Abstract
Context: Facial hirsutism is a cosmetic concern for women and can lead to significant anxiety and lack of self-esteem. Eflornithine cream is indicated for the treatment of facial hirsutism. However, limited success rate and overall patient's satisfaction, even with a long-term and high-frequency application, leave room for improvement.
Objective: The objective of this study is to test the effect of microneedle treatment on the in vitro skin permeation and the in vivo efficacy of eflornithine cream in a mouse model.
Materials and method: In vitro permeation study of eflornithine was performed using Franz diffusion cell. In vivo efficacy study was performed in a mouse model by monitoring the re-growth of hair in the lower dorsal skin of mice after the eflornithine cream was applied onto an area pretreated with microneedles. The skin and the hair follicles in the treated area were also examined histologically.
Results and discussion: The hair growth inhibitory activity of eflornithine was significantly enhanced when the eflornithine cream was applied onto a mouse skin area pretreated with microneedles, most likely because the micropores created by microneedles allowed the permeation of eflornithine into the skin, as confirmed in an in vitro permeation study. Immunohistochemistry data revealed that cell proliferation in the skin and hair follicles was also significantly inhibited when the eflornithine cream was applied onto a skin area pretreated with microneedles.
Conclusion: The integration of microneedle treatment into topical eflornithine therapy represents a potentially viable approach to increase eflornithine's ability to inhibit hair growth.
Introduction
Hirsutism is a cosmetic medical problem that is manifested by the existence of terminal hair in androgen-dependent areas in females that follows a male-like pattern (Escobar-Morreale, Citation2010; Sornalingam & Cooper, Citation2014). Terminal hair differs from vellus hair in being coarse, medullated and pigmented (e.g. the scalp, eye lashes and eyebrow hair) (Azziz, Citation2003; Onselen, Citation2011; Sornalingam & Cooper, Citation2014). The condition affects 5–10% of women at the reproductive age (Falsetti et al., Citation2000; Mofid et al., Citation2008; Harrison et al., Citation2010), and comprises a serious psychosocial problem that, in many cases, deteriorates self-esteem and may lead to social introversion and depression (Koulouri & Conway, Citation2009; Castelo-Branco & Cancelo, Citation2010; Harrison et al., Citation2010). In addition to being androgen-dependent (mainly due to polycystic ovary syndrome (PCOS)), hirsutism can also be idiopathic (Rittmaster, Citation1997; Harrison et al., Citation2010; Onselen, Citation2011; Sornalingam & Cooper, Citation2014). Meanwhile, hirsutism should be differentiated from hypertrichosis, as the hair in this case is mainly vellus, and the etiology is completely different (Mofid et al., Citation2008; Castelo-Branco & Cancelo, Citation2010; Sornalingam & Cooper, Citation2014).
Management of hirsutism usually comprises the combined use of medical treatment in addition to mechanical removal of the excessive hair (Azziz, Citation2003). Medical treatment includes the use of ovarian androgen suppression agents (e.g. oral contraceptives), peripheral androgen blockers (e.g. flutamide, spironolactone and cyproterone) and insulin sensitizers (e.g. troglitazone) (Azziz, Citation2003; Koulouri & Conway, Citation2009; Castelo-Branco & Cancelo, Citation2010). However, since hirsutism itself is more of a cosmetic issue, the risk to benefit ratio of these drugs has to be considered (Balfour & McClellan, Citation2001). In addition to being expensive (e.g. finasteride and troglitazone), most of these drugs are also associated with rather unwanted side effects, including hyperkalemia and menstrual disturbances (e.g. spironolactone) (Falsetti et al., Citation2000; Castelo-Branco & Cancelo, Citation2010; Escobar-Morreale, Citation2010), risk of hepatotoxicity (e.g. flutamide) (Koulouri & Conway, Citation2009; Escobar-Morreale, Citation2010) that can be fatal in 5% of cases (Castelo-Branco & Cancelo, Citation2010), potential teratogenecity (e.g. finasteride) (Castelo-Branco & Cancelo, Citation2010) and weight gain (e.g. cyproterone acetate) (Koulouri & Conway, Citation2009). Mechanical methods such as shaving, waxing, threading, plucking, bleaching and the use chemical depilatories (Harrison et al., Citation2010; Onselen, Citation2011) are used on a regular bases, either alone (in milder cases) or in combination with drug treatment (Balfour & McClellan, Citation2001). However, most of the aforementioned methods may create an additional nuisance to patients, either for their high frequency of applications or for their economic burden (Wolf et al., Citation2007). Permanent removal of hair using