Abstract
Context: Paclitaxel (PTX) and doxorubicin (DOX) are widely used for the combined chemotherapy of solid tumors. However, free drug combination has lower antitumor efficiency. It is necessary to design a drug delivery system to carry both of them.
Objective: This study aimed to engineer a nano-drug delivery system for co-encapsulating PTX and DOX. This system was expected to resolve the multidrug resistance caused by single drug, and the dual–drug-loaded nanostructured lipid carriers were also planned to specifically target the cancer cells without obvious influence on normal cells and tissues.
Methods: In this paper, nanostructured lipid carriers for combination delivery of PTX and DOX were prepared by the melt-emulsification technique. In vitro cytotoxicity against NCL-H460 human non-small cell lung carcinoma (NSCLS) cell line was investigated, and in vivo anti-tumor of NLC was evaluated on mice models grafting NCL-H460 cells.
Results: PTX-DOX NLC achieved the highest cytotoxic effect among all formulations in vitro, as compared to single drug delivery NLC. In vivo investigation on NSCLC animal models showed that co-delivery of PTX and DOX possessed high tumor-targeting capacity and strong anti-tumor activity.
Conclusion: The PTX-DOX NLC constructed in this research offers an effective strategy for targeted combinational lung cancer therapy.
Introduction
Combination chemotherapy for cancer patients is an important standard of care protocol for its synergistic therapeutic effects and reduced systemic toxicity by simultaneously modulating multiple cell-signaling pathways and overcoming multidrug resistance (Su et al., Citation2014). Taxanes and anthracyclines are the most common chemotherapeutic drugs in clinical application, for their excellent antitumor efficiency against various solid tumors (Piccart-Gebhart et al., Citation2008; Moreno-Aspitia & Perez, Citation2009; Feng et al., Citation2014; Langer et al., Citation2014). Paclitaxel (PTX), a semi-synthetic drug from Taxus baccata, is a novel antimicrotubule agent that promotes the assembly of microtubules from tubulin dimmers and stabilizes microtubules by preventing depolymerization. Doxorubicin (DOX) has the ability to bind DNA and inhibit nucleic acid synthesis. Because of their different action mechanism, combination of PTX and DOX was a favorable and active regimen in the treatment of many solid tumors, such as breast cancer, lung cancer, cervical cancer, etc (Sonpavde et al., Citation2000; Feng et al., Citation2014; Wang et al., Citation2014). However, combination of free drugs often brought more toxicity in clinical cancer therapy (Zhao et al., Citation2013; Lv et al., Citation2014).
Compared to free drugs, nano-scaled drug carriers have several advantages: passive targeted into the tumor site due to the enhanced permeability and retention (EPR) effect; reduced side effects, etc (Ma & Mumper, Citation2013). Therefore, nanocarriers have been widely applied for the chemotherapeutic drug delivery in cancer therapies. To date, paclitaxel albumin-bound nanoparticles (Abraxane®, Celgene Corporation, Summit, NJ) have been approved by the FDA for the treatment of metastatic breast cancer and non-small cell lung cancer (NSCLC). In addition, there are a number of novel paclitaxel nanoparticle formulations in clinical trials, such as polymeric micelle (NK 105, Genexol-PM), polymeric conjuage (CT-2013, PNU166945), liposome (Endo Tag, LEP-ETU), etc (Ma & Mumper, Citation2013). The therapy-limiting toxicity of DOX is cardiomyopathy. In order to reduce its toxicity, peglated-liposomal doxorubicin (Doxil®, ALZA Corporation, Bedford, OH) has been approved by the FDA for the treatment of ovarian cancer, etc; and the use of low-dose is another method to improve the toxicity profile of DOX (Waterhouse et al., Citation2001; Rivankar, Citation2014). Therefore, lipid nanoparticles, co-delivery of PTX and DOX for lung cancer combination therapy, were constructed to overcome these drawbacks aforementioned.
Nanostructured lipid carrier (NLC), the second-generation smarter drug carrier system of solid-lipid based nanoparticles, were developed as alternative to other colloidal carriers to overcome lipid nanoemulsions and liposomes in stability and ability to control the release of an encapsulated substance, and at the same time to be better tolerated than polymeric nanoparticles (Iqbal et al., Citation2012; Doktorovova et al., Citation2014). The NLC lipid matrix is composed of a blend of the solid lipid and the liquid lipid leading to a crystal structure with more imperfections and therefore with more room for drug accommodation especially for hydrophobic drugs (Weber et al., Citation2014). In this study, PTX and DOX (without hydrochloric acid), both have low solubility in aqueous solution. Therefore, NLC was the ideal carrier for PTX and DOX.
