Abstract
Context: Glioblastoma multiforme (GBM) is the most common malignant brain tumor originating in the central nervous system. Efficient delivery of therapeutic molecules to the cells and tissues is a difficult challenge.
Objective: Arginine-glycine-aspartic acid peptide (RGD)-modified nanostructured lipid carriers (NLCs) were used for the delivery of temozolomide (TMZ) into the GBM to provide a new paradigm in gliomatosis cerebri treatment.
Methods: RGD-conjugated polyethylene glycol-b-distearoylphosphatidylethanolamine (PEG-DSPE) was synthesized. RGD containing, TMZ-loaded NLCs (RGD-TMZ/NLCs) were prepared. Their particle size, zeta potential, drug encapsulation efficiency (EE) and drug release behavior were evaluated. In vitro cytotoxicity study of TMZ/NLCs was tested in U87 malignant glioma cells (U87MG cells). In vivo antitumor efficacy of the carriers was evaluated on mice bearing GBM model.
Results: The U87MG cells were successfully inhibited by RGD-TMZ/NLCs in vitro. RGD-TMZ/NLCs also displayed the highest antitumor efficacy in vivo than all the other formulations used for comparison.
Conclusion: RGD-TMZ/NLCs were efficient in selective delivery of TMZ into U87MG cells, and inhibition efficacy is high. These RGD-modified vectors could be a superior drug delivery nano-system to achieve therapeutic efficacy, and this research could be a new promising strategy for treatment in malignant gliomatosis cerebri.
Introduction
Glioma remains a serious threat and is widely incurable. Glioblastoma multiforme (GBM), accounting 70% of all malignant gliomas, are highly aggressive with a 5-year survival rate lower than 5% (Gao et al., Citation2014b). One of the hallmark features of GBM that makes it so difficult to treat is the tumor often has significant heterogeneity within the same foci that results in different morphological features and therefore different intratumoral behavior (Kesari, Citation2011). Current standard treatment for GBM includes surgery, radiation therapy and chemotherapy (Cavanagh & Holle, Citation2014). The most effective treatment currently being surgical resection; however, resection is not often possible due to inoperable tumor location within the brain due to existing comorbidities or poor performance status. Targeted nanoparticulate delivery system as related to brain tumors can be defined as the targeted transfer of drugs into tumor cells for therapeutic purposes (Bernal et al., Citation2014).
Lipid-based nanoparticles have attracted a large attention as possible alternatives to polymeric ones due to their highly biocompatible and biodegradable natural components (Wissing et al., Citation2004). Due to the physicochemical properties of lipids, lipid-based nanoparticles can be easily obtained by direct emulsification of the molten lipids and subsequent recrystallization, avoiding the use of potentially toxic solvents that are commonly required for the preparation of polymeric nanoparticles (Esposito et al., Citation2015; Fathi-Azarbayjani et al., Citation2015; Mattingly et al., Citation2015). Among the different types of lipid-based nanoparticles, nanostructured lipid carriers (NLCs) constituted of blends of lipids in solid and liquid state can be considered as the last generation (Shidhaye et al., Citation2008; Alam et al., Citation2013; Luanet al., Citation2013; Wang et al., Citation2013).
RGD is a peptide that was used for neovasculature targeting delivery because of its high-binding efficiency with αvβ3 and αvβ5, which is overexpressed on the endothelial cells of tumor angiogenic vessels (Gao et al., Citation2014a) as well as on GBM cells (e.g. the U87MG cell line) (Miura et al., Citation2013). Current standard of care for patients diagnosed with high-grade malignant glioma includes post-operative temozolomide (TMZ) adjuvant to radiation (Buie & Valgus, Citation2012). Accordingly, we developed RGD-modified NLCs for the targeting of GBM cells, while TMZ was used as the model drug. This system was expected to achieve stable drug-loading capacity, effectively anti-cancer ability in vitro and in vivo.
