Abstract
Context: Melanoma therapy absorbs attention because of the high morbidity and mortality. However, currently systematic administrations could take little therapeutic efficiency and severe side effects.
Objective: An effective transdermal formulation for the convenient melanoma therapy was found and evaluated.
Materials and methods: A mitoxantrone (MTO) cubic phase was prepared with glyceryl monooleate, ethanol and water. The permeation, cytotoxicity, in vivo anti-melanoma effect of the MTO cubic phases were evaluated. The anti-cancer mechanism of the MTO cubic phases was explored according to the immunohistochemistry and flow cytometry.
Results and discussion: The isotropic structure of MTO cubic phases was identified. The transdermal permeability of MTO was greatly improved by the cubic phase compared to that of the MTO solution. The MTO cubic phases showed the high cytotoxicity in B16 melanoma cells evidenced by a modified electrical cell–substrate impedance sensing system. High anti-melanoma effect of the MTO cubic phases was confirmed according to the tumor volume changes and tumor weight. The tumor inhibitory rate of the MTO cubic phases was 68.44%. The calreticulin expression of B16 cells was improved by the MTO cubic phases, and the improved cell uptake of MTO was confirmed by the flow cytometry.
Conclusion: The MTO cubic phase is a promising topical delivery system for melanoma therapy with the advantages of non-invasion and no severe side effects.
Introduction
Melanomas are malignant tumors that are derived from melanocytes and are characterized by early metastasis, rapid development, poor prognosis and high mortality. The morbidity and mortality of melanoma are increasing worldwide, particularly in the USA. About 76 100 newly diagnosed cases of melanoma were reported in the USA in 2014 with estimated 9710 expected deaths. According to the WHO, about 80% of all skin cancer-related deaths are attributed to melanoma. Despite extensive clinical research, the treatment options for this metastatic disease are limited, and melanoma is considered to be one of the most chemotherapy-resistant malignancies. Over the past 30 years, only three drugs have gained FDA approval for the treatment of melanoma; these drugs include dacarbazine, hydroxyurea and interleukin-2, and some of these drugs were approved only recently (Gogas et al., Citation2013). Recently, the nanocarriers of chemotherapeutic agents were applied to treat melanoma (Dai et al., Citation2015; Shakeel et al., Citation2015).
Mitoxantrone (MTO) is an anthraquinone anti-cancer drug to inhibit DNA synthesis and transcription (Legut et al., Citation2014). MTO is only applied via intravenous administration in clinic though some severe side effects including myelo-suppression and cardiotoxicity possibly happen (Muhonen et al., Citation1992; Podlecka-Pietowska et al., Citation2014). MTO liposomes and nanoparticles were prepared to achieve possible tumor targeting after intravenous administration (Heidari et al., Citation2013; Li et al., Citation2014; Yang et al., Citation2014).
A cubic phase consists of a curved bicontinuous lipid bilayer that extends in three dimensions and separates two congruent networks of water channels. Cubic phases exhibit isotropic and thermodynamic stability (Wu et al., Citation2008). Moreover, cubic phases can dissolve various drugs with different polarities and even encapsulate proteins, peptides and vaccines to favor permeability and stability (Li & Caffrey, Citation2014; Liu et al., Citation2014).
In the present study, a cubic phase of MTO was prepared for transdermal melanoma therapy. Highly enhanced permeation of the MTO cubic phases led to great anti-melanoma effect on animal models. Furthermore, the in vitro anti-melanoma mechanism of the MTO cubic phases was explored compared to that of the MTO solution.
Materials and methods
Materials
MTO chloride was purchased from Beijing Yikang Co., Ltd. (Beijing, China). Glyceryl monooleate (GMO, DIMODAN®MO90/D, DuPont, Wilmington, DE) was donated by Danisco (Gtindstad, Denmark). Anti-CRT antibody (primary antibody) was obtained from ABCAM (Boston, MA). Secondary antibody labeled with biotin and streptavidin/horseradish peroxidase was obtained from Zhongshan Biotical Technology Development Co., Ltd. (Beijing, China). All other materials were of pharmaceutical or analytical grade.
