Abstract
From the early sixteenth and seventeenth centuries to the present day of life, tuberculosis (TB) still is a global health threat with some new emergence of resistance. This type of emergence poses a vital challenge to control TB cases across the world. Mortality and morbidity rates are high due to this new face of TB. The newer nanotechnology-based drug-delivery approaches involving micro-metric and nano-metric carriers are much needed at this stage. These delivery systems would provide more advantages over conventional systems of treatment by producing enhanced therapeutic efficacy, uniform distribution of drug molecule to the target site, sustained and controlled release of drug molecules and lesser side effects. The main aim to develop these novel drug-delivery systems is to improve the patient compliance and reduce therapy time. This article reviews and elaborates the new concepts and drug-delivery approaches for the treatment of TB involving solid-lipid particulate drug-delivery systems (solid-lipid micro- and nanoparticles, nanostructured lipid carriers), vesicular drug-delivery systems (liposomes, niosomes and liposphere), emulsion-based drug-delivery systems (micro and nanoemulsion) and some other novel drug-delivery systems for the effective treatment of tuberculosis and role of immunomodulators as an adjuvant therapy for management of MDR-TB and XDR-TB.
Introduction
Tuberculosis (TB) is a major cause for billions of active TB cases and deaths across world. However, there are effective and cheap treatments are available by the government and non-Government health care authorities (Meena, Citation2010). Over five decades of various TB treatment programs, still the potentially effective drugs for TB have failed to reduce the prevalence of the disease. TB is mainly caused by rod shaped microorganism Mycobacterium tuberculosis. TB is an airborne infection which is a major cause of mortality and morbidity. Disease mainly affects the peoples in both developing as well as in developed countries (Zumla et al., Citation2013). Mostly, patients having active pulmonary TB may have mild/excessive coughing, fever, fatigue, loss of appetite, weight loss, night sweats, bloody sputum (Jeong & Lee, Citation2008). It is estimated that more than 200 million peoples will be affected by this disease and that expected 35 million peoples will die due to disease in this decade if effective control and preventive measures are not taken by the concerns authorities (Meena, Citation2010). Further the emergence of human immunodeficiency virus (HIV) and multidrug resistant (MDR)/extremely drug resistant (XDR) TB poses a major challenge to the control it across the world (Sarkar & Suresh, Citation2011). There are several modes of transmission of causative agents from infected persons to un-infected persons. In general, infection spreads by forming droplets by an infected person during coughing, sneezing, speaking may transmit to the air. TB is generally classified as latent TB or active TB. The latent phase of TB in which bacterium is present inside human body as inactive form without showing any kind of symptoms even shows negative result on a Mantoux tuberculin test. In the active phase, the host’s immune system becomes weakens and the bacterium becomes active and cause some secondary complications (Gupta et al., Citation2015).
Pathogenesis of tuberculosis
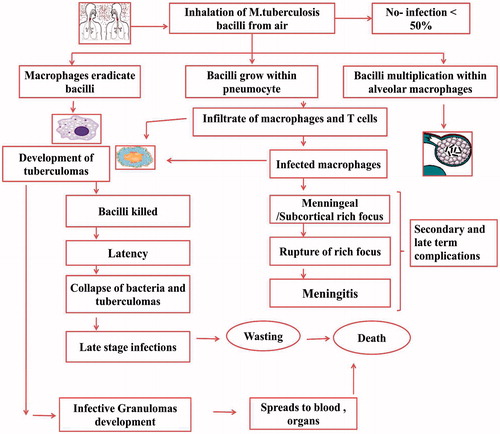
Mycobacterium tuberculosis is anaerobic, non-spore-forming, non-motile rod shaped bacteria which is highly resistant to alcohol and acid. Its mode of transmission is mainly from one infected person to another person via droplet nuclei in air due to coughing. When this mycobacterial agent reaches to the alveolar region in lungs, it invades and replicates within alveolar macrophages. Alveolar macrophages immediately take them, where they undergo phagocytosis. Further mycobacteria interacts with T lymphocytes which results in the formation of differentiated macrophages into the histocytes and epithelioid (Chaudhary & Garg, Citation2015). These epithelioid histocytes and lymphocytes aggregates into different small clusters and form granulomas. In the granulomas, CD4 cells T Lymphocytes which is also called as effector T cell, secretes different kinds of cytokines such as interferon-γ which further activates the macrophages in order to destroy the bacteria with which they are infected previously. CD8 T-Lymphocytes (cytotoxic T cells) also directly kills the infected cells (Jeong & Lee, Citation2008). Pathogenesis of tuberculosis infection is shown in .
Drugs for treatment of tuberculosis
Treatment of active TB is very complex due to the emergence of multidrug-resistant (MDR-TB) and extremely drug-resistant (XDR-TB). There are only 10 drugs approved by the US Food and Drug Administration for TB treatment. In India, available TB treatment is directly observed therapy (DOTS) which comprises the administration of four oral antibiotics for the period of 6 months or more. TB chemotherapy initially started with the discovery of streptomycin in 1944 only a very small fraction of anti-tubercular drugs reaches the alveoli which are the desired target of drug action. Some other drugs used in TB treatment include clofazimine (having direct anti-tubercular and immune-suppressive properties), amoxicillin (which inhibits the synthesis of the cell wall), rifabutin (which inhibits the synthesis of bacterial RNA) and long-acting rifamycins and inhaled interferon-gamma, clarithromycin (which inhibits the synthesis of proteins). Major drugs used for TB treatment is listed in .
Table 1. Classification of anti-TB drugs and their mechanism of action.
Nanotechnology-based drug-delivery systems in tuberculosis
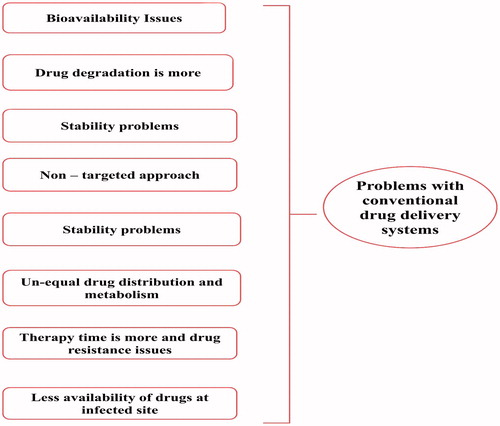
Nanotechnology-based drug-delivery systems are mainly consist of different formulations, each of them contains different functional and structural properties (Garg et al., Citation2015b). They are able to modify easily by varying or changing the composition of drug, polymers, excipients and other additives used in their preparation in order to reduce the problems associated with conventional drug-delivery systems (Garg et al., Citation2015c) which are shown in .
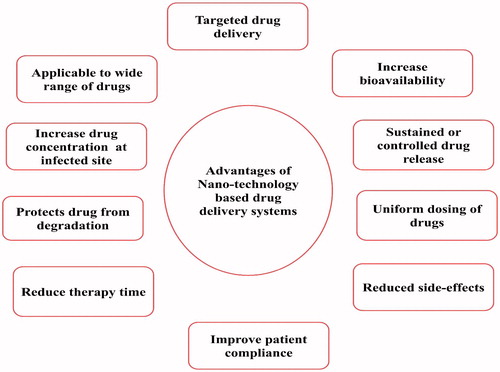
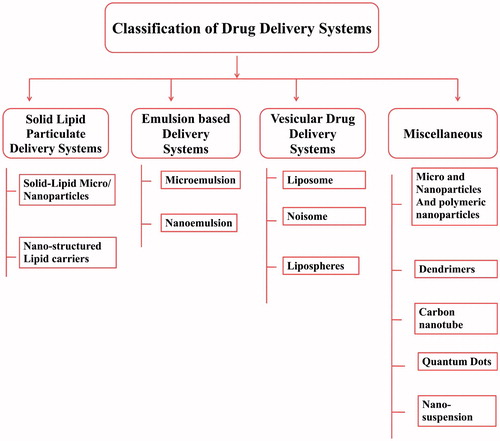
As the use of nanosized drug-delivery systems is widely accepted and commercially applicable (Garg et al., Citation2015e). In general, most of the drug-delivery systems are used as drug carrier systems due to their high stability, high carrier-loading capacity and ability to incorporate both lipophilic/hydrophilic drug substances and due to feasibility of administration through different kinds of routes like oral, topical, parenteral and pulmonary routes (Garg et al., Citation2015a; Kaur et al., Citation2015a). The advantages of nanotechnology-based system are shown in . They can also be designed for modified drug release from different carriers systems (Malik et al., Citation2015). Nanotechnology-based drug-delivery systems are broadly classified into four groups including the solid-lipid particulate dosage forms, emulsion-based systems, solid-lipid tablets and vesicular systems (Salome & Ikechukwu, Citation2013).
These formulations provide increased drug solubilization for water-insoluble drugs (Chaudhary et al., Citation2015b). Further the drug absorption is observed to be better than other conventional systems of drug delivery which might be due to the property of wetting of the hydrophobic drug particles in the presence of polymer matrix and presence of surfactants (Chaudhary et al., Citation2015a). Therefore, these drug-delivery systems represent diverse group of formulations comprising several classes of excipients for example triglyceride oils, mixed glycerides, surfactants like lipophilic surfactants, hydrophilic surfactants and water-soluble co-solvents with different kinds of properties (Salome & Ikechukwu, Citation2013). The nanotechnology-based drug-delivery systems are shown in .
