Abstract
Topical tretinoin is the most commonly used retinoid for acne. However, its irritative potential on the applied area and the barrier properties of the stratum corneum limit its use. The objective of the present study was to formulate tretinoin liposomal gel to obtain a formula with lower skin irritation potential and greater clinical effect. A statistical 24 factorial design was adopted. Sixteen formulae prepared and were properly evaluated. A candidate formula (F13G) prepared with 0.025% tretinoin, phospholipid– cholesterol–dicetylphosphate (9:1:0.01) and incorporated in 1% carbopol gel was selected for skin irritation test. Clinical study was conducted on acne patients and compared to marketed product. All liposomes formulations were spherical in shape. The addition of cholesterol in the film hydration method significantly decreased the vesicle size, and increased the percentage of incorporation efficiency at (p < 0.05). The presence of dicetylphosphate significantly increased drug release but did not affect the percentage of incorporation efficiency and vesicle size. The results of the clinical study in acne patients revealed that F13G showed significantly higher efficacy when compared to marketed product (p < 0.05).
Introduction
Tretinoin (vitamin A derivative) is essential for the treatment of skin conditions including acne, eczema, psoriasis, photoaged skin, sunburns and other skin disorders (Elbaum, Citation1988; Brisaert et al., Citation2001; Stefanaki et al., Citation2005). Due to severe side effects when systemically used (Fenaux et al., Citation2001), tretinoin is almost topically used. Application of topical retinoids has been shown to induce a manifold increase of the epidermal concentration although no significant increase of plasma retinoids was observed (Arsić & Vuleta, Citation1999). Topical tretinoin is the most commonly used retinoid for acne (Geng et al., Citation2009). However, its topical application can lead to local irritation such as erythema, peeling and burning at the application site and increased susceptibility to sunlight (Elbaum, Citation1988; Brisaert et al., Citation2001). Also tretinoin suffers from limited stability and loss during storage. Tretinoin is sensitive to air (oxygen), light and acids (Varani et al., Citation2000). In addition, delivery of drugs through topical preparations viz. creams, gels, lotions, emulsion, etc. limits the effectiveness of actives due to barrier properties of the skin which hinder the drug deposition and relative poor stability due to direct exposure of actives to UV light. These formulations can be also easily removed by wetting, movement and contacting, thus their efficacy is unpredictable (Shin et al., Citation2005). In brief, topical application of tretinoin is limited by several disadvantages such as lipophilicity, instability and local irritation. Consequently, selection of proper carrier is extremely important by considering the views in mind that they should increase drug permeation, control side effects and enhance drug stability (Arsić & Vuleta, Citation1999). One of the promising delivery systems is the use of vesicular systems, such as liposomes and niosomes. Liposomes are microscopic structures consisting of one or more concentric spheres of lipid bilayers enclosing aqueous compartments. Liposomes have been successfully used to encapsulate lipophilic and hydrophilic compounds (Eroğlu et al., Citation2014; Johal et al., 2014; Ali et al., Citation2014; El-badry et al., Citation2014; Fang et al., Citation2015). In the dermatological field, liposomes were used initially only because of their moisturizing and restoring action. Then, their capability of enclosing many different biological materials and of delivering them to the epidermal cells was investigated (Schmid & Korting, Citation1996; Lee et al., Citation2007). As drug carrier systems for topical treatment, liposomes are reported to be superior over conventional topical preparations. The study on liposomes for targeting drugs into the pilosebaceous units has suggested that liposomes are potent drug delivery systems for treating hair follicle-associated disorders, such as acne (Kumar & Katare, Citation2004; Yang et al., Citation2009). Incorporation of tretinoin in liposomes to overcome its problems has been studied by several researchers (Foong et al., Citation1990; Masini et al.,Citation1993; Patel et al., 2000; Brisaert et al., Citation2001; Sinico et al., Citation2005). It was previously found that liposomes can increase tretinoin concentration in the epidermis and dermis when compared to conventional cream or gel (Manconi et al., Citation2003). Encapsulation of tretinoin into liposomes protects it from photo degradation. Results of our previous study (Abdelrahman et al., Citation2014) demonstrated the efficacy and safety of tretinoin incorporated into proniosomal gel when compared to conventional marketed product. In fact, one of the best ways of enhancing drugs, skin penetration is the use of liposomes. The aim of our study is development of an anti-acne product of tretinion based on liposomes as a vesicular drug delivery system to obtain a formula with lower skin irritation potential and greater clinical effect. However, preparation of liposomes is not an easy task due to the number of formulation variables involved therein. In the present work, systematic statistical study for the formulation of liposomes for topical delivery of tretinoin using the factorial design approach was undertaken. Candidate formula showing small vesicle size, high incorporartion efficiency and high percentage of drug released after 5 h will be subjected to skin irritation test and comparative clinical study compared to marketed product.
Materials and methods
Materials
Tretinoin was purchased from ChemFine International Co. Ltd. (China); l-alpha lecithin (granular), cholesterol (95% stabilized) and Tween 20 were obtained from Acros Organics, Fair Lawn, NJ. Dicetyl phosphate free acid was purchased from MP Biomedical, LLC, France. Dichloromethane (analytical grade) was obtained from Fisher Scientific Loughborough, UK. Potassium di-hydrogen orthophosphate and di-sodium hydrogen orthophosphate were purchased from Fluka, Germany; triethanolamine was purchased from ADWIC, Egypt. Carbopol 934 was purchased from Goodrich Chemical Company (Charlotte, NC).