laser has proven to be successful, but is also associated with some drawbacks, including the high costs due to multiple treatments, pain, response variability and the risk of scarring (Hamzavi et al., Citation2007; Wolf et al., Citation2007). Eflornithine has been previously used intravenously for the treatment of sleeping sickness disease (i.e. Human African Trypanosomiasis) (Mpia & Pepin, Citation2002; Burri & Brun, Citation2003; Priotto et al., Citation2008). It showed marked efficacy, lower systemic toxicity compared to the standard trypanosomiasis treatment melarsoprol (Milford & Pepin, Citation1992; Chappuis et al., Citation2005), and high tolerability even when given at higher doses to children (Priotto et al., Citation2008). Eflornithine was also found to reduce facial hair growth and was approved by the U.S. Food and Drug Administration (FDA) for topical treatment of hirsutism in 2000 (Vaniqa®, Allergan, Irvine, CA) (Balfour & McClellan, Citation2001; Shapiro & Lui, Citation2001; Jackson et al., Citation2007). It functions by irreversible inhibition of ornithine decarboxylase that is responsible for the catalysis of ornithine to putrescine (Hickman et al., Citation2001), which, among other polyamines, is critical for hair follicle growth and proliferation (Shapiro & Lui, Citation2001; Azziz, Citation2003; Jobanputra et al., Citation2007; Wolf et al., Citation2007). In a randomized, double-blinded clinical study to evaluate the efficacy and safety of topical eflornithine hydrochloride 13.9% cream against hirsutism in women, it was found that twice-daily application of the cream for 24 weeks significantly reduced the length and hair mass (as area) compared to control (Wolf et al., Citation2007). Based on physician's evaluation, 32% of eflornithine-treated subjects were considered success, and only an overall 58% of the eflornithine-treated subjects were comparatively better, relative to control, independent of the method of hair removal (Wolf et al., Citation2007). In another randomized, double-blinded study, based on patient's evaluation of success, about two-third of eflornithine-treated patients reported a decrease in the overall bother at the end of the treatment period (24 weeks), compared to one-third of patients treated with the control vehicle (Jackson et al., Citation2007). However, 8 weeks after the treatment was stopped, the levels of bother in both groups were almost equal (Jackson et al., Citation2007), indicating that a lifetime of twice-daily application is required to prevent re-growth (Shapiro & Lui, Citation2005). Based on previous studies, it is clear that the success rate of topical treatment with eflornithine cream has room to improve. Hamzavi et al. (Citation2007) obtained a significantly higher success rate when eflornithine cream was combined with laser therapy, as compared to laser therapy combined with placebo cream. In the present report, we describe a method to improve the efficacy of topical eflornithine that may also enable the reduction of the frequency of application of the cream. The method described herein relies on the pre-treatment of the skin with microneedles prior to the application of the cream. Microneedles have been successfully used previously to improve the transdermal permeation of small molecules, large molecules and even nanoparticles (Prausnitz, Citation2004; Park et al., Citation2010; Kumar et al., Citation2011, Citation2012; Kim et al., Citation2012; Naguib et al., Citation2014). Microneedle-based transcutaneous drug delivery relies on creating micro-sized holes in the stratum corneum (Park et al., Citation2010; Kumar et al., Citation2011; Naguib et al., Citation2014). We hypothesized that pre-treatment of the skin with microneedles before the topical application of the eflornithine cream will augment eflornithine's ability to inhibit hair re-growth, because the microneedles can breach the stratum corneum barrier and increase the diffusion of eflornithine to the hair follicles in the viable dermis layer. The microneedle roller used in this study is already available on the market for human use for cosmetic and other dermatological applications (Kumar et al., Citation2011, Citation2012; Bariya et al., Citation2012; Naguib et al., Citation2014).
Materials and methods
Materials
Dermaroller® microneedle rollers (192 microneedles, 500 µm in length and 50 µm in base diameter) were kindly provided by Cynergy, LLC (Carson City, NV). Vaniqa® (eflornithine hydrochloride topical cream 13.9%) was purchased from SkinMedica (Carlsbad, CA). Potassium phosphate monobasic and d,l-α-difluoromethylornithine hydrochloride hydrate (eflornithine hydrochloride hydrate) were from Sigma–Aldrich (St. Louis, MO). Nair® lotion was from Church and Dwight Co (Princeton, NJ). GiGi® Honee wax was from American International Industries (Los Angeles, CA).
