Abstract
Background: Cervical cancer chemotherapy calls for the efficiently delivery of anticancer drug into cancer cells by nanoparticles. In this study, folate (FA) modified, cisplatin (CIS)-loaded nanostructured lipid carriers (NLCs) were constructed and evaluated.
Methods: FA containing polyethylene glycol (PEG)-distearoylphosphatidylethanolamine (DSPE) (FA-PEG-DSPE) was synthesized. FA-PEG-DSPE modified, CIS-loaded NLCs (FA-CIS-NLCs) were prepared. Their particle size, zeta potential, drug encapsulation efficiency (EE) and in vitro delivery behavior were evaluated. In vitro cytotoxicity study of FA-CIS-NLCs was tested in human cervix adenocarcinoma cell line (HeLa cells). In vivo anti-tumor efficacies of the carriers were evaluated on a mice-bearing cervical cancer model.
Results: The optimum FA-CIS-NLCs formulations have a particle size of 143.2 nm and a +25.7 mV surface charge. FA-CIS-NLCs displayed the best anti-tumor activity than other formulations in vitro and in vivo.
Conclusions: The results demonstrated that FA-CIS-NLCs were efficient in selective delivery to cancer cells over-expressing FA receptors (FRs). FA-CIS-NLCs targeted transfer CIS to the cervical cancer cells, enhance the anti-tumor capacity. The novel constructed NLCs could function as outstanding nanocarriers for the delivery of drugs for the targeted treatment of cervical cancers.
Introduction
Cervical cancer is the second leading cause of cancer deaths among females worldwide, with approximately half a million new cases and over 200 000 deaths each year; and prognosis for advanced stage disease is poor (Tzioras et al., Citation2007; Zong et al., Citation2015). Current treatments for cervical cancer are radiotherapy, chemotherapy and surgery; and cisplatin-based treatments are the most popular regimens (Tzioras et al., Citation2007; Diaz-Padilla et al., Citation2013). However, severe side effects such as haematological toxicity (bone-marrow depression, neutropenia, thrombocytopenia and anaemia), nephrotoxicity, neurotoxicity and chemoresistance have limited its clinical use (Maduro et al., Citation2003; Das et al., Citation2014). Therefore, an effective therapeutic strategy is imperative to enhance chemotherapeutic efficacy and decrease these side effects.
Nano-based delivery systems have attracted a great deal of attention in the past two decades as a strategy to overcome the low therapeutic index of conventional anticancer drugs and targeted delivery drugs into solid tumors. Solid lipid nanoparticles (SLNs) which were introduced in 1991 are used as alternative nanocarrier systems to traditional colloidal carriers, such as emulsions, liposomes and polymeric nanoparticles (Cassano et al., Citation2014; Rostami et al., Citation2014; Pandey et al., Citation2015). Compared with these traditional colloidal carriers, SLNs offer the following advantages: improved biocompatibility; controlled drug release; potential for site-specific drug delivery, etc. (Thukral et al., Citation2014). Nanostructured lipid carrier (NLC), the second generation SLNs, made up of physiological, biocompatible, biodegradable, non-sensitizing and non-irritating lipids (Gaba et al., Citation2014). NLCs, composed of solid and liquid lipid, were introduced to overcome the limitations of SLNs: more drug loading capacity and improved drug stability or adjustment of potential drug expulsion during storage (Zhang et al., Citation2008; Liu et al., Citation2015). In the present research, we chose NLCs as carriers to delivery cisplatin (CIS). In order to deliver CIS to the cancer tissues and improve therapeutic efficacy, targeted NLCs were designed.
Targeted NLCs have been engineered by some researchers. Both low-molecular weight (biotin) and high-molecular weight of ligands (hyaluronic acid, octreotide, vascular endothelial growth factors and Ala-Glu-Tyr-Leu-Arg peptide) can be decorated over the surface of NLCs (Liu et al., Citation2011; Han et al., Citation2013, Citation2014; Negi et al., Citation2014; Zhou et al., Citation2015). Among the various ligands researched so far, folate (FA) is one of the most promising candidates that showed immense potential to target cancer cell owing to its high affinity for folate receptors (FRs), which are normally over-expressed in various human carcinomas, such as cervix uteri, liver, breast, ovary, etc. (Dixit et al., Citation2015; Li et al., Citation2015). Therefore, FA-decorated NLCs were prepared and evaluated.
