Abstract
Targeted drug delivery is a method of delivering bioactive compounds to a patient in a manner that increases the therapeutic index. The main goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. Antibody-drug conjugates (ADC) represent an innovative therapeutic application that combines the unique properties of monoclonal antibodies with the potent cell killing activity of cytotoxic bioactive compounds. ADCs are complex molecules composed of an antibody linked, via a stable, chemical, linker with labile bonds, to a biological active cytotoxic (anticancer) payload or drug. The key components of ADC include a monoclonal antibody, a stable linker and a cytotoxic agent to target a variety of cancers. The present mini-review deals with the examination of clinical use and pharmacological properties, as well as the safety of antibody-drug conjugates that are marketed. Ado-trastuzumab emtasine and brenduximab vedotin were examined regarding their mechanism of action, pharmacology, clinical use and safety. These ADCs selectively deliver cargoes to tumor cells and provide clinical benefit by minimizing systemic toxicity.
Introduction
Nanomedicine offers delivery of potential drugs and bioactive compounds to human organs which were previously beyond reach of microscale drugs due to specific biological barriers (Singh, Citation2010; Crommelin & Florence, Citation2013). Targeted drug delivery, is a method of delivering bioactive compounds to a patient in a manner that increases the therapeutic index and decreased the adverse reactions of the medicine. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels (Singh, Citation2010; Al-Jamal, Citation2013; Crommelin & Florence, Citation2013).
As mentioned above, nanomedicine is the biomedical application of nanotechnology. The nanosize of particles/composites endows with significant properties that can be very useful for targeted drug delivery in oncology. Taking as a starting point to our note, the recent trends in bibliography, pharmaceutical nanotechnology, which is the best approach for developing innovative drugs and drug carriers, can provide challenges for producing bioinspired and self-assembled nanostructures that can be termed as advanced Drug Delivery nano Systems (aDDnSs). Systems Modulating Adverse Reactions and Toxicity or Systems More Able to Reach the Target site (SMART) nanodevices are composed of soft bionanomaterials and are characterized as chimeric or hybrid (Al-Jamal, Citation2013; Grainger, Citation2013; Kirsh et al., Citation2013; Lammers, Citation2013; Mastrobattista, Citation2013; Demetzos & Pippa, Citation2014).
Additionally, another trend in pharmacotherapy for chemotherapy is molecularly a targeted therapy, which is one of the major modalities of cancer therapy. Antibody-drug conjugates are single molecular species (or as close to single molecular species as current manufacturing can produce). It should be noted that antibody-drug conjugates are not nanoparticles but belong to molecularly targeted therapy. Monoclonal antibody therapy is a category of immunotherapy (activation/suppression immunotherapies), which uses monoclonal antibodies that specifically bind to target (cells and/or proteins) (Shefet-Carasso & Benhar, Citation2014). On the other hand, according to Beck & Reichert (Citation2014), ADCs are a sub-class of antibody-related therapeutics. Antibody-drug conjugates are a new class of highly potent biopharmaceutical medicines designed as a targeted therapy for the treatment of people mainly with cancer. ADCs are complex molecules composed of an antibody linked, via a stable, chemical, linker with labile bonds, to a biologically active cytotoxic (anticancer) payload or drug (Lianos et al., Citation2014). By combining the unique targeting capabilities of monoclonal antibodies with the cancer-killing ability of cytotoxic drugs, antibody-drug conjugates allow sensitive discrimination between healthy and diseased tissue. This means that, in contrast to traditional chemotherapeutic agents, antibody-drug conjugates target and attack the cancer cell so that healthy cells are less severely affected (Lianos et al., Citation2014). ADCs deliver highly potent cytotoxic anticancer agents to cancer cells by connecting them to monoclonal antibodies through biodegradable linkers and discriminate between cancer and normal tissue. The stability of ADCs is due to biodegradable linkers, which are either cleavable or non-cleavable. The advantages of ADCs are the increase of the cell-killing potential of monoclonal antibodies; the higher tumor selectivity, the increase of drug tolerability and limited systemic exposure (compared to standard chemotherapeutic bioactive compounds) (Haddish-Berhane et al., Citation2013; Sapra et al., Citation2013). The only disadvantage of the ADCs is the difficulties of linker technology because the design/synthesis of a bio-functional is remarkably challenging due to the requirements of specialized teams (Ornes, Citation2013).