Methodology
Design of experiments
A complete 24 factorial design was used for the preparation of tretinoin liposomes. The study involves the investigation of the four independent variables, namely presence or absence of cholesterol, drug concentration, presence or absence of charge inducer and the method of preparation at two different levels, on the average vesicle size, the percentage of incorporation efficiency of tretinoin liposomes and the percentage of tretinoin released after 5 h. The experimental design is presented in .
Table 1. Experimental plan of the 24 factorial design for the preparation of tretinoin liposomes.
Preparation of tretinoin-loaded liposomal dispersions
Preparation of tretinoin-loaded liposomal dispersions by ether injecting method. Liposomes were prepared by the modified ether injecting method (Riaz, Citation1996). The required amount of tretinoin, l-alpha-lecithin (phospholipid, Ph.), cholesterol (Ch.) (if present) and dicetylphosphate (DCP) (if present) () were dissolved in 6 ml of dichloromethane. The solution was slowly injected into 100 ml of distilled water using mechanical stirrer (Flac Instrument Treviglio (BG), Italy) at 500 rpm for 15 min at 60 °C (±5). The liposomal dispersions were obtained and then stored in refrigerator at 5 °C. The placebo liposomes were prepared in the same manner without adding the drug.
Table 2. Composition of the prepared tretinoin liposomes.
Preparation of tretinoin-loaded liposomal dispersions by hydration method
The required amount of Ph., Ch. (if present), DCP (if present) and tretinoin were dissolved in 6 ml of dichloromethane (). The solution was then evaporated at 60 °C in a rotavap (Rota-Vap, Bochi, Germany) under reduced pressure to produce a film of lipids. The lipid film was then hydrated using 100 ml distilled water and sonicated (Sonicator, Crest Ultra Sonicator Model 575DAE, Trenton, NJ) for 5 min at 60 °C (Patel et al., Citation2009). The liposomal dispersions were obtained and then stored in refrigerator at 5 °C. The placebo liposomes were prepared in the same manner without adding the drug.
Characterization of the prepared tretinoin-loaded liposomal dispersions
Transmission electron microscope (TEM)
Morphological characteristics of the vesicles were examined by the TEM (JEM-100S, Jeol Ltd., Tokyo, Japan). Samples were prepared by the negative staining technique. The samples were dispersed directly into bi-distilled water, and then copper grid coated with collodion film was put into the solution for several times. After being stained by 2% (w/v) phosphotungestic acid solution and dried at room temperature, the sample was ready for the TEM investigation at 70 kV.
Vesicle size analysis
A sample of freshly prepared liposomes dispersion was diluted with distilled water by shaking to weak opalescence dispersion. Then diluted dispersion was used to characterize the vesicle size and size distribution using laser diffraction (LD analyzer) vesicle size distribution analyzer (Horiba-LAZER Scattering vesicle size distribution analyzer LA920, Japan). The vesicle size range was set between 0.1 and 20 µm. Samples were measured in distilled water and 10% LD10, 50% LD50 and 90% LD90 were used as qualitative parameters to characterize dispersions. The LD values represent the percentage of the vesicles that are smaller than the given size, which were LD90 means that 90% (volume distribution) of the measured vesicles below the given value.
Determination of incorporation efficiency of tretinoin-loaded liposomal dispersions
The tretinoin incorporated in liposomes was separated from un-incorporated tretinoin by centrifugation (Megafuge 1.0 R, Heraeus, Germany) at 11 000 rpm for 30 min at 2 °C. The supernatant was separated from precipitate. Unincorporated tretinoin was then determined by measuring UV absorbance (UV-VIS spectrophotometer, Shimadzu UV 1601 PC, Kyoto, Japan) spectrophotometrically at λ355 nm (Moghimipour & Leis, Citation2012) using phosphate buffer (pH 5.5) containing 1% Tween 20 as blank.
The incorporation efficiency was determined relative to the original drug added. Applying the following equation (Aboelwafa et al., Citation2010):
In vitro release of tretinoin from tretinoin-loaded liposomal dispersions
In vitro release studies of tretinoin liposomal dispersions were performed using homemade Franz diffusion cell. These cells consist of donor and receptor chambers separated by a cellulose membrane with molecular weight cut-off of 12 000–14 000 (Spectrum Medical Inc., Los Angeles, CA); the area of diffusion was 1.7 cm2. The dialysis membrane was hydrated in the receptor medium, which consisted of a phosphate buffer (pH 5.5) containing 1% Tween 20, for 12 h before mounting into a Franz diffusion cell. A 0.5-ml tretinoin liposomal dispersion was placed in the donor chamber and the receptor chamber was filled with 7.5 ml receptor medium and stirred continuously at 100 rpm at 37 °C and the diffusion cells were covered with an aluminum foil to prevent light exposure. After 1, 2, 3, 4 and 5 h, samples were withdrawn from the receptor chamber through a side-arm tube. An equal volume of receptor medium was added to the receptor chamber to maintain a constant volume throughout the study. Samples were analyzed for tretinoin concentration using ultra-violet spectrophotometry at λmax 355 (Moghimipour & Leis, Citation2012). Measurements were carried out in triplicate.