In vitro permeation of eflornithine hydrochloride through mouse skin
In vitro permeation assay using Franz diffusion cell apparatus (PermeGear, Inc., Hellertown, PA) was completed as previously described (Kumar et al., Citation2011, Citation2012; Naguib et al., Citation2014) using the lower dorsal skin of C57BL/6 mice. Hair was trimmed using an electric clipper 24 h before the collection of the skin. Skin was harvested, wrapped in aluminum foil and stored at −20 °C for a maximum period of 1 month and used whenever needed. Freezing of the skin at −20 °C (without a cryo-protectant) is commonly applied in literature, and such skin samples have been used frequently for permeability studies (Stahl et al., Citation2012). Dennerlein et al. (Citation2013) showed that freezing and storing of freshly excised human skin for up to 30 days at −20 °C does not affect the skin permeability. Other researchers showed that when human skin was wrapped into aluminum foil and stored at −26 °C, the skin retained its barrier properties for up to 6 months (Badran et al., Citation2009). After the fat layer was removed, the skin was mounted onto the Franz diffusion cells with dorsal side facing upward. The receiver compartment contained 5 ml of water and was maintained at 37 °C with a Haake SC 100 Water Circulator (ThermoScientific, Wellington, NH). The hair-trimmed skin was treated with a Dermaroller® microneedle roller as previously described before it was mounted onto the Franz diffusion cells (Kumar et al., Citation2011; Naguib et al., Citation2014). The skin sample was placed onto the flat surface of a balance, and the microneedle roller was rolled in four perpendicular directions over the skin surface, 5 times each for a total of 20 times, with an applying pressure of 350–400 g, which was constantly measured using the balance while the roller was rolled. The diffusion area of the skin was 0.64 cm2. The donor compartment was loaded with 4 mg of eflornithine hydrochloride in 500 µl water and covered with parafilm to prevent evaporation. After 0, 1, 3, 6, 8 and 24 h, samples (150 µl) were withdrawn from the receiver compartment and immediately replenished with fresh medium. The samples were analyzed using HPLC following a method described previously with modifications (Saravanan et al., Citation2009). Chromatographic analysis was carried out with an Agilent 1260 Infinity HPLC station equipped with ZORBAX Eclipse Plus C18 (5 μm, 4.6 mm × 150 mm) column using a acetonitrile-buffer mixture (70%:30%, v/v) as the mobile phase. The buffer was prepared by dissolving 0.68 g of potassium phosphate monobasic in 1 l of water. The flow rate was 0.8 ml/min. The detector wavelength was 210 nm.
Animal studies
Animal studies were carried out following the U.S. National Research Council guide for the care and use of laboratory animals. The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Female C57BL/6 mice (8–10 weeks old) were from Charles River (Wilmington, MA). C57BL/6 mice are ideal for examining the physiological actions during different hair cycle phases due to the occurrence of naturally synchronized hair cycles with cyclic pigmentation (Slominski et al., Citation1991). Each experimental group was composed of three to four mice. Hair in the lower dorsal skin of anesthetized mice was either trimmed using an electric clipper, plucked using GiGi® Honee warm wax as previously described (Xiao et al., Citation2012), or chemically removed using Nair® lotion. The skin area where the hair was removed was then treated with the eflornithine hydrochloride 13.9% cream (∼50 mg per mouse per treatment) using a spatula 2 times a day in an interval of at least 8 h for a maximum period of 36 days. A group of mice whose hair in the application site was trimmed using a clipper were also treated with the microneedle roller every time before the application of eflornithine cream as previously described (Kumar et al., Citation2012). Briefly, mice were placed onto the flat surface of a balance, and the microneedle roller was rolled over the marked skin surface, 10 times parallel to mouse length, with an applying pressure of 350–400 g as indicated on the balance. In control groups, the hair in mouse dorsal skin was removed by trimming, plucking or chemical depilation with Nair®, but the area was not treated with the eflornithine cream. The hair re-growth was evaluated by taking digital photographs of the mouse skin areas for a maximum period of 36 days after the first application of the eflornithine cream. On the last day of the study, animals were euthanized and skin samples were collected from the treated areas for immunohistochemical studies.
