Abstract
Background: Delivery of anti-cancer drugs into the cancer cells or tissues by multifunctional nanocarriers may provide a new paradigm in cancer treatment. In this study, folate (FA) decorated nanostructured lipid carriers (NLCs) were constructed as nanomedicine for the delivery of curcumin (CUR).
Methods: CUR-loaded NLCs (CUR-NLCs) were prepared. FA containing polyethylene glycol (PEG)-distearoylphosphatidylethanolamine (DSPE) (FA-PEG-DSPE) was synthesized and used for the decoration of CUR-NLCs. Their particle size, zeta potential, and drug encapsulation efficiency (EE) were evaluated. In vitro cytotoxicity study FA decorated CUR-NLCs (FA-CUR-NLCs) was tested in MCF-7 human breast cancer cells (MCF-7 cells). In vivo anti-tumor efficacies of the carriers were evaluated on mice bearing breast cancer model.
Results: The optimum FA-CUR-NLCs formulations with the particle size of 127 nm and with a +13 mV surface charge. The growth of MCF-7 cells in vitro was obviously inhibited. FA-CUR-NLCs also displayed the best anti-tumor activity than other formulations in vivo.
Conclusion: The results demonstrated that FA-CUR-NLCs were efficient in selective delivery to cancer cells over-expressing FA receptors (FRs). Also FA-CUR-NLCs transfer CUR to the breast cancer cells, enhance the anti-tumor capacity. Thus, FA-CUR-NLCs could prove to be a superior nanomedicine to achieve tumor therapeutic efficacy.
Introduction
Breast cancer is one of the most common malignancies. It represents the most common malignancy in the female population worldwide, and its prevalence has increased during the last decades (Luu et al., Citation2013; Siegel et al., Citation2013). The first line chemotherapy treatment protocol for breast cancer is using anti-cancer drugs such as docetaxel, doxorubicin, paclitaxel, and so on. These drugs have been used as a single drug recipe or combination recipes of several drugs. Whereas, dose-dependent side effects and rising drug resistance of these drugs has severely limited the patient quality of life. Therefore, chemotherapy using a less toxic drug is important
Curcumin (CUR), a yellow pigment from the root of Curcuma longa Linn, is a natural inexpensive component derived from the Southeast Asian spice turmeric. CUR has been considered to have anti-oxidation; and anti-tumor effects containing cancers of breast (Yu et al., Citation2014), cervix (Saengkrit et al., Citation2014), lung (Lin et al., Citation2009), etc. Many mechanisms are involved in the biological activities of CUR including NF-κB, IκBα kinase, Akt, activator protein-1, mitogen-activated protein kinases (MAPK), cyclooxygenase-2 lipoxygenase, inducible nitric-oxide synthase, urinary plasminogen activator, tumor necrosis factor, chemokines, and cell cycle machinery have been suggested as the targets of CUR (Choi et al., Citation2008; Li et al., Citation2014). However, low bioavailability of CUR, which caused by its poor solubility and unstable at neutral and basic pH, limited in its clinical utility (Saengkrit et al., Citation2014). To enhance bioavailability and protect CUR from degradation, nanocarriers-based drug delivery systems containing CUR are considered as the promising approaches for cancer therapy (Mohanty et al., Citation2010).
It has been reported that lipid nanoparticles could improve the drug absorption and bioavailability due to their diameter and absorption enhancing effect of lipids (Hejri et al., Citation2013). Nanostructured-lipid carriers (NLCs) represent an improved generation of lipid nanoparticles produced by controlled mixing of solid lipids with spatially incompatible liquid lipids, leading to a specific nanostructure to accommodate drugs, and thus achieve higher loading capacity (Muller et al., Citation2002; Saupe et al., Citation2005).
Among the various types of ligands used for the active targeting, folate (FA) stands out to be a desirable choice for malignant tumors, since most solid tumor cells express a high level of folate receptors (FRs) on their surfaces while the levels of FRs are much lower in non-epithelial tumors and normal tissues (Xia et al., Citation2010; Tang et al., Citation2014). FA and FA-conjugates can bind to the FRs with high affinity and enter cells by receptor-mediated endocytosis, so the FA-modified drug delivery vectors can transfer the therapeutical agents to tumor cells highly expressing folate receptor (Yang et al., Citation2014). Thus, it is very necessary to explore a kind of vector containing favorable amount of FA ligands.