Solid–lipid particulate dosage forms
Solid–lipid micro and nanoparticles
In general, the colloidal particles in 10–1000 nm size range are commonly referred as nanoparticles (Momoh et al., Citation2013; Garg et al., Citation2014b). The successful use of nanoparticles in different drug-delivery systems depends on their different physicochemical properties and their ability to cross through several anatomical barriers inside body (Rohilla et al., Citation2014b; Pandey et al., Citation2015). They are effective in sustained drug release of entrapped or encapsulated drug contents (Pardeshi et al., Citation2013; Chaudhary et al., Citation2014). Solid-lipid micro/nanoparticles often shows interesting features concerning about therapeutic purposes (Jawahar et al., Citation2012; Morie et al., Citation2014). Solid-lipid nanoparticles were introduced in the early 1990s as an alternative drug-delivery system to other available traditional colloidal carrier systems such as liposomes, niosomes, emulsions and polymeric nanoparticulates (Sharma et al., Citation2014a; Weber et al., Citation2014). The hopeful strategy to overcome various problems with conventional drug-delivery system is to develop suitable drug carrier system like solid-lipid nanoparticles (Pallerla & Prabhakar, Citation2013). Solid-lipid micro/nanoparticles are aqueous colloidal dispersions comprises of melted biodegradable lipids in water or in aqueous solution of surfactant and size ranging between 50 and 1000 nm (Jeong & Lee, Citation2008; Singh et al., Citation2014a). Solid-lipid nanoparticles is an emerging field of nanotechnology with potential applications in various fields such as in effective drug delivery (Singh et al., Citation2014b), clinical medicines (Singh et al., Citation2012), cosmeceuticals (Garg et al., Citation2012b) and as well as in research (Wang & Wu, Citation2006; Garud et al., Citation2012). Solid-lipid nanoparticles containing lipid concentrations up to 2.5% do not show any cytotoxic effects in vitro (Jawahar et al., Citation2012). Commonly used lipids are triglycerides, fatty acids, partial glycerides, fats and waxes (Guo et al., Citation2012). The biggest advantages of solid-lipid nanoparticles is the use of lipids in the preparation are generally physiological lipids which have the less chances of acute/chronic toxicities (Weber et al., Citation2014). Lipophilic drugs are better suited for solid-lipid nanoparticles (Garud et al., Citation2012). Further, the use of solid-lipid instead of liquid-lipid in their preparation is more beneficial in order to control the release kinetics of encapsulated drug molecules and to improve the stability of active ingredients (Garg et al., Citation2013; Weber et al., Citation2014). SLNs mainly possess a central solid–lipid core matrix that can solubilize lipophilic molecules stabilized by use of different surfactants (Acevedo-Morantes et al., Citation2013). The melting point of lipids used must exceed body temperature (Alam et al., Citation2013). Biological membrane lipids used in solid-lipid micro/nanoparticles are lecithin or soya lecithin, biocompatible non-Ionics lipids such as ethylene oxide or propylene oxide copolymers (Swathi et al., Citation2012; Goyal et al., Citation2013).
Various advantages of solid-lipid nanoparticles are that they are effective in drug-targeting purposes (Seyfoddin et al., Citation2010). It have high drug stability (Johal et al., Citation2014), high drug-loading efficiency or high percentage encapsulation efficiency (Joshi et al., Citation2014a), ability to encapsulate both lipophilic and hydrophilic drug substances (Kaur et al., Citation2014c), less or no use of organic solvents (Liu et al., Citation2009), increased bioavailability of entrapped bioactive compounds (Goyal et al., Citation2014a), less biotoxicity of carrier system and better protection of incorporated labile compounds (Dong et al., Citation2011). Pandey et al. (Citation2005) developed SLNs, which was prepared with an encapsulation efficiency for rifampicin (51%), pyrazinamide (41%) and (45%) for isoniazid, after a single oral administration to mice. Therapeutic drug concentrations were maintained in the plasma for 8 days and in the different organs lungs, liver and spleen for 10 days where as the free drug solution were found cleared in 1–2 days (Pandey et al., Citation2005). Different examples of solid-lipid micro/nanoparticles in tuberculosis treatment are shown in .
Table 2. Examples of solid-lipid micro and nanoparticles in tuberculosis treatment.
Nanostructured lipid carrier
The colloidal drug carrier system not only offers many advantages in the case of targeted drug delivery (Luan et al., Citation2013) but also increase bioavailability of some poorly soluble drugs and protection for sensitive active compounds (Wang & Xia, Citation2014). As nanostructured lipid carriers (NLCs) were developed in the midlines of the 1990s as an alternative carrier system to the existing traditional carriers (Kamble et al., Citation2012). NLCs are the second generation of SLNs (Wang et al., Citation2013). In order to obtain the blends of particles, matrix mainly solid lipids are mixed with melted lipids in a ratio of 70:30 (Jia et al., Citation2010). They can also incorporate both lipophilic and hydrophilic drugs under the optimized conditions (Zheng et al., Citation2012). NLCs can be produced by high-pressure homogenization or any other modified techniques to yield more lipid particulate dispersions with solid contents 30–80% (Patidar et al., Citation2010). They can be administered via different routes such as oral, ocular, pulmonary and intravenous route (Parhi & Suresh, Citation2012). NLCs mainly accommodates drug due to their highly unordered lipid structures (Kamble et al., Citation2012). Song et al. (Citation2015) developed nanostructured lipid carrier to encapsulate rifampicin. The particle size was found around 160 nm. The rifampicin was found to be entrapped above 90% within the system and high uptake was observed in NR8383 cells and alveolar macrophages (Song et al., Citation2015).
Emulsion-based drug-delivery systems
Microemulsion
Microemulsions are mainly clear, transparent, thermodynamically stable, isotropic dispersions composed of an aqueous component and oily component (Nikumbh et al., Citation2015). Microemulsion also contains emulsifying agents, surfactant or co-surfactant mixtures (Kaur et al., Citation2014b). Microemulsion improves drug solubilization, absorption and permeability (Jain et al., Citation2009; Mostafa et al., Citation2014). It also provides protection against enzymatic hydrolysis (Hu et al., Citation2011). The selection of suitable surfactant combinations is a critical parameter in order to make thermodynamically stable emulsion (Wang et al., Citation2009). Establishment of a phase diagram is also necessary for the selection of multiple components in microemulsions (Narang et al., Citation2007). Agatonovic-Kustrin et al. (Citation2003) formulated a colloidal dosage form for oral delivery of isoniazid and rifampicin using artificial neural network (ANN) data modeling, which helps to understand the basic process of microemulsion formation and their stability within pseudo-ternary and ternary colloidal systems. The solubility behavior of two combined drugs and stability can be predicted based on the separation of two drugs into oil and water phases depending upon their solubility (Agatonovic-Kustrin et al., Citation2003).
Nanoemulsion
As emulsion is a biphasic dosage form with wider range of applications (Mahajan et al., Citation2014). In nanoemulsion, one phase is usually dispersed in another phase in which it is usually immiscible (Devarajan & Ravichandran, Citation2011). Nanoemulsion can be prepared by different methods, but the homogenization method is most commonly adopted (Kotta et al., Citation2015). In general, the term “nanoemulsion” is a system which is thermodynamically stable and isotropically clear dispersion of two immiscible liquids such as water and oil which are usually stabilized by use of surfactant molecules in different proportion (Thakur et al., Citation2013). In the last few decades, these o/w nanoemulsions found enormous application in various fields like healthcare, cosmetics, foods, agrochemicals, pharmaceuticals and biotechnology (Setya et al., Citation2013). Nanoemulsions can be defined as emulsion with mean droplet diameters size ranging from 50 to 1000 nm (Bhatt & Madhav, Citation2011). Nanoemulsions contains oil phase, aqueous phase, emulsifying agent and active pharmaceutical ingredients such as drugs or diagnostic agents (Abolmaali et al., Citation2011). The surfactants and co-surfactants helps to form a thermodynamically as well as kinetically stable nanoemulsion (Kumar & Singh, Citation2012). Nanoemulsion can be prepared readily and spontaneously without high-energy inputs. In general, emulsions are cloudy but the nanoemulsions have translucent and clear appearance. Nikonenko et al. (Citation2014) developed phospholipid-based nanoemulsion system of SQ-641-NE which was found to be active against Mycobacterium tuberculosis. Different examples of anti-tubercular drug loaded emulsion systems are given in .
Table 3. Examples of anti-tubercular drug loaded emulsion systems.
Vesicular drug-delivery systems
Liposome
Liposomes are the concentric bilayered vesicle in which an aqueous volume is enclosed by lipid bilayer membrane (Mansoori et al., Citation2012). Liposomes were discovered in an accidental discovery by Dr Alec. D. Banghamam, a British hematologist in 1961. When he dispersed phosphatidyl choline in water, it forms a closed bilayer-type structure which contains an aqueous phase inside which was entrapped by outer lipid bilayer (Thulasiramaraju et al., Citation2012). The name liposome is derived from two Greek words Lipos meaning fat and Soma meaning body (Mansoori et al., Citation2012). Liposome can be defined as “simple microscopic vesicles in which an aqueous volume is entirely enclosed by a membrane composed of lipid molecule” (Anwekar et al., Citation2011). They are able to encapsulate both hydrophilic and lipophilic drug substances (Utreja et al., Citation2011). It can also be used as a non-toxic vehicle to encapsulate insoluble drugs also (Patel & Panda, Citation2012). The size range is usually in 0.05–5.0 μm in diameter (Mansoori et al., Citation2012). They can be formulated and processed in different size, composition, charge and lamellarity (Patel & Panda, Citation2012). The wide range of applicability of liposomes makes them more advantageous (Garg, Citation2014). Liposome act as a vehicle to deliver the active therapeutic substances like proteins, peptide, antifungal, immunomodulators, diagnostics, enzymes, vaccines, cancer, ophthalmic, genetic material etc. (El-Badry et al., Citation2014). Liposomes are also effective in loading of water-soluble drugs in central aqueous compartment and lipid soluble agents in outer membrane (Paliwal et al., Citation2015). Due to the advancements in the methods of preparing and formulating liposomes, high-entrapment efficiencies can be made possible to incorporate different types of drugs into them (Kulkarni et al., Citation2011). Based on the structure, they are classified as unilamellar vesicles (ULVs) and multi-lamellar vesicles (MLVs). There are various advantages of liposomal system as a drug-delivery system such as they are biocompatible, biodegradable, non-toxic, flexible and non-immunogenic for systemic and non-systemic administrations, acts as a reservoir of drug and therapeutic index of drug may increase and modulates the distribution of drugs (Nasr et al., Citation2013). Basic component of liposomes are phospholipids which include natural phospholipids like phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol, synthetic phospholipids such as di-palmitoyl-phosphotidyl-choline, di-stearoylphosphotidylcholine, di-palmitoylphosphotidylglycerol, di-palmitoylphosphotidylserine, 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) are mainly used in liposomes. Di-oleaylphosphotidylcholine is also used as unsaturated lipidic phase (Rahman et al., Citation2012). Sphingolipids like Sphingomyelin and glycosphingolipids like gangliosides can also be employed as lipidic phase. Liposomes are mainly taken up by the phagocytic cells following the mechanisms like surface adsorption upon cell membrane, vesicular internalization, lysosomal fusion with endocytic vesicles and enzymatic degradation by lysosomal enzymes. After degradation, they release the encapsulated drug within phagocytic cells (Rojanarat et al., Citation2012). Liposomes are effective against intracellular pathogens as they can reach deep in lungs. Uptake and recycling of liposomal phospholipids occurs through the alveolar type II cells. Liposomes avoid decomposition of the entrapped drug molecules and release the entrapped drug molecules at designated targets in a controlled manner (Akbarzadeh et al., Citation2013). Liposomes are already developed for controlled and sustained delivery of various anti-TB drugs, proteins and peptides. Different examples of liposomal-based system in tuberculosis are given in .