In this paper, NLC for combination delivery of PTX and DOX was prepared by the melted ultrasonic dispersion technique. The particle size, zeta potential, encapsulation efficiency and in vitro release were characterized. In vitro cytotoxicity against NCL-H460 human non-small cell lung carcinoma cell line (NCL-H460 cells) was investigated, and in vivo anti-tumor of NLC was evaluated on mice models grafting NCL-H460 cells.
Materials and methods
Materials
DOX was purchased from Aladdin (Shanghai, China). The NCL-H460 cells were purchased from American Type Culture Collection (Manassas, VA). Soybean phosphatidylcholine and oleic acid were purchased from Sigma-Aldrich (St Louis, MO). N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethyl-ammonium chloride (DOTMA) was purchased from TCI (Shanghai, China). Glyceryl monostearate was purchased from Shanghai Chineway Pharmaceutical Tech. Co Ltd. (Shanghai, China). COMPRITOL® 888 ATO was generously provided by Gattefossé (Paramus, NJ). BALB/c nude mice (5 to 6-week old, 18–22 g) were purchased from the Shanghai Slack Laboratory Animal Co., Ltd, Shanghai, China. All animal experiments complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China.
Preparation of PTX- and DOX-loaded NLC
PTX- and DOX-loaded NLC (PTX-DOX NLC) was prepared by melted ultrasonic dispersion method (Taratula et al., Citation2013). PTX and DOX were dissolved in 1 mL of dimethylsulfoxide (DMSO), and then added to the hot lipid phase consisted of 100 mg COMPRITOL® 888 ATO, 100 mg oleic acid and 10 mg soybean phosphatidylcholine. Aqueous phase was prepared by dissolving 20 mg DOTMA in 10 mL of distilled water. Both phases were maintained for 15 min at 80 °C in the oil bath under magnetic stirring. Then the hot lipid phase was added slowly to the aqueous solution and dispersed using a high-speed homogenizer for 5 min at 10 000 rpm. The crude emulsion was additionally treated by a probe type ultrasonicator for 5 min at 3 W. Then, the hot emulsion was cooled at 4 °C on an ice bath, maintaining the mechanical stirring for 30 min. DMSO was removed by dialyzation: the NLC suspension dissolved with Milli-Q water and then dialyzed against Milli-Q water for 12 h. After preparation, the NLC was subjected to lyophilization. The obtained powder was stored at 4 °C until further use. Single PTX- or DOX-loaded NLC (PTX NLC, or DOX NLC), were prepared using the same method adding only one drug in the lipid phase.
The particle size (diameter, nm), polydispersity index (PDI) and surface charge (zeta potential, mV) were determined using a ZetaSizer Nano series Nano-ZS (Malvern Instruments Ltd., Malvern, UK) (Wang et al., Citation2011). Determinations were performed at 25 °C for samples appropriately diluted in distilled water.
Drug loading content and drug loading efficiency
The amount of DOX in microspheres was analyzed by a UV-Vis spectrophotometer at 480 nm by dissolving 1 mg NLC in 1 mL DMSO and employing the calibration curve of 0–100 μg/mL DOX in the same solvent (Feng et al., Citation2014). The amount of PTX in microspheres was analyzed utilizing a high performance liquid chromatography (HPLC, Waters 1525, Waters Corporation, Milford, MA) equipped with a symmetry C18 column. The NLC was dissolved in dichloromethane and flow through a 0.45 μm filter before sample inject with a 20 μL loop. The mobile phase was acetonitrile/water (v/v = 4:1) and PTX was detected with a UV detector (Waters 2489) at 227 nm.
The drug loading content (DLC) and the drug loading efficiency (DLE) of NLC loaded with DOX and PTX were calculated by the following equations (Li et al., Citation2015):
In vitro drug release behavior
The release behavior of DOX and PTX from NLC were assessed in phosphate-buffered saline (PBS) by the dialysis method (Lin et al., Citation2013; Lv et al., Citation2014). Briefly, the NLC were suspended in 5 mL of the PBS release medium and transferred into a dialysis bag (MWCO 3500 Da). The release experiments were started by placing the dialysis bag into 45 mL of release medium with continuous shaking at 100 rpm at 37 °C. At predetermined time, 4 mL of the incubated solution was withdrawn and replaced with equal volume of fresh PBS. The amount of DOX released was determined using UV-Vis spectrometer; PTX contents in the samples were measured by HPLC mentioned in the above section.
