Abstract
The clinical success of therapeutic DNA is still hindered due to the lack of effective delivery carriers. Here, we designed a tumor-targeted gene nano delivery system based on EGFR targeting strategy. Epidermal growth factor (EGF) was introduced to nano-complexes of PAMAM dendrimer and DNA via electrostatic interactions to form self-assembled PAMAM/DNA/EGF nano-complexes. The properties of self-assembled complexes were characterized by gel retardation assay and particle size and zeta potential analysis. Meanwhile, the toxicity of EGF-dendriplexes was evaluated by the MTT assay, which indicated that the complexes exhibited decreased cytotoxicity with the incorporation of EGF. We labeled polyamidoamine (PAMAM) dendrimers with FITC or a near-infrared (NIR) dye Lss670 and tested the cellular uptake in vitro and biodistribution in xenograft mouse tumor models. As compared to dendriplexes, the ternary EGF-dendriplexes showed a significantly higher cellular uptake into HepG2 cells due to the specific binding between EGF and EGF receptor (EGFR) over expressed on HepG2 cells, which resulted in the enhanced gene transfection efficiency. The biodistribution of EGF-dendriplexes in vivo was monitored with in vivo imaging technique, which indicated that EGF-dendriplexes enhanced EGFR-positive tumor-targeted biodistribution. These findings indicate that this novel nano-vector realized efficiently tumor-targeting gene delivery and high efficient gene expression in vivo, and it may possess a potential targeting gene delivery system in cancer therapy.
Introduction
Gene therapy is a promising approach in various disease therapies, especially in cancer therapy (Figueroa et al., Citation2014). Developing safe and effective non-viral vectors is urgently needed for gene therapy (Pan et al., Citation2007, Citation2009; Kang et al., Citation2010; Aalinkeel et al., Citation2012; Khurana et al., Citation2013). To improve the selectivity and efficiency of the vector, non-viral systems such as dendrimers have been modified with various targeting ligands, such as monoclonal antibodies (Chiu et al., Citation2004), peptides (Son et al., Citation2011), transferrin (Somani et al., Citation2014), galactose (Kunath et al., Citation2003), folate (Benchaala et al., Citation2014), albumin (Lu et al., Citation2006) and EGF (Yu et al., Citation2011); i.e. EGF is a small protein that binds with high affinity to EGF receptor (EGFR). EGFR over-expression occurs in various human solid tumors, including cancers of the head and neck, breast, lung, liver, bladder and brain (Mendelsohn, Citation2001).
The major approach in non-viral gene therapy is based on cationic polymers, such as polyethylenimine (PEI) (Yamada et al., Citation2014), polylysine (PLL) (Thiersch et al., Citation2013), polypropylenimine (PPI) (Kim et al., Citation2007) and polyamidoamine (PAMAM) (Zhang et al., Citation2010). Recent studies have demonstrated that polyamidoamine (PAMAM) can bind negative charged protein due to the electrostatic interaction of polymers and protein (Shcharbin et al., Citation2007; Froehlich et al., Citation2009). However, as far as we know, the studies on PAMAM-mediated gene delivery are still limited to its low efficiency in vivo (Won et al., Citation2013).
In this study, we prepared self-assembled EGF-dendriplexes via electrostatic adsorption of PAMAM-DNA complexes (dendriplexes) to negatively charged EGF. EGF-dendriplexes were characterized by gel retardation assay and particle size and zeta potential analysis, and the ability of EGF-dendriplexes to enhance transfection efficiency was evaluated in vitro, and the biodistribution of EGF-dendriplexes were monitored by in vivo imaging technique.
Materials and methods
Materials
PAMAM dendrimers (G5) and epidermal growth factor (EGF) were purchased from Sigma-Aldrich (Shanghai, China). The plasmids pLC-3.0, (Promega, Beijing, China) encoding luciferase was used in the transfections studies. Kodak X-Sight 670 Large Stokes Shift Dye was purchased form Carestream (Carestream health, Rochester, NY, USA). All other reagents were obtained from Biodee Reagent Company, Beijing, China.