Immunohistochemistry
Skin tissues were fixed with a buffered formalin (10%) solution for 24 h, washed with 0.1 M of sodium phosphate buffer (pH 7.4), dehydrated in graded ethanol, embedded in paraffin and sectioned vertically. The sections were stained using hematoxylin–eosin (H&E) or an antibody against 5-bromo-2′-deoxyuridine (BrdU) (Sigma–Aldrich, St. Louis, MO) in the Histology and Tissue Processing facility in the Dell Pediatric Research Institute at the University of Texas at Austin. Mice were injected intraperitoneally with BrdU in phosphate buffered saline (PBS, pH 7.4, 10 mM) at the dose of 100 µg/g body weight, 30 min prior to euthanasia. All skin sections were examined under an Olympus BX53 microscope (Olympus, Center Valley, PA).
Results and discussion
Eflornithine does not remove hair, but rather inhibits or actually slows down hair re-growth (Azziz, Citation2003). The evaluation of length and density of re-growing hair over time was used clinically as a tool to assess the success of eflornithine treatment (Wolf et al., Citation2007). In this report, hair on an area in the rear dorsal skin of mice was removed by trimming, plucking or chemical depilation. Vaniqa cream was then applied twice daily on the hair-removed skin area, and the hair re-growth was monitored by daily visual observation. To test the effect of pretreatment of the skin area, where the Vaniqa cream was applied, with micorneedles on the eflornithine cream ability to inhibit hair re-growth, in another hair-trimmed group of mice, the application area was pretreated with microneedles prior to the application of the Vaniqa cream. The effect of every hair removal technique and microneedle treatment on eflornithine’s ability to slow down hair regrowth was evaluated based on the time at which hair re-growth was noticeable.
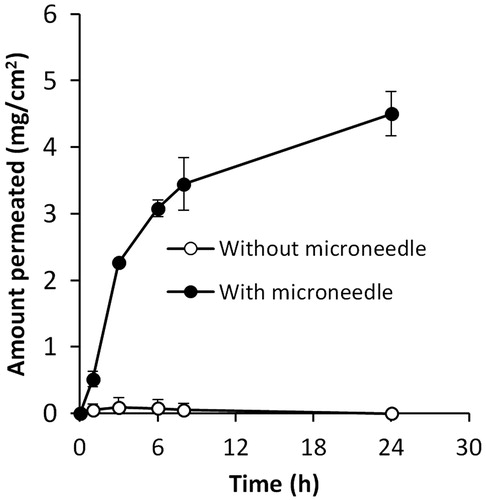
As shown in , in mice whose hair in the dorsal skin area was removed by plucking or chemical depilation without further treatment with the eflornithine cream, significant hair growth was noticeable within 12 days after hair removal. However, in mice whose hair in the dorsal skin was trimmed, with or without further treatment with microneedles and eflornithine cream, apparent hair re-growth was not observed until the 28th day following hair removal. As expected, in all groups, treatment with the eflornithine cream significantly inhibited hair re-growth (). Treatment with the eflornithine cream following pretreatment with microneedles was the most effective in inhibiting hair re-growth (). All four mice in the group where the eflornithine cream was applied following pretreatment with microneedles did not show any hair growth within 36 days, whereas in the group where the eflornithine cream was applied after hair trimming without further pretreatment with microneedles, significant hair re-growth was noticeable in three of the four mice (). In fact, in a separate experiment, it was found that significant hair re-growth was noticeable in all mice in the group where eflornithine cream was applied after hair trimming without further pretreatment with miccroneedles within 35 days (data not shown). It is likely that the micropores created in the mouse skin by the microneedles allowed the eflornithine applied onto the pretreated area to more efficiently diffuse into the skin and the hair follicles in the skin, inhibiting the hair re-growth more effectively. In fact, data from an in vitro skin permeation study showed that the permeation of eflornithine across mouse skin that was pretreated with microneedles was significantly higher than across mouse skin that was not pretreated with microneedles (). Without further studies, it is not possible to predict the percentage of the eflornithine in the Vaniqa cream that diffused into mouse skin or the hair follicles in the animal study shown in , but the increased permeation of eflornithine across mouse skin after pretreatment with microneedles as shown in clearly indicated that the microneedles had created pores or holes on the skin stratum corneum, which is the barrier that prevents eflornithine from entering the viable skin layers.
