Abstract
Cancer poses a significant threat to human health worldwide, and many therapies have been used for its palliative and curative treatments. Vincristine has been extensively used in chemotherapy. However, there are two major challenges concerning its applications in various tumors: (1) Vincristine's antitumor mechanism is cell-cycle-specific, and the duration of its exposure to tumor cells can significantly affect its antitumor activity and (2) Vincristine is widely bio-distributed and can be rapidly eliminated. One solution to these challenges is the encapsulation of vincristine into liposomes. Vincristine can be loaded into conventional liposomes, but it quickly leak out owing to its high membrane permeability. Numerous approaches have been attempted to overcome this problem. Vincristine has been loaded into PEGylated liposomes to prolong circulation time and improve tumor accumulation. These liposomes indeed prolong circulation time, but the payout characteristic of vincristine is severer, resulting in a compromised outcome rather than a better efficacy compared to conventional sphingomyelin (SM)/cholesterol (Chol) liposomes. In 2012, the USA Food and Drug Administration (FDA) approved SM/Chol liposomal vincristine (Marqibo®) for commercial use. In this review, we mainly focus on the drug's rapid leakage problem and the potentially relevant solutions that can be applied during the development of liposomal vincristine and the reason for conventional liposomal vincristine rather than PEGylated liposomes has access to the market.
Introduction
Cancer is a serious health issue globally and is the leading cause of death as well. In 2014, the international agency for research on cancer (IARC) reported that in 2012, the mortality, existing cases, and estimated incidence of cancers, excluding non-melanoma skin cancer, worldwide were 8.2 million, 32.6 million, and 14.1 million, respectively. Surgery, radiation and chemotherapy are the three classic cancer therapies (Urruticoechea et al., Citation2010). Chemotherapy plays a critical role in the treatment of lymphoma and leukemia. Many antitumor agents are available in the market and have been extensively used. However, antitumor drugs themselves cannot distinguish tumor cells from normal cells, leading to a compromised antitumor effect and severe side effects.
Drug delivery systems (DDSs) appear to be a potential solution to these problems. Liposomes are artificially prepared lipid vesicles containing an internal aqueous phase. Their characteristics, which include flexibility of composition, biocompatibility, biodegradability and non-immunogenicity, make them suitable for drug delivery. Conventional liposomes, the first generation liposomes, are attractive drug delivery systems (Cattel et al., Citation2003). However, these liposomes have a relatively short blood circulation time and mainly accumulate in the liver and spleen (Jensen & Bunch, Citation2007). Therefore, they are not suitable for tumor tissue-specific drug targeting. By averting the uptake of the mononuclear phagocyte system (MPS) and enhancing tumor accumulation by passive targeting, sterically stabilized liposomes exhibit a long circulation time compared to conventional liposomes. Doxil® is the first FDA-approved liposomal nanoparticle formulation coated with polyethylene glycol (PEG), and it exhibits prolonged retention in vivo and improved therapeutic effects (Barenholz, Citation2012). As the first commercially successful nanosized particle, Doxil® motivates current nanomedicine research related to PEGylation. Vincristine, a cell-cycle specific antitumor agent, is also loaded into PEGylated liposomes. However, the result was disappointing because its rapid leakage compromised its prolonged circulation.
In this review, we briefly describe the mechanism, clinical applications, pharmacokinetics and side effects of vincristine. Owing to its unique antitumor mechanism and pharmacokinetic behavior, vincristine is a suitable candidate for encapsulation into liposomes. Here, we introduce the development of liposomal vincristine. After successfully loading vincristine into liposomes, the retention of drug inside liposomes was found to be poor because of its high membrane permeability. Therefore, we summarize approaches to overcome this leakage problem and the mechanism of vincristine's rapid release from PEGylated liposomes in detail. Finally, we present the clinical trials of liposomal vincristine.
Vincristine
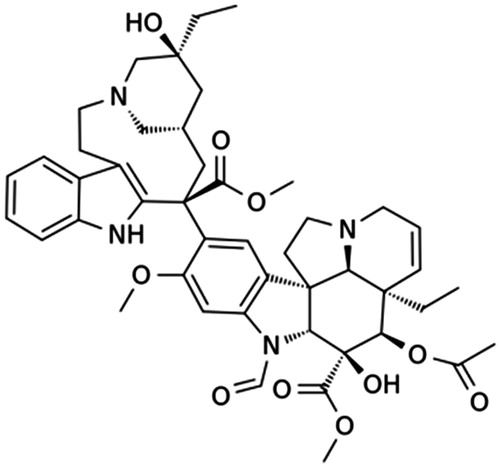
Vincristine is a naturally occurring alkaloid extracted from Catharanthus roseus leaves (Svoboda et al., Citation1962). It is a dimeric alkaloid comprising portions of catharanthine and vindoline (). Vincristine is one of the vinca alkaloids that were originally studied for their antidiabetic properties. Unfortunately, vinca alkaloids have no effect on blood sugar levels, but unexpectedly, they can produce a beneficial effect on lymphoma in mice (Noble, Citation1990). Scientists have confirmed that vincristine is one of the active compounds responsible for this effect.