In the present investigation, FA containing polyethylene glycol (PEG) – distearoylphosphatidylethanolamine (DSPE) (FA-PEG-DSPE) was synthesized and used as the ligands to further improve the targeted anti-cancer activity. The FA-PEG-DSPE modified CUR-loaded NLCs (FA-CUR-NLCs) and examined in mice bearing MCF-7 cells or MCF-7 cells model. This system was expected to achieve stable drug-loading capacity, be recognized by FRs over MCF-7 cells and finally achieve attractive anti-cancer therapeutic effects.
Materials and methods
Materials, cells and animals
Curcumin (CUR) was purchased from Tokyo Chemical Industry (TCI) Co., Ltd. (Tokyo, Japan) Folate (FA), Triethylamine (TEA), stearic acid, polyoxyethylene bisamine (H2N-PEG-NH2, Mw 3350), soybean phosphatidylcholine (SPC), oleic acid, Tween-80, (3-[4,5-dimehyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Sigma Aldrich (St. Louis, MO). Distearoyl phosphatidyl-ethanolamine (DSPE) was purchased from Avanti Polar Lipids (Alabaster, AL). Precirol ATO-5 was generously provided by Gattefossé (Paramus, NJ). Injectable soya lecithin was obtained from Shanghai Taiwei Pharmaceutical Co, Ltd (Shanghai, China). All other chemicals were of analytical grade or higher.
MCF-7 cells were obtained from the American type culture collection (Manassas, VA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Fisher Chemicals, Fairlawn, NJ) and 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma, St. Louis, MO) in a 5% CO2 fully humidified atmosphere. All experiments were performed on cells in the logarithmic phase of growth.
BALB/c nude mice (4–6 weeks old, 18–22 g weight) were purchased from the Medical Animal Test Center of Shandong Province (Ji’nan, China), and were maintained under specific pathogen-free conditions.
Synthesis of FA-PEG-DSPE
FA-PEG-DSPE was synthesized as follows: Firstly, FA and H2N-PEG-NH2 (1.5 : 1, molar ratio) were dissolved in anhydrous dimethyl sulfoxide (DMSO). DCC, NHS, and TEA (1: 1: 3, molar ratio) were then sequentially added into the DMSO solution. The reaction mixture was stirred at 400 rpm at room temperature for 4 h in the dark. The supernatant was diluted in three volumes of 50 mM sodium carbonate (Na2CO3) and passed through a PD-10 desalting column to remove low-molecular weight by-products. FA-PEG-NH2 was then collected in the void volume and lyophilized. Secondly, DSPE dissolved in anhydrous chloroform was reacted with five equivalent of TEA for 8 h at RT and then stirred with FA-PEG-NH2 in chloroform solution for 12 h at RT to finally formed FA-PEG-DSPE. FA-PEG-DSPE: IR ν/cm−1: 3391(–NH–); 3103(–CH2–, –CH–); 1667(–NH–CO–, –CO–O–); 1462(–C6H5); 1348(–CO–O–). 1H NMR (DMSO-d6, 300 mHz), δ (ppm): 1.15–1.98(–CH3), 2.13(–NH2), 4.13–4.28(–CO–O–), 4.36(s, –NH–), 7.31–8.02(m, –C6H5, Benzene ring of FA), 11.96(s, –OH). The presence of –NH–CO– and –CO–O– peaks confirmed the formation of the FA-PEG-DSPE. The production rate of FA-PEG-DSPE was 76.5%.
Preparation of NLCs
FA-CUR-NLCs, CUR-NLCs, and blank NLCs were prepared by solvent diffusion method:
Lipid dispersion was composed of Precirol ATO-5, oleic acid, plus soybean phosphatidylcholine at a ratio of 1:1:1 (w/w/w). Injectable soya lecithin (50 mg), CUR (10 mg) were dissolved in 2 ml of dimethyl formamide (DMF) and added to the lipid dispersion by heating at the temperature of 80–85 °C to form the lipid phase. Aqueous phase was prepared by added Tween-80 (50 mg) in 10 ml of water. This aqueous solution was then stirred and heated to 30 °C.
The lipid phase was rapidly injected into the stirred aqueous phase (800 rpm) at 30 °C, and the resulting suspension was then continually stirred at 600 rpm to get the CUR-loaded NLCs (CUR-NLCs) suspension. DMF was removed by dialyzed method. The CUR-NLCs suspension was dissolved with Milli-Q water and then dialyzed against Milli-Q water for 16 h to get the final CUR-NLCs.