Differential scanning calorimetry (DSC)
DSC experiments were performed with differential scanning calorimeter (model TA-60, Shimadzu, Kyoto, Japan). Samples of pure tretinoin, lecithin, cholesterol, blank liposomes and drug-loaded liposomes were submitted to DSC analysis. The analyses were performed on 5 mg samples sealed in standard aluminum pans.
The system was purged with nitrogen gas at a flow rate of 80 ml/min, and heating was performed from 30 to 300 °C at a rate of 10 °C/min.
Determination of zeta potential and formulation of tretinoin-loaded liposomal dispersion based hydrogel
On the basis of factorial design approach, a candidate formula F13 (containing 0.025% tretinoin) with small vesicle size, high percentage of incorporation efficiency and drug release was selected for further determination of zeta potential and formulation into liposomal gel. Zeta potential measurement of F13 was determined using Malvern Zetasizer (Malvern Instruments, UK). The liposomes were diluted with water and placed in the electrophoretic cell where an electric field of 80 mV was established. Measurements were carried out in triplicate at 25 °C. The selected F13 dispersion was formulated into hydrogel (F13G) by adding 1% (w/w) Carbopol 934 under magnetic stirring (DAIHAN Scientific Co., Wonju, South Korea) at 800 rpm. Stirring was continued until carbopol is dispersed. The dispersions were neutralized using triethanolamine solution (Shah et al., Citation2008). Hydrogel formulation containing 0.025% tretinoin dispersion (TG) was also prepared for comparison.
Skin irritation test
The study protocol and subject informed consent were approved by the institutional review board of Faculty of Pharmacy, Cairo University (IRB00007140) and the study was conducted according to the Declaration of Helsinki (http://www.wma.net/en/20activities/10ethics/10helsinki/, 2013) and the International Conference on Harmonization of Technical requirements for Registration of Pharmaceutics for Human Use Guidance for good clinical practice (Guidance for good clinical practice in European Medicines Agency, CPMP/ICH/135/95, July Citation2002). Ten healthy subjects (aged from 23 to 40 years) were participated in this study. The participants were briefed on the study procedures and a written informed consent was obtained from all subjects prior to conducting procedure. Each formulation (F13G, TG and marketed product) was applied once to each volunteer, at a dose of 0.3 g on a surface area of 5 cm2 on forearm. After 6 h, the test specimen was thereafter washed off by tap water and observed for any visible change such as erythema (redness). The mean erythemal scores were recorded (ranging from 0 to 4) according to Draize (Campbell & Bruce, Citation1981) where 0 means no erythema, 1 means slight erythema, 2 means moderate erythema, 3 means moderate to severe erythema and 4 means severe erythema.
Data analysis
Data are expressed as the means ± standard deviation (SD) of the mean and statistical analysis was carried out using the one-way analysis of variance. A value of p < 0.05 was considered statistically significant.
Clinical evaluation of the selected formula
Study subjects
The study was carried in the dermatology outpatient clinic in Kasr Al-Aini Teaching Hospital, Cairo, Egypt. Twelve patients who were confirmed to have acne vulgaris lesions in their faces were selected for study inclusion based on the following criteria.
Inclusion criteria
Patients could be enrolled in this planned study follow-up visits based on the following criteria:
Adult patients (>18 years old),
patients agreed to stop applying any topical product 14 days before the beginning of the study, and not to use/apply any topical product throughout the entire study period, and
patients agreed to use the provided product and were ready to comply with the treatment and follow-up procedures.
Study participants exclusion criteria
Subjects were excluded if
patients had previously received benzoyl peroxide, tretinoin and/or oral retinoid containing products,
patients had received oral antibiotics in the past 4 weeks,
patients with endocrine disorders, or with severe physical illnesses,
patients who were currently using oral contraceptives, or implantable contraceptives,
patients who were using any systemic medications likely to affect (flaring or healing it up) such as oral phenytoin, finasteride, spironolactone, flutamide, testosterone or dietary body-building protein powders or
patients who used topical, systemic, inhaled or intraocular corticosteroids within the past 4 weeks (Marcinkiewicz et al., Citation2008; Emanuele et al., Citation2012; Vender, Citation2012).
This study was approved by the Research Ethics Committee Office of Faculty of Medicine, Cairo University, which followed the tenets of the Declaration of Helsinki (http://www.wma.net/en/20activities/10ethics/10helsinki/, 2013). Each participant was informed about the purpose of the study, and signed informed consent to be photographed before and after treatment.
Study design
A prospective, randomized, double blind, parallel clinical study was conducted to evaluate the efficiency and the safety of the selected liposomal formulation. Formula F13 incorporated in 1% carbopol gel and the marketed product was tested on the skin of patients who were diagnosed with mild to moderate acne vulgaris lesions.