In this study, FA containing polyethylene glycol (PEG)-distearoylphosphatidylethanolamine (DSPE) (FA-PEG-DSPE) was synthesized as the targeting ligand. FA-PEG-DSPE modified, CIS-loaded NLCs (FA-CIS-NLCs) were prepared. Their particle size, zeta potential, drug encapsulation efficiency (EE) and in vitro delivery behavior were evaluated. The in vitro cytotoxicity against HeLa cells and in vivo therapeutic effect were investigated.
Materials and methods
Materials, cells and animal
CIS was obtained from Qilu Pharmaceutical Co Ltd. (Ji’nan, China). FA, Tween-80 and stearic acid were purchased from Sigma Aldrich (St. Louis, MO). Soya lecithin was obtained from Shanghai Advanced Vehicle Technology Co., Ltd (Shanghai, China). Soybean oil was purchased from Guangzhou Hanfang Pharmaceutical Co., Ltd. (Guangzhou, China). PEG-distearoylphosphatidylethanolamine (DSPE) was purchased from CordenPharma International (Plankstadt, Germany). All other chemicals and reagents used were of cell culture or reagent grade. HeLa cells were obtained from the American type culture collection (Manassas, VA). BALB/c nude mice (6–8 weeks old, 20–25 g weight) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China).
Synthesis of FA-PEG-DSPE
FA was conjugated to DSPE-PEG-NH2 by the amide bond (Li et al., Citation2014). Briefly, 5 mg of FA, 5 mg of EDC and 2.5 mg of NHS were dissolved in DMSO. Then, DSPE-PEG-NH2 was dissolved in dichloromethane (DCM) and triethylamine, and then added to FA solution. The mixture was stirred for 24 h under nitrogen. Finally, FA-PEG-DSPE was obtained and purified by dialyzing. The production rate of the FA-PEG-DSPE was 71.6%.
Preparation of drug-loaded NLCs
CIS-loaded NLCs (CIS-NLCs) were prepared by the emulsification method (Andey et al., Citation2015). Oil phase was composed of stearic acid, soya lecithin and soybean oil (2:1:1, w/w/w). Aqueous phase was formed by Tween-80 (1%) and double distilled water. The CIS was dissolved in dimethylsulfoxide (DMSO), added into oil phase and warmed to 65 °C. The aqueous phase was warmed to 65 °C, and then added to the oil phase under high speed homogenizing (30 000 rpm; 15 min). The resulting CIS-NLCs were purified by dialysis against phosphate buffer solution (PBS) for 4 h.
FA-modified CIS-NLCs (FA-CIS-NLCs) were prepared using the same method, except for: the FA-PEG-DSPE was dissolved in aqueous phase warmed to 65 °C, and then added to the oil phase under high-speed homogenizing, purified by dialysis against PBS to get the FA-CIS-NLCs.
Characterization of drug-loaded NLCs
Particle size and zeta potential
The measures of mean particle size and polydispersity index (PDI) of NLCs suspension were performed by the dynamic light scattering technique using a Zetasizer (Nano-ZS90, Malvern Instruments, UK) (Zhao et al., Citation2015). The zeta potential was measured by the nanoparticles electrophoresis mobility using a U-type tube at 20 °C.
Drug entrapment efficiency (EE) and loading content (LC)
EE and LC of FA-CIS-NLCs and CIS-NLCs were determined using the UV–visible spectrophotometric method of CIS with o-phenylenediamine (OPDA) (Vhora et al., Citation2014). In brief, NLCs formulations were heated at 90 °C for 30 min with OPDA solution (OPDA concentration 600 times that of CIS on molar basis) in dimethylformamide (DMF). The dilutions were made with DMF-water mixture (7:3 v/v, pH 6.2 adjusted with 0.1 N HCl), and the reaction product was estimated at 705 nm on the UV–visible spectrophotometer (UV-1800, Simadzu, Japan). The amount of CIS was calculated from the standard calibration plot. The EE and LC of drug-loaded NLCs were calculated by the following equations:
Drug release study in vitro
CIS release from NLCs was assessed in PBS by the dialysis method (Jayant et al., Citation2009). Briefly, the NLCs were suspended in 10 mL of the PBS release medium in a dialysis membrane (molecular weight cut-off: 10–14 kDa) and transferred to a glass beaker containing 90 ml of PBS (pH 7.4). The samples were incubated at 37 ± 0.5 °C with constant agitation of 200 rpm for the release studies. One milliliter of buffer was periodically withdrawn and replaced with equal volume of fresh PBS. The drug release of free CIS suspension (Free CIS) was also evaluated as contrast. The amount of CIS released was determined at 705 nm using a UV–visible spectrophotometer.
