Abstract
Context: The systemic treatment of onychomycosis has been hampered by the reported side effects of antifungals in addition to the limited blood circulation to the affected nails. Topical ungual treatment would circumvent the limitations of systemic onychomycosis treatment.
Objective: Preparation and characterization of nail penetration enhancer containing nanovesicles (nPEVs) loaded with sertaconazole for topical treatment of onychomycosis.
Materials and methods: nPEVs were prepared using different nail penetration enhancers (N-acetyl-L-cysteine, thioglycolic acid, thiourea and ethanol) by the thin film hydration method, and characterized for their particle size, zeta potential, entrapment efficiency (EE%), elasticity, viscosity, physical stability and morphology. The selected nPEVs formula and the marketed Dermofix® cream were compared in terms of nail hydration, transungual drug uptake and antifungal activity against Trichophyton rubrum.
Results: N-acetyl-l-cysteine was the optimum nail penetration enhancer for incorporation within vesicles. nPEVs showed high EE% of sertaconazole ranging from 77 to 95%, a size ranging from 38–538 nm and a zeta potential ranging from +48 to +72 mV. The selected nPEVs formula displayed spherical morphology and good storage stability. Compared to the conventional marketed cream, the selected nPEVs formula showed 1.4-folds higher hydration and drug uptake enhancement into nail clippings. Furthermore, it showed significantly higher zone of inhibition for Trichophyton rubrum (20.9 ± 0.25 mm) than the marketed cream (11.6 ± 0.44 mm).
Conclusion: Nail penetration enhancer containing nanovesicles (nPEVs) present a very promising option, worthy of clinical experimentation on onychomycotic patients.
Introduction
Onychomycosis is a troublesome nail disease characterized by increased incidence of occurrence and recurrence. It is a fungal infection that causes thickening, discoloration of the nail (Hall et al., Citation1997; Sigurgeirsson & Ghannoum, Citation2012; Barot et al., Citation2012). As infection progresses, the nail becomes brittle and starts to break or totally comes out of the toe or finger.
Treatment of onychomycosis is challenging since infection is embedded within the nail and is difficult to reach. Current studies support the theory that topical therapies alone cannot effectively treat onychomycosis because of low-penetration rates of antifungal drugs through the nail plate (Baran & Kaoukhov, Citation2005; Lim et al., Citation2014); therefore, oral antifungal medications remain a mainstay in onychomycosis treatment because of the deep-seated and persistent nature of this type of infection. However, systemic treatment of onychomycosis is undesirable owing to the reported side effects of antifungal medications, the general immune-compromised nature of patients suffering from onychomycosis with higher incidence of drug interaction and hepatotoxicity, in addition to the limited blood circulation into the affected nail bed which necessitates the presence of large amount of the drug in the blood circulation (Repka et al., Citation2002; Miron et al., Citation2014). Therefore, the use of topical therapy for treatment of onychomycosis would circumvent the aforementioned systemic limitations (Hafeez et al., Citation2013) thereby, enhance patient compliance.
The major obstacle facing the topical delivery of antifungal agent through the nail is the low permeability of the nail plate; which is a highly ordered dense epidermal structure composed of about 80% sulfur-rich keratin. The nail plate is poorly permeable to drugs owing to the physical and chemical stability of the disulfide and hydrogen bonds found in keratin. In addition, the sandwich orientation of the keratin fibers and the presence of globular proteins in the nail hold the keratin fibers together to form the toughest barrier structure of the human body. Hence, it imparts high resistance to the passage of topically applied drugs (Chouhan & Saini, Citation2012; Shivakumar et al., Citation2012).
Penetration enhancer containing vesicles (PEVs) have demonstrated promising topical skin-delivery potential (Manca et al., Citation2013; Caddeo et al., Citation2014). PEVs are analogous to liposomes, but they contain a penetration enhancer such as oleic acid, labrasol and transcutol (Bseiso et al., Citation2015). In a previous study conducted by the same authors (Bsieso et al., Citation2015), PEVs loaded with sertaconazole were successfully prepared, demonstrating effectiveness in clinical treatment of skin fungal diseases. In an attempt to develop an effective topical delivery system for treatment of onychomycosis, the authors hypothesized that sertaconazole PEVs containing a nail penetration enhancer (creating the novel vesicles nPEVs) might be useful for topical treatment of onychomycosis.