The study was designed in a way that each subject is working as his/her own control, where the face of the selected subject was divided into two zones; the right side for the application of F13 once daily, while the left side for the application of marketed tretinoin product (containing 0.025% tretinoin) once daily. On the first day of recruiting, all the patients were instructed on how to apply the formulae over the acne areas. All the subjects were asked for weekly follow-up (four consecutive follow-up visits) visits to assess the safety and efficacy of the used products.
The efficacy was determined by lesions counting during the first visits and during the four follow-up visits. The counted lesions included the papules, closed and opened comedons. The counting process was carried out by a blinded and trained dermatologist. The total number of lesions counted in the first visit was considered to be 100% and any decrease in the number of lesions was calculated accordingly and regarded as percentage reduction. Means and SD of this percentage reduction were calculated in each group of patients every week and were used for further statistical analysis for every lesion type and for total lesion count. The significant difference between percent change of the marketed product and F13 was tested by using Student t-test at p < 0.05 (Bolton & Bon, Citation2003). Percent change between before and after was calculated as:
Data were collected and analyzed using SPSS statistical package version 19 (SPSS Inc., Chicago, IL).
Results and discussion
One of the major disadvantages associated with the tretinoin therapy is skin irritation (erythema), which strongly limits its efficacy and acceptability by the patients. Preferably, the delivery system of tretinoin should be able to reduce or eliminate these erythematic side effects. On the other hand, most of the currently marketed conventional dosage forms such as creams, lotions and gels are not able to diminish the irritation caused by topical application of tretinoin. The topical effects of many drugs in the liposomal formulations were improved compared to the conventional formulations. Our objective in this study was to prepare and characterize tretinoin based liposomes in order to increase its efficiency for topical therapy of acne vulgaris. A phosphate buffer solution pH 5.5 (simulating skin pH) containing 1% Tween 20 was used for the release study and determination of incorporation efficiency.
Characterization of the prepared liposomes
Transmission electron microscope
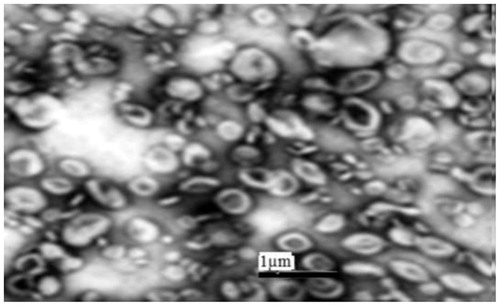
The TEM micrographs of formulation (F13) are illustrated in . It was observed that the vesicles of the liposomes were in the nanometer size range, almost spherical in shape with homogenous shading.
Vesicle size analysis
The results revealed that all prepared tretinoin-loaded liposomal dispersions showed a considerable small vesicle size with d90% less than 0.5 µm. The mean vesicle size of prepared tretinoin-loaded liposomal dispersions ranged from 0.318 ± (0.0028) µm (F13) to 0.4855 ± (0.0219) µm (F8) (). The polydispersity index (PI) as a characteristic parameter for the width of vesicle size distribution ranged from 0.43 to 0.67 (). Needless to say that small diameter is known to be advantageous to decrease irritation (Abdelrahman et al., Citation2014). All the statistical data concerning vesicle size showed that the method of preparation and presence of cholesterol had significant effects (p < 0.05) on the vesicle size.
Table 3. Physicochemical characterization of different tretinoin liposomal dispersions.
Effect of method of preparation: Film hydration method was found to significantly (p < 0.05) decrease the vesicle size when compared to ether injection method. This may be due to the use of sonication during the preparation process of liposomes in film hydration method as very high energy input based on cavitation is applied. This was in accordance to the results stated by Haskaran & Lakshmi (Citation2009) who found that film hydration method gave much smaller vesicle size than ether injection method.
Effect of cholesterol: The presence of cholesterol was found to significantly (p < 0.05) decrease the vesicle size when compared to the absence of cholesterol. These results might be attributed to cholesterol causing the bilayer to be more compact. This result is in accordance to results stated by Duangjit et al. (Citation2011) and Das& Palei (Citation2011) who found that the vesicles containing cholesterol had a lower vesicle size compared to those without cholesterol.
Effect of drug concentration: Two drug concentrations were used in the study (0.01% and 0.025%). Increasing drug concentration from 0.01% to 0.025% did not significantly change the vesicle size (p > 0.05). Gasper & Campos (Citation2003) reported that no significant difference in vesicle size of liposome appeared on using 10% and 15% paromycin.
Effect of negative charge inducer: Although the presence of negative charge slightly increased vesicle size of liposomal dispersions this increase was statistically insignificant (p > 0.05). This slight increase might be due to the inclusion of negatively charged molecules into bilayers would increase the volume of the aqueous compartment due to interaction between charged moiety and the surfactant head groups. Such interaction will develop the charge that creates mutual repulsion between phospholipid bilayers and hence increases particle size (Essa, Citation2010). Paavola et al. (Citation2000) reported slight increase in vesicle size on using negatively charged phospholipid.
Incorporation efficiency (IE%)
The incorporation efficiency of tretinoin within the different prepared liposomal dispersions is shown in . The incorporation efficiency was found to vary from 38.2 ± (3.39) for formula (1) to 73.4 ± (3.81) for formula (F13).