Figure 1. Digital photographs of C57BL/6 mouse dorsal skin with and without treatment with the Vaniqa eflornithine cream (13.9%) for up to 36 days. The hair on the application area was removed by plucking using GiGi® Honee warm wax, chemical depilation using Nair® or trimming with a clipper. In one group (bottom), following trimming, the skin area was also treated with a microneedle roller (microneedle length, 500 µm; base diameter, 50 µm) every time before the application of the eflornithine cream. The rectangles indicate the mouse skin area where the eflornithine cream was applied. For a full-color version of the figure, the reader is referred to the online version of the article.
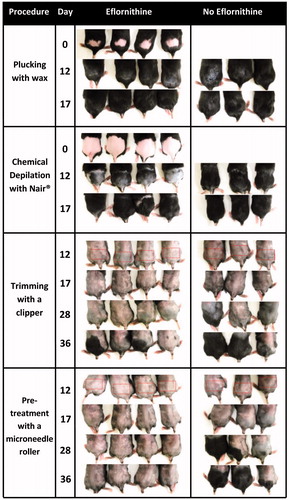
Figure 2. In vitro permeation of eflornithine hydrochloride in a solution through a mouse skin area where the hair was trimmed (without microneedle) or trimmed and then treated with microneedles (with microneedle). Data shown are mean ± SD (n = 3).
Data in showed that hair removal using an electric clipper is a better option than using waxing or a chemical depilatory cream for removing unwanted hair during the course of eflornithine cream treatment. Plucking by waxing or chemical depilation with Nair® appeared to have stimulated hair growth, which is in agreement with previous reports (Stenn & Paus, Citation2001). More importantly, eflornithine cream applied on a skin area pretreated with microneedles was the most effective in inhibiting hair re-growth ().
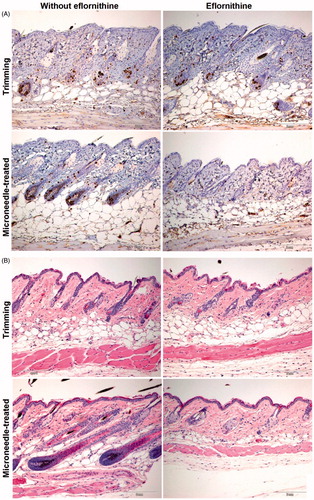
The skin and the hair follicles in the skin area that was treated with the Vaniqa cream were also examined histologically. The skin samples were collected on the last day of eflornithine cream treatment, sectioned and stained with H&E or an anti-BrdU antibody (a marker of cell proliferation). Compared with hair trimming alone, treatment with the eflornithine cream after trimming did not significantly affect the extent of anti-BrdU-positive staining in the skin and hair follicles in the treated area (). Treatment with microneedles alone without eflornithine cream apparently increased anti-BrdU positive staining, suggesting that repeated microneedle treatments stimulated cell proliferation (). In contrast, anti-BrdU positive staining was rarely detected in the hair follicles in the skin area that was treated with the eflornithine cream following pretreatment with microneedles (), which explains the lack of noticeable hair re-growth in the dorsal skin area in mice that were topically treated with the eflornithine cream following pretreatment with microneedles (). Since the main mechanism of hair growth inhibition exerted by eflornithine is due to the inhibition of cellular proliferation, it is likely that pretreatment of the mouse skin area with microneedles before the topical application of the eflornithine cream had allowed more eflornithine to reach the hair follicles. The hair follicles in the skin area that was treated with microneedles, but without the eflornithine cream, appeared larger than in other groups (). However, the hair follicles in the skin area treated with the eflornithine cream following pretreatment with microneedles were smaller and morphologically abnormal (). It seemed that treatment with microneedles alone facilitated hair follicle growth, but pretreatment with microneedles followed by eflornithine cream application significantly inhibited hair follicle growth (). Finally, it also appeared that repeated treatments with microneedles (without the eflornithine cream) increased the thickness of the skin, whereas the combination of microneedle pretreatment with the eflornithine cream treatment decreased it ().
Figure 3. Representative micrographic pictures of skin samples after anti-BrdU staining (A) or H&E staining (B). Scale bar = 2 mm. For a full-color version of the figure, the reader is referred to the online version of the article.