Cell culture
Hela, HepG2 and MDA-MB-231 cells were obtained from China Center for Type Culture Collection (Wuhan, China). MDA-MB-231-luc cells were a gift from Prof. Chen of Tsinghua University. The cells were maintained at 37 °C under 5% CO2 in Dulbecco's Modified Eagle's Medium (Dulbecco’s modified Eagle’s medium (DMEM), GIBCO-Invitrogen, Grand Island, NY, USA), supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), penicillin (100 units/mL), streptomycin (100 μg/mL) (Gibco, Beijing, China).
Plasmid preparation
Plasmid was amplified in Escherichia coli (strain DH5α), isolated and purified using an endotoxin-free Plasmid Giga Kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. Concentration and purity of plasmid were assessed using UV-6300 Spectrophotometer (Mapada, Shanghai, China) at 260 nm and 280 nm. Plasmid integrity was confirmed by 0.8% agarose gel electrophoresis and stored at −20 °C until further use.
Preparation of dendriplexes and EGF-dendriplexes
Dendriplexes with different charge ratios (N/P) were formed by incubating the two components together in PBS (150 mM NaCl, 1.9 mM NaH2PO4, 8.1 mM NaH2PO4, pH 7.4) for 15 min at 37 °C. Charge ratios (N/P) were calculated based on the number of terminal amine groups on a PAMAM dendrimer and the number of phosphate groups in the plasmid DNA. EGF-dendriplexes were prepared by adding EGF to the preformed dendriplexes at N/P ratio 20. The required amount of EGF was added to the preformed dendriplexes and vortexed in PBS. Then, EGF-dendriplexes were formed after incubated for another 15 min at 37 °C. The weight ratio of EGF and plasmid DNA was from 0.2 to 10.
Gel retardation assay
Different EGF-dendriplexes were prepared by incubating in PBS at room temperature for 30 min. Each sample was analyzed by electrophoresis on a 0.8% agarose containing EB (0.5 μg/mL) at 80 V for 1 h. The location of the DNA was identified under UV irradiation.
Characterization
Zeta potential and size of the dendriplexes prepared at different N/P ratios and EGF-dendriplexes prepared at different EGF/DNA weight ratios were measured by using Nano-ZS90 Zetasizer (Malvern Instrument, Worcs, UK). All measurements were carried out on the dendriplexes with 5 μg/mL plasmid DNA in PBS at pH 7.4.
Transfection in vitro
For transfection, cells were seeded at a density of 105 cells/well in a 24-well culture plate, grown for 24 h at 37 °C in an atmosphere of 5% CO2 and 95% relative humidity using DMEM (pH 7.4) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin solution. After this, the medium was removed and washed with PBS twice. Subsequently, 50 μL of the DNA complexes (containing 1 μg of pCL-3.0 plasmid) and 0.2 mL DMEM were added to each well, and incubated for 5 h at 37 °C with growth medium for another 48 h. Levels of luciferase activity in the transfected cells were assayed by luciferase assay kit (Promega, Madison, WI, USA), according to the manufacturer’s introduction. Levels of luciferase activity in the transfected cells were assayed by luciferase assay kit (Promega), according to the manufacturer’s introduction.
Cellular uptake
FITC-labeled dendrimers were synthesized as per the methods described in the literature. To remove the unconjugated FITC, the synthesized FITC-dendrimers were dialyzed in the dark against deionized water and replaced on a daily basis until no fluorescence was detected in the supernatant. The resultant FITC-dendrimers were dried in an oven. FITC-labeled dendriplexes were then prepared as described before to track the internalization of dendriplexes by CLSM (TCS SL, Leica, Germany). To track the internalization of dendriplexes, cells were seeded on 12-well plates with a sterile glass cover slip at 105 cells/well and incubated overnight. Subsequently, cells were rinsed twice with transfection media and transfected with FITC-labeled dendriplexes. After incubation for 1 h, test samples were aspirated. Cells were then washed twice with pre-warmed phosphate buffered saline (PBS) before they were fixed in 4% paraformaldehyde. Finally, the fixed cells were examined under a CLSM.