Blank NLCs were prepared using the same method without adding CUR.
FA-PEG-DSPE solution (10 ml) was added dropwise into 20 ml of CUR-NLCs that was stirred at 600 rpm at RT, and then stirred for 10 min to complete the modification. Subsequently, free FA-PEG-DSPE was removed from FA-CUR-NLCs by gel chromatography using a Sephadex® G-50 column (GE Healthcare Bio-Sciences, Pittsburgh, PA). The obtained complexes were filtered through a 0.45 μm membrane to obtain purified FA-CUR-NLCs.
Optimization of FA-CUR-NLCs
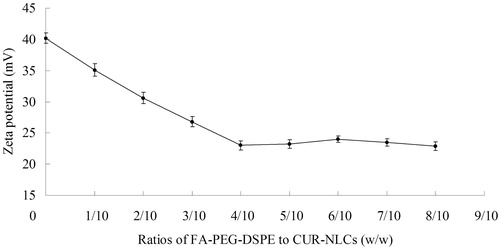
FA-PEG-DSPE was gradually decorated onto the surface of CUR-NLCs. During this process, the FA-PEG-DSPE ligands covered the surface charge of the carriers, cause the changes of the potential. The termination of the change of the potential means the end of modification. To optimize the suitable ratio (FA-PEG-DSPE to CUR-NLCs, w/w) of the modification, FA-CUR-NLCs with different weight ratios of FA-PEG-DSPE to CUR-NLCs were prepared. Zeta potentials of the obtained FA-CUR-NLCs were measured. The optimal FA-PEG-DSPE to CUR-NLCs weight ratio was confirmed by measuring the change in zeta potential and the decoration ratio of FA. The quantitation of decoration ratios of FA were determined by the HPLC method (Vahteristo et al., Citation1997).
Characterization of the NLCs
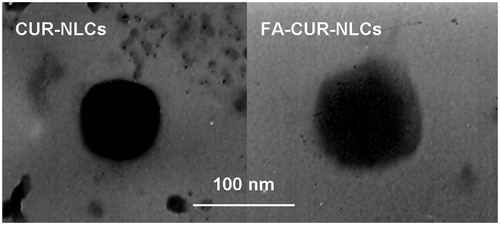
The morphology of the FA-CUR-NLCs and FA-CUR-NLCs was examined by transmission electron microscopy (TEM). Samples were prepared by placing one drop of suspension onto a copper grid and dried, followed by negative staining with one drop of a 3% aqueous solution of sodium phosphotungstate for contrast enhancement. The air-dried samples were then examined directly under the transmission electron microscope (Kong et al., Citation2012).
The mean particle size, size distribution and zeta potential of FA-CUR-NLCs, CUR-NLCs, and blank NLCs were determined by using the Malvern Zetasizer Nano ZS (Malvern Instrument Ltd., Worcestershire, UK). The average particle size was expressed as volume mean diameter.
The drug-entrapment efficiency (EE) and drug-loading efficiency (DL) of CUR were measured by absorbance using UV-Vis spectroscopy at 430 nm. Briefly, 5 ml NLCs were centrifuged (16 000 rpm and 4 °C for 30 min) separately, and the supernatants were then determined using the ICP-MS. The EE and DL were calculated as follows: EE (%) = Concentration of (total drug − free drug)/Concentration of total drug × 100. DL (%) = Concentration of (total drug − free drug)/(Concentration of drug loaded NLCs) × 100.
In vitro release study
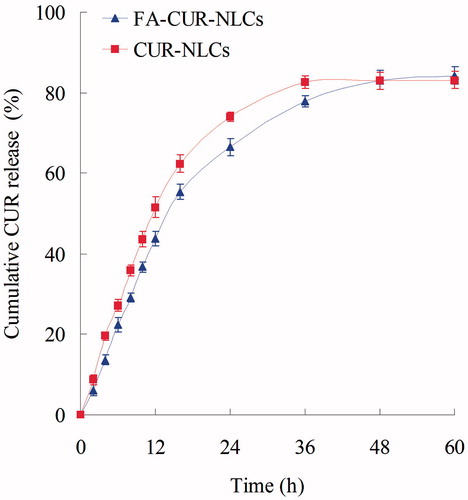
The in vitro release studies of FA-CUR-NLCs and CUR-NLCs were performed in PBS (pH 7.4) (Wang et al., Citation2012). Aliquots of complexes were suspended in 1 mL of PBS, in Eppendorf® tubes, and vortexed for 30 s. The tubes were then placed in a shaking water bath (37 °C, 100 rpm). Separate tubes were used for different data points. At predetermined time intervals, the suspensions were centrifuged (10 000 rpm, 30 min), and the amount CUR were measured by absorbance using UV-Vis spectroscopy mentioned in the “Characterization of the NLCs” section.