Animals
Male 6-week-old nude mice (weight, 17–23 g) from the Laboratory Animal Center of Beijing Institute of Radiation Medicine (BIRM) were used for the in vivo experiments. All animal handling and surgical procedures were conducted in strict accordance with the Guiding Principles for the Use of Laboratory Animals. This study was approved by the Animal Care Committee of BIRM. The mice were sacrificed to obtain the tissues. All studies were conducted in accordance with the Declaration of Helsinki. The abdominal skins of sacrificed male Wistar rats (200 ± 10 g) were excised followed by removal of the fat tissues for the permeation experiments. The skins used for cytotoxicity were immersed in penicillin/streptomycin solution (100 μg/ml penicillin and 100 units/ml streptomycin) and preserved at −20 °C for 2 days. The skins were sterilized under UV light for 15 min on both sides before the experiment.
Preparation of MTO cubic phases
An MTO solution was prepared by dissolving MTO chloride (220 mg) in water (3 ml) at 60 °C. The MTO solution was dropped into a GMO solution in ethanol at 60 °C under vortexing with the 30:64:6 w/w ratio of MTO chloride aqueous solution–GMO–ethanol. Finally, a cubic phase containing 2% (w/w) MTO was obtained. A very slow dropping rate, which was accomplished using a microsyringe, resulted in cubic phases, whereas faster dropping rates resulted in lamellar phases. The difference between the two phases was identified through polarizing light microscopy (PLM). The cubic phases were sealed in a tube and stored for 2–3 days at room temperature to remove any potential possible bubbles (Peng et al., Citation2009).
Characterization of the cubic phases
The structure of the MTO cubic phase was identified using a PLM (Leica DM2500P, Solms, Germany) and a small-angle X-ray scattering (SAXS) instrument (SAXSess, Graz, Austria) as detailed in other reports (Gan et al., Citation2010; Han et al., Citation2010). Optical observations were performed to acquire images from which the structure of the cubic phase gel could be identified using a PLM. The SAXS measurements were performed at room temperature. The scattering intensities were plotted as a function of the reciprocal spacing.
Permeation experiments
The vertical Franz-type diffusion cells (Tianjin Xinzhou Technical Co., Ltd., Tianjin, China) had a donor compartment, a receptor volume of 17 ml and a diffusion surface area of 1.96 cm2, where the skin was placed between the two compartments. Initially, the receptor compartment was filled with the warm saline (17 ml) that was kept in a bath (32 ± 0.5 °C) and stirred at 200 rpm. An MTO solution or an MTO cubic phase with the dose of 1.5 g (containing 20 mg of MTO chloride/g) was put in the donor compartment and contacted the surface of the skin stratum corneum (SC) face. At the predetermined time points (3, 6, 9, 12, 15, 18, 21 and 24 h), 5-ml aliquots of the solutions in the receptor compartment were withdrawn followed by being filled with the saline of equivalent volume. The collected solutions were filtered through a 0.45 -μm membrane and assayed by the UV–Vis spectrometry at 609 nm (Zhang et al., Citation2012).
The steady-state flux (Jss, μg cm−2 h−1) at time t represents the slope of the linear plots of the cumulative drug permeation amount (Qn, μg cm−2) as a function of time (t, h) (Dhawan et al., Citation2014). Qn is the cumulative MTO permeation amount in the receptor compartment and was calculated according to the following equation:
where V (ml) is the volume of saline in the receptor compartment, Cn (mg/ml) is the concentration of sample n, Ci (mg/ml) is the concentration of sample i, V0 (ml) is the withdrawn volume and A (cm2) is the permeation area (Zuo et al., Citation2014).