In vitro cytotoxicity
HepG2 cells were seeded at a density of 1 × 104 cells per well in 200 μL medium in a 96-well plate. After an overnight culture at 37 °C in a 5% CO2 humidified atmosphere, cells were treated with PAMAM dendrimer, dendriplexes and EGF-dendriplexes (containing 1 μg of plasmid) prepared at EGF/DNA weight ratio of 2. After 5 h incubation, 20 μL of MTT (5 mg/mL) was added and the cells were incubated for approximately 4 h. The growth medium was removed and 200 μL of dimethyl sulfoxide (DMSO) was added to dissolve the MTT crystals and the optical density was read using TECAN Safire2 Multimode microplate reader (TECAN, Shanghai, China) with 570 nm as excitation wavelength and 630 nm as the background. Viability of cells exposed to dendriplexes was expressed as a percentage of the viability of cells grown in the absence of dendriplexes.
In vivo analysis
For the circulation kinetics and biodistribution of EGF-dendriplexes and plain-dendriplexes, PAMAM dendrimers were labeled with Kodak X-Sight 670 Large Stokes Shift Dye (LSS dye) by the covalent combination between primary amino groups of PAMAM dendrimers and functional ester group of LSS dye. The labeling reaction and purification of LSS dye-labeled PAMAM dendrimer were according to the manufacturer’s introduction. Female BALB-c nude mice (4 weeks of age), were purchased from the center of experimental animal (Tsinghua University, Beijing). All animals were approval by the Committee on Animal Research at the Tsinghua University, Beijing, animal protocol No.11-ZXN3. Tumors were introduced in the armpit of the nude mice by inoculation with 1 × 106 of luciferase gene-labeled MDA-MB-231-luc cells. Palpable subcutaneous tumors had developed over a period of approximately 7 d. Distribution and metabolism was assessed by injecting LSS dye-labeled EGF-dendriplexes (EGF/DNA weight ratio of 2, N/P ratio of 20) and LSS dye-labeled plain-dendriplexes (N/P ratio of 20) into tumor-bearing mice in groups of six via the tail vein in a total volume of 200 μL (containing 30 ug pDNA per mouse). Kodak in vivo imaging system FX-Pro was used for image acquisition in vivo, and images were analyzed with the Carestream MI SE 5.0.7.23 software package. For tumor bioluminescence imaging analysis, D-luciferin was used. Mice were anesthetized with 2.5% (v/v) isoflurane and 150 mg/kg d-luciferin was injected intraperitoneally. Five minutes later, animals were placed in the dark chamber of Kodak in vivo imaging system FX-Pro for light acquisition.
Statistical analysis
Statistical analysis was performed using two-way ANOVA (Origin software, 7.5 version, Origin Lab, Northampton, MA, USA). p < 0.05 was considered statistically significant.
Results and discussion
Characterization of EGF-dendriplexes
The physicochemical properties of the non-viral vectors have a great influence on their gene delivery efficiency to target cells. Particle sizes of complexes were measured by dynamic laser light scattering in the absence or presence of EGF. As shown in , the particle sizes of the plain-dendriplexes of different N/P ratio from 5 to 30 were about 300 nm, which indicated that DNA could be effectively condensed by PAMAM at the N/P ratio higher than 5. As compared with the plain-dendriplexes, the particle sizes of the EGF-dendriplexes slightly increased with adding of EGF. This is due to the fact that the negatively charged EGF was attached to the surface of PAMAM/DNA complexes, leading to the increased sizes. Regarding of the zeta-potential of EGF-dendriplexes, all the simples were net positive charge if using N/P ratio higher than 5/1 (). Compared with plain-dendriplexes, the zeta potential of EGF-dendriplexes decreased slightly with adding of EGF. This is because the negatively charged EGF can bind the positively charged surface of dendriplexes and neutralize part of over positive charge which may cause the cytotoxicity and hemolysis in vitro and in vivo (Liu et al., Citation2012).
