Abstract
Cancer nanotherapeutics is beginning to overwhelm the global research and viewed to be the revolutionary treatment regime in the medical field. This investigation describes the development of a stable nanostructured lipid carrier (NLC) system as a carrier for curcumin (CRM). The CRM-loaded NLC developed as a particle with the size of 146.8 nm, a polydispersity index of 0.18, an entrapment efficiency (EE) of 90.86%, and the zeta potential (ZP) of −21.4 mV. Besides, the increased cytotoxicity of CRM-NLC than that of CRM to astrocytoma-glioblastoma cell line (U373MG) in the cancer cell lines was observed. Results of biodistribution studies showed higher drug concentration in brain after intranasal administration of NLCs than PDS. The results of the study also suggest that CRM-NLC is a promising drug delivery system for brain cancer therapy.
Introduction
Cancer is the most distressing and life-threatening disease that enforces severe death worldwide. Brain tumors constitute a profound and unsolved clinical problem although significant steps have been made in the treatment of many other cancer types. The treatment of brain cancer is one of the most difficult challenges in oncology. Malignant brain tumors are one of the most lethal forms of brain cancer. Median survival for patients with malignant brain tumors is only 48 weeks from diagnosis. Conventional small molecule drugs often exhibits several disadvantages including lack of sufficient specificity to the tumor, severe and toxic side effects in healthy tissues, limited delivery of hydrophobic drugs to the tumor cells, and drug resistance. This results in sub-therapeutic exposure of the tumor tissue to the drugs. The failure of chemotherapy is due to the inability of intravenously administered anticancer agents to reach the brain tissue, because of their inability to cross BBB. Another obstacle in chemotherapy is it is difficult to maintain a higher concentration of therapeutic agents at the tumor site. To enhance the drug exposure, recently the use of colloidal drug has been extensively explored. Accumulation and retention of a drug-loaded nano-scaled carrier at the brain tumor site could allow localized release of a drug, thereby enhancing tumor exposure to the cytotoxic agent. There is a clear relationship between drug lipid solubility and its central nervous system (CNS) penetration. So, the use of colloidal drug delivery system like lipid nanoparticles will be a very good approach to overcome the problem of poor penetration. Lipid nanoparticles (solid lipid nanoparticles – SLNs and nanostructured lipid carriers – NLCs) are colloidal particles composed of a biocompatible/biodegradable lipid matrix that is solid at body temperature and exhibits a size range in between 100 and 400 nm. They combine advantages of colloidal drug carrier systems like liposomes, polymeric nanoparticles, and emulsions, but at the same time, they avoid or minimize the drawbacks associated with them (Müller et al., Citation2002; Joshi & Muller, Citation2009). NLCs are composed of a binary mixture of a solid lipid and a liquid lipid and show an increased drug loading compared with SLN. NLCs are investigated for different routes of administration (Feng et al., Citation2011; Liu et al., Citation2011).
Targeted drug delivery is frequently used to deliver drugs selectively and at high concentrations to cancer tissue. One of the promising strategies to deliver neuro-therapeutics to brain is intranasal delivery. It is a non-invasive technique for bypassing blood–brain barrier (BBB) and ensures direct delivery to CNS.
The size of nanoparticles is a fundamental characteristic that determines the passive targeting of and biodistribution within brain tumors. Nanoparticles within the size range of 10–100 nm take advantage of the hyper-vascularized, leaky, and compromised lymphatic drainage system in a brain tumor to passively target and access the intratumoral space while being denied access to healthy brain tissue.
Curcumin (diferuloylmethane) is a phytochemical that has potent antiproliferative effects against a variety of tumors in vitro. It exerts its effects via diverse biological properties, including, but not limited to, suppression of nuclear factor-κB and inhibition of angiogenesis (Arbiser et al., Citation1998; Aggarwal et al., Citation2003; Li et al., Citation2005). It also enhances the antitumor effects of several classic chemotherapeutic drugs, such as doxorubicin, cis-platinum, and paclitaxel (Chan et al., Citation2003; Bava et al., Citation2005). In spite of being a ‘wonder molecule,’ the therapeutic efficacy of curcumin is limited by poor aqueous solubility, chemical instability in alkaline medium, rapid metabolism, and poor absorption from the gastrointestinal tract (Anand et al., Citation2007).