Table 4. Examples of liposomal-based preparations for tuberculosis.
Niosome
Among all types of carrier systems liposome and niosomes are very well documented in controlled drug-delivery system (Mujoriya et al., Citation2011). Niosomes have potential as a carrier system for the delivery of different types of drugs, antigens and hormones (Gopalkrishnan & Chenthilnathan, Citation2012). The first report or idea on the use of certain non-ionic surfactants vesicles was from the cosmeceuticals prepared by L’oreal (Chandu et al., Citation2012). They are usually bi-layered structures which are able to encapsulate both hydrophilic and lipophilic drug molecules either in aqueous layer or in lipidic vesicular membrane (Abdelkader et al., Citation2014). In niosomes, non-ionic surfactant such as span-60, span-80 can be used because they can be stabilized by cholesterol and some other lipid addition (Madhav & Saini, Citation2011). They behave as liposomes as they help to prolong the blood circulation of entrapped drug moieties/molecules (Mullaicharam & Murthy, Citation2004). The materials used to prepare niosomes are cheaper and make them more stable, thus niosomes have more advantages over the liposomes (Diljyot, Citation2012). Usually, the niosomes are microscopic in size (Gandhi et al., Citation2014). Niosomes also provide therapeutic activity in a well-controlled manner for a definite period of time (PravinaGurjar & Chouksey, Citation2014). They are also chosen over liposomes due to high-stability and more economical (Madhav & Saini, Citation2011) characteristics. Niosomes are now favorably used for controlled and targeted drug delivery, as the “Paul Elrich” in 1909, gives the concept of Drug-targeting and nanotechnology (Sankhyan & Pawar, Citation2012). Niosomes also helps to overcome the problems associated with liposomal system such as susceptibility to oxidation, high cost and difficulty in procuring high quality/purity levels. In noisome, hydrophobic drug molecules can be encapsulated inside surfactant bilayer and hydrophilic drug molecules inside the lipidic vesicle (PravinaGurjar & Chouksey, Citation2014). Therefore, niosomes are a promising carrier system for drug delivery being non-ionic, less toxic and it also improves the therapeutic index of drug molecules by restricting its action only towards target cells and tissues (Mullaicharam & Murthy, Citation2004). The advantages of niosomes as a drug-delivery system are like the surfactants used in niosomes are generally biodegradable, biocompatible and non-immunogenic, osmotically more active and stable, increase the oral bioavailability of drugs, improves the therapeutic performance of the drug molecules by delayed its clearance from the circulation or organs, no special storage conditions required, can be administered by oral, parenteral as well as topical routes and they are cost effective as compared to liposomes (Sharma et al., Citation2012). Basic components of niosomes are same as used in liposomes except the use of non-ionic surfactants. Different niosomal systems for incorporating anti-tubercular drugs are listed in .
Table 5. Examples of niosomes preparations in tuberculosis.
Lipospheres
Lipospheres formulation is a aqueous microdispersion of water-insoluble solid spherical particles with size range between 0.01 and 100 μm in diameter (Elgart et al., Citation2012). The lipospheres or lipidic microspheres are generally made up of solid hydrophobic triglycerides with outer monolayer of phospholipids embedded on the surface (Islan & Castro, Citation2014). Liposphere formulation is better suited for oral, parenteral and topical drug-delivery system (Barakat & Yassin, Citation2006; Rawat et al., Citation2008). The solid core in liposphere is generally used to dissolve or dispersed the drug molecules in a solid fat matrix. Several techniques such as solvent evaporation, hot and cold homogenization and high-pressure homogenization have been used for the production of liposphere (Elgart et al., Citation2012). Takenaga et al. (Citation2008) developed lipid microsphere to encapsulate rifampicin. The particle size was found around 247 nm and their size remained stable on different temperature (storage conditions) such as at 4 or 25 °C for at least 4 weeks period (Takenaga et al., Citation2008).
Miscellaneous lipid-based drug-delivery systems
Dendrimer
Dendrimers are emerging class of polymeric materials which are highly branched, mono-disperse macromolecules. The dendrimer systems have been found impactive on the physical and chemical properties of drug substances (Jana et al., Citation2012). Due to the multivalent and mono-disperse nature of dendrimers, they are emerging field of chemistry and biology especially in drug delivery, gene therapy and chemotherapy (Doshi, Citation2011). Dendrimer chemistry was first introduced in by Fritz Vogtle in 1978 (Jana et al., Citation2012). The word dendrimer derived from Greek word Dendron means tree and meros meaning part, so they are highly branched polymeric system. In general, dendrimers are synthetic nanomaterials that lie between 2 and 10 nm in diameter with defined molecular weights. Large number of functional groups can be attached on the external surface of dendrimers (Doshi, Citation2011). Dendrimers are known to have well-established entrapment properties. The dendrimer surface contains many different sites to which drug substances, ligands can be attached as well as materials such as polyethylene glycol (PEG) etc. (Mutalik et al., Citation2014). These kinds of attachments are used to modify the structure of dendrimers so that it can interact with the target receptors (Kaur et al., Citation2015b). Dendrimers generally have large interior void space which may be used to encapsulate or incorporate small drug molecules which help to reduce their toxicity and facilitate its controlled or targeted drug release (Thomas et al., Citation2008). Dendrimer solution has lower viscosity than the linear polymers (Kumar et al., Citation2015). The presence of many chain-ends or functional groups in dendrimers is responsible for high solubility, reactivity and miscibility (Sharma et al., Citation2015). The solubility of dendrimers can also influenced by the nature of groups attached on surface (Jana et al., Citation2012).
Dendrimers are significant as they have unique structural features (Garg et al., Citation2015d). Large numbers of functional groups are available at the surface of dendrimers for drug substance and ligand attachment which makes them more specific in targeting (Kaur et al., Citation2015). As the generations of dendrimer increases, the more functional groups can be attached at the branching sites (Thomas et al., Citation2008). shows the use of dendrimer-based anti-tubercular preparations.
Table 6. Dendrimer-based anti-tuberculosis preparations.
Nanoparticles/microparticles/polymeric nanoparticles/microspheres
Polymeric nanoparticles are well known to have good biocompatible/biodegradable features (Gagandeep et al., Citation2014; Kaur et al., Citation2014e). They are more sustainable and suitable candidates as drug-delivery carriers (Li et al., Citation2009; Banyal et al., Citation2013). Generally, the polymeric nanoparticles can be made structurally more stable by selecting different kinds of polymers, different surfactants and organic solvents (Cekié et al., Citation2007; Ohashi et al., Citation2009; Kaur et al., Citation2014f). They can be easily synthesized with various desirable properties like drug release, particle size/shape and zeta potential (Verma et al., Citation2008; Garg et al., Citation2014c). Polymeric nanoparticles usually have different active/functional groups depending upon polymers used and they can easily transform themselves according to either structural moiety of drugs or ligands attached (Shi et al., Citation2015). Therefore, they are more suitable as drug-targeting candidate (Banyal et al., Citation2013). Further, the lectin or guar gum, PLGA etc. conjugated nanoparticles are able to improve mucoadhesion and biorecognition on bacterial cell wall (Kaur et al., Citation2014c). They also help to induce prolonged half-life (Singh & Pai, Citation2014). Due to the size versatility of the nanoparticles, it has advantages over conventional techniques in the case of effective drug delivery (Smith, Citation2011). There are wide varieties of polymers available which can be used in tuberculosis chemotherapy via micro or nanoparticles through different routes of administration (Parikh et al., Citation2014). Microspheres are small spherical particles, with diameters in the micrometer range (typically 1–1000 μm) (Joshi et al., Citation2014b). Microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers which are biodegradable in nature (Cao et al., Citation2011). Microspheres are defined as “Monolithic sphere or therapeutic agent distributed throughout the matrix either as a molecular dispersion of particles” can be defined as structure made up of continuous phase of one or more miscible polymers in which drug particles are dispersed at the molecular or macroscopic level (Comoglu et al., Citation2008; Modgill et al., Citation2014). Microspheres can be manufactured from various natural and synthetic materials (Kalia et al., Citation2014). Glass microspheres, polymer microspheres and ceramic microspheres are commercially available (Barrow et al., Citation2007; Rohilla et al., Citation2014a). There are several studies that show nanoparticle delivery of anti-tubercular drugs in both blood plasma as well as organ tissues, some studies are listed in .