Figure 1. Characterization of EGF-dendriplexes: (a) Particle sizes of different complexes with different PAMAM/DNA N/P ratios. The weight ratio of EGF/DNA is 2. (b) Zeta potentials of different complexes with different PAMAM/DNA N/P ratios. The weight ratio of EGF/DNA is 2. (c) Agarose gel retardation assay of different complexes: Lane 1: DNA ladder; Lane 2: plasmid DNA; Lane 3–12: EGF-dendriplexes at PAMAM/DNA charge ratio of 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 30.
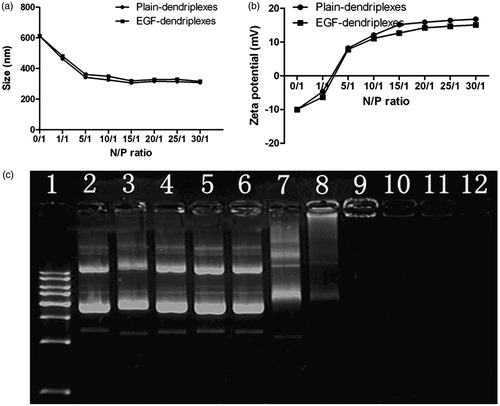
Analysis of DNA/PAMAM binding ability could be determined by the migration ability of DNA in agarose gel retardation. As shown in , an electrophoretic mobilization of DNA was retarded completely in lane 9 to lane 12, suggesting that EGF-dendriplexes of the N/P ratio from 5 to 30 could completely bind plasmid DNA and complexes were positively charged and physically stable at neutral pH.
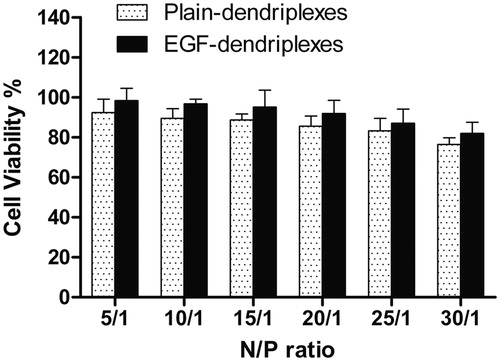
Cytotoxicity of PAMAM dendrimers–DNA complexes
The cytotoxicity of dendriplexes and EGF-dendriplexes has been evaluated by the MTT assay, and the results were illustrated in . The percentage of cell viability was reduced with the increase of N/P ratio from 5 to 30 in both two complexes. However, as compared to plain-dendriplexes, the viability of HepG2 cells treated with EGF-dendriplexes was increased relatively at each N/P ratio. As we know, the transfection efficiency and cytotoxicity is a dilemma for PAMAM mediated gene transfection (Jevprasesphant et al., Citation2003). For example, some effort has been done to increase the transfection efficiency by modification of the amino groups of PAMAM with poly-amino group such as arginine (Pisal et al., Citation2008) or ornithine (Kumar et al., Citation2010) to increase the cationic surface charge. While, with the increase of transfection efficiency, these surface-modified PAMAM dendrimers showed higher toxicity than native PAMAM (Choi et al., Citation2004). Conjugating with the poly(ethylene glycol) (PEG) chains has been considered as a method of reducing the toxicity of PAMAM dendrimers. This is because PEG is biocompatible, water-soluble, and has favorable tissue distribution. However, PEG modification can alter the binding affinity of PAMAM to DNA, drug, and protein in general (Shcharbin et al., Citation2005). In this study, negatively charged EGF was added into the PAMAM-mediated gene transfection system. With the coating of EGF, the positive charge of dendriplexes was partially neutralized by coated with negatively charged EGF, which led to be more biocompatible. Meanwhile, the decrease of zeta potential via the coating of EGF, see in , can be attributed to the decrease of the cytotoxicity of dendriplexes.