Materials and methods
Materials
TMZ was kindly provided by Laimei Pharmaceutical Co., Ltd. (Chongqing, China). Stearic acid, dimethyldioctadecylammonium bromide (DDAB), (3-[4,5-dimehyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT), soybean phosphatidylcholine (SPC) were purchased from Sigma Aldrich (St. Louis, MO). Polyethylene glycol-b-distearoylphosphatidylethanolamine (PEG-DSPE) was purchased from Avanti Polar Lipids (Alabaster, AL). COMPRITOL® 888 ATO (888 ATO) was generously provided by Gattefossé (Paramus, NJ). Polyoxyl castor oil (Cremophor ELP) was donated by BASF (Ludwigshafen, Germany). Injectable soya lecithin was obtained from Shanghai Taiwei Pharmaceutical Co, Ltd (Shanghai, China). All other chemicals were of analytical grade or higher.
Animals and cells
U87 malignant glioma cells (U87MG cells) were obtained from the American type culture collection (Manassas, VA) and cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Fisher Chemicals, Fairlawn, NJ) in a 5% CO2 fully humidified atmosphere.
BALB/c nude mice (5–6 weeks old, 18–22 g) were purchased from the Shanghai Slack Laboratory Animal Co., Ltd. All animal experiments complied with the Animal Management Rules of the Ministry of Health of the People's Republic of China.
Preparation and optimization of NLCs
RGD-conjugated polyethylene glycol-b-distearoylphosphatidylethanolamine (PEG-DSPE) was synthesized as follows (Zeng et al., Citation2014). A mixture of NH2-PEG-DSPE was dissolved in DMSO. RGD was added and stirred for another 24 h. After completion of the reaction, the solution was dialyzed successively against Milli-Q water (membrane tubing, molecular weight cutoff 1000 Da). The product after dialysis was then lyophilized, and RGD-PEG-DSPE (yield 67.3%) was obtained.
NLCs were prepared by solvent diffusion method (Tiwari & Pathak, Citation2011). In brief, the lipid dispersion was composed of 888 ATO, Cremophor ELP and SPC at a ratio of 2:1:1 (w/w/w). Injectable soya lecithin (1 g), TMZ (50 mg) and RGD-PEG-DSPE (60 mg) were dissolved in 1 ml of DMSO and added to the lipid dispersion with heating at the temperature of 70–75 °C to form the lipid phase. Aqueous phase was prepared by dissolving DDAB (50 mg) in 10 ml of water. This aqueous solution was then stirred and heated to 30 °C. The lipid phase was rapidly injected into the stirred aqueous phase (800 rpm) at 30 °C, and the resulting suspension was then dissolved with Milli-Q water and then dialyzed against Milli-Q water for 24 h to get the RGD-TMZ/NLCs. Non-RGD-modified TMZ/NLCs were prepared by the same method using PEG-DSPE instead of RGD-PEG-DSPE. Blank NLCs were prepared using the same method without adding TMZ. The NLCs obtained were stored at 2–8 °C.
To optimize the suitable RGD-PEG-DSPE ratio to the NLCs systems, different quantity of RGD-PEG-DSPE was applied in the preparation process. The resulting RGD-TMZ/NLCs were analyzed due to the data of zeta potential.
Characterization of the NLCs
The mean particle size, size distribution and zeta potential of RGD-TMZ/NLCs, TMZ/NLCs and blank NLCs were determined using the Malvern Zetasizer Nano ZS (Malvern Instrument Ltd., Worcestershire, UK). The average particle size was expressed as volume mean diameter.
The drug encapsulation efficiency (EE) and drug-loading capacity (LC) of RGD-TMZ/NLCs and TMZ/NLCs formulations were measured using the HITACHI P-4010 inductively coupled plasma mass spectrometry (ICP-MS) (Hitachi Ltd, Kyoto, Japan). Briefly, 5 ml NLCs were centrifuged (16 000 rpm and 4 °C for 30 min) separately, and the supernatants were then determined using the ICP-MS. The EE was expressed as the percentage of the amount of TMZ encapsulated in the NLCs to the total amount of TMZ initially used. EE were calculated as follows: EE (%) = Concentration of (total TMZ − free TMZ)/Concentration of total TMZ × 100. LC were calculated as follows: LC (%) = Concentration of (total TMZ − free TMZ)/Concentration of total TMZ loaded NLCs × 100.
