Abstract
Purpose: Combination anticancer therapy is promising to generate synergistic anticancer effects, to maximize the treatment effect and to overcome multi-drug resistance. Nanostructured lipid carriers (NLCs), composed of solid and liquid lipids, and surfactants are potentially good colloidal drug carriers. The aim of this study is to construct a hyaluronic acid (HA) decorated NLCs as nanocarriers for co-delivery baicalein (BCL) and doxorubicin (DOX).
Methods: BCL- and DOX-loaded NLCs (BCL/DOX-NLCs) were prepared. HA ligands were used for the decoration of BCL/DOX-NLCs to form HA decorated BCL/DOX-NLCs (HA-BCL/DOX-NLCs). The in vitro cytotoxicity studies of different formulations were evaluated on DOX drug-resistant MCF-7 breast cancer cell line (MCF-7/ADR cells). In vivo anti-tumor effects were observed on the murine bearing MCF-7/ADR cells model.
Results: HA-BCL/DOX-NLCs showed highest cytotoxicity and synergistic effect of two drugs in tumor cells in vitro. The in vivo study revealed the greatest anti-tumor activity than all the other formulations in the murine breast cancer model.
Conclusions: HA decorated NLCs could be used as a novel carrier to co-delivery BCL and DOX for breast cancer therapy. HA-BCL/DOX-NLCs could be a promising targeted and combinational therapy nanomedicine.
Introduction
Baicalein (BCL) and doxorubicin (DOX) are reported to have anti-tumor effects containing several kinds of cancers (Lee et al., Citation2005; Tsai et al., Citation2012; Dong et al., Citation2015; Guo et al., Citation2015). BCL is a bioactive flavonoid derived from the root of traditional Chinese medicine herb Scutellaria baicalensis Georgi. BCL has broad anti-tumor activity against breast, cervical and gastric cancers (Wang et al., Citation2014; Peng et al., Citation2015; Yan et al., Citation2015). The shortcomings of BCL which lead to poor clinical effect in vivo compared with its powerful efficacy in vitro, including extensive first-pass metabolism, low bioavailability, short half-life (10 min), poor water solubility and oxidized easily (Fong et al., Citation2012; Seo et al., Citation2014; Liu et al., Citation2015). DOX is an anthracycline antibiotic. It has great efficacy in the treatment of breast cancer. However, the clinical application of DOX has been severely hindered because of its critical cardiotoxicity, narrow therapeutic window and the development of multidrug resistance (MDR) (Deepa et al., Citation2014; Zhao et al., Citation2014). Combination therapy is emerging as an important strategy to overcome MDR, maximize the therapeutic effect and reduce side effects (Greco & Vicent, Citation2009; Saraswathy & Gong, Citation2013). Therefore, on the one hand, the synergistic effect of BCL to DOX has been investigated in this study on the DOX drug-resistant breast cancer cells and animal models. On the other hand, a novel drug delivery system is urgently needed to improve the solubility, prolong the in vivo circulation time, enhance the targeted effect and then reduce the systemic toxicity.
Nanocarriers using lipids to load these drugs, form a carrier system with a number of desirable features, including low toxicity, a biodegradable particulate matrix, nontoxic degradation products, a high capacity to incorporate lipophilic and hydrophilic drugs, controlled release of the incorporated drug and easy scale-up at low cost (Pardeike et al., Citation2009; Rahman et al., Citation2013). For this purpose, different types of nanocarriers, including lipid-drug conjugates, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been developed (Esposito et al., Citation2015; Trapani et al., Citation2015; Zaro, Citation2015). NLCs represent a new generation of lipid nanoparticles, which are developed through the combination of advantages from different nanocarriers, including liposomes and SLNs systems (Taratula et al., Citation2013). These carrier systems consist of aqueous dispersions of solid nanoparticles, composed of a mixture of solid and liquid lipids, and stabilized by one or two surfactants (Tichota et al., Citation2014). Based on their good biocompatibility and stability, high drug loading, and low preparation cost, NLCs systems have become promising carriers for improving the bioavailability of some of the poorly water-soluble drugs used in traditional Chinese medicine (Yuan et al., Citation2013).