Transfection in vitro
The complexes formed by different N/P ratios were used to transfect HepG2 cells with a luciferase gene to determine the optimal N/P ratio. As shown in , the luciferase activity increased significantly with increasing the N/P ratio of dendriplexes from 5 to 20. A further increase in N/P ratio did not result in an obvious improvement in the luciferase expression, which may attribute to the higher cytotoxicity of complexes with a high N/P ratio (). Thus, we fixed the N/P ratio at 20 for further study.
Figure 3. Optimization of N/P ratios and EGF/DNA weight ratios: a. Luciferase expression in HepG2 cells transfected with different complexes with different PAMAM/DNA N/P ratios. The weight ratio of EGF/DNA is 2. b. Luciferase expression in HepG2 cells transfected with different complexes with different EGF/DNA weight ratios. The PAMAM/DNA N/P weight ratio of is 20.
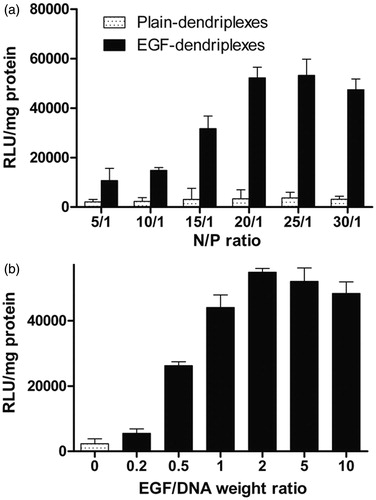
Compared with plain-dendriplexes, gene expression mediated by EGF was significantly higher in each N/P ratio. We examined the effect of EGF on transfection mediated by PAMAM/DNA complexes at different EGF/DNA weight ratios. The enhancement of PAMAM-mediated gene transfer by EGF-dendriplexes was found in transfected cells with an increase of the EGF/DNA weight ratio (). These data suggested that the more EGF available on the surface of the complexes, the higher gene expression can be obtained. However, a plateau and slightly decrease in transfection occurred with higher EGF amounts.
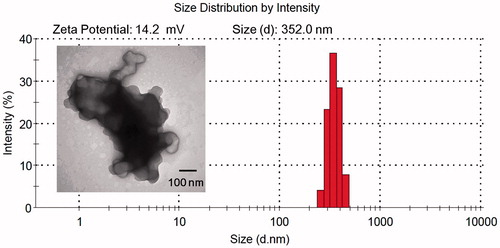
Thus, the complexes formed by N/P ratios of 20 and EGF/DNA weight ratios of 2 were used to transfect cells for further study. TEM image of the morphology of EGF-dendriplexes were shown in . The size observed was similar to the measurements obtained by dynamic light scattering. Zeta-potential measurements showed that the EGF-dendriplexes were positive charged complexes with the zeta potential of 14.2 mV.
Cellular uptake
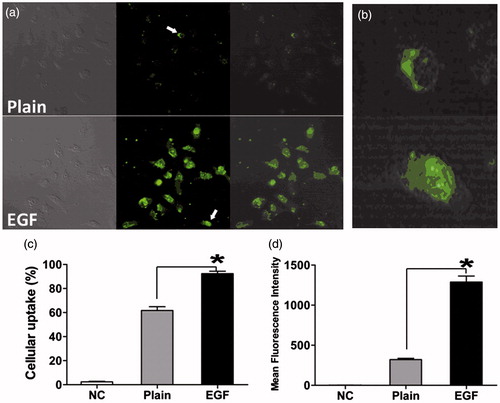
To further confirm the mechanism of the improvement of transfection in the presence of EGF moiety, we used confocal microscopy to visualize the cellular uptake of different complexes. As shown in and , after co-incubation with plain-dendriplexes for 1 h, the intensity of fluorescence was higher for the cells treated with EGF-dendriplexes, indicating that the existence of the EGF moiety played an important role in the cell uptake and EGF-dendriplexes could enter the cells through specific interactions between EGF and its receptors. Cells incubated with EGF-dendriplexes showed strong nuclei-associated green fluorescence, indicating that EGF facilitated the nuclear uptake which might contribute to the enhancement of gene transfection capability. As shown in , the percentage of cells that treated with EGF-dendriplexes was significantly higher than those treated with dendriplexes. Besides, the intensity of fluorescence observed in the group treated with EGF-dendriplexes was significantly stronger than those transfected with dendriplexes (p < 0.05, ). These results were in good agreement with the results of the in vitro transfection.