In vitro cytotoxicity studies
NCL-H460 cells were used to investigate the cytotoxicity of drug-loaded NLC and solution formulations in vitro by MTT assay (Yuan et al., Citation2013; Duan et al., Citation2015). The NCL-H460 cells were seeded into 96-well culture plates at a density of 1 × 104, and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Fisher Chemicals, Fairlawn, NJ) in a 5% CO2 fully humidified atmosphere for 24 h. The cells were then treated with the PTX-DOX NLC, PTX NLC, DOX NLC, free PTX suspension (DMSO, 0.1%) (free PTX) and free DOX suspension (free DOX). To determine the optimal ratio of PTX and DOX in PTX-DOX NLC, DOX and PTX combinations at various weight ratios (PTX/BCL = 1/5, 1/2, 1/1, 2/1, 5/1; w/w) were prepared. All samples were performed at different concentrations. Culture medium was used as a blank control. After 48 h of incubation, MTT solution (5 mg/mL) was added to each well and the cells were incubated for another 4 h. The culture medium was then removed and 100 µL of DMSO was added to each well to dissolve the formazan crystals. The optical density (OD) was measured at 570 nm using a Multiskan MK3 Microplate Reader (Thermo Fisher Scientific, Waltham, MA). Cell viability was calculated using the following formula:
The drug concentration causing 50% inhibition (IC50) was calculated using Statistical Package for the Social Sciences version 17 software (SPSS Inc., Chicago, IL).
In vivo antitumor efficiency
A human NSCLC xenograft tumor model was generated by the subcutaneous injection of NCL-H460 cells (1.5 × 106, 100 µL in PBS) in the right flank of each mouse. When the tumor volume reached approximately 50 mm3, mice were randomly divided into 6 groups (n = 8). Mice were treated with PTX-DOX NLC, PTX NLC, DOX NLC, free PTX, free DOX and 0.9% saline intravenously via tail vein every 4 days. Tumor volume and body weight were measured every 4 days to evaluate the antitumor activities and systematic toxicities of various formulations. The tumor volume was calculated based on the following equation (Yadav et al., Citation2010):
where L is the longest dimension and W is the dimension.
The antitumor efficacy of each formulation was evaluated by tumor inhibition rate, and was calculated using the following formula (Nekkanti et al., Citation2011):
where Wt and Wc represent the mean tumor weight of the treated and control groups, respectively.
Results and discussion
Particle size, surface charge and drug loading
Particle size and the PDI are important features of NLC, from which the stability of these nanoparticles when drug-loaded can be predicted (Rahman et al., Citation2013). Particle size can influence the distribution of nanocarriers, small particle size is an advantage for NLC because it decreases uptake by the liver, prolongs circulation time in the blood, and improves bioavailability. The mean particle size and PDI of different NLC formulations produced in this study are summarized in . The diameters from blank NLC to single drug-loaded NLC and PTX-DOX NLC were around 125–130 nm. These results could be the evidence that the adding of drug(s) did not affect the size of NLC.
Table 1. Particle size, surface charge and drug loading of NLC.
The zeta potential is a key factor in prediction and evaluation of the stability of a colloidal dispersion (Zhang et al., Citation2013). The +27 mV of potential could ensure the stability of the PTX-DOX NLC and also facilitate the delivery of NLC to the negatively charged cancer cells. The DLE of PTX and DOX encapsulated in PTX-DOX NLC were 82 and 84%, separately. The DLC of PTX- and DOX-loaded in PTX-DOX NLC were 13 and 10%, respectively.