Table 7. Examples of microparticles/nanoparticles/polymeric nanoparticle-based anti-TB preparations.
Carbon nanotubes
Carbon nanotube is an emerging field of nano-biotechnology which has wide potential applications in drug delivery. They are known to have several micrometers in length with cross-sectional diameter in the range of 1–100 nm (Petersen et al., Citation2011; Hussain et al., Citation2014). They are mainly of two types, single-walled and multi-walled (Kaur et al., Citation2014a). The biggest advantage of carbon nanotubes is that they can be easily functionalized with a number of different chemical/functional groups and drug moieties to their surface according to the desired range of properties (Banyal et al., Citation2013). Carbon nanotubes can behave in both metallic and non-metallic manner (Kaur et al., Citation2014h). By increasing their biochemical utility manifolds and different functional groups such as proteins/peptides and nucleic acids can be coupled to their surface to makes them easily available to cells, organs and various tissues for targeting purpose (Yang & Han, Citation2000; Pabreja et al., Citation2014).
Quantum dots
Quantum dots are semi-conductor particles which generally have size ranging from 1 to 10 nm. That can be made to fluorescent in different colors depending on their size (Sharma et al., Citation2014b). As they are very good fluorophore, considering their wide emission spectra, they may be utilized for the detection and estimation of TB-infected cells easily and faster with high efficacy in different targeting organs (Banyal et al., Citation2013).
Nanosuspension
Nanosuspensions are generally micron-sized, colloidal dispersions (Rana & Murthy, Citation2013). Nanosuspension usually stabilized with use of different surfactants (Wang et al., Citation2015). Nanonization is a also useful methodology to improve the solubility of profile of drugs (Patel et al., Citation2011). A nanosuspension is a “very fine solid particles which are dispersed in a suitable aqueous vehicle” intended to be used through different routes like oral, topical, parenteral etc. (Kumar & Kumar, Citation2011; Kaur et al., Citation2014d). The size of particles in nanosuspension generally should be between 200 and 600 nm (Feng et al., Citation2011; Goyal et al., Citation2014b). Peters et al. (Citation2000) developed a clofazimine-loaded nanocrystalline suspension in order to reduce their toxicity and the low solubility (0.3 μg/mL) of the drug. The bacterial counts were found less in all organs. The results show that the clofazimine-loaded nanosuspension is suitable for IV injection. The drug concentration in liver, spleen and lungs were found to be high, so nanosuspension was found effective against Mycobacterium avium strains (Peters et al., Citation2000). Reverchon et al. (Citation2002) developed supercritical carbon-di-oxide assisted atomization process to produce rifampicin-loaded nanosuspension (sub-micron sized particles) (Reverchon et al., Citation2002).
Drug resistance in tuberculosis
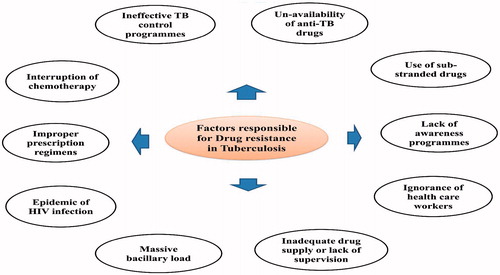
Multidrug resistant (MDR) tuberculosis and XDR tuberculosis are the emerging threats in management and treatment of TB. MDR-TB is the TB strains in which the resistance mainly produced to first line drugs such as isoniazid and rifampicin (Thakare et al., Citation2012). Whereas the extensively drug-resistant tuberculosis (XDR-TB) is a new TB strain which is mainly resistant to fluoro-quinolone agents and one or more second line injectable anti-tubercular agents such as amikacin and kanamycin capreomycin (Denholm et al., Citation2012). In general, the drug resistance is a man-made problem which results from misuse of available anti-tubercular drugs and due to the poor management of disease course or therapy (Yashodhara et al., Citation2010). Drug resistant strains are now accounts about 10% deaths of all TB cases. Now a days, India and China are those countries having more cases of patients with multi-drug-resistant TB. In India, “Revised National Tuberculosis Control Program” (RNTCP) has been introduced DOTS therapy which is effective and safe (Thakare et al., Citation2012). Most of the second line drugs used in MDR-TB is costly and toxic. Whole therapy lasts up to 2 years resulted patient stops taking medication which leads to more severity in TB. Major factors responsible for drug resistance in tuberculosis are shown in .
Current treatments available for drug-resistant tuberculosis are inadequate and outcomes are less effective than for drug-susceptible TB patients previously treated with second-line drugs (Zumla et al., Citation2013). As per the WHO report among 7063 patients from 13 different countries who started second-line treatment for MDR-TB in 2007 only 37% cases were successfully treated and 20% cases either died or undergoes failure of treatment (Thakare et al., Citation2012). As per WHO report 2014, anti-TB drug resistances were evaluated in 144 countries which account 95% of the World’s population. In 2013, six countries out of the 36 with high MDR-TB cases completed drug resistance surveys these countries includes Azerbaijan, Thailand and Vietnam, Myanmar, Pakistan, Philippines. In mid-2014, other surveys were conducted in five of these countries which include India, South Africa, China, Kenya and Ukraine. Extensively drug-resistant TB (XDR-TB) has been reported by 100 countries on average 9.0% of people with MDR-TB have XDR-TB. Treatment options for patients with MDR-TB and XDR-TB are extremely limited. Further, the poor efficacy, tolerability, treatment discontinuation in many of cases leads to resistance which in turn increases the risk of high mortality rate (Zhang & Yew, Citation2009). shows the first line drugs and their resistance.
Table 8. First line drugs and their resistance.
Implications of nanotechnology in MDR-TB and XDR-TB
As there are no first line drugs or treatment available for drug resistance in the case of TB. However, nanotechnology-based approaches can also be utilized to incorporate several first and second line anti-tubercular drugs. The lack of nanotechnological-based studies in resistance profiles in drug resistant tuberculosis is that the resistance vary greatly on individual treatment basis (Jassal & Bishai, Citation2009). There is no treatment options are available against the TB and drug resistance TB. However, our current strategy in various TB programs is that, drug resistance programs, research, and drug development is based on the hypothesis/believes that we will develop a suitable carrier system against rising drug resistance TB epidemics in future. The development of novel TB drugs is on priority basis to fight with TB-resistance.
Novel carrier system in MDR-TB and XDR-TB
Nanodispersions
Nanodispersions are submicron dispersions which are generally colloidal in nature of pure drugs which is stabilized via use of different surfactants (Newa et al., Citation2008; Malik et al., Citation2014). They also improve the solubility profile drugs (Chokshi et al., Citation2007). They are thermodynamically stable with the size of droplet mostly ranges between 10 and 100 nm (Varshosaz et al., Citation2006). They can host hydrophilic drugs inside core and lipophilic drugs within hydrophobic domains respectively (Jores, Citation2004; Kaur et al., Citation2014g).
Polymeric micelles
These are nanocarrier systems which are generated by the self-assembly or self-arrangement of a polymers in water above its critical micellar concentration (CMC) (Lu et al., Citation2010; Garg et al., Citation2014a). They are also effective in high drug-loading and drug release, specifically in the case of anti-tubercular drugs (Halperin & Alexander, Citation1989). They are also effective in drug targeting. Moretton et al. (Citation2010) developed polymeric micelles for rifampicin. It was found during study that large micelles were found by increasing the poly-ethylene glycol concentration. Rifampicin was found to be entrapped within the system appropriately (Moretton et al., Citation2010).
Future prospective in the treatment of MDR-TB and XDR-TB
Some newer drug candidates in tuberculosis treatment must be needed in order to reduce the burden of different kinds of resistance in tuberculosis and some other severe complications. Some of new drug candidates are listed in .
Table 9. Newer drug candidates in tuberculosis treatment (Mohan et al., Citation2013).
Further, the management of treatment for MDR-TB and XDR-TB should be in a proper way in order to reduce the burden of these resistances and secondary complications in disease. Some of second line anti-tubercular drugs used in the treatment of MDR-TB and XDR-TB are listed in .
Table 10. Second line anti-tubercular drugs used in the treatment of MDR-TB and XDR-TB (Prasad, Citation2007).
Role of immunomodulators in treatment of MDR-TB and XDR-TB
Immunomodulation is the emerging and promising therapeutic alternate, specifically in the tuberculosis management and treatment (Thakare et al., Citation2012). The weak immune response of host is accountable for the relapse of infection. Immunomodulators may be effective for this purpose such as immune stimulatory can be used for stimulation of weak immune response. Some of immunostimulant are listed in .
Table 11. Examples of immunostimulant (Patil et al., Citation2012).
Similarly, the immune suppressors are also useful in decreasing an elevated immune response in individual to get beneficial results. Some of examples of immune suppressants are given in .
Table 12. Examples of immunosupressants (Patil et al., Citation2012).
Therefore, immunomodulation is an approach based on the fact or principle that a particular microorganism causes disease conditions in a host organism due to the host’s susceptibility rather than its capability or characteristics alone. This principle may be utilized to obtain marked improved results in management and treatment of tuberculosis (Wallis & Johnson, Citation2009). Therefore, impairment of immune response of the host may thus effective. For this, combination of an immune potentiating agent along with chemotherapy in tuberculosis would be beneficial to prevent such severe disease conditions (Patil et al., Citation2012). The immunomodulators therapy is useful in tuberculosis due to their efficacy in:
preventing infections to proceed further in disease conditions and secondary complications (Garg & Goyal, Citation2012);
preventing the re-occurrence of later stage infections (Garg & Goyal, Citation2014b,Citationc);
helps to achieve early controls and measures of infections when given in conjunction with specific chemotherapeutic agents in tuberculosis (Garg & Goyal, Citation2014a);
they also help to achieve early clinical response like weight gain etc.