Vincristine has many effects on actively dividing cells. The most well-known mechanism of its antitumor activity is its ability to bind to tubulins, which are the basic constituents of mitotic spindle microtubules (Himes, Citation1991; Jordan et al., Citation1991). Vincristine inhibits the polymerization of mitotic spindle microtubules, resulting in the suppression of mitosis. However, it was recently found that the lowest effective concentration exerts anti-proliferative effects by subtly changing the addition and loss of tubulins at mitotic spindle microtubule ends and has little or no microtubule depolymerization or spindle disorganization effects (Jordan et al., Citation1991). Therefore, vincristine stabilizes mitotic spindle assembly and disassembly processes that lead to metaphase arrest at low concentrations, while it totally depolymerizes the microtubules at high concentration. Furthermore, vincristine inhibits solid tumor blood flow in animal bearing colon 38 and two multidrug-resistant P388 cell lines and induces solid tumors necrosis after intraperitoneal administration (Baguley et al., Citation1991). Its other antitumor mechanisms have been reviewed in the literature (Rowinsky & Donehower, Citation1991).
In 1963, the FDA approved the vincristine preparation Oncovin® for commercial use. Vincristine is widely employed in cancer chemotherapy for palliative and curative treatments (Rowinsky & Donehower, Citation1991). It is primarily administered as an intravenous injection at a dose of 1.4 mg/m2 weekly, with a capping dose of 2.0 mg/m2 for adults, and a dose range of 1.5–2.0 mg/m2 with a maximum dose of 2.0–2.5 mg/m2 for children. Vincristine generally exhibits better efficacy when administered in combination with other antitumor agents. Combination chemotherapy can enhance the destruction of tumor cells and decrease toxicity and drug resistance with drugs exhibiting different mechanisms of action (Lee & Nan, Citation2012). It is extensively applied to treat various adult and pediatric malignant cancers and solid tumors, such as acute lymphocytic leukemia (ALL), lymphomas including Hodgkin's and non-Hodgkin's lymphoma (HL and NHL) as a part of MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone), CVF (cyclopnosphamide, vincristine and prednisone), CHOP (cyclopnosphamide, doxorubicin, vincristine and prednisone), plasma cell dyscrasia and pediatric and adult solid malignancies.
Although the applications of vincristine are extensive, the side effects of vincristine cannot be ignored. The main adverse reaction is neurotoxicity, which is dose-dependent (Carbone et al., Citation1963). The incidence and severity of neuropathy increase with elevated doses. Other toxicities include constipation, abdominal cramps, nausea, vomiting, diarrhea, hair loss, myelosuppression and fever.
Liposomal vincristine
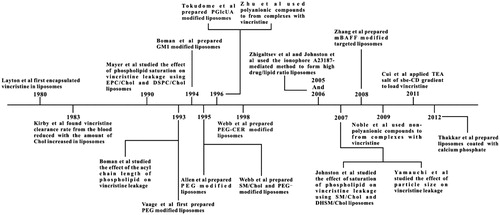
The pharmacokinetic behavior of vincristine in human blood is consistent with a three-compartment system. The values of t1/2 α, t1/2 β, and t1/2 γ are 1.9 min (less than 5 min), 19.2 min, and 22.6 h, respectively (Sethi et al., Citation1981). Other literatures have also reported similar results, but the t1/2 γ is longer [133 and 155 h (Nelson et al., Citation1977)]. Although the elimination phase is quite long, the clearance rate is fast. The apparent volume of distribution is 167.6 L/1.73 m2, which means that vincristine distributes extensively in body. Vincristine's characteristic pharmacokinetic behaviors include its rapid clearance rate and wide bio-distribution. In addition, vincristine is a schedule-dependent drug, and it is advantageous to prolong its exposure intervals to tumor tissues. Therefore, vincristine is a good candidate for encapsulation into liposomes. The development of liposomal vincrinstine is summarized in .
Conventional liposomal vincristine
Conventional liposomes typically consist of only phospholipids and/or Chol. Layton et al. (Citation1980) first encapsulated vincristine into liposomes to reduce its toxicity. Compared to vincristine solution, its liposomes displayed neither reduced toxicity nor improved therapeutic effects for the treatment of lymphocytic leukemia, which might be due to toxicity caused by the addition of stearylamine in lipids (Nishiya et al., Citation1995). However, the finding that liposomal vincristine lowers the plasma clearance rate should not been overlooked.
Vincristine is a drug with high membrane permeability [log P = 2.82 (Leo et al., Citation1971)], which leads to its rapid release from liposomes in circulation. Since lipid compositions can affect drug retention properties, many researchers have focused on this factor. Drug circulation time increases with increasing acyl chain length or the degree of phospholipid molecules saturations. Boman et al. (Citation1993) showed that dibehenoylphosphatidylcholine (DBPC, C22)/Chol liposomes exhibited the slowest release rate in vitro compared to shorter acyl chains. Regarding the degree of saturation of phospholipids, Mayer et al. (Citation1990) demonstrated that vincristine leakage from egg phosphatidylcholine (EPC)/Chol liposome encapsulations was faster than that from distearoylphosphatidylcholine (DSPC)/Chol liposomes. In addition, dihydrosphingomyelin (DHSM)/Chol liposomes exhibited the slowest drug payout characteristics in vitro and in vivo among egg SM, bovine milk SM, bovine brain SM and DHSM (Johnston et al., Citation2007). The above results are consistent with the fact that increasing the acyl chain length or the degree of phospholipid saturation leads to a higher phase transition temperature (Shimshick et al., Citation1973).