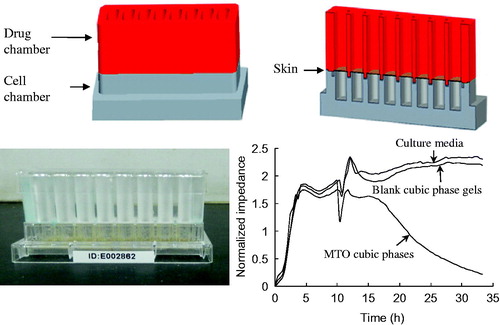
Cellular electrical impedance experiment
Electrical cell–substrate impedance sensing (ECIS) systems are used to continuously measure the growth states of adherent cells via the monitoring of changes in cell impedance, which is a non-invasive and label-free technique of cytotoxicity (Wegener et al., Citation2000; Solly et al., Citation2004). The ECIS system (ACEA, San Diego, CA) comprised an ECIS device and a modified chip that was composed of a cell chamber and a drug chamber. The two champers were separated by a piece of skin, which was our novel design to adapt the ECIS and the need of transdermal investigation. DMEM solution supplemented with 10% (v/v) fetal bovine serum and penicillin/streptomycin solution (culture Medium A, 100 μl, 37 °C) were placed in each cell chamber for 1 min to achieve baseline equilibrium. The mouse melanoma B16 cell suspension (300 μl, 8 × 103 cells, 37 °C) was added to each cell chamber. The ECIS device was placed in a humidified incubator at 37 °C containing a 5% CO2 atmosphere. Impedance data were recorded every 25 min. Medium A in the cell chambers was replaced with fresh Medium A after 10 h. Subsequently, Medium A, blank cubic phases and MTO cubic phases with the dose of 1.5 g were separately added to the drug chambers. The ECIS device was placed in the incubator, and impedance data were continually recorded. The normalized impedance was the average signal of triplicates.
Pharmacodynamic study
Every Balb/C nude mouse was subcutaneously injected in the hip with the B16 cell suspension (0.2 ml, 1 × 106 cells/ml). The xenograft volume (V) is measured with a caliper and calculated as 0.5 L × W2, where L is the largest superficial diameter and W is the smallest superficial diameter of the xenograft (Du et al., Citation2013). The tumor sizes were measured and recorded every day. The formulations were initially applied to the mice at the tumor sites topically once daily after the tumor size reached about 50–100 mm3. The melanoma-bearing mice were divided to three groups of six mice in each group. Group I was composed of tumor-bearing mice without treatment and served as the control. The other groups included the tumor-bearing mice that were treated with the MTO solution (Group II) and the MTO cubic phase (Group III). The mice were sacrificed after 10 days. The tumors were dissected and weighed to calculate the tumor inhibitory rates, which were defined as (Wblank − Wtest)/Wblank × 100%, where Wblank and Wtest were the average tumor weights of the control group and the therapeutic groups, respectively. The data are expressed as the means ± SDs. The Student’s t-test was used for all the statistical evaluations. Statistical significance was defined as a p value <0.05.
Immunohistochemistry
Some of the tumor tissues were preserved in formalin solutions for immunohistochemical study. The CRT expression on the cell surfaces of the B16 cells was evaluated using the anti-CRT antibody (Bisht et al., Citation2010). Tumor sections on the silane-coated slides were deparaffinized with xylene and dehydrated with ethanol. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. The sections were transferred to normal goat serum for 10 min at 37 °C. After removal of the goat serum, 1:50 (v/v) water-diluted primary anti-CRT antibodies were applied according to the manufacturer’s instructions. The secondary anti-CRT antibodies labeled with biotin were added to the above samples. The samples were incubated for 30 min and then washed with phosphate-buffered solutions (pH 7.4). Streptavidin/HRP was added. Diaminobenzidine was used as the chromogenic agent for visualization.