In vitro release study
The release of RGD-TMZ/NLCs and TMZ/NLCs was evaluated by the dialysis method. Briefly, samples were added to the dialysis bag separately. Then, the dialysis bags were incubated with 30 ml of release medium (PBS, pH 7.4) at 37 °C with stirring at 100 rpm. At the predetermined time intervals, 1 ml of solution was removed and 1 ml of fresh medium was filled to replace the remaining release medium. The amount of TMZ released from the samples was then determined by the same method described in the “Characterization of the NLCs” section.
In vitro cytotoxicity study
The cytotoxicity of RGD-TMZ/NLCs was tested in U87 MG cells using the MTT assay (Choi, Citation2014). Briefly, cells were seeded in a 24-well plate at a density of 1 × 104 cells/well and allowed to adhere for 24 h prior to the assay. Cells were exposed to various concentrations of RGD-TMZ/NLCs, TMZ/NLCs, free TMZ solution and blank NLCs, respectively. Culture medium was used as a blank control. After 48 h of incubation, MTT solution (5 mg/ml) was added to each well, and the cells were incubated for another 4 h. Cellular viability was assessed according to the MTT manufacturer's procedures, and the absorbance at 570 nm was measured using a microplate reader (Model 680, Bio-Rad Laboratories Inc., Philadelphia, PA). Cells without the addition of MTT reagents were used as a blank control. The drug concentration causing 50% inhibition (IC50) was calculated using the CalcuSyn software (Biosoft, Ferguson, MO) (Casagrande et al., Citation2013).
In vivo therapeutic studies
BALB/c nude mice were inoculating subcutaneously (s.c.) in the right armpit with U87 MG cells suspended in PBS for 24 h for the preparation of malignant glioma bearing animal models. Mice were then divided into five groups (8 mice per group). The RGD-TMZ/NLCs, TMZ/NLCs, free TMZ solution, blank NLCs and 0.9% sodium chloride solution were prepared and then injected intravenously into the mice via the tail vein (i.v.). The initial day of i.v. administration was defined as day 0, and administration was then repeated once every 3 days (d) over a 21-d therapeutic period. The measurements were taken in two perpendicular dimensions, and the tumor volumes (mm3) were calculated by applying the formula: Tumor volumes (mm3) = (L × W2)/2, where L is the longest dimension and W is the dimension perpendicular to L (Sarisozen et al., Citation2014). The antitumor efficacy of each formulation was evaluated by tumor inhibition rate and was calculated using the following formula: Tumor inhibition rate (%) = (Wc − Wt)/Wc × 100. Wt and Wc represent the mean tumor weight of the treated and control groups, respectively.
Statistical analysis
Quantitative data were presented as mean ± standard deviation (SD). Statistical significance was analyzed using the Student's t test with the p value less than 0.05 (p < 0.05) indicating significance.
Results
Characterization and optimization of NLCs
The mean particle size, size distribution, zeta potential and EE of RGD-TMZ/NLCs, TMZ/NLCs and blank NLCs are characterized and summarized in . The RGD-TMZ/NLCs has a size of 118.3 nm, with a potential of 28.9 mV. The EE of RGD-TMZ/NLCs and TMZ/NLCs was 85.3 and 84.7%, respectively. It is reported that the positive surface charge and proper particle size of nanocarriers were important for efficient drug delivery, so it was expected that the obtained cationic NLCs could achieve efficient cellular endocytosis (Bothun et al., Citation2011).
Table 1. Characterization of different vectors.
To optimize the suitable RGD–PEG–DSPE ratio to the NLCs systems, different quantity of RGD–PEG–DSPE was applied in the preparation process. The positively charged RGD was coated onto the NLCs and cause the increase of zeta potential. The more amount of RGD increased in the formulation, the more increase of zeta potential of the system should be found. No obvious change of zeta potential indicates the suitable RGD–PEG–DSPE ratio. The zeta potential increased from 23 to 29 mV when the quantity of RGD-PEG-DSPE increased to 60 mg in 1 ml solution, after that the potential did not change obviously (). Therefore, the quantity of RGD–PEG–DSPE was fixed (60 mg).
Table 2. Optimization of RGD-TMZ/NLCs.