Cell viability assays in vitro
HeLa cells were plated at a density of 1 × 104 cells/well in 96-well plates. Cells were cultured in DMEM medium (supplemented with 10% fetal bovine serum, 100 U/mL of penicillin and 100 µg/mL streptomycin) and maintained at 37 °C in a 5% CO2 atmosphere. After the overnight incubation, the medium was replaced with medium containing CIS-loaded NLC at concentrations ranging from 1 to 100 µg/mL of CIS. Then the cells were incubated at 37 °C, 5% CO2 for additional 72 h (Skidan et al., Citation2009). The cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method according to manufacturer’s instructions.
Anti-tumor efficiency studies in vivo
BALB/c mice were subcutaneously inoculating with HeLa cells suspended in PBS. When tumor volume (TV) reached about 50 mm3, transplanted mice were randomly divided into five groups containing six animals each (Negi et al., Citation2014). FA-CIS-NLCs, CIS-NLCs, Free CIS, NLCs without CIS (NLC) and normal saline (NS) were injected every six days, respectively. Tumor length and width were measured every three days by digital vernier caliper to depict the late stage of the tumor progression. The TV was calculated by the following equation:
where length is the longest dimension and width is the dimension perpendicular to length.
The anti-tumor efficacy of each formulation was evaluated by tumor inhibition rate (TIR), which was calculated using the following equation:
The body weight changes of different groups were also recorded.
Statistical analysis
All studies were repeated three times and all measurements were carried out in triplicate. Results were reported as means ± SD (SD = standard deviation). Statistical significance was preformed by two-tailed Student's t-test at p value less than 0.05 (p < 0.05).
Results and discussion
Characterization of drug-loaded NLCs
summarized the characteristic of NLCs. The mean particle size of FA-CIS-NLCs was 143.2 ± 5.3 nm (PDI 0.15 ± 0.02) with zeta potential of +25.7 ± 2.3 mV, EE 87.5 ± 3.2 %, LC 9.3 ± 0.6%. CIS-NLCs have a particle size of 119.6 ± 3.6 nm (PDI 0.11 ± 0.01) with zeta potential of +34.5 ± 3.1 mV, EE 89.2 ± 2.9%, LC 11.3 ± 0.8%. As control, blank NLCs without drugs showed the mean particle size 116.2 ± 2.9 nm (PDI 0.08 ± 0.01) with zeta potential of +11.3 ± 1.2 mV.
Table 1. Characterization of different vectors.
Particle size and the PDI are important factors for the nanocarriers, which can influence the distribution of carriers (Jin et al., Citation2007). Particle size lower than 200 nm could decreases uptake by the liver, prolongs circulation time in the blood and improves bioavailability. No significant change in diameter was found from blank NLC to s CIS-NLCs; however, the size of FA-CIS-NLCs was larger due to the addition of the FA ligands. The zeta potential is a key factor for the stability of nanocarrier systems (Xu et al., Citation2009). The positive charge of the NLCs and also facilitates the delivery of drug to the cancer cells.
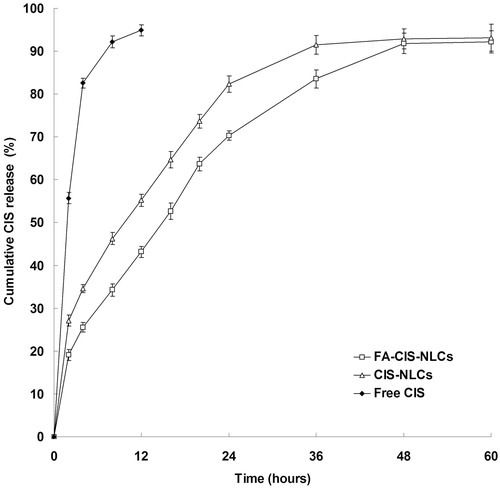
CIS release profiles are described in . The results showed that the CIS release from Free CIS formulation was fast, complete within 12 h. The releases from NLCs were sustained: FA-CIS-NLCs and CIS-NLCs obtained over 90% of drug release after 48 and 36 h. CIS release from FA-CIS-NLCs was even slower, which may because the modification of FA hindered the drug release. The sustained release of drug from nanocarriers may due to homogenous entrapment of the drug (Rahman et al., Citation2013). The release profile showed that FA-CIS-NLCs formulation had the capacity to release CIS at a sustained rate, let the anticancer drug to maintain continuous efficiency.