Flow cytometry
Some of the tumor tissues of Groups I (the mice without treatment) and III (the mice treated with the MTO cubic phases) were cut into fragments and filtered through 70 -μm cell screens. The obtained B16 cell suspensions were detected using a flow cytometric instrument (BDFACS Calibur, Franklin Lake, NJ) at an excitation wavelength of 635 nm and an emission wavelength of 661 nm (Kim et al., Citation2012). The fluorescence intensities of the samples (1 × 106 cells/µl) were assessed and analyzed with the CellQuest Pro software (BD, Franklin Lake, NJ).
Results and discussion
Formulation and characteristics of the MTO cubic phase
In our preliminary experiments, various ratios of the components in MTO cubic phases were tried. The fraction of MTO solution was constant and the ratio of GMO/ethanol was adjusted. When the 30:60:10 ratio of MTO solution/GMO/ethanol was used, a lamellar liquid crystal was obtained. After a detailed screening process, the 30:64:6 ratio was optimal and a homogeneous cubic phase was obtained. When the ethanol ratio decreased, even down to 0%, the product was a rigid gel. Therefore, the formulation of the MTO cubic phase was confirmed.
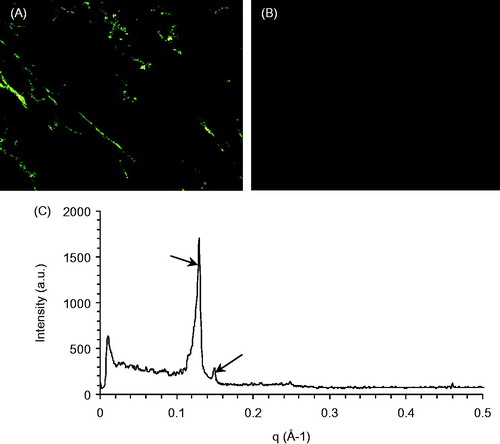
The MTO cubic phases was a viscous semi-solid and appeared to be blackish-blue in color. The structure of the cubic phases can be identified through the PLM and SAXS methods (Kraineva et al., Citation2005). In the PLM images, the lamellar phase exhibited some reflected light due to its anisotropy (). In contrast, only a dark background appeared in the PLM images () of the cubic phase due to its isotropy. Indeed, rapid agitation and homogeneous mixing were the key aspects of the MTO cubic phase preparation. Non-homogeneous mixing of the MTO and the excipients produces anisotropic lamellar phases. The spacing ratio of the scattering peaks for the MTO cubic phase was on the SAXS graph (), which is in accordance with the cubic G-type (Ia3d space group) (Han et al., Citation2010).
In vitro permeation of the MTO cubic phases
The Franz diffusion method is the method that is typically used to investigate the permeability of a drug into the skin. In this study, the MTO aqueous solution hardly penetrated the rat skins because no MTO was detected in the receptor compartment within 24 h. Therefore, the permeability of MTO itself was very weak due to its hydrophilicity (Tsai et al., Citation2014). However, MTO was quickly detected in the diffusion experiments with the cubic phase (). The steady state flux (Jss = 28.11 μg cm−2 h−1) of the MTO cubic phase was markedly high.
Figure 2. Cumulative amounts of MTO released from the cubic phases crossing through the rat skins as a function of time. The data are presented as the means ± SDs (n = 3).
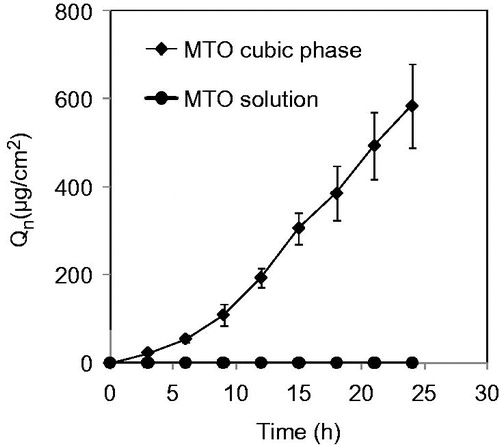
The state of MTO in transdermal delivery formulations should be a key factor that influences its transdermal efficiency. MTO should be highly dispersive and sufficient MTO amount should be supplied. High transdermal efficiency can only be achieved when both of these conditions are satisfied. In the present study, the bicontinuous structures of the cubic phases were prone to enter the SC due to high hydration, which improved the penetration of the cargo (i.e. MTO) into the skin. In contrast, the MTO solutions exhibited very low or even no permeability. Obviously, MTO transdermal delivery depends on the form of the dosage. The key excipient GMO intercalated with the extracellular lipid matrix of the skin and disrupted the highly compacted structures (Kwon & Kim, Citation2010; Luo et al., Citation2011). These comprehensive actions resulted in the MTO cubic phase exhibiting great transdermal efficiency. Furthermore, the in vitro cytotoxicity and in vivo anti-cancer effect must be related to the actual penetrating amount of MTO.