Effect of preparation method: The percentage of drug incorporated in the prepared liposomes was determined and was found to vary from 38.2 ± (3.39)% to 70.5 ± (0.81)% for liposomes prepared using ether injection method (F1–F8, ), and from 51.3 ± (4.49)% to 73.4 ± (4.49)% for those prepared using film hydration method (F9–F16, ). Concerning the preparation method, the percentage of drug entrapped in case of film hydration method was significantly higher (p < 0.05) than in case of liposomes prepared by ether injecting method. This increase in the percentage of incorporation efficiency could be attributed to smaller vesicle size of the prepared liposomes using film hydration method so vesicles entrap smaller amount of aqueous content this led to higher affinity of vesicles to entrap the hydrophobic drug (tretinoin). This result was in agreement to the results reported by Haskaran & Lakshmi (Citation2009) and Rangasamy et al. (Citation2008), who reported that film hydration method with or without sonication give higher entrapment percentage than those prepared by ether injection method.
Effect of presence of cholesterol: The incorporation efficiency of tretinoin significantly increased upon addition of cholesterol p < 0.05. This might be attributed to that cholesterol incorporation increased hydrophobicity of liposome bilayer that led to enhanced drug entrapment (tretinoin being a lipophilic drug) beside the cementing effect of cholesterol on the membrane packing. This result was in accordance to the results reported by Ramana et al. (Citation2010) who stated that addition of cholesterol in 9:1 (phospholipid–cholesterol) ratio enhanced incorporation efficiency of nevirapine but further increase in the cholesterol content did not enhance drug incorporation capacity of the liposomes instead, in fact reduced the encapsulation efficiency ratio enhanced the entrapment efficiency. Similarly, Fatouros et al. (Citation2001) stated that addition of cholesterol significantly increased the entrapment percentage of both free and B-cyclodextrin complex prednisolone.
Effect of drug concentration: Concerning drug concentration, increasing drug concentration (0.025% tretinoin; F5–F8 and F13–F16) led to significant increase in the percentage of drug incorporation efficiency when compared to the lower concentration (0.01%; F1–F4 and F9–F12; p < 0.05). This result could be attributed to the saturation of the media with tretinoin at higher concentration that forces the drug to be encapsulated into vesicles. This result was in agreement to that previously reported by Aboelwafa et al. (Citation2010) and Keservani et al. (Citation2011).
Effect of presence of negative charge inducer: Regarding the charge inducer (dicetylphosphate), no significant effect on the percentage of entrapment efficiency was found (p > 0.05). This result was in agreement with results reported by Kumar & Katare (Citation2004), who stated that use of charge imparting agent, i.e. dicetyl phosphate, did not influence the entrapment of tamoxifen in the liposomal compartments.
Release study
and () represents the release pattern of tretinoin from liposomes prepared by ether injection and film hydration methods, respectively, for 5 h. The percentage of tretinoin released from liposomes ranged from 22% ± (4.4)% to 53% ± (5.4)% after 5 h. Liposomes prepared by film hydration showed higher percentage release than those prepared using ether injection method.
Figure 2. Release of tretinoin from liposome formulations prepared by (a) ether injection method and (b) film hydration method after 5 h (N = 3).
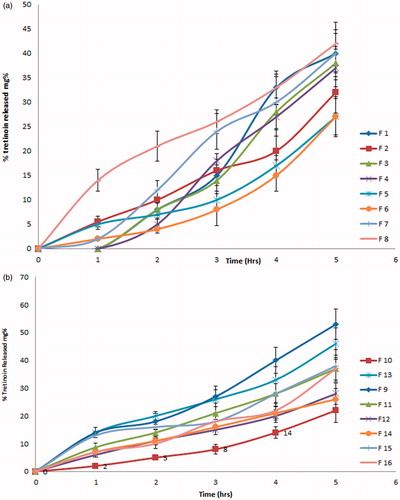
Effect of method of preparation: Concerning the method of preparation, the percentage of tretinoin released from the liposomes prepared using film hydration method was slightly higher than those prepared using ether injection method but this slight increase was found to be statistically insignificant (p > 0.05). This could be due to the lower vesicle size shown by liposomes prepared by film hydration method.
Effect of cholesterol: Presence of cholesterol significantly lowered the percentage of tretinoin released from liposomes after 5 h. This may be due to that cholesterol produced more rigid vesicles and might act as cement material that decreased permeability for the encapsulated drug causing retarded drug release. This result was in accordance to that reported by Keservani et al. (Citation2011), Duangjit et al. (Citation2011) and Begum et al. (Citation2011), who stated that cholesterol led to lower release and skin permeation. Similarly, Sankar et al. (Citation2010) stated that increasing cholesterol markedly reduced the efflux of the drug where cholesterol filled the pores in vesicular bilayers and abolished the gel-liquid phase transition of liposomal system resulting in less leaky liposomes. Cholesterol in the formulation acts as a membrane stabilizing agent that helped to sustain drug release.