Current therapy of tuberculosis consists of use of ordinary first line anti-tubercular drugs and specific antibiotics either for a particular period of time or for long period (Garg et al., Citation2012a).
This increases the chances for generation of resistance and MDR strains. All the countries in the world besides their social or economic status are suffering from MDR-TB and XDR-TB. The continuous development of MDR-TB and XDR-TB is considerably reducing the outcome of anti-tubercular drugs which in turn causes therapy failure (Bhattacharya et al., Citation2013). Furthermore, there is a chance of TB re-activation and re-infection which also suggests that these chemotherapeutics and antibiotics cause impairment in immune response of host (Bhattacharya et al., Citation2013). Therefore, an approach for TB treatment reduces chances of immune impairment, able to reduce therapy time of treatment and able to diminish toxicities.
Immunomodulation using immune therapeutic vaccines for tuberculosis
Immune therapeutic vaccines are one of the first immune therapeutic applications of vaccines to show some promise approach in a clinical trial was the heat-killed Mycobacterium vaccae. Its mode of action has been proposed to be an enhancement of Th1 and down-regulation of Th2 cytokine expression (Reljic & Ivanyi, Citation2012). shows the vaccines used in tuberculosis immunotherapy.
Table 13. Vaccines used in immunotherapy in tuberculosis.
Immunomodulation using cytokines in TB-immunotherapy
Cytokines are highly pleiotropic proteins which are effective to promote host immune defense mechanisms. For the effective treatment of mycobacterial infections, the administered cytokines must first reach their target cells after binding to the specific receptors and finally to activate an intact signal transduction pathway to produce cellular response in host cell. The dose and route of administration of cytokines must be carefully chosen in order to avoid the risk of toxicity/unwanted pharmacological responses or effects. Several cytokines have been considered for treatment of mycobacterial infections, including IFN-gamma, IL-2, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony stimulating factor (G-CSF) (Reljic & Ivanyi, Citation2012).
Immunomodulation using antibodies in TB-immunotherapy
Antibodies could have played a vital role in immunotherapy since normal human sera contain high antibody titers for mycobacterial heat shock proteins. Some of examples regarding the use of antibodies in TB-immunotherapy are given in . shows the role of immunomodulators as adjuvant to chemotherapy in treatment of tuberculosis.
Table 14. Antibodies in tuberculosis immunotherapy.
Table 15. Studies showing role of immunomodulators as adjuvant to chemotherapy in the treatment of tuberculosis.
Conclusion
As the pulmonary tuberculosis is a second leading cause of death after AIDS since time immemorial and it continues to cause immense human misery today up to greater extent. The emergence of MDR-TB and XDR-TB has been threatening and imposing various problems to destabilize TB control globally in developing as well as developed countries. TB has remained a neglected disease, but there is an urgent need for new anti-TB drugs that should be more effective and should have less side effects and toxicity. There is also a need for newer and innovative anti-TB drug-delivery systems in the better management of tuberculosis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The author Dr. Amit K. Goyal (AMR/43/2011-ECD-1 dated 1 July 2013) is thankful to the Indian Council of Medical Research (ICMR), New Delhi, India, for providing financial assistance to carry out research work.
References
- Abdelkader H, Alani AW, Alany RG. (2014). Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv 21:87–100
- Abolmaali SS, Tamaddon AM, Farvadi FS, et al. (2011). Pharmaceutical nanoemulsions and their potential topical and transdermal applications. Iran J Pharm Sci 7:139–50
- Aboutaleb E, Noori M, Gandomi N, et al. (2012). Improved antimycobacterial activity of rifampin using solid-lipid nanoparticles. Int Nano Lett 2:1–8
- Acevedo-Morantes CY, Acevedo-Morantes MT, Suleiman-Rosado D, Ramírez-Vick JE. (2013). Evaluation of the cytotoxic effect of camptothecin solid-lipid nanoparticles on MCF7 cells. Drug Deliv 20:338–48
- Agatonovic-Kustrin S, Glass BD, Wisch MH, Alany RG. (2003). Prediction of a stable microemulsion formulation for the oral delivery of a combination of antitubercular drugs using ANN methodology. Pharm Res 20:1760–5
- Ahmad Z, Pandey R, Sharma S, Khuller G. (2006). Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci 48:171–6
- Ahmed M, Ramadan W, Rambhu D, Shakeel F. (2008). Potential of nanoemulsions for intravenous delivery of rifampicin. Int J Pharm Sci 63:806–11
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. (2013). Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102
- Alam MI, Baboota S, Ahuja A, et al. (2013). Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv 20:247–51
- Allen B, Mitchison D, Chan Y, et al. (1983). Amikacin in the treatment of pulmonary tuberculosis. Tubercle 64:111–18
- Anwekar H, Patel S, Singhai A. (2011). Liposome as drug carriers. Int J Pharm Life Sci 2:945–51
- Banyal S, Malik P, Tuli HS, Mukherjee TK. (2013). Advances in nanotechnology for diagnosis and treatment of tuberculosis. Curr Opin Pulmon Med 19:289–97
- Barakat NS, Yassin AEB. (2006). In vitro characterization of carbamazepine-loaded precifac lipospheres. Drug Deliv 13:95–104
- Barrow EL, Barrow WW, Quenelle DC, et al. (2007). Efficacy of rifabutin-loaded microspheres for treatment of Mycobacterium avium-infected macrophages and mice. Drug Deliv 14:119–27
- Bhandari R, Kaur IP. (2013). Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid-lipid nanoparticles. Int J Pharm 441:202–12
- Bhardwaj A, Kumar L, Narang R, Murthy R. (2013). Development and characterization of ligand-appended liposomes for multiple drug therapy for pulmonary tuberculosis. Artif Cells Nanomedicine Biotechnol 41:52–9
- Bhatt P, Madhav S. (2011). A detailed review on nanoemulsion drug delivery system. Int J Pharm Sci Res 2:2292–8
- Bhattacharya D, Dwivedi VP, Das G. (2013). Revisiting immunotherapy in tuberculosis. J Mycobac Dis 4:e123
- Caceres NE, Harris NB, Wellehan JF, et al. (1997). Overexpression of the D-alanine racemase gene confers resistance to D-cycloserine in Mycobacterium smegmatis. J Bacteriol 179:5046–55
- Cao F, Ding B, Sun M, et al. (2011). Lung-targeted delivery system of curcumin loaded gelatin microspheres. Drug Deliv 18:545–54
- Cekié ND, Savić SD, Milić J, et al. (2007). Preparation and characterisation of phenytoin-loaded alginate and alginate-chitosan microparticles. Drug Deliv 14:483–90
- Chandu VP, Arunachalam A, Jeganath S, et al. (2012). Niosomes: a novel drug delivery system. Int J Novel Trends Pharm Sci 2:25–31
- Chaudhary C, Garg T. (2015). Scaffolds: a novel carrier and potential wound healer. Crit Rev Ther Drug Carrier Syst 32:277–321
- Chaudhary S, Garg T, Murthy R, et al. (2015a). Development, optimization and evaluation of long chain nanolipid carrier for hepatic delivery of silymarin through lymphatic transport pathway. Int J Pharm 485:108–21
- Chaudhary S, Garg T, Murthy RS, et al. (2014). Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target 22:871–82
- Chaudhary S, Garg T, Rath G, et al. (2015b). Enhancing the bioavailability of mebendazole by integrating the principles solid dispersion and nanocrystal techniques, for safe and effective management of human echinococcosis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.1000493
- Chimote G, Banerjee R. (2010). In vitro evaluation of inhalable isoniazid-loaded surfactant liposomes as an adjunct therapy in pulmonary tuberculosis. J Biomed Mater Res B Appl Biomater 94:1–10
- Chokshi RJ, Zia H, Sandhu HK, et al. (2007). Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution – pros and cons. Drug Deliv 14:33–45
- Comoglu T, Gonul N, Dogan A, Basci N. (2008). Development and in vitro evaluation of pantoprazole-loaded microspheres. Drug Deliv 15:295–302
- Dawson R, Condos R, Tse D, et al. (2009). Immunomodulation with recombinant interferon-γ1b in pulmonary tuberculosis. PLoS One 4:e6984
- Denholm J, Street A, McBryde E, Eisen D. (2012). Management of tuberculosis. Lulu.com
- Deol P, Khuller G. (1997). Lung specific stealth liposomes: stability, biodistribution and toxicity of liposomal antitubercular drugs in mice. Biochim Biophys Acta Gen Subjects 1334:161–72
- Devarajan V, Ravichandran V. (2011). Nanoemulsions: as modified drug delivery tool. Int J Compr Pharm 2:1–6
- Diljyot K. (2012). Niosomes: a new approach to targeted drug delivery. Int J Pharm Phytopharmacol Res 2:53–9
- Doan T, Olivier J. (2009). Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int J Pharm 382:61–6
- Domingo M, Gil O, Serrano E, et al. (2009). Effectiveness and safety of a treatment regimen based on isoniazid plus vaccination with Mycobacterium tuberculosis cells’ fragments: field-study with naturally Mycobacterium caprae-infected goats. Scand J Immunol 69:500–7
- Dong Z, Xie S, Zhu L, et al. (2011). Preparation and in vitro, in vivo evaluations of norfloxacin-loaded solid-lipid nanopartices for oral delivery. Drug Deliv 18:441–50
- Doshi M. (2011). Dendrimer and its application. Int J Pharm Sci Rev Res 7:104–11
- Dutt M, Khuller G. (2001). Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in poly (DL-lactide-co-glycolide) microparticles. J Antimicrob Chemother 47:829–35