In order to solubilize curcumin in a physiologic stable medium and possibly to improve their brain targeting, a nanoparticulate system appears as the optimal solution. Lipid-based nanoparticles can be considered as a versatile tool with a high potential of applications; in fact they can solubilize a number of molecules with different physicochemical properties in a biocompatible and biodegradable matrix with well-established safety profiles (Battaglia & Gallarate, Citation2012; Nyström & Fadeel, Citation2012).
The goal of this study was to design and produce NLC containing curcumin for intranasal delivery to CNS. The objectives include identification of formulation variables affecting NLC formulation and its characteristics like size and zeta potential (ZP). Further objective was to carry out in vitro cyto-toxicity and brain distribution studies of the developed formulations.
Materials and methods
Materials
Curcumin was obtained from Hi Media Lab. Pvt. Ltd, Mumbai, India (Batch no. 155108). Precirol OTO5 was obtained from Gattefosse (Nanterre, France), Capmul MCM was obtained from ABITEC Corporation (Columbus, OH), Tween 80 was obtained from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India), Soya lecithin PHOSPHOLIPID was obtained from GmbH, Krefeld, Germany. All other reagents used were of analytical grade.
Methods
In the present work, two chemically different lipids precirol (solid lipid) and capmul MCM (liquid lipid) were selected from lipid-screening tests. Tween 80 and soya lecithin (SL) were chosen as a surfactant and a stabilizer, respectively. NLCs were prepared by hot high pressure homogenization (HPH) (How et al., Citation2011). Briefly, the lipid phase containing CRM, precirol, and capmul MCM was heated up to 85 ± 0.5 °C to melt the lipids. Pre-emulsion was prepared by dispersing the hot aqueous surfactant (Tween-80 and SL) solution to above-melted lipid phase under mechanical stirring (Remi Instruments Ltd, Mumbai, India) at 1500 rpm maintained at 85 ± 0.5 °C for 20 min. The resultant pre-emulsion was then passed through HPH (PANDA 2K, Niro Soavi, Italy) at 600 bar pressure and for four cycles. During processing of the samples, machine was coated with a heater tape and the temperature of the homogenization circuit was maintained at 85 ± 0.5 °C to avoid the risk of boiling, in the sample-collecting deposit, a digital thermometer was placed. Ultimately, the resulting hot o/w nanoemulsion was cooled to 4 ± 0.5 °C, recrystallization of the lipid and CRM-loaded NLCs (CRM-NLCs) were generated (Araújo et al., Citation2010). The CRM-NLCs' aqueous dispersion was centrifuge at 45 000 rpm for 35 min at room temperature by using Optima “MAX-XP” ultracentrifuge (Beckman Coulter, Nyon, Switzerland) for the separation of the nano-particulate system. Deposited particulate was redispersed in little amount of water. The CRM-NLCs' aqueous dispersion with 5% cryoprotectant (mannitol) was frozen in a refrigerator at −70 °C for 12 h. Then the samples were lyophilized using a lab freeze dryer (Vir-Tis Benchtop, SP Scientific, Warminster, PA). After freeze drying for 28 h, the vials were sealed with rubber caps (Li, 2002; Kakkar et al., Citation2013).
Characterization of NLCs
Photon correlation spectroscopy
The mean particle size (MPS) and poly dispersity index (PDI) were determined by photon correlation spectroscopy (PCS) using a Malvern Zetasizer (Nano ZS 90, Malvern Ltd., Malvern, UK). The measurement using PCS is based on the light-scattering phenomena in which the statistical intensity fluctuations of the scattered light from the particles in the measuring cells are measured. Prior to the measurements, all samples were diluted with double-distilled water to produce a suitable scattering intensity. The z-average and PDI values were obtained at an angle of 90° using disposable polystyrene cells having 10 mm diameter cells at 25 °C, which were equilibrating for 120 s. Refractive index (RI), for size measurement of lipid NPs dispersion, was set as RI = 1.330 (abs = 0.01). All measurements were performed in triplicate at 25 °C (Araújo et al., Citation2010; Liu et al., Citation2011).
Zeta potential
The ZP, reflecting the electric charge on the particle surface and indicating the physical stability of colloidal systems, was measured by determining the electrophoretic mobility using the Malvern Zetasizer (Nano ZS 90, Malvern Ltd., Malvern, UK). The measurements were performed following dilution in double-distilled water. It was measured using the Dip cell by applying a field strength of 20 V/cm and the average of the ZP was given from 30 runs.