Effect of presence of negative charge inducer: A significant increase in release of tretinoin was observed upon incorporation of dicetyl phosphate as a negative charge inducer (p < 0.05). This could be attributed to the higher affinity of charged vesicles for water hydration leading to lower affinity to hydrophobic drug. The presence of negative charge led to liposome–liposome interaction, there by stabilizing the bilayer. The result is in agreement with those reported by Bajoria & Contractor (Citation1997) who stated that anionic liposomes were significantly more (p < 0.05) leaky than the neutral.
Effect of drug concentration: Increasing drug concentration from 0.01% to 0.025% did not significantly affect the percentage of tretinoin released from the prepared liposomes after 5 h (p > 0.05).
The result was in agreement with results reported by Abo-Elwafa et al. (Citation2010) who found that the release rate was not greatly affected by increasing the amount of drug added.
Differential scanning calorimetry
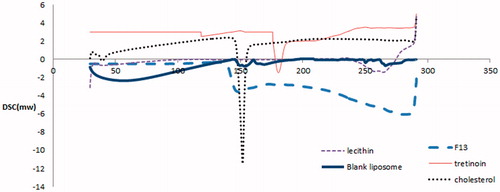
In , the DSC thermogram of tretinoin showed a sharp endothermic peak at 178.86 °C. DSC thermogram of cholesterol showed an endothermic peak at about 148.3 °C and DSC thermogram of l-alpha lecithin showed an endothermic peaks at about 263 °C. DSC thermogram of blank liposomes showed broadening of cholesterol peak and slight shift in l-alpha lecithin peak from 263 to 267.46 °C. The DSC thermogram of tretinoin-loaded liposomes composed of l-alpha lecithin–cholesterol (9:1) weight ratio interestingly showed disappearance of the melting endotherm of tretinoin. The melting endotherm of l-alpha lecithin was found to be shifted from 263 to 281.02 °C, signifying that all the lipid components might interact with each other to an extent, while forming the lipid bilayer. Absence of the melting endotherm of tretinoin and shifting of the lipid bilayer components endotherm suggested possible interaction of tretinoin with bilayers, leading to enhanced entrapment of the drug. The DSC results of liposomes suggest enhanced incorporation efficiency of tretinoin in the lipid bilayer. These results were in accordance to results obtained by Hathout et al. (Citation2007) who recorded that absence of the melting incorporation of acetazolamide and shifting of the lipid bilayer components endotherm suggested interaction of acetazolamide with bilayers.
Zeta potential measurements and irritation test
Based on the previously mentioned characterization, and the results of the main effects of the adopted factorial design a candidate formula F13 (containing 0.025% tretinoin) with smallest vesicle size (0.318 ± (0.003)), highest incorporation efficiency (73.4 ± (3.81)) and high percentage of drug released after 5 h (46% ± (5.6)) was selected and subjected to zeta potential measurement and to be incorporated into 1% carbopol gel to be tested for skin irritation. The zeta potential of F13 was found to be −41.2 ± 1.2 mV. This high value indicates the stability of the prepared liposomes because strong electrostatic repulsion interaction prevents the aggregation of liposomes. It was previously published that suspension with zeta potentials >+30 or <−30 mV are normally considered stable (Laouini et al., Citation2012). It is worth noting that incorporation of the liposomal formulation in gel will further enhance stability as viscosity of gel will inhibit aggregation of particles. Regarding the skin irritation test (), very slight erythema (score 0.2 ± 0.37) was observed for F13 gel. Conversely, 0.025% TRT gel showed a well-defined erythema score of 1.70 ± 0.751. Similarly marketed product showed an erythema score of 1.40 ± 0.534 and was not able to diminish the irritation caused by topical application of TRT. This could be attributed to the small vesicle size and the role of liposomes in protecting the skin from direct contact with the drug which was embedded in the vesicles. Incorporation of TRT in vesicles would reduce the contact of the acidic group (–COOH) of TRT with the stratum corneum and allowed gradual delivery of TRT to epidermal, therefore resulting in reduced the erythematic events and improve skin tolerability (Gupta et al., Citation2009). The small vesicle size and the high incorporation efficiency of TRT in this candidate formula could be beneficial to reduce the skin irritation.
Table 4. Skin irritation test of tretinoin in different formulations.
Clinical study
The study population comprised 12 Egyptian patients aged >18 years (2 males and 10 females) being diagnosed with acne (papules, closed comedons and open comedons) on their face.
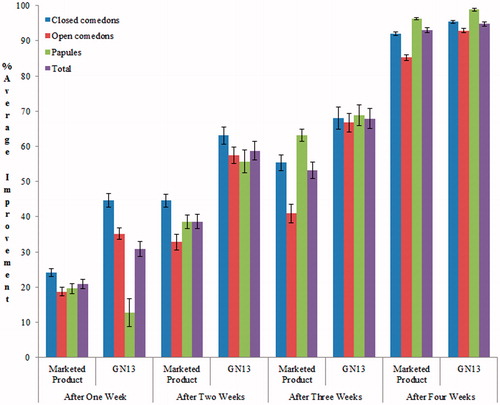
Formula 13 composed of [(0.025% tretinoin, phospholipid– cholesterol–dicetylphosphate, 9–1–0.01) and prepared by film hydration method] and incorporated into 1% carbopol gel showed a significantly (p < 0.05) superior efficacy compared to the marketed product over a 4- week period. Also, the overall lesion improvement during the whole study period was in favor of F13 formulae ( and ). These results could be attributed to the enhanced penetration of tretinoin from liposomes across the stratum courneum when compared to conventional dosage form (Fang et al., Citation2001; Jung et al., Citation2006; Lee et al., Citation2007; Jaafari et al., Citation2009; Duangjit et al., Citation2011).