In vitro cytotoxicity study
The cytotoxicity of FA-CUR-NLCs and other formulations was tested in MCF-7 cells using the MTT assay. Briefly, cells were seeded in a 96-well plate at a density of 3000 cells/well and allowed to adhere for 24 h prior to the assay. Cells were exposed to various concentrations of 0.9% saline (the control group), CUR solution (CUR dissolved in DMSO), CUR-NLCs, CUR-NLCs, and FA-CUR-NLCs at various concentrations for 48 h at 37 °C and 5% CO2 atmosphere, respectively. Culture medium was used as the blank group. Then, MTT solution (5 mg/ml) was added to each well and cells were incubated for 4 h. 200 μl of DMSO was added to each well to dissolve the MTT formazan crystals. The optical density (OD) of formazan product was measured using a microplate reader (Model 680, BIO-RAD, Hercules, CA) at 570 nm. The relative cell viability (CV) was calculated by the below equation: CV (%) = (ODsample − ODblank)/(ODcontrol − ODblank) × 100. Cells without the addition of MTT reagents were used as a blank control. The drug concentration causing 50% inhibition (IC50) was calculated.
In vivo anti-tumor efficacy
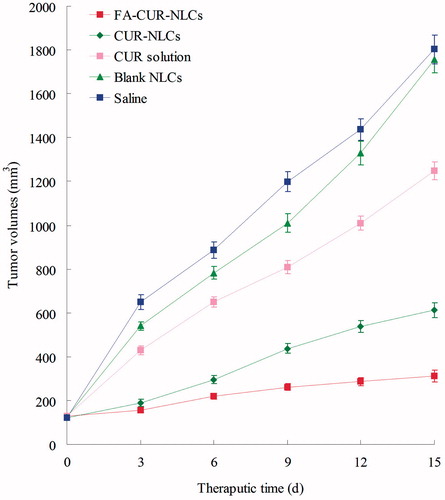
BALB/c nude mice were housed at a temperature of 25 ± 2 °C and a relative humidity of 70 ± 5% under natural light/dark conditions for one week before dosing. Then the mice were inoculating subcutaneously (s.c.) in the right armpit with MCF-7 cells suspended in PBS for 24 h for the preparation of breast cancer bearing animal models. Mice were then divided into six groups (aix mice per group). The FA-CUR-NLCs, CUR-NLCs, CUR-NLCs, free CUR solution, blank NLCs, and 0.9% sodium chloride solution were prepared and then injected intravenously into the mice via the tail vein (i.v.). The initial day of i.v. administration was defined as day 0, and administration was then repeated once every 3 days (d) over a 15-day therapeutic period. The body weights of mice and tumor sizes were also measured tumor growth was determined by caliper measurement every 3 d. The measurements were taken in two perpendicular dimensions and tumor volumes (mm3) were calculated by applying the formula (L × W2)/2, where L is the longest dimension and W is the dimension perpendicular to L (Sarisozen et al., Citation2014). The anti-tumor efficacy of each formulation was evaluated by tumor inhibition rate, and was calculated using the following formula (Yuan et al., Citation2013): Tumor inhibition rate (%) = (Wc − Wt)/Wc × 100. Wt and Wc represent the mean tumor weight of the treated and control groups, respectively. The body weights of mice and tumor sizes were also measured every 3 d.
Statistical analysis
All studies were repeated three times and all measurements were carried out in triplicate. Quantitative data were presented as means ± standard deviation (SD). Statistical significance was analyzed using the Student’s t-test or one-way ANOVA. Values were considered statistically significant at p < 0.05.