Cell viability assays in vitro
In order to know the activity of drug-loaded NLC against cervical cancer cells, in vitro HeLa cell cytotoxicity was evaluated by MTT assay. The IC50 values of FA-CIS-NLCs, CIS-NLCs and Free CIS are presented in . All the three samples showed a dose-dependent cytotoxicity against HeLa cells at the studied CIS concentrations. CIS-NLCs significantly decreased the IC50 value by one-third compared with Free CIS (p < 0.05), implying that NLCs formulations shown higher cytotoxicity against cervical cancer cells. FA-CIS-NLCs also decreased the IC50 value by one-third over CIS-NLCs (p < 0.05).
Table 2. IC50 of FA-CIS-NLCs, CIS-NLCs and Free CIS evaluated in HeLa cells.
A possible mechanism underlying the enhanced efficacy of CIS against tumor cells may include the enhanced intracellular drug accumulation by ligand-receptor recognition, and by nanocarrier uptake (Liu et al., Citation2011). Conventional cancer chemotherapy delivers the toxic anticancer agent indiscriminately, to cancers or normal tissues, causing undesirable systemic side effects. One way of tackling these problems is to deliver anticancer drugs selectively to the cancer sites. Utilizing the enhanced permeability and retention (EPR) effect of the cancer vasculature is one of the most effective strategies (Yokoyama, Citation2005). By harnessing this unique characteristic (EPR effect) of solid tumors, the selective delivery of nanocarrier-based anticancer drugs to the cancer site has become a reality. The EPR effect will confer most nano-sized drug delivery systems such as nanoparticle and liposomal or micellar drugs with selective cancer-targeting characteristics. Once delivered to the tumor, these nanocarrier-based drugs will remain in the tumor tissues for long periods of time (Iyer et al., Citation2006; Torchilin, Citation2007). Therefore, the use of nanocarrier-based drug delivery systems has taken a predominant role in anticancer drug delivery by nanocarrier uptake.
On the other hand, the major limitation of these nanocarriers is poor transfection efficiency at the target site after systemic administration due to uptake by the cells of reticuloendothelial system (He et al., Citation2015). Thus, surface-modification of nanocarriers is the appropriate method to overcome this shortage. Ligand conjugation can also be used to favorably modify the intracellular disposition of nanocarriers. Surface-modification of the nanocarriers is employed to control their biological properties in a desirable fashion such as longevity, target ability, intracellular penetration and contrast loading, thus allows them to simultaneously perform various therapeutically or diagnostically important functions (Torchilin, Citation2006). Folates are low-molecular weight pterin-based vitamins required by eukaryotic cells for one-carbon metabolism and de novo nucleotide synthesis. The fact that the high-affinity FR is expressed at much higher levels mainly on cancer cells might provide the cells with a growth advantage relative to neighboring normal tissue (Zhang & Zhang, Citation2005).