It is worth noting that although mouse skin and hair growth on mouse skin do not resemble human skin in many aspects, studies with many hair growth inhibitors and hair growth promoting drugs have been performed with different strains of mice, including the C57BL/6 mice used in the present study (Jo et al., Citation2013; Kang et al., Citation2013), and it is expected that the information learned using mice and mouse skin in the present study will likely be useful in designing improved eflornithine therapy of unwanted hair growth in humans in the future. Further investigation is still needed to evaluate the safety of this microneedle-based modality in enhancing the eflornithine cream’s ability to inhibit hair re-growth. Safety concerns may arise as a result of the expected increase in the systemic absorption of eflornithine across the skin, as well as the repeated life-long microneedle application. Eflornithine is well-tolerated when given systemically by i.v. infusion for the treatment of trypanosomiasis in adults and children, even when given at high doses (Chappuis et al., Citation2005; Priotto et al., Citation2008). Transdermal absorption is usually very limited (<1%), and most of the absorbed eflornithine is excreted unchanged in the urine (Malhotra et al., Citation2001). The improved hair growth inhibition by the combined treatment with microneedles and eflornithine may also allow less frequent application/exposure. Inactive ingredients in the Vaniqa cream include ceteareth-20, cetearyl alcohol, dimethicone, glyceryl stearate, PEG-100 stearate, mineral oil and parabens, which are all FDA-approved inactive ingredients for topical use. It is unclear whether pretreatment of skin with microneedles affects the safety or toxicity profiles of those inactive ingredients after topical application. Previous studies showed that microneedle application was painless and caused no skin irritation (Kaushik et al., 2001). In the present study, no noticeable irritation was observed on the mouse skin area during the 36 days of twice daily eflornithine treatments following microneedle application. No visible signs of local adverse effects, such as inflammation or swelling, were observed on the skin surface even when skin was examined under a microscope after the completion of the study.
The microneedle rollers used herein and other similar ones are already used by humans. Compared to other microneedle-mediated drug delivery methods (e.g. solid microneedles coated with drugs, dissolvable microneedles with drugs incorporated in microneedles and hollow microneedles for injection), the microneedle roller is a preferred design because of the roller's ability to cover a larger area of the skin, where the eflornithine cream can be readily applied prior to or after the microneedle roller treatment. Of course, innovative microneedle designs that take consideration of the “topography” of the facial skin surface may be needed to make long-term microneedle treatment more friendly and convenient to patients.
Conclusion
In the present study, it was shown that pretreatment of mouse skin with microneedles before topically applying eflornithine cream significantly enhanced eflornithine ability to inhibit hair growth. This finding likely has clinical implications because it may be beneficial to recommend patients who are prescribed with topical eflornithine cream to gently trim the unwanted hair with an electric clipper and then treat the desired skin area with microneedles using a self-applying device such as the microneedle roller before applying the eflornithine cream.
Acknowledgements
The authors acknowledge Cynergy, LLC (Carson City, NV) for generously providing Dermaroller® microneedle rollers free of charge. This work was supported in part by a NIH grant (AI078304 to ZC) and The University of Texas at Austin College of Pharmacy (to ZC).
Declaration of interest
The authors declare that they have no conflicts of interest to disclose. Y.W.N. was supported by a doctoral scholarship from the Egyptian Ministry of Higher Education.