Furthermore, antibodies are classified into five classes based on the sequence of their heavy chain constant regions: IgM, IgD, IgG, IgE and IgA. IgG is most frequently used in cancer therapeutics. Most common are IgG1 antibodies (Trastuzumab, Bevacizumab, Cetuximab, etc.); however, IgG2 (Panitumumab) and IgG4 (Gemtuzumab ozogamicin) antibodies are also available. The use of IgG antibodies maybe be based on the fact that subclasses of IgG, most notably IgG1 and IgG3, are potent activators of the classical complement pathway. The binding of two or more IgG molecules to the cell surface leads to high-affinity binding of C1q to the Fc domain, followed by activation of C1r enzymatic activity and subsequent activation of downstream complement proteins and finally to tumor cell lysis (Weiner et al., Citation2010; Scott et al., Citation2012).
The main goal of the present review is to examine the clinical use and pharmacological properties, as well as the safety of antibody-drug conjugates that are marketed. Ado-trastuzumab emtasine and brenduximab vedotin were examined regarding their mechanism of action, pharmacology, clinical use and safety.
Methods
Systemic search and review of papers regarding the approval of antibody-drug conjugates took place via MedLine and abstract presentations of international conferences.
Ado-trastuzumab emtasine
Ado-trastuzumab emtasine (T-DM1) is a an antibody-drug conjugate consisting of the HER2 monoclonal antibody trastuzumab conjugated to the maytansinoid DM1 via a non-reducible thioether linkage () with a potential antineoplastic activity. It was approved for use in advance breast cancer in February 2013 and in November 2013 by FDA and EMA, respectively, under the brand name Kadcyla® (Krop et al., 2012). Clinical development of free DM1 parent agent was stopped because of its very narrow therapeutic index Kandcyla, Genetch, Int (San Francisco, CA) (Remillard et al., Citation1975; Cabanillas et al., Citation1979; Cassady et al., Citation2004; Erickson et al., Citation2006). It was approved with a dose of 3.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity in patients with HER2-positive, unrespectable locally advanced or metastatic breast cancer, who have been previously treated with trastuzumab and a taxane, separately or in combination (Kadcyla package insert, August 2013).
Figure 1. Schematic of trastuzumab-Maytansine (T-DM1) including the [N-maleimidomethyl]cyclohexane-1-carboxylate (MCC) linker. An average of 3.5 DM1 molecules are conjugated to the Fc region of trastuzumab (Adapted from Krop et al., 2012).
![Figure 1. Schematic of trastuzumab-Maytansine (T-DM1) including the [N-maleimidomethyl]cyclohexane-1-carboxylate (MCC) linker. An average of 3.5 DM1 molecules are conjugated to the Fc region of trastuzumab (Adapted from Krop et al., 2012).](/cms/asset/c792c55e-3961-4ab9-ae21-b228a681a436/idrd_a_998323_f0001_c.jpg)
Mechanism of action
Ado-trastuzumab emtasine incorporates the HER2 targeted actions of trastuzumab with the microtubule inhibition action of DM1. Moreover, its special pharmaceutical design allows intracellular drug delivery specifically to HER2-overexpressing cells, resulting in cell cycle arrest and apoptosis, thereby improving the therapeutic index and minimizing exposure of normal tissue (Lewis et al., Citation2008; Junttila et al., Citation2011).
Pharmacokinetics
The peak levels of T-DM1 are achieved near to the end of the administration (90 min for the first infusion and 30 min for the next infusions), it presents a volume of distribution 3.13 L and half-life elimination of 4 days. DM1 also presents high protein binding 93% and it undergoes hepatic metabolism via CYP3A4/5 (Girish et al., Citation2012).