Results and discussion
Determination of FA decoration
Since breast cancer cells express a high level of FRs on their surfaces, FA-modified drug delivery vectors can efficiently transfer the therapeutic agents to MCF-7 cells. FA-PEG-DSPE was synthesized and used as the ligands to modify the NLCs. FA-PEG-DSPE solution was added dropwise into CUR-NLCs, during this process, FA-PEG-DSPE was gradually decorated onto the surface of CUR-NLCs, neutralized the surface charge of the carriers, and caused the changes of the potential. The termination of the change of the potential means the end of modification.
FA-CUR-NLCs with different weight ratios of FA-PEG-DSPE to CUR-NLCs were prepared and the zeta potentials were illustrated in . The zeta potential was decreased gradually until the ratio of 2/5 (w/w). The decoration ratio of FA was also increased to 36.2 μg/mg between 1/5 and 2/5 (L/C, w/w). When L/C was up to 2/5, the decoration ratio was nearly constant (). Therefore, the optimum ligand to carrier ratio of FA-CUR-NLCs was obtained at 2/5 (w/w). This ratio was fixed and used for the preparation of FA-CUR-NLCs.
Table 1. Quantification of decoration ratios of FA to NLCs’ surface.
Characterization of FA-CUR-NLCs
TEM images of the vectors are shown in . The mean particle size, size distribution, zeta potential, and EE of FA-CUR-NLCs, CUR-NLCs, and blank NLCs are characterized and summarized in . The FA-CUR-NLCs has a size of 127 nm, with a potential of +13 mV. The EE CUR in FA-CUR-NLCs and CUR-NLCs was 83 and 84%, respectively. It is reported that the positive surface charge and proper particle size of nanocarriers were important for efficient drug delivery, so it was expected that FA-CUR-NLCs with the size of around 127 nm and +13 mV could obtain for efficient cellular endocytosis (Bothun et al., Citation2011). No significance variations of EE were observed in FA-CUR-NLCs and CUR-NLCs (), suggesting a negligible amount of CUR was leaked during the process of modifying FA-PEG-DSPE to the surface of CUR-NLCs, and the modified vectors are stable.
Table 2. Characterization of different NLCs.
In vitro release study
The in vitro release profiles are illustrated in . Both FA-CUR-NLCs and CUR-NLCs have sustained release behavior. The release of CUR-NLCs was faster than FA-CUR-NLCs. The complete release of the FA-CUR-NLCs was 48 h, 12 h later than CUR-NLCs. These may indicate that the FA coating retained the drugs inside the carriers for longer time. Could this help with the anti-tumor effect of the CUR? The following in vitro and in vivo studies will give the answer.
In vitro cytotoxicity
shows the cytotoxicity of FA-CUR-NLCs and other vectors in MCF-7 cells. The IC50 values for the NLCs formulations were about three times dose advantage over free drug solutions (p < 0.05). The IC50 value of FA-CUR-NLCs was over 3.5-fold over CUR-NLCs in reducing viability of breast cancer cells, accounting for the highest anti-tumor activity. This could be explained by the active targeting ability of FA which could cell internalization through specific FRs on the surface of MCF-7 cells (Wu et al., Citation2014). The results also indicated that the enhanced permeability and retention (EPR) effect could appeared effect in highly fenestrated tumor endothelial cells, uptake of NLCs by MCF-7 cells might be enhanced by across the cell membrane via endocytosis or direct penetration may enhance intracellular drug accumulation (Guo et al., Citation2014).
Table 3. IC50 of formulations to MCF-7 cells.
In vivo anti-cancer therapy and systemic toxicity
The in vivo anti-tumor efficiency was evaluated in MCF-7 solid tumors in mice. As shown in , although tumor growth was suppressed to some extent after administration of free-drug solution, while in contrast, tumor growth was significantly inhibited when NLCs formulations were administered intravenously. The most obviously tumor regressions were observed in the FA-CUR-NLCs group, the tumor growth was prominently inhibited (p < 0.05), which attained only 312 mm3 on 15 d, while in saline treated group, tumor volume grew rapidly to 1803 mm3 during 15-day therapeutic period. The tumor inhibition rates of tumor-bearing mice treated with FA-CUR-NLCs, CUR-NLCs and CUR solution were 83, 66, and 31% compared with control.