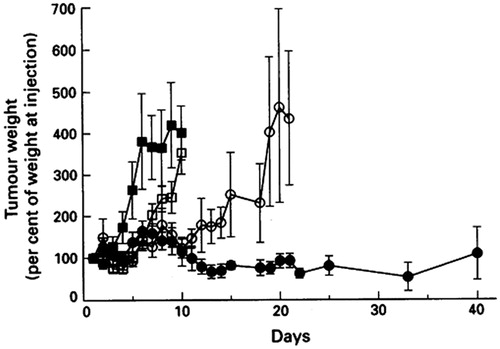
Webb et al. (Citation1995) substituted SM for DSPC to prepare liposomes entrapped with vincristine. The mean diameter of these liposomes was 120–130 nm and the encapsulation efficiency was more than 95% with the active loading method. The in vivo pharmacokinetic studies showed that the retention of vincristine in SM/Chol liposomes improved compared to that in DSPC/Chol liposomes. As a result, plasma drug levels of SM/Chol liposomes were 7-fold higher than that of DSPC/Chol liposomes after 72 h. The improved behavior of SM/Chol liposomes in vivo might be due to a reduction in the hydrolysis rate of SM and the reduced blood component absorption of SM/Chol liposomes. Decreased vincristine leakage from SM/Chol liposomes in plasma was well correlated with an increase in drug-loaded liposomes delivered to ascitic P388 and solid A431 tumors, as well as with its enhancement in its antitumor efficacy against these tumors (). Krishna et al. (Citation2001) showed that the AUC of vincristine in SM/Chol liposomes at tumor sites was approximately 2-fold higher than that of vincristine encapsulated in DSPC/Chol liposomes by using the Lewis lung carcinoma solid tumor model. This observation was in accordance with the improved antitumor effect displayed by Webb et al. (Citation1995).
Figure 3. The antitumor effect of vincristine formulations in SCID mice bearing A431 tumors. The control group (▪) received no treatment and vincristine formulations (vincristine solution (□), DSPC/Chol (○) or SM/Chol (•) liposomal vincristine) were intravenously injected at a dose of 2.0 vincristine mg/kg. Reprinted by permission from Macmillan Publishers Ltd: British Journal of Cancer, 72(4), Webb MS, Harasym TO, Masin D, Bally MB, Mayer LD, Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumor models, 896–904, copyright (Citation1995). We acknowledge Nature Publishing Group's permission (http://www.nature.com/bjc/index.html).
In addition, a variety of approaches have been proposed to improve drug retention in liposomes, such as a high drug/lipid ratio, large particle size and calcium phosphate (CP) nanoshell. Zhigaltsev et al. (Citation2005) encapsulated vincristine into SM/Chol liposomes using the ionophore A23187-mediated method. It was found that the retention of vincristine increased upon increasing the drug/lipid ratio using ionophore loading technology rather than the transmembrane pH gradient created by citrate. Although A23187 can drive Mg2+ outside liposomes, there must be some Mg2+ left inside. As bivalent ions can form complexes with bioamines, the intraliposomal Mg2+ might play a critical role in precipitation (Cui et al., Citation2011). Cryo-transmission electron micrography showed that there were more electron-dense liposomes with high drug/lipid ratios, which could suggest internal vincristine precipitation (Zhigaltsev et al., Citation2005). Johnston et al. (Citation2006) studied the in vivo pharmacokinetics and the therapeutic efficacy of the above formulations at various drug/lipid ratios in mice. The results demonstrated that the retention of vincristine improved, but the antitumor activity reduced with high drug/lipid ratio (>0.1, w/w) because of the slow rate of drug release from liposomes at tumor sites.
The leakage rate of vincristine loaded in EPC/Chol liposomes reduced when the particle size larger than 120 nm (Yamauchi et al., Citation2007). One reason for this is that the curvature of small liposomes was greater, and thus the packing of lipids in the bilayer was not compact; another reason was that the lamellarity of liposomes was greater than one in larger liposomes, which was confirmed by the calculation of trap volume and the measurement of 31P-NMR, and the multilayers resulted in slower vincristine diffusion. However, we should not neglect the fact that a particle size of less than 200 nm could result in effective accumulation in tumor tissues by avoiding the uptake of the MPS and with the help of the enhanced permeability and retention (EPR) effect (Albanese et al., Citation2012). Thakkar et al. (Citation2012) prepared liposomes coated with CP, and the in vitro release rate of vincristine from CP-covered liposomes was lower than that from uncoated ones.
Besides phospholipids, another basic component of liposomes is Chol. Sufficient Chol contained in membrane bilayers can maximize the longevity of liposomes in the blood (Chow et al., Citation1989). Chol can also reduce the leakage of encapsulated drug from liposomes caused by the disruption of liposomes via lipid exchange (Damen et al., Citation1981). It was shown by Kirby et al. (Citation1983) that the vincristine clearance rate from the blood was reduced when the amount of Chol increased in liposomes.
Sterically-stabilized liposomal vincristine
Since vincristine is a cell-cycle specific drug, it is advantageous to have a long half-life in circulation, which would improve the therapeutic effect. In order to prolong the circulation time, researchers assessed sterically-stabilized liposomes that display long circulating times. There are two approaches that improve circulation time: using techniques that mimic the cells in the body, such as erythrocytes, and reducing the opsonization of liposomes, such as PEG coating.