Entrapment efficiency and drug loading
Percent entrapment efficiency (EE) is defined as the percentage of drug incorporated into the lipid nanoparticles relative to the total drug added. It specifies how much percent of drug is included in the particles and how much percent of free drug is still present in the dispersion medium. For this, CRM-NLCs' dispersion was centrifuge at 45 000 rpm for 35 min; 1.0 ml of the supernatant collected after centrifugation was diluted with 3.0 ml of DMSO and methanol was measured spectrophotometrically at 423 nm using a UV–Visible spectrophotometer (UV 1700, Shimadzu, Kyoto, Japan) (Liu & Wu, Citation2010).
Loading capacity/drug loading (DL) refers to the percentage of drug incorporated into the lipid nanoparticles relative to the total weight of the lipoidal phase (i.e. lipid + drug). For this, CRM from lyophilized powder was extracted with DMSO and methanol. The CRM content was analyzed spectrophotometrically (UV 1700, Shimadzu, Kyoto, Japan) at 423 nm, against the DMSO and methanol mixture as a blank.
The %EE and% DL were calculated using the following equations:
(1)
(2)
Morphological study
The NLCs were examined morphologically by scanning electron microscope (JSM-6390LV, JEOL, Tokyo, Japan) with 20 kV accelerating voltage. Samples were prepared by placing NLCs onto an aluminum specimen stub, dried overnight, and sputter coated with gold prior to imaging.
Differential scanning calorimetry
Thermal analysis was performed using a differential scanning calorimetry (DSC) (Mettler-Toledo, Greifensee, Switzerland). The instrument was calibrated with indium. DSC thermograms were recorded for pure CRM, lipid-CRM physical mixture, and CRM-loaded NLCs. The samples, weighing 2 mg, were analyzed in sealed and pin-holed standard 40 μl aluminum pan, with a heating rate of 10 °C/min from 30 °C to 300 °C and, during the measurement, the sample cell was continuously purged with nitrogen at a flow rate of 40 ml/min.
X-ray diffraction studies
X-ray diffraction (XRD) patterns of CRM, freeze-dried blank NLCs, and CRM-loaded NLCs' formulation were obtained using the X-ray diffractometer (Brucker Axs, D8 Advance, Karlsruhe, Germany) in which the Cu-Kα line was used as a source of radiation by operating at a voltage of 40 kV and the current applied was 30 mA. All samples were measured in the 2θ angle range between 10° and 60° with a scanning rate of 3°/min and a step size of 0.02°.
In vitro drug release
In vitro drug release from lyophilized powder was carried out by the Franz diffusion cell having a diameter of 2.0 cm and a capacity of 25 ml. Dialysis membrane (Himedia, Mumbai, India) having molecular weight cut-off range of 12 000–14 000 kDa was used as a diffusion membrane. Pieces of dialysis membrane were soaked in simulated nasal fluid (SNF) of pH 6.4 for 24 h prior to experiment. The diffusion cell was filled with SNF of pH 6.4 and the dialysis membrane was mounted on a cell. The temperature was maintained at 37 °C. After a pre-incubation time of 20 min, the lyophilized powder equivalent to 50 mg of CRM was dispersed in 3 ml of SNF and was placed in the donor compartment. Samples were periodically withdrawn from the receptor compartment and replaced with an equivalent quantity of fresh SNF, and assayed by a UV spectrophotometer at 423 nm.
Ex vivo permeation study
Fresh nasal tissues were carefully removed from the nasal cavity of sheep obtained from the local slaughterhouse. Tissue samples were inserted in Franz diffusion cells displaying a permeation area of 0.785 cm2. SNF of pH 6.4 with 1% SLS (25 ml) at 37 °C was added to the acceptor compartment. The temperature within the chambers was maintained at 37 °C. After a pre-incubation time of 20 min lyophilized powder equivalent to 50 mg of CRM was dispersed in 3 ml of SNF of pH 6.4 and placed in the donor chamber. At predetermined time points, 3 ml of the samples were withdrawn from the acceptor compartment, replacing the sampled volume with SNF of pH 6.4 after each sampling, for a period of 11 h. The samples withdrawn were filtered and used for analysis. Blank samples (without drug) were run simultaneously throughout the experiment to check for any interference. The amount of permeated drug was determined using a UV–Visible spectrophotometer at 423 nm.
The permeation coefficient was calculated by using the following formula:
(3)
where Jss is the permeability coefficient, Co is the initial concentration in the donor compartment, A is the area of mucosal surface, dc/dt is the rate of permeability.