Cytotoxicity of the MTO cubic phases
A novel designed chip was used in the ECIS system to well explore the cytotoxicity of MTO from the cubic phases (). The culture medium and the blank cubic phase maintained high impedances, indicating no cytotoxicity. The MTO cubic phase showed obvious cytotoxicity according to decreasing impedances (). Therefore, the MTO cubic phase released much MTO into the medium. The impedance profiles of the formulations were in accordance with the in vitro permeation profiles detailed above. Moreover, the modified ECIS system provides a novel, convenient cytotoxicity detection method, which would be applied for the evaluation and high throughput screening of transdermal delivery systems.
Anti-cancer effects of the MTO cubic phases
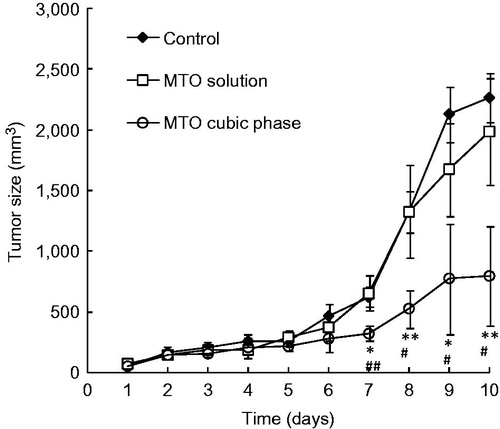
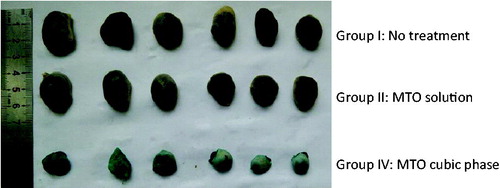
A significant difference initially appeared on day 7 (). A significant difference initially appeared between Group III (MTO cubic phase) and Group I (control) on day 7 (p < 0.01). Comparatively, a significant difference between Group III (MTO cubic phase) and Group II (MTO solution) began to appear on day 8 (p < 0.01) and maintained to the final day. The tumor sizes of Group II were close to those of Group I in the whole experimental field, indicating that the MTO solution had almost no therapeutic effect on melanoma. Obviously, the therapeutic results were strongly related to the MTO permeation efficiencies of the formulations. The tumor weights of Groups I, II and III were 2.13 ± 0.18 , 2.10 ± 0.35 and 0.67 ± 0.15 g, respectively, with the tumor inhibitory rates of 1.80%, 25.70% and 68.44%. The appearances of the tumors also exhibited significant differences between the groups (). Therefore, the anti-cancer effect of the MTO cubic phase was markedly high.
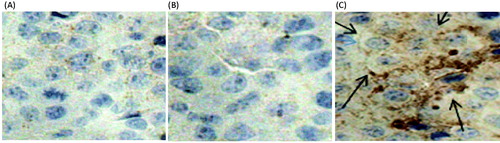
CRT expression improved by the MTO cubic phases
Dendritic cells recognize and eliminate tumor cells based on the number of outer CRT on the cell surface transferring from the intracellular sites. MTO can improve CRT expression on the membranes of B16 cells, resulting in increasing of the numbers of dendritic cells and T cells in the tumors. The growth of melanoma is then inhibited due to the induction of an anti-cancer immune response (Obeid et al., Citation2007a, Citationb; Cao et al., Citation2009). In this study, the extent of CRT expression reflected the permeation efficiency of MTO transdermal formulations. The MTO solution had no influence on CRT expression as well as the control (). However, the MTO cubic phase obviously improved CRT expression, which was in accordance with the high permeation efficiency of the MTO cubic phase.
