained: Span-based niosomes showed smaller vesicle size than Tween-based niosomes which are more hydrophilic. The photo microphotograph of the optimized formulation finally concluded that the vesicles were finely distributed (). The micrographs revealed the formation of well-identified spherical niosomal vesicles with sharp boundaries after hydration.
Ex vivo transport study
The ex vivo transport study profile of all the formulation showed enhanced skin permeation data in compare to control formulation. The maximum transdermal flux value was 93.21 ± 13.14 µg/cm2/h over control formulation (35.04 ± 14.43 µg/cm2/h) with an enhancement ratio of 2.66 across rat skin (). The reason for this better performance of niosomal formulations penetration enhancing effect of non-ionic surfactant and vesicle–skin interaction may contribute to the enhancing mechanisms for permeation (Fang et al., Citation2001). The enhancement in permeation of drugs across skin may be due to structure modification of the stratum corneum. It has been reported that the intercellular lipid barrier in the stratum corneum would be looser and more permeable following treatment with liposomes and niosomes (Ogiso et al., Citation1996; Barry, Citation2001). Both phospholipids and non-ionic surfactants in the formulation can act as penetration enhancers, and presents higher flux of the drug due to direct transfer of drug from vesicles to the skin (Ogiso et al., Citation1996).
In vitro release studies
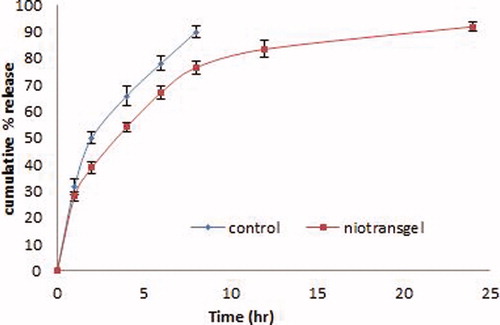
The results of in vitro release from LUT niotransgel and control gel formulation are illustrated in . It was apparent that the incorporation of LUT in niotransgel led to significantly slower release profiles (p < 0.01) compared to its control gel formulation where 98% was released in 6 h. The kinetic analysis of release profiles followed diffusion-controlled mechanism with an initial relative fast-release phase followed by a slower release one. The initial phase was due to desorption of LUT from the surface of niosomes while the drug release in the slower phase was regulated by diffusion through the swollen niosomal bilayers (Pardakhty et al., Citation2007). This profile could be advantageous if we considered the importance of epidermis saturation with initial fast drug released to achieve high concentration gradient required for successful drug delivery (Csoka et al. Citation2007). This may be attributed to the niosomal which makes it act as a solubilizing agent for the drug, thus, facilitating drug release from the gel base.
Biological evaluation
In vitro anti-arthritic activity
Denaturation of protein is one of the manifestations of arthritis. Production of autoantigens as in the case of rheumatoid arthritis may be due to denaturation of proteins. Diclofenac gel (Omnigel) was taken as standard gel to compare the result with developed formulation. The results revealed that standard gel showed percentage inhibition of 85.21%, whereas niotransgel showed percent inhibition of 81.82%. The comparison of result showed that developed formulation data was comparable with standard gel.
Analgesic activity
The result of analgesic activity in rats revealed that animals receiving negative control group showed thermal stimulus time 14.33 s, whereas standard gel formulation showed time 26.56 s. Carbopol-loaded niotransgel group animals showed thermal stimulus time 32.61 s which is higher than standard (p < 0.05). The control formulation showed only for 21.61 s which is higher than negative control. The increase in activity in developed formulation is due to higher penetration of drug into skin in compared to control and standard. As evident from the above explanation, analgesic activity of niotransgel formulation was higher to that of standard gel (Omnigel).
CFA-induced anti-arthritic model
It was evaluated after 12 days treatment with LUT-niotransgel formulation. There paw volume and haematological parameters of the animals were assessed to observe anti-arthritic activity. The radiographical analyses of all groups were performed to confirm the anti-arthritic activity.
Paw volume
This parameter was assessed on 0, 5th, 12th and 21st day from the initiation of the study. Statistical analysis was applied on the results obtained with respect to paw volume using Dunnett’s test. The result revealed that there was an increment in paw volume of all groups from Day 0–5. LUT-niotransgel formulation showed comparable results to those of the standard marketed gel which supports the therapeutic potential of developed formulation (). Whereas, toxic group showed continuous increase in paw volume due to no-treatment.