Histopathological studies
Histopathological studies were carried out using isolated sheep nasal mucosa. Three sheep nasal mucosa pieces (A, B, and C) with a uniform thickness were selected and mounted on Franz diffusion cells. A was treated with SNF of pH 6.4 (negative control), B was treated with CRM-loaded NLC, and C was treated with isopropyl alcohol (positive control). After treatment for 6 h, all the samples were washed properly with double-distilled water, sectioned, and stained with hematoxylin and eosin. The mucosa was dissected out, then subjected to histological studies to evaluate the toxicities of NLC and photographed by optical microscope (Jiang et al., Citation1995).
In vitro cytotoxicity assay
Cytotoxicity assay of CRM-NLCs was carried out using astrocytoma–glioblastoma cell line (U373MG). The cells were seeded into 96-well plates at a density of 1.0 × 104 cells per well. The cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 µg/ml penicillin, 200 µg/ml streptomycin, 2 mM l-glutamine, and the culture was maintained in a humidified atmosphere with 5% CO2. The cells were divided into two treatment groups: (a) CRM suspension and (b) freeze dried CRM-NLCs' suspension and the % inhibition was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay based on the mitochondrial reduction of yellow MTT tetrazolium dye to highly colored blue formazan product. The plates were incubated with compounds with series of concentrations tested for 48 h at 37 ± 0.5 °C in RPMI/DMEM/MEM with 10% FBS medium. Then the above media were replaced with 90 µl of fresh serum free media and 10 µl of MTT reagent (5 mg/ml) and plates were incubated at 37 ± 0.5 °C for 4 h, there after the above media was replaced with 200 µl of DMSO and incubated at 37 ± 0.5 °C for 10 min. The absorbance at 423 nm was measured on a spectrophotometer (Spectra max, Molecular Devices, Silicon Valley, CA). IC50 values were determined by plotting a graph of % inhibition versus concentration.
Biodistribution studies
All animal experiments were approved and performed in accordance with the guidelines by Institutional Animal Ethics Committee of R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur, resolution number RCPIPER/IAEC/2011-12/25. Male Wistar rats weighing 250–270 g were selected for the biodistribution studies which were divided into two groups: one for NLC formulation and another for plain drug suspension. The rats were anesthetized with an intraperitoneal injection of pentobarbital (40 mg/kg) and kept on a heating pad to maintain the body temperature. To group I, 100 μl of dispersion containing lyophilized powder equivalent to 50 mg CRM were instilled into the nostrils with the help of micropipette attached with LDPE tubing, having 0.1 mm internal diameter at the delivery site. The rats were manually fixed in supine position during intranasal administration. To group II, the plain drug suspension (PDS) (dose equivalent to 50 mg/ml) was instilled into the nostrils in a similar manner. The rats were sacrificed humanely at different time intervals and the skull was cut open and the brain was carefully excised. Each brain tissue was quickly rinsed with saline and blotted up with a filter paper to get rid of blood-taint and macroscopic blood vessels as much as possible and weighed. After weighing, the brain tissue samples were homogenized with one volume of saline in a tissue homogenizer (Teflon homogenizer, Thomas Scientific, Swedesboro, NJ). At each time point, three rats were taken for measurements. All brain homogenates were stored for up to 48 h in a deep freezer (−70 °C) until HPLC analysis.
Processing of samples
The whole procedure was carried out at room temperature. To a 100 μl of brain homogenate, 50 μl IS (4-hydroxybenzophenone, 10 μg/ml) was spiked and vortex mixed for 30 s. Then 250 µl of acetonitrile was added and vortex mixed for 3 min. The sample was centrifuged at 3500 rpm for 10 min in an ultracentrifuge. The supernatant layer was transferred to a 15-ml glass test tube, and then 4.5 ml of extraction solvent and methyl t-butyl ether:n-hexane (9:1) were added. The sample was vortex mixed for 3 min using a multi-tube vortex mixer. The organic layer was quantitatively transferred to a 6-ml glass tube and evaporated to dryness using an evaporator at 40 °C under a stream of nitrogen. Then the dried extract was reconstituted in 100 μl of water–methanol (50:50, v/v; diluent) and 20 μl aliquot was injected into the chromatographic system.