In vitro release study
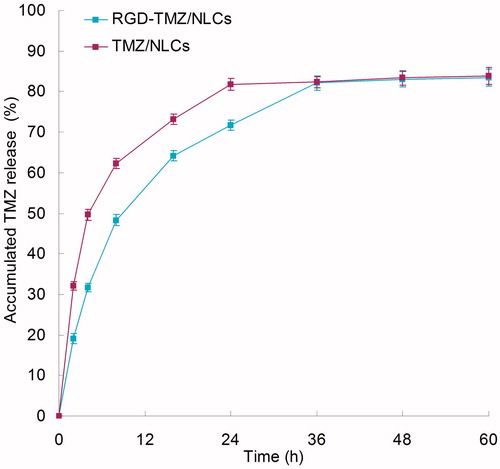
exhibits the drug release profiles of the samples, respectively. The TMZ was released from NLCs at a sustained rate. The release of RGD-TMZ/NLCs was slower than TMZ/NLCs. The complete release of the RGD-TMZ/NLCs and TMZ/NLCs were about 24 and 36 h, respectively. These may indicate that the lipid structure of NLCs helps to retain the drugs inside the carriers, and the RGD coating bring about more persistent therapeutic effect than the unmodified NLCs.
In vitro cytotoxicity
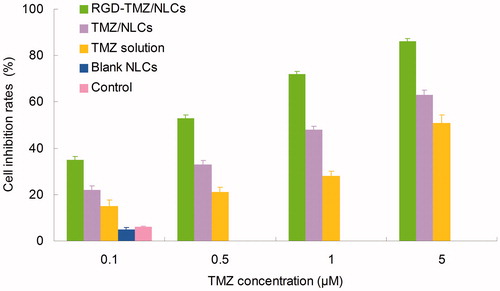
The cytotoxicity of RGD-TMZ/NLCs and other formulas in U87MG cells at different concentrations after 48 h of incubation are illustrated in . The cell inhibition rates of all formulas conformed to concentration-dependent patterns. RGD-TMZ/NLCs showed significantly higher cytotoxicity than TMZ/NLCs (p < 0.05). TMZ/NLCs exhibited remarkably higher cell inhibition effect than TMZ solution (p < 0.05). The IC50 values of RGD-TMZ/NLCs, TMZ/NLCs and TMZ solution were 0.48, 1.02 and 4.96 µM, respectively. The IC50 value of RGD-TMZ/NLCs was 2-fold over RGD-TMZ/NLCs and 10-fold over TMZ solution in reducing viability of malignant glioma cells, accounting for the highest antitumor activity. Cytotoxicity deeply influenced the ability of the nanocarriers (Yu et al., Citation2010). The results demonstrated the higher cytotoxicity of NLCs formulas than free drug solution. Also the presence of RGD could let the carriers more efficient due to the target ability of the ligands to the receptors.
In vivo therapeutic studies
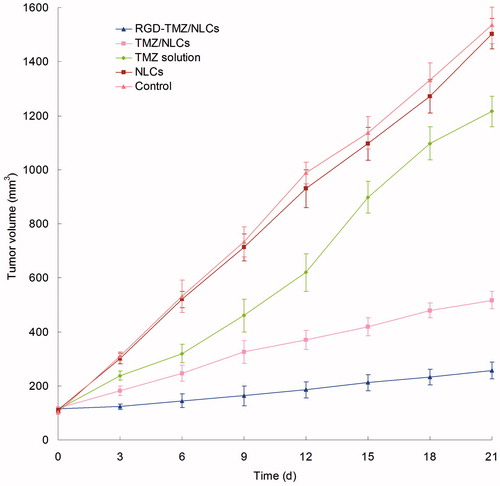
The in vivo antitumor therapeutic effect was evaluated in U87 MG solid tumors in mice. As shown in , tumor growth was significantly inhibited by NLCs formulas than free solution group (p < 0.05). The most obvious tumor regressions were observed in the RGD-TMZ/NLCs group, where the tumor growth was prominently inhibited. After 21 d of administration, the tumor inhibition rates of tumor bearing mice treated with RGD-TMZ/NLCs, TMZ/NLCs and free TMZ were 83.3, 66.3 and 20.8%. TMZ/NLCs inhibited tumor growth three times higher than that treated with free TMZ solution, for RGD-TMZ/NLCs groups, four times. For the free drugs and the drug-loaded NLC-treated groups, two conclusions could be summarized as follows: (1) the containing of RGD was more effective than the use of non-RGD contained TMZ/NLCs. (2) The TMZ-loaded NLCs showed better antitumor effect compared with free drug (Xiao et al., Citation2012; Lv et al., Citation2014). The NLCs formulation is good, and the modified NLCs formula is excellent. RGD-TMZ/NLCs constructed in this research could be very useful for in vivo drug delivery and proved to be efficient for the treatment of GBM.