Glycolipid-modified liposomal vincristine
Erythrocytes are good models for cells that exhibit long circulation times (120 days) (Bratosin et al., Citation1998). The surface of an erythrocyte is covered with a dense layer of glycocalyx, such as gangliosides, which forms a hydration shell to partially protect it from immune system recognition (Bratosin et al., Citation1998; Cattel et al., Citation2003). Monosialotetrahexosylganglioside (GM1) is one of the gangliosides whose terminal sialic acid residues might be responsible for the long life span of erythrocytes (Bratosin et al., Citation1998). This hypothesis is based on the fact that sialidase-treated erythrocytes are rapidly cleared from the bloodstream (Aminoff et al., Citation1977). Boman et al. (Citation1994) demonstrated that vincristine trapped in liposomes containing 10 mol% GM1 with an intraliposomal pH of 2.0 was highly effective against P388 leukemia. However, at pH 2.0 the DSPC in liposomes could hydrolyze at a rapid rate [9.11% per hour (Webb et al., Citation1995)], and the uptake of GM1-modified liposomes was weakened only in mice but not in rats or rabbits (Hitoshi et al., Citation1995). Besides this, other drawbacks, such as the cost and the difficulty of its purification, blocked its application in DDSs. Another glycolipid, palmityl-d-glucuronide (PGlcUA), was grafted on the surface of liposomes and resulted in a long circulation time in mice (Oku et al., Citation1992) and rats (Namba et al., Citation1990). Tokudome et al. (Citation1996) tested the long circulation activity and therapeutic efficacy of vincristine in liposomes containing PGlcUA. PGlcUA-modified liposomes were most efficient at suppressing Meth A sarcoma growth compared to free vincristine and conventional liposomes. However, we should not ignore the fact that the dose of vincristine used in all the liposomes was 2-fold higher than that in the drug solution.
PEGylated liposomal vincristine
PEGylation is a milestone in DDSs and has been applied in the modification of proteins, peptides, and vehicles (Veronese & Pasut, Citation2005). PEGylated nanoparticles can prolong bloodstream circulation time by averting opsonization and uptake by the MPS (Owens & Peppas, Citation1996) and consequently accumulate in tumor tissue rather than normal tissues through the EPR effect (Maeda et al., Citation2000). Moreover, the PEG modification avoids liposome aggregation improving stability of preparations.
Vaage et al. (Citation1993) formulated vincristine-loaded PEGylated liposomes. Compared to SM/Chol liposomes, the particle size (about 100 nm) and trapping efficiency (>95%) was similar to conventional liposomes, but the surface charges of these two formulations were different (the electrophoretic mobility of SM/Chol and PEGylated liposomes was 0.0 and −0.83 × 10−4 cm2/Vs) at pH 7.0 (Webb et al., Citation1998). This PEG-coating formulation resulted in improved therapeutic effects against mammary carcinoma MC2 compared to the drug solution. This effect might be due to longer circulating time of liposomal vincristine, consequently resulting in increased drug accumulation in the tumor. Allen et al. (Citation1995) confirmed this finding and they found that liposomal vincristine was more effective against s.c. or i.p. implanted tumors than i.v. disseminated leukemia, which suggests that the site of tumor implantation has a marked impact on the therapeutic index.
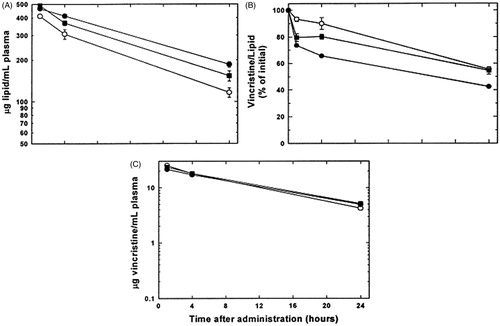
Webb et al. (Citation1995) found that the circulation time of PEG-DSPE/SM/Chol liposomes (t1/2 = 24.0 h) was longer than non-PEG liposomes (t1/2 = 18.9 h), but the leakage rate of vincristine from PEG liposomes was faster than that from non-PEG liposomes, which resulted in nearly the same vincristine levels in the blood and similar antitumor effects for both formulations. These pharmacokinetic properties () of PEGylated SM/Chol liposomal vincristine were confirmed by subsequent research by the same team (Webb et al., Citation1998). The results showed that the circulation time of SM/Chol liposomes was comparable to that of PEG-modified liposomes.
Figure 4. The pharmacokinetics of SM/Chol (○), SM/Chol/PEG-DSPE (•) and SM/Chol/PEG-CER (▪) liposomal vincristine. Concentrations of liposomal lipid (A), vincristine/lipid ratio (B), concentrations of vincristine (C) in plasma at various times after intravenous administration. Reprinted from Copyright Webb et al., (Citation1998), with permission from Elsevier.
Webb et al. (Citation1998) attempted to interpret the above phenomenon, therefore, they synthesized a novel neutral lipid PEG-CER, which is the conjugation of PEG and ceramide. PEG-DSPE was substituted with PEG-CER to determine whether the surface charge of liposomes was the cause of the increased drug leakage. The retention properties of vincristine in PEG-CER liposomes were enhanced compared to liposomes with negatively charged lipids, such as PEG-DSPE, DOPS and DSPG at physiological pH. When PEG grafting density increased, the vincristine half-life decreased (Cui et al., Citation2011). Negatively charged liposomes are speculated to interact with cationic vincristine [pKa1 = 5.0and pKa2 = 7.4 (Johnson et al., Citation1963)] inside liposomes (intraliposomal pH 4.0) resulting in elevated local drug concentrations at the inner surface and a raised transmembrane vincristine concentration gradient.