- Düzgüneş N, Flasher D, Reddy MV, et al. (1996). Treatment of intracellular Mycobacterium avium complex infection by free and liposome-encapsulated sparfloxacin. Antimicrob Agents Chemother 40:2618–21
- El-Badry M, Fetih G, Shakeel F. (2014). Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv 21:34–43
- El-Ridy M, Mostafa D, Shehab A, et al. (2007). Biological evaluation of pyrazinamide liposomes for treatment of Mycobacterium tuberculosis. Int J Pharm 330:82–8
- El-Ridy MS, Abdelbary A, Nasr EA, et al. (2011). Niosomal encapsulation of the antitubercular drug, pyrazinamide. Drug Dev Ind Pharm 37:1110–18
- El-Ridy MS, Yehia SA, Kassem MA-E-M, et al. (2015). Niosomal encapsulation of ethambutol hydrochloride for increasing its efficacy and safety. Drug Deliv 22:21–36
- Elgart A, Cherniakov I, Aldouby Y, et al. (2012). Lipospheres and pro-nano lipospheres for delivery of poorly water soluble compounds. Chem Phys Lipids 165:438–53
- Feng F-F, Zhang D-R, Tian K-L, et al. (2011). Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by oridonin nanosuspension. Drug Deliv 18:265–71
- Feng R, Zhang Z, Li Z, Huang G. (2014). Preparation and in vitro evaluation of etoposide-loaded PLGA microspheres for pulmonary drug delivery. Drug Deliv 21:185–92
- Gagandeep Garg T, Malik B, Rath G, Goyal AK. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–16
- Gandhi M, Paralkar S, Sonule M, et al. (2014). Niosomes: novel drug delivery system. Int J Pure Appl Biosci 2:267–74
- Gangadharam P, Ashtekar DR, Flasher DL, Düzgüneş N. (1995). Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically stabilized liposomes. Antimicrob Agents Chemother 39:725–30
- Gao X-F, Yang Z-W, Li J. (2011). Adjunctive therapy with interferon-gamma for the treatment of pulmonary tuberculosis: a systematic review. Int J Infect Dis 15:e594–600
- Garg T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.916715
- Garg T, Bhandari S, Rath G, Goyal AK. (2015a). Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J Drug Target. [Epub ahead of print]. doi: 10.3109/1061186x.2015.1029930
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Goyal AK. (2014c). Medicated chewing gum: patient compliance oral drug delivery system. Drug Deliv Lett 4:72–8
- Garg T, Goyal AK, Arora S, Murthy R. (2012a). Development, optimization, evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett 2:251–61
- Garg T, Kumar A, Rath G, Goyal AK. (2014a). Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst 31:531–57
- Garg T, Rath G, Goyal AK. (2014b). Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst 31:331–64
- Garg T, Rath G, Goyal AK. (2014c). Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J Drug Target 23:202–21
- Garg T, Rath G, Goyal AK. (2015b). Colloidal drug delivery systems: current status and future directions. Crit Rev Ther Drug Carrier Syst 32:89–147
- Garg T, Rath G, Murthy RR, et al. (2015c). Current nanotechnological approaches for an effective delivery of bioactive drug molecules to overcome drug resistance tuberculosis. Curr Pharm Des 21:3076–89
- Garg T, Rath G, Goyal AK. (2015d). Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1008508
- Garg T, Rath G, Goyal AK. (2015e). Nanotechnological approaches for the effective management of psoriasis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1037885
- Garg T, Singh O, Arora S, Murthy R. (2012b). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Garud A, Singh D, Garud N. (2012). Solid-lipid nanoparticles (SLN): method, characterization and applications. Int Curr Pharm J 1:384–93
- Gaspar M, Cruz A, Penha A, et al. (2008). Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int J Antimicrob Agents 31:37–45
- Gaspar MM, Neves S, Portaels F, et al. (2000). Therapeutic efficacy of liposomal rifabutin in a Mycobacterium avium model of infection. Antimicrob Agents Chemother 44:2424–30
- Gopalkrishnan S, Chenthilnathan AN. (2012). A novel drug delivery device. Res J Pharm Biol Chem Sci 3:1090–8
- Goyal G, Garg T, Malik B, et al. (2013). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2013.855277
- Goyal G, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst 31:89–119
- Goyal G, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst 31:365–405
- Guirado E, Amat I, Gil O, et al. (2006). Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect 8:1252–9
- Guo X, Xing Y, Zhang X, et al. (2012). In vivo controlled release and prolonged antitumor effects of 2-methoxyestradiol solid-lipid nanoparticles incorporated into a thermosensitive hydrogel. Drug Deliv 19:188–93
- Gupta P, Garg T, Tanmay M, Arora S. (2015). Polymeric drug-delivery systems: role in P-gp efflux system inhibition. Crit Rev Ther Drug Carrier Syst 32:247–75
- Halperin A, Alexander S. (1989). Polymeric micelles: their relaxation kinetics. Macromolecules 22:2403–12
- Hari BV, Chitra KP, Bhimavarapu R, et al. (2010). Novel technologies: a weapon against tuberculosis. Indian J Pharmacol 42:338–44
- Hu L, Yang J, Liu W, Li L. (2011). Preparation and evaluation of ibuprofen-loaded microemulsion for improvement of oral bioavailability. Drug Deliv 18:90–5
- Hussain T, Garg T, Goyal AK, Rath G. (2014). Biomedical applications of nanofiber scaffolds in tissue engineering. J Biomater Tissue Eng 4:600–23
- Islan GA, Castro GR. (2014). Tailoring of alginate–gelatin microspheres properties for oral Ciprofloxacin-controlled release against Pseudomonas aeruginosa. Drug Deliv 21:615–26
- Jain C, Vyas S, Dixit V. (2006). Niosomal system for delivery of rifampicin to lymphatics. Indian J Pharm Sci 68:575–8
- Jain V, Prasad V, Jadhav P, Mishra P. (2009). Preparation and performance evaluation of saquinavir laden cationic submicron emulsions. Drug Deliv 16:37–44
- Jana S, Gandhi A, Sen KK, Basu SK. (2012). Dendrimers: synthesis, properties, biomedical and drug delivery applications. Am J Pharm Tech Res 2:32–55
- Jassal M, Bishai WR. (2009). Extensively drug-resistant tuberculosis. Lancet Infect Dis 9:19–30
- Jawahar N, Meyyanathan S, Reddy G, Sood S. (2012). Solid-lipid nanoparticles for oral delivery of poorly soluble drugs. J Pharm Sci Res 4:1848–55
- Jeong YJ, Lee KS. (2008). Pulmonary tuberculosis: up-to-date imaging and management. Am J Roentgenol 191:834–44
- Jia L-J, Zhang D-R, Li Z-Y, et al. (2010). Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv 17:11–18
- Johal HS, Garg T, Rath G, Goyal AK. (2014). Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.928760
- Jores K. (2004). Lipid nanodispersions as drug carrier systems-a physicochemical characterization. Wittenberg: Martin-Luther Universität Halle
- Joshi D, Garg T, Goyal AK, Rath G. (2014a). Advanced drug delivery approaches against periodontitis. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.935531
- Joshi D, Garg T, Goyal AK, Rath G. (2014b). Development and characterization of novel medicated nanofibers against periodontitis. Curr Drug Deliv 12:1–5
- Justo OR, Moraes AM. (2005). Kanamycin incorporation in lipid vesicles prepared by ethanol injection designed for tuberculosis treatment. J Pharm Pharmacol 57:23–30
- Justo OR, Moraes ÂM. (2003). Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Deliv 10:201–7
- Kalia V, Garg T, Rath G, Goyal AK. (2014). Development and evaluation of a sublingual film of the antiemetic granisetron hydrochloride. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.984303
- Kamble MS, Vaidya KK, Bhosale AV, Chaudhari PD. (2012). Solid-lipid nanoparticles and nanostructured lipid carriers – an overview. Int J Pharm Chem Biol Sci 2:681–91
- Kaur G, Garg T, Rath G, Goyal AK. (2015a). Archaeosomes: an excellent carrier for drug and cell delivery. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2015.1019653
- Kaur G, Mehta SK. (2014). Probing location of anti-TB drugs loaded in Brij 96 microemulsions using thermoanalytical and photophysical approach. J Pharm Sci 103:937–44
- Kaur M, Garg T, Narang RK. (2014a). A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.962745
- Kaur M, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014c). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv 22:328–34
- Kaur N, Garg T, Goyal AK, Rath G. (2014d). Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.963900
- Kaur P, Garg T, Rath G, Goyal AK. (2015b). In situ nasal gel drug delivery: a novel approach for brain targeting through the mucosal membrane. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1012260
- Kaur P, Garg T, Rath G, et al. (2014e). Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.993486
- Kaur P, Garg T, Vaidya B, et al. (2014f). Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: behavioral and biochemical assessment. J Drug Target 23:275–86
- Kaur R, Garg T, Goyal AK, Rath G. (2015). Development, optimization and evaluation ofelectrospun nanofibers: tool fortargeted vaginal delivery of antimicrobials against urinary tract infections. Curr Drug Deliv 22:328–34
- Kaur R, Garg T, Rath G, Goyal AK. (2014g). Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst 31:495–530
- Kaur V, Garg T, Rath G, Goyal AK. (2014h). Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target 22:859–70
- Kochi A, Vareldzis B, Styblo K. (1993). Multidrug-resistant tuberculosis and its control. Res Microbiol 144:104–10
- Kotta S, Khan AW, Ansari S, et al. (2015). Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv 22:455–66
- Kulkarni PR, Yadav J, Vaidya KA. (2011). Liposomes: a novel drug delivery system. Int J Curr Pharm Res 3:10–18