Table 3. Effect of application of formulation on paw edema in rats.
Haematological parameters
It has been reported that there is a significant decrease in RBC count in arthritis due to anaemic condition. The more important causes are the abnormal storage of iron in the reticuloendothelial system and synovial tissue and the failure of bone marrow to respond to anaemia (Yoshikawa et al., Citation1985). In arthritic condition, there is an increase in WBC count due to stimulation of immune system against the invading antigens. Also, ESR increases in arthritic condition (Ekambaram & Perumal, Citation2010). At the end of the study, on 22nd day RBC count, WBC count and ESR of all groups was determined (). RBC count decreased in toxic group. However, there was an increment in RBC count of marketed formulation and LUT-loaded carbopol niotransgel but lesser than that of control group. Also, the RBC count of marketed formulation and LUT-niotransgel groups were nearly same but more than that of CFA control group indicating good anti-arthritic potential of standard gel. The WBC count was found to be lesser than that of CFA group, indicating lesser inflammation than groups treated with standard gel.
Table 4. Effect on haematological parameters.
Radiological analysis
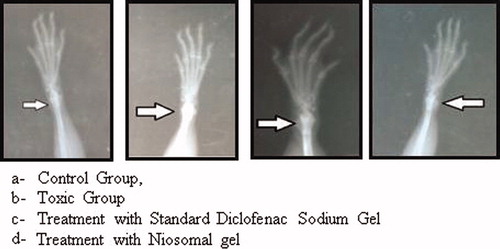
Hind paw X-rays of all groups of animals were taken for evaluating bone damage (). The summary of study given below:
: the radiographical analysis indicates no arthritic condition.
: this group did not receive any treatment so severe arthritic condition was seen in this group. The arrow indicates the inflamed joint and the degree of whiteness shows the severity of arthritis.
: X-ray of rat showed good recovery from arthritis which was treated with standard diclofenac gel and LUT niotransgel. The arrow indicates the inflamed joint with slight arthritic condition with a good recovery is evident from the X-ray. The good recovery is assigned in condition due to good therapeutic potential of LUT in arthritis and high penetration of niosomal gel into skin.
Conclusion
LUT-loaded niosomes were prepared using different non-ionic surfactants and characterized for in-vitro and in vivo anti-arthritic activity. The optimized niosomal formulation was converted into gel using carbopol as gelling agent. From the presented study, it was found that LUT-loaded niosomes provides both the improved EE and enhanced transdermal flux value across rat skin. The in vivo data revealed that analgesic effect of LUT niotransgel was comparable to that of standard gel (Omnigel). Rat paw volume showed a depriving effect with enhanced RBC count and decreased WBC count. The above observations make it evident our in-house LUT niotransgel was effective in arthritis management. Further corroboration was done by radiographical analysis which depicted a good recovery phase in group treated with developed formulation.
Acknowledgements
The authors would like to thank the Head of the Department of Pharmacognosy, Jamia Hamdard University, New Delhi, India for providing necessary facilities. A special mention of Abidin Medical Centre, AIIMS, New Delhi, India, for providing with the facilities required for radiographical analysis of rat paw and TEM study.
Declaration of interest
The authors report no conflicts of interest.