In addition, the intermolecular and intramolecular hydrogen bonding present in SM bilayers result in a more rigid structure (Schmidt et al., Citation1977). The addition of PEG-DSPE might disrupt the formation of intramolecular hydrogen bonding between SM, and consequently, the leakage rate of vincristine increases.
Except for changing the compositions of liposomes, developing novel strategies for stabilizing liposomes, such as forming complexes with polyanionic or non-polyanionic compounds inside liposomes or using the sulfobutylether cyclodextrin (sbe-CD) gradient, can also reduce the leakage of vincristine from PEG liposomes. Zhu et al. (Citation1996) employed suramin, heparin and dextran sulfate to precipitate vincristine inside liposomes. The results indicate that the plasma distribution half-lives of liposomes with complexes inside was longer than that of the formulation without polyanionic compounds, but the antitumor activity was almost the same for all PEG-coated liposomes. Noble et al. (Citation2009) used non-polymer multivalent anionic agent, sucroseoctasulfate, to prepare stabilized liposomes by forming the electrostatic complexes inside the aqueous phase. This formulation could prolong the pharmacokinetics, and the efficacy was similar to that of free vincristine against nude mice bearing a BT-474-M2 tumor. Cui et al. (Citation2011) applied triethylamine (TEA) salt of sbe-CD gradient to load vincristine. During this process, sbe-CD serves as a trapping agent, and triethylamine serves as a driving force. The UV and FT-IR spectra show that the drug and sbe-CD did not form stable inclusion complexes, and their interaction was similar to that observed in the above experiments owing to the sulfonic group of sbe-CD and the amine group of vincristine at intraliposomal pH 5.
Although the leakage rate of vincristine decreased owing to these strategies, the antitumor activity reduced. It should be noted that the release and retention of liposomal drug could be optimally balanced. This case was well illustrated by sterically stabilized liposomal cisplatin. Bandak et al. (Citation1999) showed that PEGylated liposomal cisplatin increased circulation longevity and enhanced tumor accumulation after intravenous injection. However, there was a poor therapeutic effect in three murine tumor models owing to the slow release rate of cisplatin from liposomes. A similar result was observed by Zamboni et al. (Citation2004) after mice bearing melanoma tumors were administered cisplatin loaded PEG-modified liposomes.
Ligand-modified PEGylated liposomal vincristine
In DDSs, PEGylation has been applied mainly to increase the circulation longevity of nanoparticles. Although PEGylation can prolong blood circulation, it also reduces the drug efficacy, resulting in compromised drug activity. Ligands are frequently connected with the ends of PEG chains for targeted drug delivery. The receptors of B cell activating factor (BAFF belonging to the tumor necrosis factor family) are expressed in numerous B cell malignancies. mBAFF, a mutant of BAFF, can compete with BAFF in the treatment of relevant hematologic diseases. Zhang et al. (Citation2008) conjugated the amine group of mBAFF to the sulfhydryl group of PEG and decorated it on DSPC/Chol liposomes. The results illustrated that mBAFF-modified liposomal vincristine was more effective than non-targeted liposomal vincristine against Raji lymphoma cells in both in vitro cytotoxicity and in vivo therapy studies. However, when the ligand is a small molecule, it should be taken into account that ligand-modified PEG liposomes can severely inhibit the molecule's affinity for its receptors (Hennig et al., Citation2014).
Although PEGylated liposomes have favorable characteristics, evidence suggests that caution needs to be exercised, especially for multiple dose administration of PEGylated formulation. Recent reports have shown that intravenously injected PEGylated liposomes have a marked impact on the pharmacokinetics and bio-distribution of the second dose within a certain time frame. This phenomenon is called the ‘‘accelerated blood clearance (ABC) phenomenon’’ which is a potential challenge for the PEGylated formulations (Dams et al., Citation2000; Laverman et al., Citation2001; Xu et al., Citation2014).
Clinical trials
The clinical trials for liposomal vincristine are summarized in .
Table 1. The clinical trials for liposomal vincristine.
Phase I clinical trials
Monotherapy
Embree et al. (Citation1997) conducted the first clinical trial of liposomal vincristine (DSPC/Chol liposomes) to determine the pharmacokinetic behavior of this preparation by intravenous infusion over 1 h. The t1/2 values of 2.0, 2.4, and 2.8 mg/m2 were 117 ± 403, 317 ± 472 and 434 ± 286 min, respectively, which were significantly prolonged compared to the vincristine solution. In addition, there was a significant increase (about 45-fold) of vincristine levels in plasma.
In another research study, the maximum-tolerated dose (MTD), recommended phase II dose, pharmacokinetics, and toxicity of the above-mentioned liposomes were determined by Gelmon et al. (Citation1999) through an intravenous infusion every three weeks. The results showed that the MTD was 2.4 mg/m2, and the dose recommended for subsequent phase II clinical trials was 2.0 mg/m2, which indicated that a higher dose could be used for treatment by loading vincristine into liposomes. The plasma concentration of vincristine was 2.5- to 154-fold higher than its free form. Although the drug AUC increased, the toxicity was not severer because vincristine remained inside liposomes in the bloodstream. The most frequently occurring side effects were myalgias, constipation, peripheral neuropathy, fever, and mild anemia, and these were comparable to the vincristine solution.