Table 5. Effect of the two tested formulae (commercial versus F13) on different types of lesions (comedons and papules) during 4 weeks study period (mean ± SE).
It was previously published that the maximum comedolytic activity of tretinoin was reached at a concentration of 5–10 times lower when tretinoin is incorporated into liposomes, compared to the conventional alcoholic gels (Nikouei et al., Citation2011).
Conclusion
Tretinoin-loaded liposomal dispersion F13 composed of [(0.025% tretinoin, phospholipid–cholesterol–dicetylphosphate 9:1:0.01) and prepared by film hydration mehod] and incorporated into 1% carbopol gel possess remarkable advantage over marketed formulation in enhancing the effect of tretinoin against non-inflammatory acne lesions thereby improving skin tolerability and patient compliance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abdelrahman S, AbdelMalak NS, Badawi A, et al. (2014). Formulation of tretinoin-loaded topical proniosomes for treatment of acne: in-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv 3:1–9
- Aboelwafa A, El-Setouhy DA, Elmeshad AN. (2010). Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech 11:1591–602
- Ali J, Fazil M, Qumbar M, et al. (2014). Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv 6:1–17
- Arsić I, Vuleta G. (1999). Influence of liposomes on the stability of Vitamin A incorporated in polyacrylate hydrogel. Int J Cosmet Sci 21:219–25
- Bajoria R, Contractor SF. (1997). Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta. Ped Res 42:520–7
- Begum MY, Abbulu K, Sudhakar M. (2011). Design and evaluation of flurbiprofen liposomes. J Pharm Res 4:653–5
- Bolton S, Bon C. (2003). Pharmaceutical statistics: practical and clinical applications. Edition Number: 4. Boca Raton, FL: Taylor & Francis, Inc
- Brisaert M, Gabriels M, Matthijs V, Plaizier-Vercammen J. (2001). Liposomes with tretinoin: a physical and chemical evaluation. J Pharm Biomed Anal 26:909–17
- Campbell RL, Bruce RD. (1981). Direct comparison of rabbit and human primary skin irritation responses to isopropylmyristate. Toxicol Appl Pharmacol 59:555–63
- Das M, Palei NN. (2011). Sorbitan ester niosomes for topical delivery of rofecoxib. Ind Exp Biol 49:438–45
- Declaration of Helsinki. World Medical Association. Available from: http://www.wma.net/en/20activities/10ethics/10helsinki/ [last accessed Feb 2013]
- Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. (2011). Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. Drug Deliv. DOI: http://dx.doi.org/10.1155/2011/418316
- Elbaum DJ. (1988). Comparison of the stability of topical isotretinoin and topical tretinoin and their efficacy in acne. J Am Acad Dermatol 19:486–91
- El-Badry M, Fetih G, Shakeel F. (2014). Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv 21:34–43
- Emanuele E, Bertona M, Altabas K, Alessandrini G. (2012). Anti-Inflammatory effects of a topical preparation containing nicotinamide, retinol, and 7-dehydrocholesterol in patients with acne: a gene expression study. Clin Cosmet Investig Dermatol 5:33–7
- Eroğlu I, Azizoğlu I, Özyazıcı M, et al. (2014). Effective topical delivery systems for corticosteroids: dermatological and histological evaluations. Drug Deliv 9:1–11
- Essa A. (2010). Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J Pharm 4:227–33
- Fang JY, Hang CT, Chiu WT, Wang YY. (2001). Effect of liposome and niosomes on skin permeation of Enoxacin. IJP 219:61–72
- Fang S, Niu Y, Zhu W, et al. (2015). Liposomes assembled from a dual drug-tailed phospholipid for cancer therapy. Chem Asian J 10:1232–1238
- Fatouros DG, Hatzidimitriou K, Antimisiaris SG. (2001). Liposomes encapsulating prednisolone and prednisolone–cyclodextrin complexes: comparison of membrane integrity and drug released. Eur J Pharm Sci 13:287–96
- Fenaux P, Chomienne C, Degos L. (2001). Treatment of acute promyelocytic leukaemia. Best Pract Res Clin Haematol 14:153–74
- Foong WC, Harsanyi BB, Mezei M. (1990). Biodisposition and histological evaluation of topically applied retinoic acid in liposomal, cream and gel dosage forms. In Hanin I, Pepeu G, eds. Phospholipids. New York: Plenum Press, 139–54
- Gasper LR, Campos M. (2003). Rheological behavior and the SPF of sunscreens. IJP 250:35–44
- Geng A, Weinstock MA, Hall R, et al. (2009). Tolerability of high-dose topical tretinoin: the veterans affairs topical tretinoin chemoprevention trial. Br J Dermatol 161:918–24
- Guidance for good clinical practice European Medicines Agency, CPMP/ICH/135/95 July 2002