Anti-tumor efficiency studies in vivo
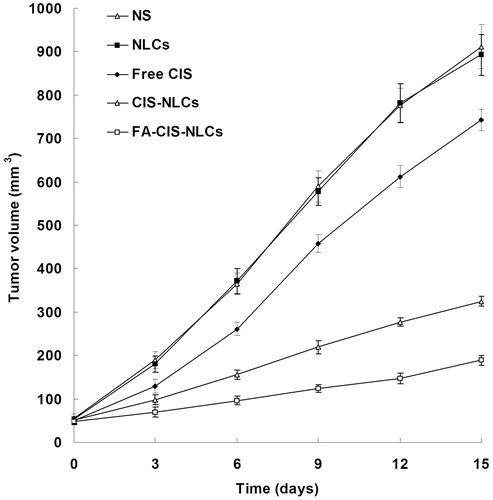
Anti-tumor efficiency was evaluated in mice-bearing human cervical cancer xenograft. The anti-tumor effect in terms of tumor growth inhibition is shown in . Obvious tumor regression was observed in mice treated with FA-CIS-NLCs, CIS-NLCs and Free CIS, while no anti-tumor effect was observed in the NLC and NS groups. At the end of the test, TV of CIS-NLCs group was 325 mm3 which was significantly smaller than the Free CIS (743 mm3) and control group (912 mm3) (p < 0.05). The TV of FA-CIS-NLCs group was the smallest (189 mm3). The TIRs of different groups are illustrated in . FA-CIS-NLCs group exhibited the highest TIR (79.3%), followed by CIS-NLCs (64.4%) and Free CIS (18.5%). The TIR of FA-CIS-NLCs formulation was significantly higher than the non-modified CIS-NLCs (p < 0.05); and the NLCs formulations have the higher TIR than free CIS solution formula (p < 0.05). The results suggested that FA-CIS-NLCs, as a target drug delivery system, could specifically target chemotherapeutic agents to tumors which over-express HA receptor.
Table 3. TIRs of FA-CIS-NLCs, CIS-NLCs and Free CIS.
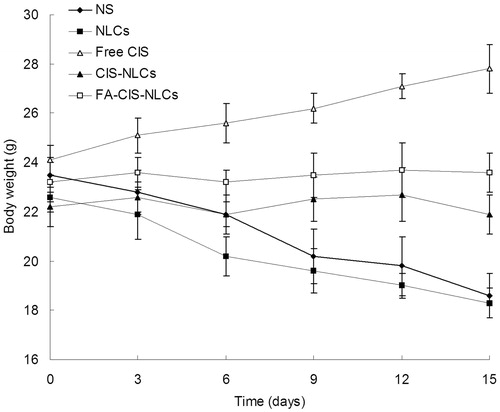
Body weight changes are illustrated in . No significant changes were found in FA-CIS-NLCs and CIS-NLCs. However, decrease in mean body weights were found in NLCs and NS groups, with the reduced foods intake, and moving inactively. The results suggest the less systemic toxic side effect of the NLCs formulations for the in vivo cancer treatment.
Conclusions
The efficiency of FA modified, CIS-loaded NLCs for cervical cancer chemotherapy was assessed in human cervical cells and HeLa cells treated a mice cancer model. This drug delivery system could achieve targeted delivery of drug, reduce the systemic toxicity and reach the best anti-tumor efficiency. The novel constructed NLCs could function as outstanding nanocarriers for the delivery of drugs for the targeted treatment of cancers.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Andey T, Sudhakar G, Marepally S, et al. (2015). Lipid nanocarriers of a lipid-conjugated estrogenic derivative inhibit tumor growth and enhance cisplatin activity against triple-negative breast cancer: pharmacokinetic and efficacy evaluation. Mol Pharm 12:1105–20
- Cassano R, Ferrarelli T, Mauro MV, et al. (2014). Preparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal delivery. Drug Deliv . [Epub ahead of print]. doi: 10.3109/10717544.2014.932862
- Das R, Bhattacharya K, Samanta SK, et al. (2014). Improved chemosensitivity in cervical cancer to cisplatin: synergistic activity of mahanine through STAT3 inhibition. Cancer Lett 351:81–90
- Diaz-Padilla I, Monk BJ, Mackay HJ, et al. (2013). Treatment of metastatic cervical cancer: future directions involving targeted agents. Crit Rev Oncol Hematol 85:303–14
- Dixit N, Vaibhav K, Pandey RS, et al. (2015). Improved cisplatin delivery in cervical cancer cells by utilizing folate-grafted non-aggregated gelatin nanoparticles. Biomed Pharmacother 69:1–10
- Gaba B, Fazil M, Ali A, et al. (2014). Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Deliv . [Epub ahead of print]. doi: 10.3109/10717544.2014.898110
- Han C, Li Y, Sun M, et al. (2014). Small peptide-modified nanostructured lipid carriers distribution and targeting to EGFR-overexpressing tumor in vivo. Artif Cells Nanomed Biotechnol 42:161–6
- Han CY, Yue LL, Tai LY, et al. (2013). A novel small peptide as an epidermal growth factor receptor targeting ligand for nanodelivery in vitro. Int J Nanomedicine 8:1541–9
- He X, Li L, Su H, et al. (2015). Poly(ethylene glycol)-block-poly(ɛ-caprolactone) and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int J Nanomedicine 10:1791–804