Hyaluronic acid (HA) is a member of the glycosaminoglycan family composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine and has been expanded into various biomedical applications due to its biocompatibility, biodegradability and non-immunogenicity (Zhang et al., Citation2014). Cluster of differentiation 44 (CD44), a family of multifunctional trans-membrane glycoproteins which is over-expressed in breast cancer cells, has been demonstrated to interact with HA at the N terminus of its extracellular domain, and therefore serves as a major cell surface receptor for HA (Lee et al., Citation2013; Yang et al., Citation2015). Therefore, HA has been used as a drug carrier and a ligand on liposomes or nanoparticles to target drugs to CD44 over-expressing cells (Platt & Szoka, Citation2008).
In this study, HA decorated, BCL- and DOX-loaded NLCs were constructed. The synergistic anti-tumor efficacy was assessed in MCF-7/ADR cells and mice bearing MCF-7/ADR cells breast cancer models. This system was expected to achieve targeted co-delivery of BCL and DOX, overcome the MDR of DOX and reduce the systemic toxicity.
Materials and methods
Animals
Baicalein was purchased from Ci Yuan Biotechnology Co., Ltd. (Shaanxi, China). Doxorubicin hydrochloride (DOX·HCl), stearic acid, soybean phosphatidylcholine (SPC) and Roswell Park Memorial Institute (RPMI) 1640 were purchased from Sigma-Aldrich Co., Ltd (St Louis, MO). Precirol ATO 5 was generously provided by Gattefossé USA (Paramus, NJ). Polyoxyl castor oil (Cremophor ELP) was donated by BASF (Ludwigshafen, Germany). Didecyldimethylammonium bromide (DDAB) was purchased from Ziyi Reagent factory (Shanghai, China). HA was provided by Freda Biochem Co., Ltd. (Shandong, China). MCF-7/ADR cells were obtained from the American type culture collection (Manassas, VA). All the solvents in use were analytical reagent grade.
Kunming mice (4–6 weeks old, 18–22 g weight) were purchased from the Medical Animal Test Center of Shandong University. All animal experiments complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China.
Preparation of BCL/DOX-NLCs
BCL/DOX-NLCs were prepared by emulsion evaporation–solidification at the low temperature method (Zhang et al., Citation2011; Fan et al., Citation2013). Prior to the NLCs preparation, the DOX·HCl was stirred with twice the molar amount of TEA in DMSO for 12 h to obtain lipophilic DOX base (Taratula et al., Citation2013). Stearic acid, SPC, Precirol ATO 5, Cremophor ELP, BCL and DOX base (BCL/DOX = 2/1, w/w) were melting and dissolving at 60 °C as the lipid phase. The lipid phase was rapidly injected into the aqueous phase containing 0.1% DDAB at the same temperature and stirred (800 rpm) for 1 h. The obtained warm nanoemulsion was then added into 20 ml distilled water at 0 °C in an ice bath immediately. BCL/DOX-NLCs were harvested by stirring in an ice bath for another 20 min.
Single drug containing NLCs or no drug containing blank NLCs was prepared by the same method above by adding single drug or no drug, presented as BCL-NLCs, DOX-NLCs and NLCs. All kinds of NLCs obtained were stored at 2–8 °C.
Preparation of HA-BCL/DOX-NLCs
HA decoration was carried out by electrostatic attraction and the decoration ratio was optimized. Ten milliliter of BCL/DOX-NLCs dispersion was added dropwise into 90 mL HA solution under the stirring at 400 rpm at room temperature. Two hour of agitation was followed to complete the decoration. To select the suitable ratio of HA ligands used in the formula, HA solution containing 100, 200, 300, 400 and 500 mg of HA were applied and 5 kinds of solutions were prepared separately. HA-BCL/DOX-NLCs obtained were stored at 2–8 °C.