In vitro drug release behavior
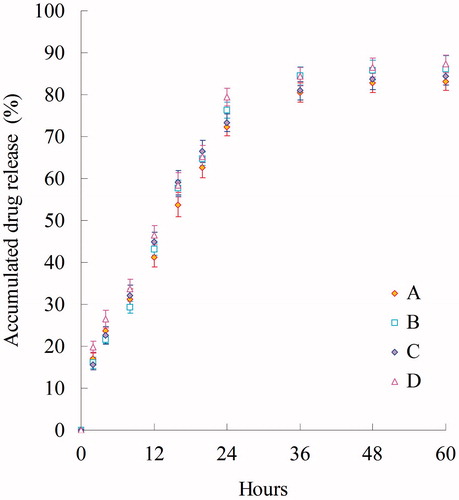
In vitro PTX and DOX release behaviors of PTX-DOX NLC, PTX NLC, DOX NLC were performed using the dialysis technique and data are shown in . The results showed that the PTX and DOX in the NLC was released gradually over the 48-hour period from the NLC formulations, indicating that the drug release from nanoparticles generally takes place by several mechanisms, including surface and bulk erosion, disintegration, diffusion and desorption (Rahman et al., Citation2013). This sustained release of drug from nanoparticles is due to homogenous entrapment of the drug. At high lipid concentration, a drug-enriched core can develop that will slow down release of the drug. Slow release of drug from NLC has been found to occur predominantly by diffusion from the lipid matrix. The in vitro release profile shows that PTX-DOX NLC formulation has the capacity to release PTX or DOX at a sustained rate.
In vitro cytotoxicity studies
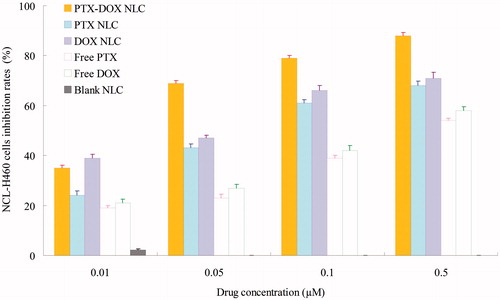
MTT assay were used to investigate the cytotoxicity of drug-loaded NLC and solution formulations in vitro (). The viability of NCL-H460 cells treated with various concentrations of free drugs and drug(s) loaded NLC for 48 h decreased in a dose-dependent manner. The IC50 value for PTX and DOX of different formulations are summarized in . The IC50 of PTX-DOX NLC at the PTX/BCL weight ratio of 1/1 was the lowest compared with other weight ratios. So this ratio was determined. The IC50 for PTX of PTX-DOX NLC, PTX NLC and free PTX was 0.021, 0.062 and 0.193 µM, respectively; while the IC50 for DOX of PTX-DOX NLC, DOX NLC and free DOX was 0.021, 0.059 and 0.176 µM, separately. The cytotoxic effect of PTX-DOX NLC on lung cancer cells was significantly higher than single drug NLC (3-fold), and free drug formula (9-fold) (p < 0.05).
Table 2. IC50 values of free drugs and NLC on NCL-H460 cells.
In vivo antitumor efficiency
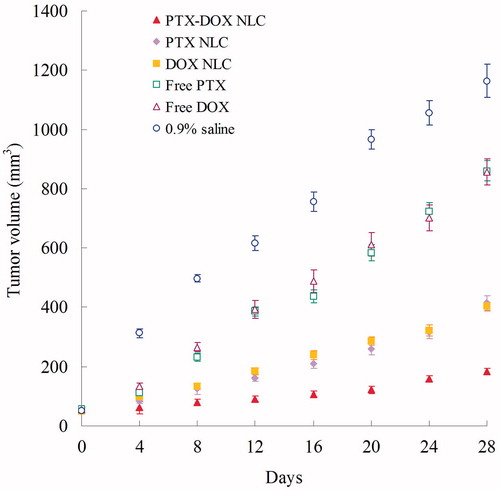
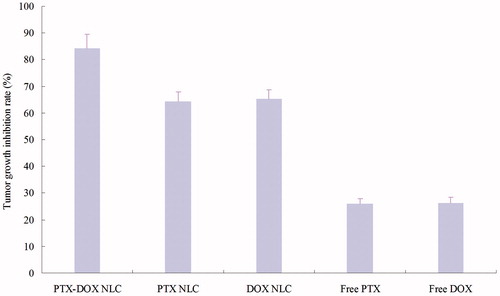
The in vivo anti-tumor efficiency of drug(s)-loaded NLC and free drugs were evaluated in mice bearing human lung cancer xenograft by measuring the volumes of the solid tumors. shows the tumor growth curves of different formulations in vivo. Tumor growth was significantly inhibited by PTX-DOX NLC, than PTX NLC and DOX NLCs formulations (p < 0.05). The obviously better tumor inhibition effects were observed in the NLC groups than the free drug solution groups. The tumor inhibition rates of different groups are illustrated in . PTX-DOX NLC exhibited the highest inhibition rate of 84%, followed by DOX NLC (65%) and PTX NLC (64%). Free DOX and PTX got about 26% tumor inhibition rates. Also, no obvious body weight change were found in three NLC groups; however, a progressive and irregular decrease in mean body weight was found in the free drug and saline group, with the reductively intake of foods, inactive in moving. The results suggested the best anti-tumor effect of double drugs contained NLC due to the synergetic effect of the two drugs, and the least systemic toxic side effect of the NLC formulations for the lung cancer treatment.