Clinical trials overview
Phase II and III clinical trials have already studied and proved the efficacies as well as they have demonstrated the safety profile of T-DM1. More specifically, Burris et al. (Citation2011) studied the effect of T-DM1 in patients who had been previously treated with HER2 directed therapy and concluded that T-DM1 at a dose of 3.6 mg/kg every 3 weeks is well tolerated and has a robust single-agent activity with reported response rate of 25.9% and progression free survival of 4.6 months. Another phase II trial published by Hurvitz et al. (Citation2013) compared T-DM1 to trastuzumab and docetaxel regimen as first-line treatment and concluded that T-DM1 arm presented significant improvement in progression free survival with 14.2 months versus 9.2 months and favorable safety profile. Krop et al. (Citation2012) examined the effect of T-DM1 in heavily pre-treated patients and they reported that T-DM1 is a well-tolerated and effective new treatment for those patients. Finally, the international, randomized, open-label phase II trial EMILIA compared, in patients previously treated with trastuzumab and taxanes, T-DM1 with the standard regimen lapatinib plus capecitabine. Their results significantly showed higher response rates in T-DM1 arm with 43.6 versus 30.8%, significantly prolonged PFS of 9.6 versus 4.6 months and overall survival of 30.9 versus 25.1 months (Verma et al., Citation2012). The most common significant adverse reactions observed with T-DM1 are skin rash, nausea, constipation or diarrhea, thrombocytopenia, anemia, increased serum transminases and increased serum bilirubin (Verma et al., Citation2012; Hurvitz et al., Citation2013; Kadcyla package insert, August 2013).
Clinical development
Clinical Trials.gov search for clinical development of T-DM1 presented many ongoing clinical trials that studied T-DM1 in metastatic breast cancer in combination with pertuzumab, with pertuzumab plus paclitaxel, with capecitabine and with standard endocrine therapy. In addition, it is under study for use in adjuvant or neo-adjuvant setting. Finally, the combination with capecitabine is also being studied in gastric cancer (ClinicalTrials.gov).
Brenduximab vedotin
Brenduximab vedotin is an antibody drug conjugate consisting of cAC10 anti-CD30-specific chimeric IgG1 antibody, monomethylauristatin E (MMAE) a microtubule-disrupting agent and a protease cleavable dipeptide linker (). It was approved for use in refractory Hodgkin and systemic anaplastic large cell lymphoma in August 2011 and in October 2012 by FDA and EMA, respectively, under the brand name Adcetris® (Fierce Biotech, EMA).
Figure 2. Composition of brentuximab vedotin (cAC10-vcMMAE, SGN-35). SGN-30 (cAC10, the parent naked antibody) is a chimeric anti-CD30 monoclonal antibody derived from the fusion of the variable heavy and light region of the murine anti-CD30 antibody AC10, with the constant gamma1-heavy and kappa-light region of the human immunoglobulin (Adapted from Wahl et al., Citation2002; Doronina et al., Citation2006).
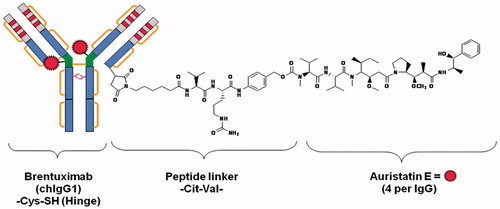
It was approved with a dose of 1.8 mg/kg (maximum dose: 180 mg) every 3 weeks until disease progression or unacceptable toxicity in patients with Hodgkin lymphoma, ineligible for transplant or after autologous stem cell transplant failure, of at least two prior chemotherapy regimens and in patients with anaplastic large cell lymphoma after a failure of at least one prior chemotherapy regimen (Adcetris prescribing information, September 2013).
Mechanism of action
The conjugate binds to cells that express CD30 and forms a complex, which is internalized within the cell and releases MMAE that binds to the tubules and disrupts the cellular microtubule network, including cell cycle arrest (G2/M phase) and apoptosis (Oflazoglu et al., Citation2008).