Physicochemical characterization of NLCs
The mean diameter and zeta potential of different NLCs formulations were measured using a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK). The entrapment efficiency (EE) was determined by HPLC (LC-20A, Shimadzu, Japan) and calculated by the following equation (Liu et al., Citation2014a):
Release assay in vitro
A dialysis technique was conducted to compare the release of different NLCs formulations (Liu et al., Citation2014b). Preparations were added to a pretreated dialysis bag. Then, the dialysis bags were incubated with 30 ml of release medium (1 M sodium salicylate in PBS, pH 7.4) at 37 °C and stirred at the rate of 100 rpm. At predetermined time intervals, the NLCs suspensions were centrifuged and the amount of drug released in the supernatant was analyzed by HPLC.
In vitro cytotoxicity
The cytotoxicity of HA-BCL/DOX-NLCs, BCL/DOX-NLCs, BCL-NLCs, DOX-NLCs, BCL and DOX mixed solution (BCL/DOX solution, BCL/DOX = 2/1, w/w), BCL solution, and DOX solution was evaluated by MTT assay (How et al., Citation2013). The MCF-7/ADR cells were grown in the RPMI-1640 medium supplemented with 10% fetal bovine serum at 37 °C in 5% CO2 humidified atmosphere. The confluent monolayer cells were harvested and seeded into 96-well microplates at a density of 6000 cells/well. The cultures were allowed to stand for 24 h in the incubator for cell attachment and cell recovery after trypsinization before subjected to NLCs treatment and incubation for 72 h. Then, MTT solution (5 mg/ml) was added to each well and cells were incubated for 4 h. Two hundred microliter of DMSO was added to each well to dissolve the MTT formazan crystals. The optical density of formazan product was measured using a microplate reader (Model 680, BIO-RAD, Hercules, CA, USA) at 570 nm. Cells without the addition of MTT reagents were used, as a blank, to calibrate the spectrophotometer to zero absorbance. Cell viability was expressed as the percentage of MTT counts of treated cells relative to those without MTT treatment (Sun et al., Citation2014). The IC50 was the concentration that caused 50% inhibition of cell viability and was calculated by the Logit method (Armutlu et al., Citation2008).
Combination index (CI) determination was measured to study the synergy in BCL and DOX in the combination system (De Jong & Borm, Citation2008). CI was calculated by the equation: where (D)1 and (D)2 represent the concentration of BCL and DOX in the combination system at the IC50 value, respectively. (D50)1 and (D50)2 represent the IC50 value of BCL and DOX alone, respectively. CI50 < 1 represents synergism and CI50 > 1 represents antagonism.
In vivo anti-tumor efficacy
MCF-7/ADR cells (2 × 106 cells) were inoculated into the right armpit of Kunming mice to establish a breast cancer model. When tumor volume reached around 100 mm3, mice were randomly divided into six groups (eight per group) separately. The mice were injected intravenously with HA-BCL/DOX-NLCs, BCL/DOX-NLCs, BCL-NLCs, DOX-NLCs, BCL/DOX solution and 0.9% saline weekly. Three weeks later, all the mice were sacrificed by cervical dislocation and the tumor tissue samples were taken out. The volumes of the solid tumor were measured with a digital caliper every three days, and were calculated by the formula (W2 × L)/2, where W is the tumor measurement at the widest point and L is the tumor dimension at the longest point. The anti-tumor efficacy of each formulation was evaluated by tumor inhibition rate (TIR), which was calculated using the following formula: where C and T represent the tumor weight of the control and treated groups, respectively.
Statistical analysis
Results were presented as mean ± SD. Statistical comparisons were made by Student’s t-test or ANOVA analysis. The accepted level of significance was p < 0.05.