Figure 5. Cellular uptake of EGF-dendriplexes and dendriplexes: (a) Confocal images of HepG2 cells after incubation with Plain-dendriplexes and EGF-dendriplexes. The PAMAM/DNA N/P ratio is 20, and the weight ratio of EGF/DNA is 2. (b) Single typical HepG2 cell treated with Plain-dendriplexes and EGF-dendriplexes. (c) The percentage of cellular uptake of Plain-dendriplexes and EGF-dendriplexes. (d) The mean fluorescence intensity of HepG2 cells treated with plain-dendriplexes and EGF-dendriplexes. *means p < 0.05.
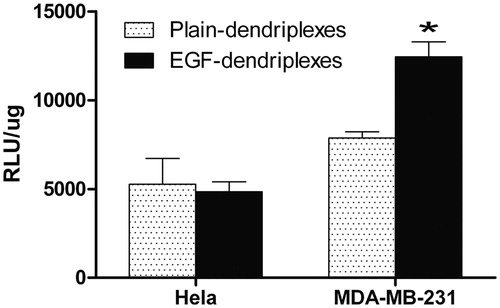
It is well known that transfection efficiency of the synthetic vectors depends on the nature of the cell line. This may be because of the difference in cellular binding, internalization mechanism and the properties of the cell line such as doubling time, rate of uptake of nutrients and related biochemical processes (Mo & Lim, Citation2004; Douglas et al., Citation2008). In our study, the in vitro transfections were carried out in both Hela cells and MDA-MB-231 cells (). The transfection efficiency and luciferase expression levels with EGF-dendriplexes were significantly higher than the plain-dendriplexes in MDA-MB-231 cell lines, which over-express EGF receptors. As most tumor cell types over-express EGFR (Mendelsohn, Citation2001), these results suggested that the presence of EGF was favorable for the transfection in these cells. In this study, the transfection activity of EGF-dendriplexes was almost the same as that of plain-dendriplexes in Hela cells which low-express EGFR. The presence of EGF on the surface of the complexes could not enhance the transfection, suggesting that EGF-dendriplexes would not increase the gene expression in non-tumor cells, which EGF receptors were not overexpressed. Our results clearly indicated that EGF-dendriplexes was a better tumor-targeting gene delivery vector for EGFR over-expressed tumor cells.
In vivo and ex vivo NIR (near infrared ray) fluorescence imaging
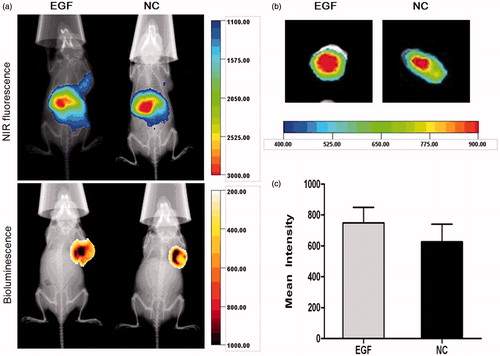
In order to investigate whether EGF-dendriplexes could effectively target to tumor, we performed in vivo and ex vivo NIR fluorescence imaging. The direct and real-time of tumor-targeting effect and biodistribution of LSS dye-labeled complexes could be analyzed by non-invasive NIR fluorescence optical imaging technology. As shown in , the location and possible metastasis of MDA-MB-231-luc tumor were determined with bioluminescence. Within the selected scale range of fluorescence counts, stronger signals and clearer boundary of NIR fluorescence were observed 2-h post treatment at tumor site in the group treated with EGF-dendriplexes. High signals were observed at abdomens of mice from both groups. indicated the representative ex vivo NIR fluorescence images of tumor in 2-h post injection. Strong NIR fluorescence intensity were observed in the mice tumor with EGF-dendriplexes treated, which showed direct evidence of in vivo tumor accumulation of EGF-dendriplexes in mice tumor.