- Iyer AK, Khaled G, Fang J, et al. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 11:812–18
- Jayant RD, McShane MJ, Srivastava R. (2009). Polyelectrolyte-coated alginate microspheres as drug delivery carriers for dexamethasone release. Drug Deliv 16:331–40
- Jin W, Xu P, Zhan Y, et al. (2007). Degradable cisplatin-releasing core-shell nanogels from zwitterionic poly(beta-aminoester)-graft-PEG for cancer chemotherapy. Drug Deliv 14:279–86
- Li Y, Wu H, Jia M, et al. (2014). Therapeutic effect of folate-targeted and PEGylated phytosomes loaded with a mitomycin C-soybean phosphatidyhlcholine complex. Mol Pharm 11:3017–26
- Li YJ, Dong M, Kong FM, et al. (2015). Folate-decorated anticancer drug and magnetic nanoparticles encapsulated polymeric carrier for liver cancer therapeutics. Int J Pharm 489:83--90
- Liu D, Liu F, Liu Z, et al. (2011). Tumor specific delivery and therapy by double-targeted nanostructured lipid carriers with anti-VEGFR-2 antibody. Mol Pharm 8:2291–301
- Liu Q, Li J, Pu G, et al. (2015). Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv . [Epub ahead of print]. doi: 10.3109/10717544.2015.1031295
- Maduro JH, Pras E, Willemse PH, et al. (2003). Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat Rev 29:471–88
- Negi LM, Talegaonkar S, Jaggi M, et al. (2014). Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part II. In vivo biodistribution, pharmacodynamic and hematological toxicity studies. Colloids Surf B Biointerfaces 123:610–15
- Pandey V, Gajbhiye KR, Soni V. (2015). Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv 22:199–205
- Rahman HS, Rasedee A, How CW, et al. (2013). Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomedicine 8:2769–81
- Rostami E, Kashanian S, Azandaryani AH, et al. (2014). Drug targeting using solid lipid nanoparticles. Chem Phys Lipids 181:56–61
- Skidan I, Miao B, Thekkedath RV, et al. (2009). In vitro cytotoxicity of novel pro-apoptotic agent DM-PIT-1 in PEG-PE-based micelles alone and in combination with TRAIL. Drug Deliv 16:45–51
- Thukral DK, Dumoga S, Mishra AK. (2014). Solid lipid nanoparticles: promising therapeutic nanocarriers for drug delivery. Curr Drug Deliv 11:771–91
- Torchilin VP. (2006). Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–55
- Torchilin VP. (2007). Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J 9:E128–47
- Tzioras S, Pavlidis N, Paraskevaidis E, et al. (2007). Effects of different chemotherapy regimens on survival for advanced cervical cancer: systematic review and meta-analysis. Cancer Treat Rev 33:24–38
- Vhora I, Khatri N, Desai J, et al. (2014). Caprylate-conjugated cisplatin for the development of novel liposomal formulation. AAPS PharmSciTech 15:845–57
- Xu X, Zhao C, Yang H, et al. (2009). Anti-inflammatory activity of injectable dexamethasone acetate-loaded nanostructured lipid carriers. Drug Deliv 18:485–92
- Yokoyama M. (2005). Drug targeting with nano-sized carrier systems. J Artif Organs 8:77–84
- Zhang XG, Miao J, Dai YQ, et al. (2008). Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharm 361:239–44
- Zhang Y, Zhang J. (2005). Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Colloid Interface Sci 283:352–7
- Zhao S, Zhang Y, Han Y, et al. (2015). Preparation and characterization of cisplatin magnetic solid lipid nanoparticles (MSLNs): effects of loading procedures of Fe3O4 nanoparticles. Pharm Res 32:482–91
- Zhou X, Zhang X, Ye Y, et al. (2015). Nanostructured lipid carriers used for oral delivery of oridonin: an effect of ligand modification on absorption. Int J Pharm 479:391–8
- Zong S, Wang X, Yang Y, et al. (2015). The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur J Pharm Biopharm 93:127--35