Conclusion
RGD-modified, TMZ-loaded NLCs were fabricated for the GBM chemotherapy. The well-constructed RGD-TMZ/NLCs exhibited small particle size, reasonable positive charges and best tumor cells inhibition effect in vitro. It significantly increased the in vitro and in vivo drug delivery efficiency. These RGD-modified vectors could be a superior nano-system to achieve therapeutic efficacy, and this research could be a new promising strategy for treatment in malignant gliomatosis cerebri.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alam MI, Baboota S, Ahuja A, et al. (2013). Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv 20:247–51
- Bernal GM, LaRiviere MJ, Mansour N, et al. (2014). Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine 10:149–57
- Bothun GD, Lelis A, Chen Y, et al. (2011). Multicomponent folate-targeted magnetoliposomes: design, characterization, and cellular uptake. Nanomedicine 7:797–805
- Buie LW, Valgus JM. (2012). Current treatment options for the management of glioblastoma multiforme. Hematol Oncol Pharm 2:57–63
- Casagrande N, De Paoli M, Celegato M, et al. (2013). Preclinical evaluation of a new liposomal formulation of cisplatin, lipoplatin, to treat cisplatin-resistant cervical cancer. Gynecol Oncol 131:744–52
- Cavanagh TT, Holle LM. (2014). Potential new gene therapy option with sitimagene ceradenovec for newly diagnosed patients with glioblastoma multiforme. Cancer Biol Ther 15:263–5
- Choi YH. (2014). Linoleic acid-induced growth inhibition of human gastric epithelial adenocarcinoma AGS cells is associated with down-regulation of prostaglandin E2 synthesis and telomerase activity. J Cancer Prev 19:31–8
- Esposito E, Boschi A, Ravani L, et al. (2015). Biodistribution of nanostructured lipid carriers: a tomographic study. Eur J Pharm Biopharm 89:145–56
- Fathi-Azarbayjani A, Ng KX, Chan YW, Chan SY. (2015). Lipid vesicles for the skin delivery of diclofenac: cerosomes vs. other lipid suspensions. Adv Pharm Bull 5:25–33
- Gao H, Xiong Y, Zhang S, et al. (2014a). RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm 11:1042–52
- Gao H, Yang Z, Cao S, et al. (2014b). Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials 35:374–82
- Kesari S. (2011). Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol 38:S2–10
- Luan J, Zhang D, Hao L, et al. (2013). Design and characterization of Amoitone B-loaded nanostructured lipid carriers for controlled drug release. Drug Deliv 20:324–30
- Lv S, Tang Z, Li M, et al. (2014). Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 35:6118–29
- Mattingly SJ, O'Toole MG, James KT, et al. (2015). Magnetic nanoparticle-supported lipid bilayers for drug delivery. Langmuir 31:3326–32
- Miura Y, Takenaka T, Toh K, et al. (2013). Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano 7:8583–92
- Sarisozen C, Abouzeid AH, Torchilin VP. (2014). The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm 88:539–50
- Shidhaye SS, Vaidya R, Sutar S, et al. (2008). Solid lipid nanoparticles and nanostructured lipid carriers – innovative generations of solid lipid carriers. Curr Drug Deliv 5:324–31
- Tiwari R, Pathak K. (2011). Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm 415:232–43
- Wang Q, Cheng H, Zhou K, et al. (2013). Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv 20:331–7
- Wissing SA, Kayser O, Müller RH. (2004). Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56:1257–72
- Xiao H, Song H, Yang Q, et al. (2012). A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials 33:6507–19
- Yu W, Liu C, Liu Y, et al. (2010). Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res 27:1584–96
- Zeng S, Wu F, Li B, et al. (2014). Synthesis, characterization, and evaluation of a novel amphiphilic polymer RGD-PEG-Chol for target drug delivery system. Sci World J 2014:546176