Conclusions
This study demonstrated that PTX-DOX NLC effectively inhibited the lung tumor growth and killed the lung cancer cells in vivo and in vitro. The synergistic anti-tumor efficacy was assessed in NCL-H460 and NCL-H460 cell-treated mice lung cancer model. This system could achieve co-delivery of PTX and DOX, exert the ability of the two drugs, reduce the systemic toxicity, and get the significant better tumor inhibition efficiency. The resulting NLC formulation could function as promising nanocarriers for the delivery of dual drugs in the synergistic treatment of cancers.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Doktorovova S, Souto EB, Silva AM. (2014). Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. Eur J Pharm Biopharm 87:1–18
- Duan Y, Wang J, Yang X, et al. (2015). Curcumin-loaded mixed micelles: preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv 22:50–7
- Feng T, Tian H, Xu C, et al. (2014). Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur J Pharm Biopharm 88:1086–93
- Iqbal MA, Md S, Sahni JK, et al. (2012). Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target 20:813–30
- Langer CJ, Novello S, Park K, et al. (2014). Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 32:2059–66
- Li M, Liu P, Gao G, et al. (2015). Smac therapeutic Peptide nanoparticles inducing apoptosis of cancer cells for combination chemotherapy with Doxorubicin. ACS Appl Mater Interfaces 7:8005–12
- Lin T, Fang Q, Peng D, et al. (2013). PEGylated non-ionic surfactant vesicles as drug delivery systems for Gambogenic acid. Drug Deliv 20:277–84
- Lv S, Tang Z, Li M, et al. (2014). Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 35:6118–29
- Ma P, Mumper RJ. (2013). Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol 4:1000164
- Moreno-Aspitia A, Perez EA. (2009). Anthracycline- and/or taxane-resistant breast cancer: results of a literature review to determine the clinical challenges and current treatment trends. Clin Ther 31:1619–40
- Nekkanti V, Karatgi P, Paruchuri S, et al. (2011). Drug product development and pharmacological evaluation of a sparingly soluble novel camptothecin analog for peroral administration. Drug Deliv 18:294–303
- Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. (2008). Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 26:1980–6
- Rahman HS, Rasedee A, How CW, et al. (2013). Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomedicine 8:2769–81
- Rivankar S. (2014). An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther 10:853–8
- Sonpavde G, Ansari R, Walker P, et al. (2000). Phase II study of doxorubicin and paclitaxel as second-line chemotherapy of small-cell lung cancer: a Hoosier Oncology Group Trial. Am J Clin Oncol 23:68–70
- Su CW, Chiang CS, Li WM, et al. (2014). Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine (Lond) 9:1499–515
- Taratula O, Kuzmov A, Shah M, et al. (2013). Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release 171:349–57
- Wang H, Zhao Y, Wu Y, et al. (2011). Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 32:8281–90
- Wang Y, Ma S, Xie Z, et al. (2014). A synergistic combination therapy with paclitaxel and doxorubicin loaded micellar nanoparticles. Colloids Surf B Biointerfaces 116:41–8
- Waterhouse DN, Tardi PG, Mayer LD, et al. (2001). A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf 24:903–20
- Weber S, Zimmer A, Pardeike J. (2014). Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm 86:7–22
- Yadav AK, Agarwal A, Rai G, et al. (2010). Development and characterization of hyaluronic acid decorated PLGA nanoparticles for delivery of 5-fluorouracil. Drug Deliv 17:561–72
- Yuan L, Liu C, Chen Y, et al. (2013). Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int J Nanomedicine 8:4339–50
- Zhang ZH, Wang XP, Ayman WY, et al. (2013). Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv 20:86–93
- Zhao Y, Alakhova DY, Kabanov AV. (2013). Can nanomedicines kill cancer stem cells? Adv Drug Deliv Rev 65:1763–83