Figure 7. X-ray and in vivo NIR fluorescence overlay images of tumor-bearing mice treated with plain-dendriplexes and EGF-dendriplexes. (a) The location and possible metastasis of MDA-MB-231-luc tumor were determined with bioluminescence by injecting 150 mg/kg d-luciferin. The optical imaging was obtained using Kodak multimodal imaging system FX-Pro equipped with an excitation bandpass filter at 690 nm and an emission at 790 nm. (b) X-ray and ex vivo NIR fluorescence overlay images of tumors treated with plain-dendriplexes and EGF-dendriplexes. (c) Mean ± standard deviation (n = 3) of fluorescence intensity of plain-dendriplexes and EGF-dendriplexes ex vivo.
In our previous studies, Renilla luciferase was transfected by intravenous administrated non-modified and EGF modified-dendriplexes (activated) of EGF/DNA weight ratio of 2 (Yin et al., Citation2012). Our result shows that such self-assembled EGF modification can significantly enhance the expression levels of dendriplexes transfected Renilla luciferase in tumor tissue. Activated EGF-dendriplexes group with weight ratio of EGF/DNA = 2 exhibited higher luciferase expression (3865 ± 484 RLU/mg protein) than the non-EGF modified group (1845 ± 245 RLU/mg protein) in vivo. This observation can be explained by competitive binding between pre-assembled EGF and other serum component. Positively charged dendriplexes can interact with negatively charged molecules in the plasma post-intravenous injection, some of which may even dissociate DNA from the complexes (Zelphati et al., Citation1998).
Conclusion
In conclusion, the EGF-dendriplexes, a novel nano-non-viral gene delivery system, designed in this study showed a remarkably transfection performance on the tumor-targeted delivery, cellular uptake and high gene expression in vivo. N/P ratio and EGF/DNA weight ratio are two key factors on size and zeta potential of EGF-dendriplexes, which contribute to the high efficient gene delivery and expression in vitro and in vivo. Additionally, these self-assembled complexes in this work were prepared by simple and quality-controlled mixing of PAMAM dendrimer, plasmid DNA and the EGF ligand without complicated chemical reaction. These findings indicate that EGF-dendriplexes promise a novel non-viral nano gene delivery system for tumor-targeted therapy.
Declaration of interest
The authors report no conflict of interest in this research. This work was supported by grants from the National Science and Technology Major Project (No.2012ZX09103301-031), National Natural Science Foundation of China (No.31271070), National High Technology Research and Development Program of China (863) (No.2014AA020708) and China Postdoctoral Science Foundation (No.2013M530647).