References
- Azziz R. (2003). The evaluation and management of hirsutism. Obstet Gynecol 101(Pt 1):995–1007
- Badran MM, Kuntsche J, Fahr A. (2009). Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci 36:511–23
- Balfour JA, McClellan K. (2001). Topical eflornithine. Am J Clin Dermatol 2:197–201
- Bariya SH, Gohel MC, Mehta TA, Sharma OP. (2012). Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 64:11–29
- Burri C, Brun R. (2003). Eflornithine for the treatment of human African trypanosomiasis. Parasitol Res 90(Supp 1):S49–52
- Castelo-Branco C, Cancelo MJ. (2010). Comprehensive clinical management of hirsutism. Gynecol Endocrinol 26:484–93
- Chappuis F, Udayraj N, Stietenroth K, et al. (2005). Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis. Clin Infect Dis 41:748–51
- Dennerlein K, Schneider D, Goen T, et al. (2013). Studies on percutaneous penetration of chemicals – impact of storage conditions for excised human skin. Toxicol In Vitro 27:708–13
- Escobar-Morreale HF. (2010). Diagnosis and management of hirsutism. Ann N Y Acad Sci 1205:166–74
- Falsetti L, Gambera A, Platto C, Legrenzi L. (2000). Management of hirsutism. Am J Clin Dermatol 1:89–99
- Hamzavi I, Tan E, Shapiro J, Lui H. (2007). A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol 57:54–9
- Harrison S, Somani N, Bergfeld WF. (2010). Update on the management of hirsutism. Cleve Clin J Med 77:388–98
- Hickman JG, Huber F, Palmisano M. (2001). Human dermal safety studies with eflornithine HCl 13.9% cream (Vaniqa), a novel treatment for excessive facial hair. Curr Med Res Opin 16:235–44
- Jackson J, Caro JJ, Caro G, et al. (2007). The effect of eflornithine 13.9% cream on the bother and discomfort due to hirsutism. Int J Dermatol 46:976–81
- Jo SJ, Choi SJ, Yoon SY, et al. (2013). Valproic acid promotes human hair growth in in vitro culture model. J Dermatol Sci 72:16–24
- Jobanputra KS, Rajpal AV, Nagpur NG. (2007). Eflornithine. Indian J Dermatol Venereol Leprol 73:365–6
- Kang JI, Kim EJ, Kim MK, et al. (2013). The promoting effect of Ishige sinicola on hair growth. Mar Drugs 11:1783–99
- Kaushik S, Hord AH, Denson DD, et al. (2001). Lack of pain associated with microfabricated microneedles. Anesth Analg 92:502–4
- Kim YC, Park JH, Prausnitz MR. (2012). Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64:1547–68
- Koulouri O, Conway GS. (2009). Management of hirsutism. BMJ 338:823–6
- Kumar A, Li X, Sandoval MA, et al. (2011). Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomed 6:1253–64
- Kumar A, Wonganan P, Sandoval MA, et al. (2012). Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J Control Release 163:230–9
- Malhotra B, Noveck R, Behr D, Palmisano M. (2001). Percutaneous absorption and pharmacokinetics of eflornithine HCl 13.9% cream in women with unwanted facial hair. J Clin Pharmacol 41:972–8
- Milford E, Pepin J. (1992). Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping. Lancet 340:652–655
- Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. (2008). Hirsutism. Int J Clin Pract 62:433–43
- Mpia B, Pepin J. (2002). Combination of eflornithine and melarsoprol for melarsoprol-resistant Gambian trypanosomiasis. Trop Med Int Health 7:775–9
- Naguib YW, Kumar A, Cui Z. (2014). The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm Sin B 4:94–9
- Onselen JV. (2011). Hirsutism: causes and treatment for women. Br J Nurs 20:985–6,988,990
- Park JH, Choi SO, Seo S, et al. (2010). A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm 76:282–9
- Prausnitz MR. (2004). Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 56:581–7
- Priotto G, Pinoges L, Fursa IB, et al. (2008). Safety and effectiveness of first line eflornithine for Trypanosoma brucei gambiense sleeping sickness in Sudan: cohort study. BMJ 336:705–8
- Rittmaster RS. (1997). Hirsutism. Lancet 349:191–5
- Saravanan C, Kumudhavalli MV, Kumar M, Jayakar B. (2009). A new validated RP-HPLC method for estimation of eflornithine hydrochloride in tablet dosage form. J Pharm Res 2:1730–1
- Shapiro J, Lui H. (2005). Treatments for unwanted facial hair. Skin Ther Lett 10:1–4
- Shapiro J, Lui H. (2001). Vaniqa – eflornithine 13.9% cream. Skin Ther Lett 6:1–3, 5
- Slominski A, Paus R, Costantino R. (1991). Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol 96:172–9
- Sornalingam S, Cooper M. (2014). Hirsutism. InnovAiT Educ Inspir Gen Pract 7:160–7
- Stahl J, Wohlert M, Kietzmann M. (2012). Microneedle pretreatment enhances the percutaneous permeation of hydrophilic compounds with high melting points. BMC Pharmacol Toxicol 13:5
- Stenn KS, Paus R. (2001). Controls of hair follicle cycling. Physiol Rev 81:449–94
- Wolf JE Jr, Shander D, Huber F, et al. (2007). Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol 46:94–8
- Xiao G, Li X, Kumar A, Cui Z. (2012). Transcutaneous DNA immunization following waxing-based hair depilation elicits both humoral and cellular immune responses. Eur J Pharm Biopharm 82:212–17