Chromatographic conditions
CRM concentrations in the brain homogenate were determined using the HPLC system as reported by Pawar et al. (Citation2012). The drug concentration in plasma was analyzed by a high-performance liquid chromatography (HPLC) method using the Rheodine type manual injector. The HPLC system (Agilent 1200 Series, Agilent Technologies, Santa Clara, CA) consisted of C18 column (Eclipsed XDB 5 μm, 4.6 mm × 150 mm, Agilent Technologies, Yishun, Singapore), Ezchrome Elite Software, quaternary pump, Model G1354 A, and ultraviolet variable wavelength diode array detector, Model G1315D. The detection wavelength was 423 nm. The mobile phase was composed of ACN–citric acid buffer (pH 3.0; 55:45). Elution was performed isocratically at 40 °C at a flow rate of 1.0 ml/min.
Calibration curves of CRM in brain homogenate
Calibration curves of CRM were prepared with brain tissue spiked with known amounts of the drug utilizing its HPLC peak area ratio to the internal standard (4-hydroxybenzophenone). The linear range of CRM was 200–2000 ng/ml in the brain tissue. The detection limits were 200 ng/ml (or 100 ng/g) for brain tissue samples.
Data analysis
The non-compartmental analysis was considered as a best suited model for the calculation of different pharmacokinetic parameters. The Cmax and Tmax were directly computed from the concentration versus the time plot. The trapezoidal method was used to calculate the concentration–time curve from time 0 to 48 h (AUC0→48). The Kinetica 5® (Thermo Fisher Scientific Demo version, Thermo Fisher Scientific, Waltham, MA) software was employed for the study.
Statistical analysis
Results are expressed as the mean ± SD (standard deviation) of at least three experiments. Statistical significance was assessed using the Student t-test for multiple comparisons with p < 0.05 as the minimal level of significance.
Results and discussion
The CRM-loaded NLCs were prepared using hot high pressure homogenization technique. For the preparation of CRM-NLCs, Precirol ATO5 was selected as a solid matrix and capmul MCM as a liquid lipid. Tween 80 and soya lecithin were selected as a surfactant and a stabilizer, respectively. The balance of emulsifiers is required at the oil–water interface for the stability of dispersions. NLCs can be prepared by various methods including hot and cold homogenization, solvent diffusion, and microemulsion. The hot high pressure homogenization method was simple and quick at laboratory scale; also there is no use of organic solvents in the development of formulation. Therefore, for the preparation of CRM-NLCs, hot HPH technique was selected with precirol and capmul MCM, which provided the highest drug pay-load and there was no problem of drug leakage out (Zhang et al., Citation2010). The homogenizer pressure was optimized to 600 bar for four cycles at 85 ± 0.5 °C (Obeidat, 2012).
Photon correlation spectroscopy
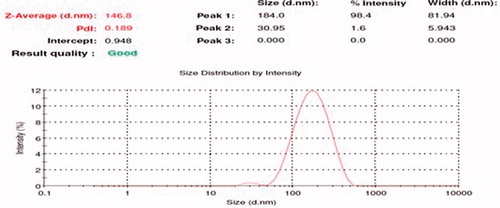
In , a small peak is followed by a broader peak, indicating two populations of particles: the first one can observe a negligible first population of particles having a diameter of 35.95 nm and a second broader peak represented one with a larger diameter of 146.8 nm with polydispersity indexes (PDI) of 0.18 (). The particle size of the NLCs' dispersion is a crucial factor because it determines the rate and the extent of drug release as well as drug absorption. The smaller droplet size provides a larger interfacial surface area for drug absorption. The particles having average diameter up to 200 nm could be easily transported transcellularly via intranasal route. In addition, it was suggested that the smaller size permit a faster release rate. Also, it has been reported that the smaller particle size may lead to more rapid absorption and improve the bioavailability. Also, PDI measures the width of particle size distribution. If PDI is lower than 0.5, it might be associated with a high homogeneity in the particle population, whereas high PDI values suggest a broad size distribution.
Zeta potential
The ZP represents the electrical charge to the NLCs' surface. The greater the ZP value, the more likely the suspension is to be stable because the charged particles repel one another and thus overcome the natural tendency to aggregate. It is currently admitted that higher ZP values, either positively or negatively charged, mean that dispersion will have greater long-term stability. The ZP value was found to be −21.4 ± 1.87.
EE and drug loading
The use of lipid NPs as drug carriers is important as they have capacity for drug loading. For CRM-NLCs, increase in EE was observed as the amount of precirol was increased. But when the capmul MCM concentration was increased above 35%, it was observed that the EE was decreased. This may be due to lipid precipitation which occurs during particle production (Aggarwal et al., 2003). After NLCs' formulation, when it was cooled, recrystallization of lipids occurs resulting into a drug-free core or a core with the reduced drug content. Therefore, increase in the lipid beyond certain extent leads to poor EE. Additionally, a significant effect was observed with Tween-80. As the concentration of Tween 80 was increased, the EE of the formulation was increased. EE of the NLC dispersion was found to be 90.86%.