Pharmacokinetics
The volume of distribution of ADC is 6–10 L and the terminal half-life elimination time is 4–6 days. The peak levels of ADC are achieved at the end of the 30-minutes infusion and the peak levels of MMAE are achieved within 1–3 days. MMAE undergoes primarily a minimal oxidation by CYP3A4/5 metabolism and it is excreted in feces (72%, mainly unchanged) and in urine (Younes et al., Citation2010).
Clinical trials overview
Younes et al. (Citation2010) conducted two phase I dose-escalation studies. They concluded that the maximum tolerated dose of single-agent Brentuximab Vedotin was 1.58 mg/kg administrated every 3 weeks with durable objective responses in patients with relapsed CD-30 positive lymphomas. In the other phase I trial, they studied the safety of Brentuximab Vedotin combined with standard regimens ABVD or AVD in newly diagnosed patients with advanced stage Hodgkin lymphoma. In that study, the aim of maximum tolerated dose of 1.8 mg/kg was not reached. Moreover, Chen et al. (Citation2011) studied the safety and efficacy of Brentuximab Vedotin in patients with relapsed or refractory Hodgkin lymphoma in a phase II, single-arm, multicenter trial. They reported manageable adverse events and objective responses in 75% of the patients. In a phase II, single-arm, multicenter trial that studied Brentuximab vedotin in relapsed or refractory systemic anaplastic large cell lymphoma, the authors reported objective responses in 86% of the patients and high proportion of complete responses (Pro et al., Citation2011). Finally, the most common significantly reported adverse reactions were rash, nausea, diarrhea, neutropenia, anemia and thrombocytopenia (Younes et al., Citation2010; Chen et al., Citation2011; Pro et al., Citation2011; Adcetris prescribing information, September 2013)
Clinical development
There are many on-going studies on the effect of Brentuximab Vedotin in other malignancies and in combination with other agents. More specifically, it is studied in CD-30 positive non-lymphomatous malignancies and CD-30 positive germ cell tumors. In addition, for use in non-Hodgkin lymphoma, in refractory chronic Graft versus Host disease and in acute myeloid leukemia in combination with azacytidine. The combination of drugs that are studied are rituximab and bendamustine (ClinicalTrials.gov).
Lessons learned
In conclusion, a large number of the ADCs, which are currently under research and development or in clinical trials, are for anticancer indications (Beck et al., Citation2010; Beck & Reichert, Citation2014). This is primarily driven by the bioavailability of monoclonal antibodies targeting various types of cancer. However, some drug developers are also looking for expanding the application of ADCs beyond oncology, like hematology and other important disease areas. At this time, these two marketed ADCs are only the first steps towards new therapeutic targeted approaches.
Conclusions
Antibody-drug conjugates represent an innovative therapeutic application that combines the unique properties of monoclonal antibodies with the potent cell killing activity of cytotoxic bioactive compounds. Ado-trastuzumab emtasine is an antibody-drug conjugate consisting of the HER2 monoclonal antibody trastuzumab conjugated to the maytansinoid DM1 via a non-deductible thioether linkage with potential antineoplastic activity. It was approved for use in advance breast cancer. On the other hand, Brenduximab vedotin is an antibody drug conjugate consisting of cAC10 anti-CD30-specific chimeric IgG1 antibody, monomethylauristatin E (MMAE) a microtubule-disrupting agent and a protease cleavable dipeptide linker. It was approved for use in refractory Hodgkin and systemic anaplastic large cell lymphoma. In conclusion, the unique properties of the above-mentioned antibody–drug conjugates are the so-called armed antibodies that selectively dispatch highly potent cytotoxic anticancer chemotherapies directly to tumor tissues while, at the same time, leaving healthy cells unaffected.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Al-Jamal KT. (2013). Active drug targeting: lessons learned and new things to consider. Int J Pharm 454:525–6
- Beck A, Reichert JM. (2014). Antibody-drug conjugates: present and future. MAbs 6:15–17