Results and discussion
Determination of HA decoration
HA could specifically recognize CD44 receptors and has been identified as a potent targeting ligand for CD44 over-expressing breast cancer cells. Also because of its favorable properties such as good biocompatibility, high biodegradability, low toxicity and non immunotoxicity, HA was welcomed. HA is a polyanion electrolyte molecule with a large number of negative charges. During the decoration process, the amount of HA increased on cationic NLC surface, zeta potential of the cationic particles should decreased. No obvious decrease in potential may indicate the complete of coating. At the same time, the modification of HA should not reduce the EE of the BCL/DOX-NLCs. Different amount of HA (100, 200, 300, 400 and 500 mg) was used in the volume fixed solution (90 mL), the zeta potential and EE were measured to determine the optimum HA dosage in the HA-BCL/DOX-NLCs formulation.
As summarized in , zeta potential decreased from 22 to 13 mV when the HA dosage increased to 300 mg, after that the potential was stable. The EE of BCL was significantly decreased from 91% to 68% and 55% at the HA dosage of 400 and 500 mg. Finally, HA solution was determined as 300 mg solved in 90 mL distilled water.
Table 1. Determination of HA decoration.
Characterization of NLCs
The mean diameter and zeta potential of different NLCs were analyzed and summarized in . Nano-sized vectors are also minimally phagocytosed by macrophages, so destruction and clearance by the body is minimized (De Jong & Borm, Citation2008). The size of NLCs without drugs was around 80 nm. With the loading of drugs, the size increased to 90 nm. The size of HA-BCL/DOX-NLCs was 104 nm due to the surface HA decoration. The zeta potentials of HA-BCL/DOX-NLCs were positive (+13 mV). Cationic surface charge of vectors could exploit the negative charges present at the cell surface for increased cellular uptake due to the electrostatic interactions, thus facilitate the internalization process (Harush-Frenkel et al., Citation2008). We used DDAB as cationic surfactant in the liquid phase of the NLCs preparation. The zeta potentials of HA-BCL/DOX-NLCs were positive (+13 mV). EE is an important parameter in the determination of drug-release characteristics, so its determination is an integral part of formulation development. The EE of both drugs loaded in HA-BCL/DOX-NLCs were over 90%.
Table 2. Physicochemical characterization of different NLCs complexes.
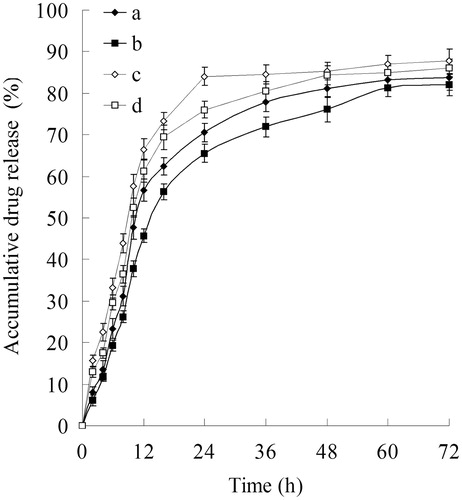
The in vitro BCL and DOX release profiles of different NLCs formulations are depicted in . HA-BCL/DOX-NLCs and BCL/DOX-NLCs showed the sustained-release behavior of both drugs. Nearly complete of BCL releases were achieved at 36 and 60 h, respectively. DOX release was faster, over 80% of accumulated releases were observed at 24 and 36 h for HA-BCL/DOX-NLCs and BCL/DOX-NLCs, respectively. The sustained-release of NLCs may bring about continuous anti-tumor effect over the therapeutic process.
Cytotoxicity assays
In vitro cytotoxicity of HA-BCL/DOX-NLCs, BCL-NLCs, DOX-NLCs, BCL/DOX solution, BCL solution and DOX solution were evaluated by MTT assay in MCF-7/ADR cells.
showed the IC50 and CI50 values of drugs solutions and NLCs formulations. The IC50 values of BCL solution and DOX solution on MCF-7/ADR cells were 0.876 and 6.271 μg/mL, respectively. The IC50 values of BCL/DOX solution were 0.662/0.331, with a CI50 of 0.808, exhibited the synergy effect of this two drugs in mixed solution. The IC50 values of HA-BCL/DOX-NLCs were the lowest (0.056), this value was about 12-fold dose advantage over BCL/DOX solution. This can contribute to the advantage of NLCs that improved the drug bioavailability. More than two fold of IC50 values of HA-BCL/DOX-NLCs over BCL/DOX-NLCs in reducing viability of breast cancer cells was also found, accounting for the highest anti-tumor activity of the HA decorated NLCs formula. This may due to the HA ligands on the particle surface which could target the HA receptors on the tumor cell surface, thus facilitate the entrance of BCL and DOX into the cancer cells.