References
- Ahad A, Aqil M, Ali A. (2014). Investigation of antihypertensive activity of carbopol valsartan transdermal gel containing 1,8-cineole. Int J Bio Macrom 64:144–9
- Ammar HO, Ghorab M, El-Nahhas SA, Higazy IM. (2011). Proniosomes as carrier system for transdermal delivery of tenoxicam. Int J Pharm 405:142–52
- Arunothayanun P, Bernard MS, Craig DQM, et al. (2000). The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 201:7–14
- Balakrishnan P, Shanmugam S, Lee WS, et al. (2009). Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm 377:1–8
- Barry BW. (2001). Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14:101–14
- Chahar KM, Sharma N, Dobhal MP, Joshi YC. (2011). Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 5:1–12
- Chaudhary H, Rohilla A, Rathee P, Kumar V. (2013). Optimization and formulation design of carbopol loaded Piroxicam gel using novel penetration enhancers. Int J Biol Macrom 55:246–53
- Cotelle N, Bernier JL, Catteau JP, et al. (1996). Antioxidant properties of hydroxyl-flavones. Free Radic Biol Med 20:35–45
- Csoka G, Marton S, Zelko R, et al. (2007). Application of sucrose fatty acid esters in transdermal therapeutic systems. Eur J Pharm Biopharm 65:233–7
- Deshpande V, Jadhav VM, Kadam VJ. (2009). In-vitro antiarthritic activity of Abutilon indicum (Linn.) Sweet. J Pharm Res 2:644–5
- Ekambaram S, Perumal SS. (2010). Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complement Altern Med 10:1–9
- El-Laithy MH, Omar Shoukry S, Mahran LG. (2011). Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J of Pharm and Biopharm 77:43–55
- El-Nabarawi MA, Bendas ER, El Rehem RTA, Abary MYS. (2013). Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int J Pharm 443:307–17
- Fang JY, Yu SY, Wu PC, et al. (2001). In vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm 215:91–9
- Guinedi AS, Mortada ND, Mansour S, Hathout RM. (2005). Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm 306:71–82
- Hiremath SP, Badami S, Hunasagatta SK, Patil SB. (2000). Antifertility and hormonal properties of flavones of Striga orobanchioides. Eur J Pharmacol 391:193–7
- Hu CJ, Rhodes DG. (1999). Proniosomes: a novel drug carrier preparation. Int J Pharm 185:23–35
- Imam SS, Aqil M, Akhtar M, et al. (2014). Formulation by design based proniosome for accentuated transdermal delivery of risperidone: in vitro characterization and in vivo pharmacokinetic study. Drug Delivery, Early Online: 1–12, DOI: 10.3109/10717544.2013.870260
- Khanna D, Sethi G, Ahn KS, et al. (2007). Natural products as gold mine for arthritis treatment. Curr Opin Pharmacol 7:344–51
- Kibbe AH. (Ed.). (2000). Handbook of pharmaceutical excepients, 3rd ed. Washington, DC: American Pharmaceutical Association, 511–14
- Middleton EJ, Kandaswami C, Theoharides TC. (2000). Effects of plant flavonoids on mammalian cells:implications for inflammation, heart disease and cancer. Pharmacol Rev 52:673–751
- Mohammed AR, Weston N, Coombes AGA, et al. (2004). Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm 285:23–34
- Mokhtar M, Sammour OA, Hammad MA, et al. (2008). Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm 361:104–11
- Niemiec SM, Ramachandran C, Weiner N. (1995). Influence of non-ionic liposomal composition on topical delivery of peptide drugs into pilosebaceous units: an in vivo study using the hamster ear model. Pharm Res 12:1184–8
- Pardakhty J, Varshosaz A, Rouholamini A. (2007). In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm 328:130–41
- Rui L, Xi L, Jian Y, Guan-Hua D. (2010). Protective effects of luteolin against amyloid β25-35-induced toxicity on rat cerebral microvascular endothelial cells. Chin J Nat Med 8:223–7
- Shailajan S, Yeragi M. (2011). Optimization of microwave assisted extraction of luteolin from leaves of Vitex negundo Linn and its comparison with conventional extraction methods. Int J Pharm Res Dev 3:128–34
- Siewert M, Dressman J, Brown CK, Shah VP. (2003). FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 4:1–10
- Simoes CM, Schenkel EP, Bauer L, Langeloh A. (1988). Pharmacological investigation on Achyrocline satureioides (Lam.) D.C. Compositae. J Ethanopharm 22:281–93
- Singh M, Soni P, Upmanyu N, Shivhare Y. (2011). In-vitro anti-arthritic activity of Manikara zapota Linn. Asian J Pharm Tech 1:123–4
- Ogiso T, Niinaka N, Iwaki M. (1996). Mechanism for enhancement effect of lipid disperse system on percutaneous absorption. J Pharm Sci 85:57–64
- Tsou MF, Chen GW, Hung CF, et al. (2001). Luteolin inhibits the growth and arylamine N-acetyl-transferase activity Neisseria gonorrhoeae. Microbios 104:87–97
- Yoshikawa T, Tanaka H, Kondo M. (1985). The increase of lipid peroxidation in rat adjuvant arthritis and its inhibition by superoxide dismutase. Biochem Med 33:320–6