- Kumar A, Garg T, Sarma GS, et al. (2015). Optimization of combinational intranasal drug delivery system for the management of migraine by using statistical design. Eur J Pharm Sci 70C:140–51
- Kumar G, Sharma S, Shafiq N, et al. (2011). Pharmacokinetics and tissue distribution studies of orally administered nanoparticles encapsulated ethionamide used as potential drug delivery system in management of multi-drug resistant tuberculosis. Drug Deliv 18:65–73
- Kumar N, Kumar M. (2011). Nanotechnology: a focus on treatment of tuberculosis. Int J Drug Deliv 3:25–42
- Kumar PV, Asthana A, Dutta T, Jain NK. (2006). Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target 14:546–56
- Kumar S, Singh V. (2012). Nanoemulsification – a novel targeted drug delivery tool. J Drug Deliv Ther 2:40–5
- Labana S, Pandey R, Sharma S, Khuller G. (2002). Chemotherapeutic activity against murine tuberculosis of once weekly administered drugs (isoniazid and rifampicin) encapsulated in liposomes. Int J Antimicrob Agents 20:301–4
- Leitzke S, Bucke W, Borner K, et al. (1998). Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrob Agents Chemother 42:459–61
- Li X, Guo Q, Zheng X, et al. (2009). Preparation of honokiol-loaded chitosan microparticles via spray-drying method intended for pulmonary delivery. Drug Deliv 16:160–6
- Liang Y, Wu X, Zhang J, et al. (2011). Treatment of multi-drug-resistant tuberculosis in mice with DNA vaccines alone or in combination with chemotherapeutic drugs. Scand J Immunol 74:42–6
- Liu Z, Zhong Z, Peng G, et al. (2009). Folate receptor mediated intracellular gene delivery using the charge changing solid-lipid nanoparticles. Drug Deliv 16:341–7
- Lu X, Zhang F, Qin L, et al. (2010). Polymeric micelles as a drug delivery system enhance cytotoxicity of vinorelbine through more intercellular accumulation. Drug Deliv 17:255–62
- Luan J, Zhang D, Hao L, et al. (2013). Design and characterization of Amoitone B-loaded nanostructured lipid carriers for controlled drug release. Drug Deliv 20:324–30
- Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. (2014). Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv 21:148–54
- Malik R, Garg T, Goyal AK, Rath G. (2014). Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target 23:109–24
- Malik R, Garg T, Goyal AK, Rath G. (2015). Diacerein-loaded novel gastroretentive nanofiber system using PLLA: development and in vitro characterization. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.1000492
- Mansoori M, Agrawal S, Jawade S, Khan M. (2012). A review on liposome. Int J Adv Res Pharm Biosci 2:453–64
- Maretti E, Rossi T, Bondi M, et al. (2014). Inhaled solid-lipid microparticles to target alveolar macrophages for tuberculosis. Int J Pharm 462:74–82
- Masungi C, Temmerman S, van Vooren J-P, et al. (2002). Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J Infect Dis 185:513–20
- Meena LS. (2010). Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J 277:2416–27
- Mehta S, Kaur G, Bhasin K. (2007). Analysis of Tween based microemulsion in the presence of TB drug rifampicin. Colloids Surf B Biointerfaces 60:95–104
- Mehta S, Kaur G, Bhasin K. (2008). Incorporation of antitubercular drug isoniazid in pharmaceutically accepted microemulsion: effect on microstructure and physical parameters. Pharm Res 25:227–36
- Mehta S, Kaur G, Bhasin K. (2010). Entrapment of multiple anti-Tb drugs in microemulsion system: quantitative analysis, stability, and in vitro release studies. J Pharm Sci 99:1896–911
- Mehta SK, Jindal N, Kaur G. (2011). Quantitative investigation, stability and in vitro release studies of anti-TB drugs in Triton niosomes. Colloids Surf B Biointerfaces 87:173–9
- Mikusová K, Slayden RA, Besra GS, Brennan PJ. (1995). Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother 39:2484–9
- Modgill V, Garg T, Goyal AK, Rath G. (2014). Transmucosal delivery of linagliptin for the treatment of type-2 diabetes mellitus by ultra-thin nanofibers. Curr Drug Deliv 12:323–32
- Mohan A, Kumar DP, Harikrishna J. (2013). Newer anti-TB drugs and drug delivery systems. In: Muruganathan A, ed. Medicine update. New Delhi: Jaypee Brothers Medical Publishers (for The Association of Physicians of India), 388–92
- Momoh M, Kenechukwu F, Attama A. (2013). Formulation and evaluation of novel solid-lipid microparticles as a sustained release system for the delivery of metformin hydrochloride. Drug Deliv 20:102–11
- Moretton MA, Glisoni RJ, Chiappetta DA, Sosnik A. (2010). Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf B Biointerfaces 79:467–79
- Morie A, Garg T, Goyal AK, Rath G. (2014). Nanofibers as novel drug carrier – an overview. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.927879
- Morlock GP, Metchock B, Sikes D, et al. (2003). ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 47:3799–805
- Mostafa DM, Ammar NM, Abd El-Alim SH, El-Anssary AA. (2014). Transdermal microemulsions of Glycyrrhiza glabra L.: characterization, stability and evaluation of antioxidant potential. Drug Deliv 21:130–9
- Mujoriya RZ, Dhamandeb K, Bodla R. (2011). Niosomal drug delivery systems – a review. Int J Appl Pharm 3:7–10
- Mullaicharam A, Murthy R. (2004). Lung accumulation of niosome-entrapped rifampicin following intravenous and intratracheal administration in the rat. J Drug Deliv Sci Technol 14:99–104
- Mutalik S, Shetty PK, Kumar A, et al. (2014). Enhancement in deposition and permeation of 5-fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv 21:44–54
- Muttil P, Kaur J, Kumar K, et al. (2007). Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur J Pharm Sci 32:140–50
- Nair R, Arun Kumar K, Badivaddin TM, Sevukarajan M. (2011). Formulation and evaluation of solid-lipid nanoparticles of water soluble drug: isoniazid. J Pharm Sci Res 3:1256–64
- Narang AS, Delmarre D, Gao D. (2007). Stable drug encapsulation in micelles and microemulsions. Int J Pharm 345:9–25
- Nasr M, Taha I, Hathout RM. (2013). Suitability of liposomal carriers for systemic delivery of risedronate using the pulmonary route. Drug Deliv 20:311–18
- Newa M, Bhandari KH, Kim J-A, et al. (2008). Preparation and evaluation of fast dissolving ibuprofen-polyethylene glycol 6000 solid dispersions. Drug Deliv 15:355–64
- Nikolaeva LG, Maystat TV, Pylypchuk VS, et al. (2008a). Cytokine profiles of HIV patients with pulmonary tuberculosis resulting from adjunct immunotherapy with herbal phytoconcentrates Dzherelo and Anemin. Cytokine 44:392–6
- Nikolaeva LG, Maystat TV, Pylypchuk VS, et al. (2008b). Effect of oral immunomodulator Dzherelo in TB/HIV co-infected patients receiving anti-tuberculosis therapy under DOTS. Int J Immunopharmacol 8:845–51
- Nikonenko B, Reddy VM, Bogatcheva E, et al. (2014). Therapeutic efficacy of SQ641-NE against Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:587–9
- Nikumbh KV, Sevankar SG, Patil MP. (2015). Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv 22:509–15
- Nimje N, Agarwal A, Saraogi GK, et al. (2009). Mannosylated nanoparticulate carriers of rifabutin for alveolar targeting. J Drug Target 17:777–87
- Madhav NVS, Saini A. (2011). Niosomes: a novel drug delivery system. Int J Res Pharm Chem 3:498–511
- O'Hara P, Hickey AJ. (2000). Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm Res 17:955–61
- Ohashi K, Kabasawa T, Ozeki T, Okada H. (2009). One-step preparation of rifampicin/poly (lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release 135:19–24
- Onoshita T, Shimizu Y, Yamaya N, et al. (2010). The behavior of PLGA microspheres containing rifampicin in alveolar macrophages. Colloids Surf B Biointerfaces 76:151–7
- Pabreja S, Garg T, Rath G, Goyal AK. (2014). Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.966195
- Paliwal SR, Paliwal R, Vyas SP. (2015). A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv 22:231–42
- Pallerla SM, Prabhakar B. (2013). A review on solid-lipid nanoparticles. Int J Pharm Sci Rev Res 20:196–206
- Palmero D, Eiguchi K, Rendo P, et al. (1999). Phase II trial of recombinant interferon-α2b in patients with advanced intractable multidrug-resistant pulmonary tuberculosis: long-term follow-up. Int J Tuberc Lung Dis 3:214–18
- Palomino JC, Martin A. (2014). Drug resistance mechanisms in Mycobacterium tuberculosis. Antibiotics 3:317–40
- Pandey R, Khuller G. (2006a). Oral nanoparticle-based antituberculosis drug delivery to the brain in an experimental model. J Antimicrob Chemother 57:1146–52
- Pandey R, Khuller GK. (2006b). Nanoparticle-based oral drug delivery system for an injectable antibiotic-streptomycin. Evaluation in a murine tuberculosis model. Chemotherapy 53:437–41
- Pandey R, Sharma S, Khuller G. (2004). Lung specific stealth liposomes as antitubercular drug carriers in guinea pigs. Indian J Exp Biol 42:562–6
- Pandey R, Sharma S, Khuller G. (2005). Oral solid-lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis 85:415–20
- Pandey V, Gajbhiye KR, Soni V. (2015). Lactoferrin-appended solid-lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv 22:199–205
- Pardeshi CV, Rajput PV, Belgamwar VS, et al. (2013). Novel surface modified solid-lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv 20:47–56
- Parhi R, Suresh P. (2012). Preparation and characterization of solid-lipid nanoparticles – a review. Curr Drug Discov Technol 9:2–16
- Parikh R, Patel L, Dalwadi S. (2014). Microparticles of rifampicin: comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Deliv 21:406–11
- Patel M, Shah A, Patel N, et al. (2011). Nanosuspension: a novel approach for drug delivery system. J Pharm Sci Biosci Res 1:1–10