Table 3. IC50 and CI50 values of drugs solutions and NLCs on MCF-7/ADR cells.
In vivo anti-tumor efficacy
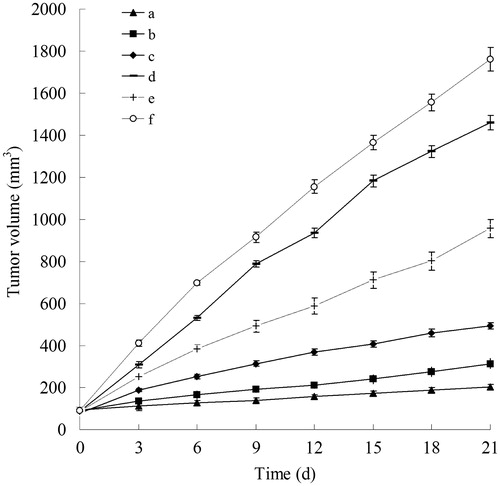
The in vivo anti-tumor efficiency of HA-BCL/DOX-NLCs, BCL/DOX-NLCs, BCL-NLCs, DOX-NLCs and BCL/DOX solution was evaluated in mice bearing human breast cancer by measuring the volumes of the solid tumors. showed the tumor growth curves of different formulations in vivo. Tumor growth was significantly inhibited by HA-BCL/DOX-NLCs, BCL/DOX-NLCs and BCL-NLCs formulations, although was suppressed to some extent by other groups. The most obviously tumor inhibition were observed in the HA-BCL/DOX-NLCs group, the tumor growth attained only 202 mm3 on day 21, in contrast, in 0.9% saline treated group, tumor volume grew all the way to 1763 mm3. The tumor volume of BCL/DOX-NLCs, and BCL-NLCs reached 315, and 493 mm3, also good results. The TIR of HA-BCL/DOX-NLCs, BCL/DOX-NLCs, BCL-NLCs, DOX-NLCs and BCL/DOX solution was 88%, 72%, 62%, 17% and 45%, respectively. The results suggested that the best anti-tumor effect of HA decorated two drugs contained NLCs in vitro and in vivo, showed the synergy effect of BCL to DOX in the MDR breast cancer cells.
Conclusions
HA-BCL/DOX-NLCs were effective in breast cancer cells over-expressing HA receptors, showed cytotoxicity of tumor cells in vitro and significantly tumor growth inhibition ability of in vivo. The synergistic anti-tumor efficacy was assessed in MCF-7/ADR cells and mice bearing MCF-7/ADR cells breast cancer models. This system could achieve targeted co-delivery of BCL and DOX, overcome the MDR of DOX, and reduce the systemic toxicity. HA-BCL/DOX-NLCs could function as promising active targeting nanomedicine for the delivery double drugs for the treatment of cancers.
Declaration of interest
The authors have no declarations of interest to report.