- Gupta V, Ahirwar D, Sharma N, Jhade D. (2009). Proniosomal gel as a carrier for improved transdermal delivery of griseofulvin: preparation and in-vitro characterization. Res J Pharm Dosage Forms Technol 1:33–7
- Haskaran BS, Lakshmi PK. (2009). Comparative evaluation of niosome formulations prepared by different techniques. Pharm Sci 51:27–31
- Hathout RM, Mansour S, Mortada ND, Guinedi AS. (2007). Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharm SciTech 8:E1–E12
- Jaafari MR, Bavarsad N, Bazzaz BSF, et al. (2009). Effect of topical liposomes containing paromomycin sulfate in the course of leishmania major Infection in susceptible BALB/mice antimicrobial agents and chemotherapy. Am Soc Microbiol 53:2259–65
- Johal HS, Garg T, Rath G, Goyal AK. (2014). Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv 6:1–14
- Jung S, Otberg N, Thiede G, et al. (2006). Innovative liposomes as a transfollicular drug delivery system: penetration into porcine hair follicles. J Inv Dermatol 126:1728–32
- Keservani K, Sharma K, Shailesh J. (2011). Effect of different process variables on the preparation of Baclofen niosomes. Int J Univ Pharm Life Sci 1:2249–6793
- Kumar R, Katare O. (2004). Tamoxifen in topical liposomes; development, characterization and in-vitro evaluation. JPPS 7:252–9
- Laouini A, Jaafar-Maalej C, Limayem-Blouza I, et al. (2012). Preparation, characterization and applications of liposomes: state of the art. J Coll Sci Biotechnol 1:147–68
- Lee S, Lee J, Choi YW. (2007). Skin permeation enhancement of ascorbyl palmitate by liposomal hydrogel (lipogel) formulation and electrical assistance. Biol Pharm Bull 30:393–6
- Manconi M, Valenti D, Sinico C, et al. (2003). Niosomes as carriers for tretinoin: II. Influence of vesicular incorporation on tretinoin photostability. Int J Pharm 260:261–72
- Marcinkiewicz J, Wojas-Pelc A, Walczewska M, et al. (2008). Topical taurine bromine, a new candidate in the treatment of moderate inflammatory acne vulgaris – a pilot study. Eur J Dermatol 18:433–940
- Masini V, Bonte F, Meybeck A, et al. (1993). Cutaneous bioavailability in hairless rats of tretinoin in liposomes or gel. J Pharm Sci 82:17–21
- Moghimipour E, Leis ASF. (2012). Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv Pharm Bull 2:141–7
- Nikouei BM, Golmohammadzadeh S, Hosseini NM. (2011). Preparation and characterization of liposomes encapsulated with clindamycin and tretinoin. Int J Comp Pharmacy 2:1–4
- Paavola A, Kilpeläinen I, Yliruusia J, Rosenberg P. (2000). Controlled release injectable liposomal gel of ibuprofen for epidural analgesia. IJP 199:85–93
- Patel RP, Patel H, Baria AH. (2009). Formulation and evaluation of liposomes of Ketoconazole. Int J Drug Deliv Technol 1:16–23
- Ramana LN, Sethuraman S, Ranga U, Krishnan U. (2010). Development of a liposomal nano-delivery system for nevirapine. J Biomed Sci 17:57–65
- Rangasamy M, Ayyasamy B, Raju S, et al. (2008). Formulation and in vitro evaluation of niosome encapsulated Acyclovir. J Pharm Res 1:163–6
- Riaz M. (1996). Liposomes preparation methods. J Pharm Sci 19:65–77
- Sankar V, Ruckmani K, Durga S, Jailani S. (2010). Proniosomes as drug carriers. Pak J Pharm Sci 23:103–7
- Schmid MH, Korting HC. (1996). Therapeutic progress with topical liposome drugs for skin disease. Adv Drug Deliv Rev 18:335–42
- Shah KA, Date AA, Joshi MD, Patravale VB. (2008). Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. IJP 345:163–71
- Shin S, Kim H, Oh I, et al. (2005). Development of tretinoin gels for enhanced transdermal delivery. Eur J Pharm Biopharm 60:67–71
- Sinico C, Manconi M, Peppi M, et al. (2005). Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle–skin interaction. J Control Release 103:123–36
- Suresh K, Singh P, Saraf S. (2013). Novel topical drug carriers as a tool for treatment of psoriasis: progress and advances. Afr J Pharm Pharmacol 7:138–147
- Stefanaki C, Stratigos A, Katsambas A. (2005). Topical retinoids in the treatment of photoaging. J Cosmet Dermatol 4:130–4
- Varani J, Warner R, Kermani MG, et al. (2000). Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human Skin. J Investig Dermatol 114:480–6
- Vender R. (2012).Clinical study, double-blinded, vehicle-controlled proof of concept study to investigate the recurrence of inflammatory and non-inflammatory acne lesions using tretinoin gel (Microsphere) 0.04% in male patients after oral isotretinoin use. Dermatol Res Pract 4:1–5
- Yang J, Pornpattananangkul D, Nakatsuji T, et al. (2009). The antimicrobial activity of liposomal lauric acids against propionibacterium acnes. Biomaterials 30:6035–40