- Patel N, Panda S. (2012). Liposome drug delivery system: a critic review. J Pharm Sci Biosci Res 2:162–94
- Patidar A, Thakur DS, Kumar P, Verma J. (2010). A review on novel lipid based nanocarriers. Int J Pharm Pharm Sci 2:30–5
- Patil U, Jaydeokar A, Bandawane D. (2012). Immunomodulators: a pharmacological review. Int J Pharm Pharm Sci 4:30–6
- Peters K, Leitzke S, Diederichs J, et al. (2000). Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother 45:77–83
- Petersen EJ, Zhang L, Mattison NT, et al. (2011). Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ Sci Technol 45:9837–56
- Pourshahab PS, Gilani K, Moazeni E, et al. (2011). Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J Microencapsul 28:605–13
- Prasad R. (2007). Management of multi-drug resistant tuberculosis: practitioner's view point. Indian J Tuberc 54:3–11
- Pravinagurjar N, Chouksey S. (2014). Niosome: a promising pharmaceutical drug delivery. Int J Pharm Drug Anal 2:425–31
- Prihoda N, Arjanova OV, Yurchenko LV, et al. (2009). Adjuvant immunotherapy of extensively drug-resistant tuberculosis (XDR-TB) in Ukraine. Curr Res Tuberc 1:1–6
- Rahman S, Cao S, Steadman KJ, et al. (2012). Native and β-cyclodextrin-enclosed curcumin: entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Deliv 19:346–53
- Rana P, Murthy R. (2013). Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: a potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv 20:224–35
- Rawat M, Singh D, Saraf S, Saraf S. (2008). Lipid carriers: a versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi 128:269–80
- Reljic R, Ivanyi J. (2012). Immunotherapy of tuberculosis with IgA and cytokines, Croatia: INTECH Open Access Publisher
- Reverchon E, de Marco I, della Porta G. (2002). Rifampicin microparticles production by supercritical antisolvent precipitation. Int J Pharm 243:83–91
- Ricci M, Giovagnoli S, Blasi P, et al. (2006). Development of liposomal capreomycin sulfate formulations: effects of formulation variables on peptide encapsulation. Int J Pharm 311:172–81
- Rohilla R, Garg T, Bariwal J, et al. (2014a). Development, optimization and characterization of glycyrrhetinic acid-chitosan nanoparticles of atorvastatin for liver targeting. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.977460
- Rohilla R, Garg T, Goyal AK, Rath G. (2014b). Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.945018
- Rojanarat W, Nakpheng T, Thawithong E, et al. (2012). Inhaled pyrazinamide proliposome for targeting alveolar macrophages. Drug Deliv 19:334–45
- Rosada RS, Torre LG, Frantz FG, et al. (2008). Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol 9:38
- Salome AC, Ikechukwu VO. (2013). Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol 7:3034–59
- Sankhyan A, Pawar P. (2012). Recent trends in niosome as vesicular drug delivery system. J Appl Pharm Sci 2:20–32
- Saraogi GK, Sharma B, Joshi B, et al. (2011). Mannosylated gelatin nanoparticles bearing isoniazid for effective management of tuberculosis. J Drug Target 19:219–27
- Sarkar S, Suresh MR. (2011). An overview of tuberculosis chemotherapy – a literature review. J Pharm Pharm Sci 14:148–61
- Sensi P. (1983). History of the development of rifampin. Rev Infect Dis 5:S402–6
- Setya S, Talegaonkar S, Razdan B. (2013). Nanoemulsions: formulation methods and stability aspects. World J Pharm Pharm Sci 3:2214–28
- Seyfoddin A, Shaw J, Al-Kassas R. (2010). Solid-lipid nanoparticles for ocular drug delivery. Drug Deliv 17:467–89
- Sharma A, Garg T, Aman A, et al. (2014a). Nanogel – an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.930745
- Sharma AK, Garg T, Goyal AK, Rath G. (2015). Role of microemuslsions in advanced drug delivery. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1012261
- Sharma R, Garg T, Goyal AK, Rath G. (2014b). Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi:10.3109/21691401.2014.966194
- Sharma R, Mahatma R, Bharkatiya M, Goyal A. (2012). Niosomes – as potential drug delivery system. Int J Drug Res Technol 2:422–9
- Sharma R, Saxena D, Dwivedi AK, Misra A. (2001). Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm Res 18:1405–10
- Shi J, Zhang J, Shen Y, et al. (2015). Arginine-stabilized mPEG-PDLLA (50/50) polymeric micelles of docetaxel by electrostatic mechanism for tumor-targeted delivery. Drug Deliv 22:168–81
- Singh B, Garg T, Goyal AK, Rath G. (2014a). Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.937868
- Singh G, Dwivedi H, Saraf SK, Saraf SA. (2011). Niosomal delivery of isoniazid-development and characterization. Trop J Pharm Res 10:203–10
- Singh G, Pai RS. (2014). Pharmacokinetics and in vivo biodistribution of optimized PLGA nanoparticulate drug delivery system for controlled release of emtricitabine. Drug Deliv 21:627–35
- Singh H, Bhandari R, Kaur IP. (2013). Encapsulation of Rifampicin in a solid-lipid nanoparticulate system to limit its degradation and interaction with Isoniazid at acidic pH. Int J Pharm 446:106–11
- Singh K, Arora N, Garg T. (2012). Superbug: antimicrobial resistance due to NDM-1. Int J Inst Pharm Life Sci 2:58–66
- Singh O, Garg T, Rath G, Goyal AK. (2014b). Microbicides for the treatment of sexually transmitted HIV infections. J Pharm. [Epub ahead of print]. doi: 10.1155/2014/352425
- Smith JP. (2011). Nanoparticle delivery of anti-tuberculosis chemotherapy as a potential mediator against drug-resistant tuberculosis. Yale J Biol Med 84:361–9
- Song X, Lin Q, Guo L, et al. (2015). Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm Res 32:1741–51
- Suarez S, O'Hara P, Kazantseva M, et al. (2001). Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J Antimicrob Chemother 48:431–4
- Sung JC, Padilla DJ, Garcia-Contreras L, et al. (2009). Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res 26:1847–55
- Swathi G, Prasanthi N, Manikiran S, Ramarao N. (2012). Solid-lipid nanoparticles: colloidal carrier systems for drug delivery. ChemInform 43:1–15
- Takenaga M, Ohta Y, Tokura Y, et al. (2008). Lipid microsphere formulation containing rifampicin targets alveolar macrophages. Drug Deliv 15:169–75
- Thakare V, Nayak U, Lacchiramka P. (2012). Newer drugs for multi drug resistant tuberculosis and special emphasis on linezolid. Biol Sci 2:37–46
- Thakur A, Walia MK, Kumar S. (2013). Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore 4:15–25
- Thomas TP, Shukla R, Kotlyar A, et al. (2008). Dendrimer − epidermal growth factor conjugate displays superagonist activity. Biomacromolecules 9:603–9
- Thulasiramaraju T, Babu AS, Arunachalam A, et al. (2012). Liposomes: a novel drug delivery system. Int J Curr Pharm Res 3:10–18
- Utreja P, Jain S, Tiwary A. (2011). Localized delivery of paclitaxel using elastic liposomes: formulation development and evaluation. Drug Deliv 18:367–76
- Varshosaz J, Faghihian H, Rastgoo K. (2006). Preparation and characterization of metoprolol controlled-release solid dispersions. Drug Deliv 13:295–302
- Verma RK, Kaur J, Kumar K, et al. (2008). Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob Agents Chemother 52:3195–201
- Vijayaraj Kumar P, Agashe H, Dutta T, Jain NK. (2007). PEGylated dendritic architecture for development of a prolonged drug delivery system for an antitubercular drug. Curr Drug Deliv 4:11–19
- Wade MM, Zhang Y. (2004). Mechanisms of drug resistance in Mycobacterium tuberculosis. Front Biosci 9:975–94
- Wallis RS, Johnson JL. (2009). Immunotherapy of tuberculosis. In: Schaaf HS, Zumla A, eds. Tuberculosis. A comprehensive clinical reference. 1st ed. London: Saunders, Elsevier, 718–26
- Wang J, Xia Q. (2014). Alpha-lipoic acid-loaded nanostructured lipid carrier: sustained release and biocompatibility to HaCaT cells in vitro. Drug Deliv 21:328–41
- Wang Q, Cheng H, Zhou K, et al. (2013). Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv 20:331–7
- Wang S, Yang R, Yao H, et al. (2009). In vivo lymphatic targeting of methylene blue with microemulsion and multiple microemulsion. Drug Deliv 16:371–7
- Wang Y, Wu W. (2006). In situ evading of phagocytic uptake of stealth solid-lipid nanoparticles by mouse peritoneal macrophages. Drug Deliv 13:189–92
- Wang Z, Li Z, Zhang D, et al. (2015). Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv 22:79–85
- Weber S, Zimmer A, Pardeike J. (2014). Solid-lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm 86:7–22
- Yang L, Han J. (2000). Electronic structure of deformed carbon nanotubes. Phys Rev Lett 85:154–62
- Yashodhara B, Huat CB, Naik LN, et al. (2010). Multidrug and extensively drug-resistant tuberculosis from a general practice perspective. Infect Drug Resist 3:115–22
- Zahoor A, Sharma S, Khuller G. (2005). Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents 26:298–303
- Zhang Y, Liu J, Wang Y, et al. (2012). Immunotherapy using IL-2 and GM-CSF is a potential treatment for multidrug-resistant Mycobacterium tuberculosis. Sci China Life Sci 55:800–6
- Zhang Y, Wade MM, Scorpio A, et al. (2003). Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother 52:790–5
- Zhang Y, Yew W. (2009). Mechanisms of drug resistance in Mycobacterium tuberculosis. In: Chiang CY, ed. State of the art series. Drug-resistant tuberculosis. Number 1 in the series. Int J Tuberc Lung Dis 13:1320–30
- Zheng D, Dai W, Zhang D, et al. (2012). In vivo studies on the oridonin-loaded nanostructured lipid carriers. Drug Deliv 19:286–91
- Zumla A, Raviglione M, Hafner R, von Reyn CF. (2013). Current concepts. N Engl J Med 368:745–55