Scanning electron microscopy
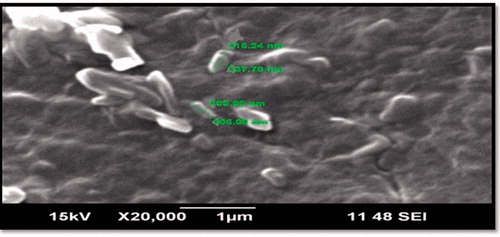
Morphological study of NLC formulation was carried by taking scanning electron microscopy (SEM) images of freeze-dried NLCs. It was revealed that they were ovoid in shape (). Size analysis results are in agreement with the report produced by photon correlation spectroscopy.
Differential scanning calorimetry
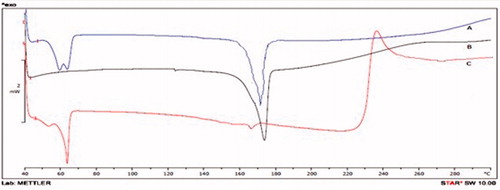
DSC was a basic method to investigate the crystallization or amorphous state of drug in the compounds and NLCs by determining the variation of temperature and energy at phase transition. shows the DSC curve of CRM, physical mixture, and lyophilized powder of CRM-NLCs. The thermogram of CRM showed a melting peak of CRM at around 173 °C. For PRE, the melting process took place with a maximum peak at 60.93 °C. All the melting peaks appeared and had almost the same value in the curve of the physical mixture. However, the melting peak for the CRM was not detected in the thermograms of the lyophilized CRM-NLCs. The disappearance of the endothermic peak of CRM in the CRM-NLCs powder suggests that the drug was molecularly dispersed in the lipid matrix and converted to an amorphous state from the crystalline state (Montenegro et al., Citation2011).
X-ray diffraction studies
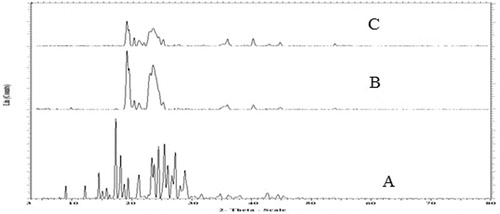
XRD was used to study the crystalline nature of lipid structure and of drug-loaded and drug-free NLCs. Sharp high intensity reflections are generated by highly crystalline lipids whereas low intensity reflections are created by amorphous background of imperfect lattice of lipids. Lipids which are mixtures of mono-, di-, and triglycerides and containing fatty acids of different chain lengths like precirol usually form crystals with many imperfections, offering space to accommodate more drug molecules. shows the XRD diffractograms of CRM, lyophilized powder of blank NLCs, and lyophilized powder of CRM-loaded NLCs. Pure CRM reflections were sharp while high intensity reflection was produced by blank NLCs whereas lyophilized CRM-loaded NLCs showed low intensity diffractograms than placebo, demonstrating the amorphous nature of NLCs.
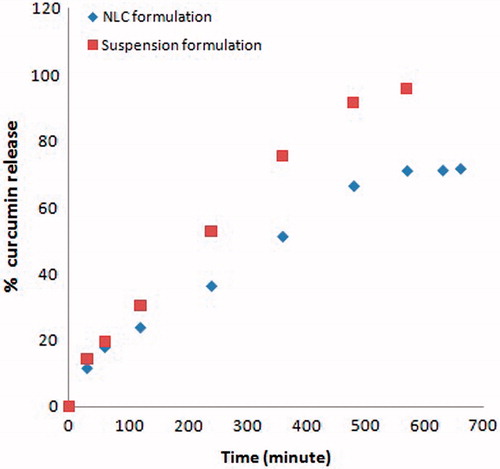
In vitro drug release
The release profile of CRM from drug-loaded NLC and plain drug suspension (PDS) through the dialysis membrane in SNF (pH 6.4) is shown in . NLC formulations showed biphasic release pattern, which was burst release at the initial stage and followed by sustained release at the constant rate. Possible explanation for this behavior is the difference in melting point of solid lipid and liquid lipid. Solid lipid which has a higher melting point could crystallize first forming a liquid lipid free or little lipid core. Finally, most of the liquid lipids located at the outer shell of the nanoparticles lead to drug-enriched shell causing burst release at the initial stage.