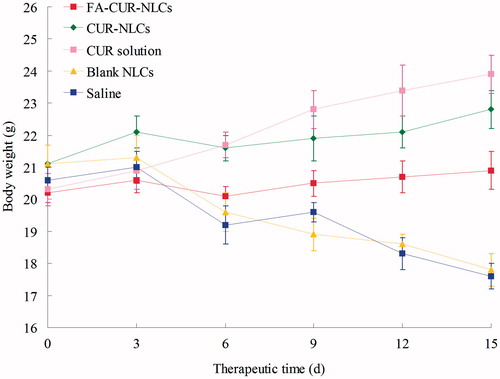
Systemic toxicity is a key factor during the cancer therapy. Body weight loss is an indicator of systemic toxicity. The obviously body weight loss could be observed in the free drug solutions, and the control groups (), denoting systemic toxicity of the two anti-cancer drugs. The body weights of NLCs groups slight increased, which might due to the lower systemic toxicity and the tumor growth. Comparatively, FA-CUR-NLCs did not lead to significant body weight loss and increase, demonstrating the reduced systemic toxicity, and effective tumor inhibition.
Conclusion
To deliver drugs efficiently to breast cancer cells, FA modified CUR-loaded NLCs were fabricated. The well-formed FA-CUR-NLCs formulation exhibited small particle size, reasonable positive charges, and high cancer cell inhibition capacity in vitro. FA mediated targeting provided clear advantages and significantly increased the in vivo anti-tumor efficacy. The effect of FA targeting and delivery ability of this NLCs formulation might be an effective tumor therapy strategy for treatment in breast carcinoma.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bothun GD, Lelis A, Chen Y, et al. (2011). Multicomponent folate-targeted magnetoliposomes: design, characterization, and cellular uptake. Nanomedicine 7:797–805
- Choi BH, Kim CG, Lim Y, et al. (2008). Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett 259:111–18
- Guo S, Wang Y, Miao L, et al. (2014). Turning a water and oil insoluble cisplatin derivative into a nanoparticle formulation for cancer therapy. Biomaterials 35:7647–53
- Hejri A, Khosravi A, Gharanjig K, et al. (2013). Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem 141:117–23
- Kong F, Zhou F, Ge L, et al. (2012). Mannosylated liposomes for targeted gene delivery. Int J Nanomedicine 7:1079–89
- Li Y, Zhang T. (2014). Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett 346:197–205
- Lin SS, Lai KC, Hsu SC, et al. (2009). Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 285:127–33
- Luu HN, Dahlstrom KR, Mullen PD, et al. (2013). Comparison of the accuracy of Hybrid Capture II and polymerase chain reaction in detecting clinically important cervical dysplasia: a systematic review and meta-analysis. Cancer Med 2:367–90
- Mohanty C, Sahoo SK. (2010). The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 31:6597–611
- Muller RH, Radtke M, Wissing SA. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:S131–55
- Saengkrit N, Saesoo S, Srinuanchai W, et al. (2014). Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B 114:349–56
- Sarisozen C, Abouzeid AH, Torchilin VP. (2014). The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm 88:539–50
- Saupe A, Wissing SA, Lenk A, et al. (2005). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) – structural investigations on two different carrier systems. Bio-Med Mater Eng 15:393–402
- Siegel R, Naishadham D, Jemal A. (2013). Cancer statistics, 2013. CA Cancer J Clin 63:11–30
- Tang Z, Li D, Sun H, et al. (2014). Quantitative control of active targeting of nanocarriers to tumor cells through optimization of folate ligand density. Biomaterials 35:8015–27
- Vahteristo L, Lehikoinen K, Ollilainen V, et al. (1997). Application of an HPLC assay for the determination of folate derivatives in some vegetables, fruits and berries consumed in Finland. Food Chem 59:589–97
- Wang W, Zhou F, Ge L, et al. (2012). Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid carrier mediated gene delivery system for tumor-targeted transfection. Int J Nanomedicine 7:2513–22
- Wu W, Wang J, Lin Z, et al. (2014). Tumor-acidity activated surface charge-conversion of polymeric nanocarriers for enhanced cell adhesion and targeted drug release. Macromol Rapid Commun 35:1679–84
- Xia W, Low PS. (2010). Folate-targeted therapies for cancer. J Med Chem 53:6811–24
- Yang C, Chen H, Zhao J, et al. (2014). Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloids Surf B Biointerfaces 121:206–13
- Yu Y, Zhang X, Qiu L. (2014). The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(β-amino ester) derivates. Biomaterials 35:3467–79
- Yuan L, Liu C, Chen Y. (2013). Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int J Nanomedicine 8:4339–50