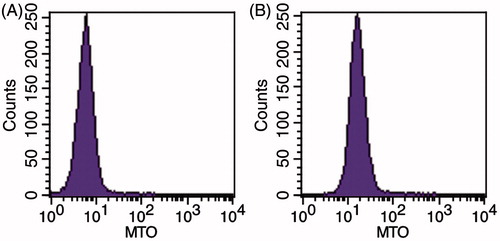
Cell uptake of MTO from the cubic phase
The uptake of MTO by B16 cells was investigated through flow cytometry. The geometric mean fluorescence intensity (GMFI) value of the control without treatment was 5.58 ± 0.75 (), indicating that almost no MTO entered into the cells. In contrast, the GMFI value of the MTO cubic phase group was 16.13 ± 3.05 (n = 3) with statistic difference compared to the control (p < 0.05). Therefore, the cell uptake of MTO from the cubic phase was high. The results are also in accordance with the other experiments.
Conclusions
Melanoma therapy is an emergent need for clinic because of the high morbidity and mortality and increasing drug resistance. An effective transdermal melanoma therapy is convenient and artificially controlled. More importantly, the side effect of transdermal drug delivery may be little compared to other systematic administrations. A high effective and permeable transdermal MTO formulation – MTO cubic phase was prepared in this study. The unique structure of cubic phases takes the hydrophilic MTO high permeation crossing through the skin and penetrating into the melanoma tissue. The MTO cubic phases will take hope for effective and convenient melanoma treatment.
Declaration of interest
The work was supported by the National Key Technologies R&D Program for New Drugs (No. 2012ZX09301003-001-009) and the National Natural Science Foundation of China (No. 81441095). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Bisht S, Mizuma M, Feldmann G, et al. (2010). Systemic administration of polymeric nanoparticle-encapsulated curcumin (nanocurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther 9:2255–64
- Cao C, Han Y, Ren Y, et al. (2009). Mitoxantrone-mediated apoptotic b16-f1 cells induce specific anti-tumor immune response. Cell Mol Immunol 6:469–75
- Dai W, Fan Y, Zhang H, et al. (2015). A comprehensive study of irgd-modified liposomes with improved chemotherapeutic efficacy on b16 melanoma. Drug Deliv 22:10–20
- Dhawan B, Aggarwal G, Harikumar S. (2014). Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int J Pharm Invest 4:65–76
- Du L, Jin Y, Yang J, et al. (2013). A functionalized poly(amidoamine) nanocarrier loading 5-fluorouracil: ph-responsive drug release and enhanced anticancer effect. Anti-Cancer Drugs 24:172–80
- Gan L, Han S, Shen J, et al. (2010). Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int J Pharm 396:179–87
- Gogas H, Polyzos A, Kirkwood J. (2013). Immunotherapy for advanced melanoma: fulfilling the promise. Cancer Treat Rev 39:879–85
- Han S, Shen JQ, Gan Y, et al. (2010). Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharm Sin 31:990–8
- Heidari M, Asgari D, Barar J, et al. (2013). Specific targeting of cancer cells by multifunctional mitoxantrone-conjugated magnetic nanoparticles. J Drug Target 21:328–40
- Kim HP, Bernard L, Berkowitz J, et al. (2012). Flow cytometry-based assessment of mitoxantrone efflux from leukemic blasts varies with response to induction chemotherapy in acute myeloid leukemia. Cytomet B Clin Cytomet 82:283–94
- Kraineva J, Narayanan RA, Kondrashkina E, et al. (2005). Kinetics of lamellar-to-cubic and intercubic phase transitions of pure and cytochrome c containing monoolein dispersions monitored by time-resolved small-angle X-ray diffraction. Langmuir 21:3559–71