Ex vivo permeation studies
The drug was diffused at a faster rate and the total percentage diffusion was much higher from the nanostructured lipid carrier system than PDS. About 76.71% of the drug was diffused from drug-loaded NLC at the end of 11 h. The permeability coefficient was found to be 3.8 cm2/min.
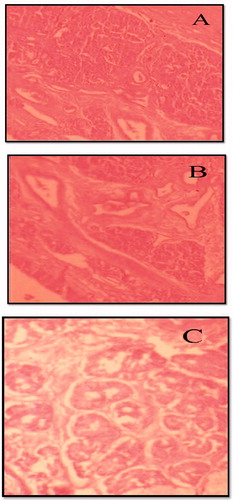
Histopathological studies
It is necessary to examine histological changes in nasal mucosa caused by formulations, if it is to be considered for practical use. Histological studies show negative control mucosa (normal nasal mucosa) and positive control mucosa stained with hematoxylin–eosin and the effect of formulation on sheep nasal mucosa, 6 h after applying the formulations (). No change in mucosal structure was seen when treated with drug-loaded NLC as compared with the positive control. The section of mucosa treated with formulation CRM-NLC showed no changes in nasal epithelium. There was no sign of remarkable destructive effect of formulations on the treated nasal mucosa.
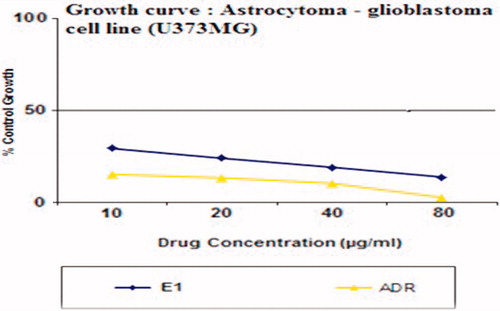
In vitro cytotoxicity assay of CRM-NLCs
The in vitro cytotoxicity of freeze-dried CRM-NLCs' suspension was examined and compared with the standard (adrenomycin) by the cell viability test. shows a graph of % inhibition of astrocytoma-glioblastoma cell line (U373MG) incubated with the CRM-NLCs and adrenomycin versus different drug concentrations. From the results, we can see that at the same concentration, CRM-NLCs produced higher inhibition on the cells. The IC50 values were found to be 9.8 ng/ml for CRM-NLCs and 13.6 ng/ml for adrenomycin, demonstrating effectiveness of CRM-NLCs against the gliblastoma cell line. Thus, CRM-NLCs show lower cellular viability which means that it increased the cytotoxicity of formulation towards cells. To explain the cytotoxic effect of NLCs, it was hypothesized that NLCs absorbed to cell surface and released CRM close to the membrane, leading to a high local drug concentration gradient or was brought in the cells and then released from the NLCs (Zhao & Feng, Citation2010).
Biodistribution study in rats
The profiles of the CRM level in brain displayed an initial absorption phase and maximum concentration achieved after about 180 min in brain after IN administration of CRM-NLC. These findings are in good agreement with that previously reported by Chow et al. (Citation1999) for the intranasal administration of cocaine and support the existence of a nose–brain direct pathway. After the initial 30 min, the drug concentration in the brain was found to be higher for IN-delivered CRM-NLC (20 183 ± 298.1 ng/g) than PDS (10 920 ± 283.1 ng/g). As time progressed, the concentration increased and thus, after 90 min, IN-delivered CRM-NLC showed higher accumulation (39 451 ± 8901.1 ng/g) of drug in the brain compared with PDS (20 986 ± 632.10 ng/g; ). The highest concentration was observed in the brain after IN administration of CRM-NLC, the Cmax was 86 201 ± 8182.1 ng/g at tmax of 120 min; whereas for PDS, the Cmax was 54 321 ± 2098.8 ng/g at tmax of 180 min, after IN administration. A statistically significant difference (p < 0.05) between the two formulations was found from the Student t-test analysis. Significant enhancement of curcumin delivery from CRM-NLC (146.8 nm diameter) to the CNS was observed on nasal administration as compared with the PDS. This could relate to the rapid absorption and longer residence time of the CRM-NLC in rat nasal cavity, which provides the opportunity for intranasal delivery to the brain. Also, their small diameter potentially allows CRM-NLC to be transported transcellularly through olfactory neurones to the brain via the various endocytic pathways of neuronal cells in the olfactory membrane (Mistry et al., Citation2009). Thus, the results of the present investigation prove that drug could be transported directly to the CNS after intranasal delivery of CRM-NLC, thereby enhancing drug concentration in the brain and also enhancing the nasal bioavailability of curcumin.