Bedikian et al. (Citation2008) evaluated the safety and activity of liposomal vincristine (SM/Chol liposomes) in patients with metastatic melanoma. The dose of vincristine was 2 mg/m2 without a capping dose, and it was infused over 1 h every two weeks. This formulation was safe, and no grade 4 adverse events were found. The disease control rate was 31% with 4% complete response (CR), 8% partial response (PR) and 19% stable disease (SD), which was better than the corresponding values observed with the vincristine solution (16% partial or better response).
Combination therapy
The efficacy of liposomal vincristine (SM/Chol liposomes) combined with pulse dexamethasone for the treatment of relapsed or refractory ALL was tested by Thomas et al. (Citation2009). The MTD was 2.25 mg/m2 based on the toxicity and the overall response rate (ORR), composed of CR (19%) and partial response (PR=3%), was 22%. The most common toxicities were constipation, fatigue, peripheral neuropathy, anemia and fever.
Phase II clinical trials
Monotherapy
The subsequent phase II clinical trials were carried out using liposomal vincristine (SM/Chol liposomes) against relapsed NHL and ALL by Sarris et al. (Citation2000). Patients were treated with 2.0 mg/m2 by intravenous infusion over 1 h every two weeks, and responders received a maximum of 12 injections. The ORR was 41% for NHL, and all patients with NHL had previously been treated with vincristine. No grade 3 or 4 nausea, vomiting, constipation, fever and grade 3–4 motor or sensory neuropathy was observed. The curative effect of sphingosomal vincristine in patients with recurrent or refractory ALL was also studied by Thomas et al. (Citation2006) at a dose of 2.0 mg/m2 every two weeks. The ORR was 14%.
In the pivotal phase II study of SM/Chol liposomal vincristine, 119 patients with multiple relapsed or refractory aggressive NHL were treated intravenously at 2 mg/m2 every two weeks to estimate the therapeutic effect and tolerability of this formulation (Rodriguez et al., Citation2009). The ORR was 25% and showed approximately twice the dose intensity tolerability compared to vincristine solution. Unfortunately, the Oncology Drugs Advisory Committee (ODAC) of the FDA vetoed recommending accelerated approval for sphingosomal vincristine as a treatment for relapsed aggressive NHL.
In another pivotal phase II trial of liposomal vincristine (SM/Chol liposomes), 65 patients with advanced, relapsed, and refractory adult Philadelphia chromosome (Ph)-negative ALL were infused intravenously with a high dose (2.25 mg/m2) every week (O'Brien et al., Citation2013). The ORR was 35% and generally well tolerated. The toxicity of SM/Chol liposome formulation of vincristine was predictable, manageable, and comparable to that of vincristine solution. This study was the basis for accelerated FDA approval granted on 9 August 2012.
The efficacy of vincristine-loaded SM/Chol liposomes in patients with metastatic uvel melanoma was investigated by Bedikian et al. (Citation2009). The formulation was administered at 2.25 mg/m2 by 1 h intravenous infusion every two weeks. The SD was 57%, and the median OS was 6.4 months. A grade 1 or 2 toxicity and a grade 3 peripheral neuropathy were observed (only 18% of patients).
Combination therapy
Combination therapy was conducted by substituting sphingosomal vincristine for vincristine solution in CHOP plus rituximab (no rituximab for T-cell histology) in patients with previously untreated aggressive B cell NHL (Rodriguez et al., Citation2005). The dose of vincristine was 2.0 mg/m2 every three weeks. The ORR was 92.6%, and the OS was 94%. The toxicity was grade 3–4 neutropenia and thrombocytopenia and grade 3 anemia.
The efficacy of palliative therapy of vincristine-loaded SM/Chol liposomes plus rituximab for patients with advanced, relapsed, and refractory diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) was confirmed by Kaplan et al. (Citation2014). The dose of vincristine was 2.0 mg/m2 every two weeks, and the dose of rituximab was 375 mg/m2 every week. The ORR was 59%. Median response duration, time to progression (TTP), and median OS of this liposomal vincristine were 147 days, 121 days, and 322 days, respectively.
Based on the results of the pivotal trials conducted by O'Brien, in 2012, the FDA approved vincristine-encapsulated SM/Chol liposomes (Marqibo®) for the treatment of adult patients with Ph-negative ALL in second or greater relapse or with progressive disease following two or more anti-leukemia therapies. The administration route is intravenous infusion for 1 h weekly at a dose of 2.25 mg/m2.
Although the remarkable anti-leukemic effect has been seen in Phase II clinical trials, the role of Marqibo® in ALL remains unclear. Currently, Phase III clinical trials are in progress to evaluate the safety and efficacy of Marqibo® in the treatment of patients older than 60 with newly diagnosed ALL, and further clinical trials are needed to assess the significant role of Marqibo® in the treatment of ALL or other tumors.
Conclusions
SM/Chol liposomal vincristine displays relative long circulation time, reduced leakage rate from liposomes, and an improved antitumor effect compared to PEGylated liposomal vincristine, which has finally been approved by the FDA. This fact reminds us that although PEGylation has many advantages, such as prolonged residence in the circulatory system and enhanced tumor accumulation, conventional liposomes with rigid structures like SM/Chol liposomes may be more suitable for high membrane permeability drugs.
Declaration of interest
The authors report no declarations of interest.