- Kwon TK, Kim JC. (2010). In vitro skin permeation of monoolein nanoparticles containing hydroxypropyl beta-cyclodextrin/minoxidil complex. Int J Pharm 392:268–73
- Legut M, Lipka D, Filipczak N, et al. (2014). Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines – in vitro studies. Int J Nanomed 9:653–68
- Li D, Caffrey M. (2014). Renaturing membrane proteins in the lipid cubic phase, a nanoporous membrane mimetic. Sci Rep 4:5806. http://dx.doi.org/10.1038/srep05806
- Li C, Zhao X, Deng C, et al. (2014). Pegylated liposomal mitoxantrone is more therapeutically active than mitoxantrone in l1210 ascitic tumor and exhibits dose-dependent activity saturation effect. Int J Pharm 460:165–72
- Liu W, Wacker D, Wang C, et al. (2014). Femtosecond crystallography of membrane proteins in the lipidic cubic phase. Phil Trans R Soc B 369:20130314
- Luo M, Shen Q, Chen J. (2011). Transdermal delivery of paeonol using cubic gel and microemulsion gel. Int J Nanomed 6:1603–10
- Muhonen TT, Wiklund TA, Blomqvist CP, et al. (1992). Unexpected prolonged myelosuppression after mitomycin, mitoxantrone and methotrexate. Eur J Cancer 28A:1974–6
- Obeid M, Tesniere A, Ghiringhelli F, et al. (2007a). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
- Obeid M, Tesniere A, Panaretakis T, et al. (2007b). Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev 220:22–34
- Peng X, Yang Z, Chen M, et al. (2009). Preparation, characterization and content determination of cubic phase gel containing capsaicin. Chin J Clin Mater Med 35:3123–6
- Podlecka-Pietowska A, Kochanowski J, Zakrzewska-Pniewska B, et al. (2014). The n-terminal pro-brain natriuretic peptide as a marker of mitoxantrone-induced cardiotoxicity in multiple sclerosis patients. Neurol Neurochir Pol 48:111–15
- Shakeel F, Haq N, Al-Dhfyan A, et al. (2013). Chemoprevention of skin cancer using low hlb surfactant nanoemulsion of 5-fluorouracil: a preliminary study. Drug Deliv. Early Onlnie: 1–8. doi:10.3109/10717544.2013.868557
- Solly K, Wang X, Xu X, et al. (2004). Application of real-time cell electronic sensing (rt-ces) technology to cell-based assays. Assay Drug Dev Technol 2:363–72
- Tsai MJ, Fu YS, Lin YH, et al. (2014). The effect of nanoemulsion as a carrier of hydrophilic compound for transdermal delivery. PLoS One 9:e102850
- Wegener J, Keese CR, Giaever I. (2000). Electric cell–substrate impedance sensing (ecis) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 259:158–66
- Wu HB, Huo DF, Jiang XG. (2008). Advances in the study of lipid-based cubic liquid crystalline nanoparticles as drug delivery system. Acta Pharm Sin 43:450–5
- Yang J, Shi Y, Li C, et al. (2014). Phase i clinical trial of pegylated liposomal mitoxantrone plm60-s: pharmacokinetics, toxicity and preliminary efficacy. Cancer Chemother Pharmacol 74:637–46
- Zhang P, Ling G, Pan X, et al. (2012). Novel nanostructured lipid-dextran sulfate hybrid carriers overcome tumor multidrug resistance of mitoxantrone hydrochloride. Nanomedicine 8:185–93
- Zuo J, Du L, Li M, et al. (2014). Transdermal enhancement effect and mechanism of iontophoresis for non-steroidal anti-inflammatory drugs. Int J Pharm 466:76–82