- Beck A, Wurch T, Bailly C, Corvaia N. (2010). Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 10:345–52
- Burris HA, Rugo HS, Vukelja SJ, et al. (2011). Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29:398–405
- Cabanillas F, Bodey GP, Burgess MA, et al. (1979). Results of a phase II study of maytansine in patients with breast carcinoma and melanoma. Cancer Treat Rep 63:507–9
- Cassady JM, Chan KK, Floss HG, et al. (2004). Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull 52:1–26
- Chen RW, Gopal AK, Smith SE, et al. (2011). Results from a pivitol phase II study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL). J Clin Oncol 29:8031–9
- Crommelin DJ, Florence AT. (2013). Towards more effective advanced drug delivery systems. Int J Pharm 454:496–511
- Demetzos C, Pippa N. (2014). Advanced drug delivery nanosystems (aDDnSs): a mini-review. Drug Deliv 21:250–7
- Doronina SO, Mendelsohn BA, Bovee TD, et al. (2006). Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem 17:114–24
- Erickson HK, Park PU, Widdison WC, et al. (2006). Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 66:4426–33
- Girish S, Gupta M, Wang B, et al. (2012). Clinical pharmacology of trastuzumab emtasine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive breast cancer. Cancer Chemother Pharmacol 69:1229–40
- Grainger DW. (2013). Connecting drug delivery reality to smart materials design. Int J Pharm 454:521–4
- Haddish-Berhane N, Shah DK, Ma D, et al. (2013). On translation of antibody drug conjugates efficacy from mouse experimental tumors to the clinic: a PK/PD approach. J Pharmacokinet Pharmacodyn 40:557–71
- Hurvitz SA, Dirix L, Kocsis J, et al. (2013). Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 31:1157–63
- Junttila TT, Li G, Parsons K, et al. (2011). Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128:347–56
- Kirsh, R, Hood, S, Brook C, et al. (2013). Will nanomedicine deliver on its promise of changing therapeutics or remain an interesting and important research tool in cell biology and physiology? Int J Pharm 545:530–1
- Krop IE, LoRusso P, Miller KD, et al. (2012). A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, ataxane, and capecitabine. J Clin Oncol 30:3234–41
- Lammers T. (2013). Smart drug delivery systems: back to the future vs. clinical reality. Int J Pharm 545:527–9
- Lewis PGD, Li G, Dugger DL, et al. (2008). Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68:9280–90
- Lianos GD, Vlachos K, Zoras O, et al. (2014). Potential of antibody-drug conjugates and novel therapeutics in breast cancer management. Onco Targets Ther 7:491–500
- Mastrobattista E. (2013). Advanced drug delivery in motion. Int J Pharm 545:517–20
- Oflazoglu E, Kissler KM, Sievers EL, et al. (2008). Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol 142:69–73
- Ornes S. (2013). Antibody-drug conjugates. Proc Natl Acad Sci USA 110:13695
- Pro B, Avandi R, Brice P, et al. (2011). Durable remissions with brentuximab vedotin (SG-35): updated results of a phase II study in patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). J Clin Oncol 29:8032
- Remillard S, Rebhun LI, Howie GA, et al. (1975). Antimitotic activity of the potent tumor inhibitor maytansine. Science 189:1002–5
- Sapra P, Betts A, Boni J. (2013). Preclinical and clinical pharmacokinetic/pharmacodynamic considerations for antibody-drug conjugates. Expert Rev Clin Pharmacol 6:541–54
- Scott AM, Wolchok JD, Old LJ. (2012). Antibody therapy of cancer. Nat Rev Cancer 12:278–87
- Shefet-Carasso L, Benhar I. (2014). Antibody-targeted drugs and drug resistance – challenges and solutions. Drug Resist Updat. doi: 10.1016/j.drup.2014.11.001
- Singh S. (2010). Naomedicine-nanoscale drugs and delivery systems. J Nanosci. Nanotechnol 10:7906–18
- Verma S, Miles D, Gianni L, et al. (2012). Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–91
- Wahl AF, Klussman K, Thompson JD, et al. (2002). The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin's disease. Cancer Res 62:3736–42
- Weiner LM, Surana R, Wang S. (2010). Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 10:317–27
- Younes A, Bartlett NL, Leonard JP, et al. (2010). Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363:1812–21