References
- Armutlu P, Ozdemir ME, Uney-Yuksektepe F, et al. (2008). Classification of drug molecules considering their IC50 values using mixed-integer linear programming based hyper-boxes method. BMC Bioinformatics 9:411
- Deepa K, Singha S, Panda T. (2014). Doxorubicin nanoconjugates. J Nanosci Nanotechnol 14:892–904
- De Jong WH, Borm PJ. (2008). Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 3:133–49
- Dong D, Gao W, Liu Y, et al. (2015). Therapeutic potential of targeted multifunctional nanocomplex co-delivery of siRNA and low-dose doxorubicin in breast cancer. Cancer Lett 359:178–86
- Esposito E, Ravani L, Drechsler M, et al. (2015). Cannabinoid antagonist in nanostructured lipid carriers (NLCs): design, characterization and in vivo study. Mater Sci Eng C Mater Biol Appl 48:328–36
- Fan X, Chen J, Shen Q. (2013). Docetaxel-nicotinamide complex-loaded nanostructured lipid carriers for transdermal delivery. Int J Pharm 458:296–304
- Fong YK, Li CR, Wo SK, et al. (2012). In vitro and in situ evaluation of herb-drug interactions during intestinal metabolism and absorption of baicalein. J Ethnopharmacol 141:742–53
- Greco F, Vicent MJ. (2009). Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev 61:1203–13
- Guo Y, Wang L, Lv P, et al. (2015). Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett 9:1065–72
- Harush-Frenkel O, Rozentur E, Benita S, et al. (2008). Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules 9:435–43
- How CW, Rasedee A, Manickam S, et al. (2013). Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: characterization, stability assessment and cytotoxicity. Colloids Surf B Biointerfaces 112:393–9
- Lee HZ, Leung HW, Lai MY, et al. (2005). Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res 25:959–64
- Lee T, Lim EK, Lee J, et al. (2013). Efficient CD44-targeted magnetic resonance imaging (MRI) of breast cancer cells using hyaluronic acid (HA)-modified MnFe2O4 nanocrystals. Nanoscale Res Lett 8:149
- Liu L, Tang Y, Gao C, et al. (2014a). Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf B Biointerfaces 115:125–31
- Liu Y, Wang L, Zhao Y, et al. (2014b). Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int J Pharm 476:169–77
- Liu TY, Gong W, Tan ZJ, et al. (2015). Baicalein inhibits progression of gallbladder cancer cells by downregulating ZFX. PLoS One 10:e0114851
- Pardeike J, Hommoss A, Müller RH. (2009). Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm 366:170–84
- Peng Y, Guo C, Yang Y, et al. (2015). Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol Med Rep 11:2129–34
- Platt VM, Szoka FC Jr. (2008). Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm 5:474–86
- Rahman HS, Rasedee A, How CW, et al. (2013). Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomed 8:2769–81
- Saraswathy M, Gong S. (2013). Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv 31:1397–407
- Seo MJ, Choi HS, Jeon HJ, et al. (2014). Baicalein inhibits lipid accumulation by regulating early adipogenesis and m-TOR signaling. Food Chem Toxicol 67:57–64
- Sun M, Nie S, Pan X, et al. (2014). Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces 113:15–24
- Taratula O, Kuzmov A, Shah M, et al. (2013). Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release 171:349–57
- Tichota DM, Silva AC, Sousa Lobo JM, et al. (2014). Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int J Nanomed 9:3855–64
- Trapani A, Mandracchia D, Di Franco C, et al. (2015). In vitro characterization of 6-Coumarin loaded solid lipid nanoparticles and their uptake by immunocompetent fish cells. Colloids Surf B Biointerfaces 127C:79–88
- Tsai MJ, Wu PC, Huang YB, et al. (2012). Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int J Pharm 423:461–70
- Wang N, Ren D, Deng S, et al. (2014). Differential effects of baicalein and its sulfated derivatives in inhibiting proliferation of human breast cancer MCF-7 cells. Chem Biol Interact 221:99–108
- Yan X, Rui X, Zhang K. (2015). Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep 33:737–43
- Yang X, Lyer AK, Singh A, et al. (2015). MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci Rep 5:8509
- Yuan L, Liu C, Chen Y, et al. (2013). Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int J Nanomed 8:4339–50
- Zaro JL. (2015). Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J 17:83–92
- Zhao X, Chen Q, Liu W, et al. (2014). Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int J Nanomed 10:257–70
- Zhang TC, Chen JN, Zhang Y, et al. (2011). Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur J Pharm Sci 43:174–9
- Zhang H, Li J, Sun W, et al. (2014). Hyaluronic acid-modified magnetic iron oxide nanoparticles for MR imaging of surgically induced endometriosis model in rats. PLoS One 9:e94718