References
- Aalinkeel R, Nair B, Reynolds JL, et al. (2012). Quantum rods as nanocarriers of gene therapy. Drug Deliv 19:220–31
- Benchaala I, Mishra MK, Wykes SM, et al. (2014). Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int J Pharm 466:258–65
- Chiu SJ, Ueno NT, Lee RJ, et al. (2004). Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin®) conjugated polyethylenimine. J Control Rel 97:357–69
- Choi JS, Nam K, Park JY, et al. (2004). Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-arginine. J Control Rel 99:445–56
- Douglas KL, Piccirillo CA, Tabrizian M. (2008). Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur J Pharm Biopharm 68:676–87
- Figueroa ER, Lin AY, Yan J, et al. (2014). Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 35:1725–34
- Froehlich E, Mandeville JS, Jennings C. (2009). Dendrimers Bind Human Serum Albumin. J Phys Chem B 113:6986–93
- Jevprasesphant R, Penny J, Attwood D, et al. (2003). Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm Res 20:1543–50
- Kang JH, Toita R, Katayama Y. (2010). Bio and nanotechnological strategies for tumor-targeted gene therapy. Biotechnol Adv 28:757–63
- Khurana B, Goyal AK, Budhiraja A. (2013). Lipoplexes versus nanoparticles: pDNA/siRNA delivery. Drug Deliv 20:57–64
- Kim TI, Baek JU, Zhe BC, et al. (2007). Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials 28: 2061–67
- Kumar A, Yellepeddi VK, Davies GE, et al. (2010). Enhanced gene transfection efficiency by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. Int J Pharm 392:294–303
- Kunath K, von Harpe A, Fischer D, et al. (2003). Galactose-PEI-DNA complexes for targeted gene delivery: degree of substitution affects complex size and transfection efficiency. J Control Rel 88:159–72
- Liu N, Hao Y, Yin Z, et al. (2012). Self-assembled human serum albumin-coated complexes for gene delivery with improved transfection. Die Pharmazie 67: 174–81
- Lu W, Sun Q, Wan J, et al. (2006). Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res 66:11878–87
- Mendelsohn, J. (2001). The epidermal growth factor receptor as a target for cancer therapy. Endocrine-Relat Cancer 8:3–9
- Mo Y, Lim L-Y. (2004). Mechanistic study of the uptake of wheat germ agglutinin-conjugated PLGA nanoparticles by A549 cells. J Pharm Sci 93:20–8
- Pan BF, Cui DX, Sheng Y, et al. (2007). Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res 67: 8156–63
- Pan BF, Cui DX, Xu P, et al. (2009). Synthesis and characterization of polyamidoamine dendrimer-coated multi-walled carbon nanotubes and their application in gene delivery systems. Nanotechnology 20:125101–9
- Pisal DS, Yellepeddi VK, Kumar A, et al. (2008). Transport of surface engineered polyamidoamine (PAMAM) dendrimers across IPEC-J2 cell monolayers. Drug Deliv 15:515–22
- Shcharbin D, Janicka M, Wasiak M, et al. (2007). Serum albumins have five sites for binding of cationic dendrimers. BBA-Proteins Proteom 1774:946–51
- Shcharbin D, Klajnert B, Mazhul V, et al. (2005). Dendrimer interactions with hydrophobic fluorescent probes and human serum albumin. J Fluoresc 15:21–8
- Somani S, Blatchford DR, Millington O, et al. (2014). Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J Control Rel 188:78–86
- Son S, Hwang do W, Singha K, et al. (2011). RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J Control Rel 155:18–25
- Thiersch M, Rimann M, Panagiotopoulou V, et al. (2013). The angiogenic response to PLL-g-PEG-mediated HIF-1α plasmid DNA delivery in healthy and diabetic rats. Biomaterials 34:4173–82
- Won YW, Lee M, Kim HA, et al. (2013). Synergistically combined gene delivery for enhanced VEGF secretion and antiapoptosis. Mol Pharmaceutics 10:3676–83
- Yamada H, Loretz B, Lehr CM. (2014). Design of starch-graft-PEI polymers: an effective and biodegradable gene delivery platform. Biomacromolecules 15: 1753–61
- Yin Z, Liu N, Ma M, et al. (2012). A novel EGFR-targeted gene delivery system based on complexes self-assembled by EGF, DNA, and activated PAMAM dendrimers. Int J Nanomed 7:4625–35
- Yu H, Nie Y, Dohmen C, et al. (2011). Epidermal growth factor-PEG functionalized PAMAM-pentaethylenehexamine dendron for targeted gene delivery produced by click chemistry. Biomacromolecules 12:2039–47
- Zelphati O, Uyechi LS, Barron LG, et al. (1998). Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochimica et Biophysica Acta 1390:119–33
- Zhang Q, Chen S, Zhuo RX, et al. (2010). Self-assembled terplexes for targeted gene delivery with improved transfection. Bioconjugate Chem 21:2086–92