Table 1. Pharmacokinetic parameters following intranasal administration of CRM-loaded NLC and plain drug suspension.
Conclusion
This study clearly describes a new formulation of CRM-NLCs with antitumor properties. A hot high pressure homogenization method was employed to prepare the CRM-NLC with improved drug incorporation and release properties. Using DSC analysis and XRD, the drug was found to be in its amorphous state in the NLC particles. These results show that the NLC prepared in this study could be developed as a carrier. NLC was found to have an extremely low level of toxicity to that astrocytoma-glioblastoma cells. Significant increase of curcumin in CNS following CRM-NLC after nasal administration highlights an alternative strategy for therapy of CNS diseases. This study portends a potential breakthrough in the treatment of brain cancer by using novel, biodegradable and biocompatible CRM-NLC with enhanced anti-cancer activities through nasal administration.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Aggarwal BB, Kumar A, Bharti AC. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–98
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–18
- Araújo J, Gonzalez-Mira E, Egea MA, et al. (2010). Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm 393:167–75
- Arbiser JL, Klauber N, Rohan R. (1998). Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4:376–83
- Battaglia L, Gallarate M. (2012). Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv 9:497–508
- Bava SV, Puliappadamba VT, Deepti A, et al. (2005). Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-nB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J Biol Chem 280:6301–8
- Chan MM, Fong D, Soprano KJ, et al. (2003). Inhibition of growth and sensitization to cisplatin-mediated killing of ovarian cancer cells by polyphenolic chemopreventive agent. J Cell Physiol 194:63–70
- Chow HHS, Chen Z, Matsuura GT. (1999). Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci 88:754–8
- Feng F, Zheng D, Zhang D, et al. (2011). Preparation, characterization and biodistribution of nanostructured lipid carriers for parenteral delivery of bifendate. J Microencapsul 28:280–5
- How CW, Abdullah R, Abbasalipourkabir R. (2011). Physicochemical properties of nanostructured lipid carriers as colloidal carrier system stabilized with polysorbate 20 and polysorbate 80. Afr J Biotechnol 10:1684–9
- Jiang XG, Cui JB, Fang XL, et al. (1995). Toxicity of drugs on nasal mucocilia and the method of its evaluation. Acta Pharmacol Sin 30:848–53
- Joshi MD, Müller RH. (2009). Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm 71:161–72
- Kakkar WM, Schwabe K, Müller RH, Keck CM. (2013). Preservation of nanostructured lipid carriers (NLC). Eur J Pharm Biopharm 76:56–67
- Li C. (2002). Poly (l-glutamic acid)–anticancer drug conjugates. Adv Drug Deliv Rev 54:695–713
- Li L, Braiteh FS, Kurzrock R. (2005). Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104:1322–31
- Liu CH, Wu CT. (2010). Optimization of nanostructured lipid carriers for lutein. Colloids Surf A: Physicochem Eng Asp 353:149–56
- Liu D, Liu Z, Wang L, et al. (2011). Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf B Biointerfaces 85:262–9
- Liu Z, Zhang X, Wu H, et al. (2011). Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev Ind Pharm 37:475–81
- Mistry A, Stolnik S, Illum L. (2009). Nanoparticles for direct nose to brain delivery of drugs. Int J Pharm 379:146–57
- Montenegro L, Campisi A, Sarpietro MG, et al. (2011). In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev Ind Pharm 37:737–46
- Müller RH, Radtke M, Wissing SA. (2002). Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 242:121–8
- Nyström AM, Fadeel B. (2012). Safety assessment of nanomaterials: implications for nanomedicine. J Control Release 16:403–8
- Obeidat WM. (2012). Investigation of temperature-induced physical instability of preserved coenzyme Q10-loaded (NLC): a comparative study at different temperatures. Afr J Pharm Pharmacol 6:2413–23
- Pawar YB, Purohit H, Valicherla GR, et al. (2012). Novel lipid based oral formulation of curcumin: development and optimization by design of experiments approach. IntJ Pharm 436:617–23
- Zhang X, Liu J, Qiao H, et al. (2010). Formulation and optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol 197:120–8
- Zhao L, Feng S. (2010). Enhanced oral bioavailability of paclitaxel formulated in vitamin E-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J Pharm Sci 99:3552–60