References
- Albanese A, Tang PS, Chan WC. (2012). The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
- Allen TM, Newman MS, Woodle MC, et al. (1995). Pharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomes. Int J Cancer 62:199–204
- Aminoff D, Bruegge WF, Bell WC, et al. (1977). Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Natl Acad Sci USA 74:1521–4
- Baguley BC, Holdaway KM, Thomsen LL, et al. (1991). Inhibition of growth of colon 38 adenocarcinoma by vinblastine and colchicine: evidence for a vascular mechanism. Eur J Cancer 27:482–7
- Bandak S, Goren D, Horowitz A, et al. (1999). Pharmacological studies of cisplatin encapsulated in long-circulating liposomes in mouse tumor models. Anticancer Drugs 10:911–20
- Barenholz Y. (2012). Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release 160:117–34
- Bedikian AY, Papadopoulos NE, Kim KB, et al. (2008). A pilot study with vincristine sulfate liposome infusion in patients with metastatic melanoma. Melanoma Res 18:400–4
- Bedikian AY, Sato T, Kim KB, et al. (2009). Phase II study of vincristine sulfate liposomes injection in patients with metastatic uveal melanoma. J Clin Oncol (2009 ASCO Annual Meeting) 27:15
- Boman NL, Masin D, Mayer LD, et al. (1994). Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res 54:2830–3
- Boman NL, Mayer LD, Cullis PR. (1993). Optimization of the retention properties of vincristine in liposomal systems. Biochim Biophys Acta 1152:253–8
- Bratosin D, Mazurier J, Tissier JP, et al. (1998). Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages: a review. Biochimie 80:173–95
- Carbone PP, Vincent B, Frei E III, Brindley CO. (1963). Clinical studies with vincristine. Blood 21:640–7
- Cattel L, Ceruti M, Dosio F. (2003). From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori 89:237–49
- Chow DD, Essien HE, Padki MM, Hwang KJ. (1989). Targeting small unilamellar liposomes to hepatic parenchymal cells by dose effect. J Pharmacol Exp Ther 248:506–13
- Cui J, LiC, Wang C, et al. (2011). Development of pegylated liposomal vincristine using novel sulfobutyl ether cyclodextrin gradient: is improved drug retention sufficient to surpass DSPE-PEG-induced drug leakage? J Pharm Sci 100:2835–48
- Damen J, Regts J, Scherphof G. (1981). Transfer and exchange of phospholipid between small unilamellar liposomes and rat plasma high density lipoproteins. Dependence on cholesterol content and phospholipid composition. Biochim Biophys Acta 665:538–45
- Dams ET, Laverman P, Oyen WJ, et al. (2000). Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 292:1071–9
- Embree L, Gelmon K, Tolcher A, et al. (1997). Pharmacokinetic behavior of vincristine sulfate following administration of vincristine sulfate liposome injection. Cancer Chemother Pharmacol 41:347–52
- Gelmon KA, Tolcher A, Diab AR, et al. (1999). Phase I study of liposomal vincristine. J Clin Oncol 17:697–705
- Hennig R, Pollinger K, Veser A, et al. (2014). Nanoparticle multivalency counterbalances the ligand affinity loss upon PEGylation. J Control Release 194:20–7
- Himes RH. (1991). Interactions of the catharanthus (Vinca) alkaloids with tubulin and microtubules. Pharmacol Ther 51:257–67
- Hitoshi Y, ToshiroY, Takashi K, et al. (1995). Effects of sialic acid derivative on long circulation time and tumor concentration of liposomes. Int J Pharm 113:141–8
- Jensen GM, Bunch T. (2007). Conventional liposome performance and evaluation: lessons from the development of Vescan. J Liposome Res 17:121–37
- Johnson IS, Armstrong J, Gorman M, Burnett JP Jr. (1963). The vinca alkaloids: a new class of oncolytic agents. Cancer Res 23:1390–427
- Johnston MJ, Semple SC, Klimuk SK, et al. (2006). Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta 1758:55–64
- Johnston MJ, Semple SC, Klimuk SK, et al. (2007). Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta 1768:1121–7
- Jordan MA, Thrower D, Wilson L. (1991). Mechanism of inhibition of cell proliferation by vinca alkaloids. Cancer Res 51:2212–22
- Kaplan LD, Deitcher S, Silverman JA, Morgan G. (2014). Phase II study of vincristine sulfate liposome injection (Marqibo) and rituximab for patients with relapsed and refractory diffuse large B-Cell lymphoma or mantle cell lymphoma in need of palliative therapy. Clin Lymphoma Myeloma Leuk 14:37–42
- Kirby C, Gregoriadis G. (1983). The effect of lipid composition of small unilamellar liposomes containing melphalan and vincristine on drug clearance after injection into mice. Biochem Pharmacol 32:609–15
- Krishna R, Webb M, St Onge, Mayer LD. (2001). Liposomal and non-liposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther 298:1206–12
- Laverman P, Carstens M, Boerman OC, et al. (2001). Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther 298:607–12
- Layton D, Trouet A. (1980). A comparison of the therapeutic effects of free and liposomally encapsulated vincristine in leukemic mice. Eur J Cancer 16:945–50
- Lee JH, Nan A. (2012). Combination drug delivery approaches in metastatic breast cancer. Drug Deliv, Early Online: 1-17. doi: http://dx.doi.org/10.1155/2012/915375
- Leo A, Hansch C, Elkins D. (1971). Partition coefficients and their uses. Chem Rev 71:525–616
- Maeda H, Wu J, Sawa T, et al. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–84
- Mayer LD, Bally M, Loughrey H, et al. (1990). Liposomal vincristine preparations which exhibit decreased drug toxicity and increased activity against murine L1210 and P388 tumors. Cancer Res 50:575–9
- Namba Y, Sakakibara T, Masada M, et al. (1990). Glucuronate-modified liposomes with prolonged circulation time. Chem Pharm Bull 38:1663–6
- Nelson RL, Dyke RW, Root MA. (1977). Comparative pharmacokinetics of the vinca alkaloids in man. Clin Pharmacol Ther 21:112
- Nishiya T, Lam R, Eng F, et al. (1995). Mechanistic study on toxicity of positively charged liposomes containing stearylamine to blood. Artif Cells Blood Substit Immobil Biotechnol 23:505–12
- Noble CO, Guo Z, Hayes ME, et al. (2009). Characterization of highly stable liposomal and immunoliposomal formulations of vincristine and vinblastine. Cancer Chemother Pharmacol 64:741–51
- Noble RL. (1990). The discovery of the vinca alkaloids-chemotherapeutic agents against cancer. Biochem Cell Biol 68:1344–51
- O'brien S, Schiller G, Lister J, et al. (2013). High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol 31:676–83
- Oku N, NambaY, Okada S. (1992). Tumor accumulation of novel RES-avoiding liposomes. Biochim Biophys Acta 1126:255–60
- Owens DE 3rd, Peppas NA. (1996). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307:93–102
- Rodriguez MA, Pytlik R, Kozak T, et al. (2009). Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study. Cancer 115:3475–82
- Rodriguez, Maria A, Fayad L, et al. (2005). Phase II study of sphingosomal vincristine in CHOP+/− rixtuximab for patients with aggressive non-Hodgkin's lymphoma (NHL): promising 3 year follow-up results in elderly patients. Blood (ASH Annual Meeting Abstracts) 106:943
- Rowinsky EK, Donehower RC. (1991). The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther 52:35–84
- Sarris AH, Hagemeister F, Romaguera J, et al. (2000). Liposomal vincristine in relapsed non-Hodgkin's lymphomas: early results of an ongoing phase II trial. Ann Oncol 11:69–72
- Schmidt CF, BarenholzY, Thompson TE. (1977). A nuclear magnetic resonance study of sphingomyelin in bilayer systems. Biochemistry 16:2649–56
- Sethi VS, Jackson DV Jr, White DR, et al. (1981). Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res 41:3551–5
- Shimshick EJ, McConnell H. (1973). Lateral phase separation in phospholipid membranes. Biochemistry 12:2351–60
- Svoboda GH, Johnson Is, Gorman M, Neuss N. (1962). Current status of research on the alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don). J Pharm Sci 51:707–20
- Thakkar HP, Baser A, Parmar MP, et al. (2012). Vincristine-sulphate-loaded liposome-templated calcium phosphate nanoshell as potential tumor-targeting delivery system. J Liposome Res 22:139–47
- Thomas DA, Kantarjian H, Stock W, et al. (2009). Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer 115:5490–8
- Thomas DA, Sarris A, Cortes J, et al. (2006). Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer 106:120–7
- Tokudome Y, Oku N, Doi K, et al. (1996). Antitumor activity of vincristine encapsulated in glucuronide-modified long-circulating liposomes in mice bearing Meth A sarcoma. Biochim Biophys Acta 1279:70–4
- Urruticoechea A, Alemany R, Balart J, et al. (2010). Recent advances in cancer therapy: an overview. Curr Pharm Des 16:3–10
- Vaage J, Donovan D, Mayhew E, et al. (1993). Therapy of mouse mammary carcinomas with vincristine and doxorubicin encapsulated in sterically stabilized liposomes. Int J Cancer 54:959–64
- Veronese FM, Pasut G. (2005). PEGylation, successful approach to drug delivery. Drug Discov Today 10:1451–8
- Webb MS, Harasym T, Masin D, et al. (1995). Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br J Cancer 72:896–904
- Webb MS, Saxon D, Wong FM, et al. (1998). Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): effects on the pharmacokinetics of liposomal vincristine. Biochim Biophys Acta 1372:272–82
- Xu H, Ye F, Hu M, et al. (2014). Influence of phospholipid types and animal models on the accelerated blood clearance phenomenon of PEGylated liposomes upon repeated injection. Drug Deliv, Early Online: 1-10. doi: http://dx.doi.org/10.3109/10717544.2014.885998
- Yamauchi M, Tsutsumi K, Abe M, et al. (2007). Release of drugs from liposomes varies with particle size. Biol Pharm Bull 30:963–6
- Zamboni WC, Gervais A, Egorin MJ, et al. (2004). Systemic and tumor disposition of platinum after administration of cisplatin or STEALTH liposomal-cisplatin formulations (SPI-077 and SPI-077 B103) in a preclinical tumor model of melanoma. Cancer Chemother Pharmacol 53:329–36
- Zhang L, Gao H, Chen L, et al. (2008). Tumor targeting of vincristine by mBAFF-modified PEG liposomes in B lymphoma cells. Cancer Lett 269:26–36
- Zhigaltsev IV, Maurer N, Akhong QF, et al. (2005). Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release 104:103–11
- Zhu G, Oto E, Vaage J, et al. (1996). The effect of vincristine-polyanion complexes in STEALTH liposomes on pharmacokinetics, toxicity and anti tumor activity. Cancer Chemother Pharmacol 39:138–42