Therefore, the aim of the current manuscript is to develop an effective topical delivery system suitable for treatment of onychomycosis (nPEVs), to eliminate the need for systemic treatment. The chosen nail penetration enhancers were N-acetyl-L-cysteine, thioglycolic acid, thiourea and ethanol. The prepared sertaconazole nPEVs were characterized for their EE%, particle size, zeta potential. Selected formula(e) were tested for their relative deformability, viscosity, morphology. Ex vivo nail hydration and transungual drug uptake were also tested in nail clippings collected from healthy human volunteers. The microbiological efficacy of nPEVs was tested in vitro on Trichophyton rubrum using the agar diffusion method. The nPEVs were also tested for their physical stability after refrigeration storage for 90 days.
Materials and Methods
Materials
Sertaconazole was supplied as a gift from Ferrer International Company, Barcelona, Spain. Phosphatidylcholine (Epikuron 200) was kindly supplied by Cargill Texturizing solutions, Deutschland GmbH & Co., Hamburg, Germany. Transcutol P® was kindly provided by Gattefosse' Co., France. Stearylamine and N-acetyl-l-cysteine were purchased from Sigma Chemical Co., St. Louis, MO. Spectra/Por dialysis membrane, 12 000–14 000 molecular weight cut off was purchased from Spectrum Laboratories Inc., Rancho Dominguez, Ontario, Canada. HPLC grade methanol and water were purchased from Fisher Scientific Co., Leicestershire, UK. Thioglycolic acid was purchased from Fine-Chem. Limited, India. Cellulose nitrate Whatman membrane filters (Millipore) with pore size 220 nm were purchased from Whatman Limited, Maidstone, England. ISOPORE™ membrane filters 50 nm VMTP02500 were purchased from EMD Millipore, Ireland. Dermofix® cream containing 2% sertaconazole nitrate was purchased from October Pharma, Egypt under production license of Ferrer International Co., Barcelona, Spain. Sabouraud dextrose agar (SDA) was purchased from LAB M Limited, Lancashire, UK. Uranyl acetate was purchased from Allied signal, Riedel-de Haen, Germany. All other chemicals were purchased from El-Nasr Pharmaceutical Co., Cairo, Egypt.
Methodology
Preparation of nPEVs using the thin film hydration method
Sertaconazole nPEVs were prepared using the thin-film hydration technique followed by sonication (Nasr et al., Citation2008a; Bsieso et al., Citation2015). Three nail penetration enhancers were chosen for the current study; N-acetyl-l-cysteine, thioglycolic acid and thiourea. The composition of the differently prepared nPEVs is shown in . In all prepared nPEVs, 100 mg of sertaconazole, 400 mg of phosphatidylcholine, 200 mg of transutol and 20 mg stearylamine in addition to a certain amount of nail penetration enhancer (as described in ) were accurately weighed and dissolved in chloroform: methanol mixture (2:1, v/v). The organic solvent mixture was evaporated (Rotary evaporator, Heidolph WB 2000, Germany) under reduced pressure at 40 °C and 150 rpm, so that a thin film of dry lipid with the drug was formed on the inner wall of the flask. The dry lipid film was hydrated with 5 ml of phosphate buffer (pH 7.4) with or without ethanol, as shown in through portion-wise addition. The dispersion was mechanically rotated for 30 minutes at 40 °C, followed by sonication (Julabo USR 3, Germany) for 15 minutes to reduce the size of the vesicles, and storage at 4 °C.
Table1. Composition of the prepared nPEVs.
Separation of unentrapped sertaconazole from nPEVs
The unentrapped sertaconazole was separated from nPEVs using the exhaustive dialysis method (Manconi et al., Citation2011, Citation2012). nPEVs were incorporated in dialysis tubings (Mwt cut off 12 000–14 000) and dialyzed against 1 liter of distilled water (pH = 7.04) at room temperature, which was changed twice during 24 hours. These conditions were chosen based on preliminary dialysis experiments (Bsieso et al., Citation2015).
Characterization of the prepared nPEVs
Determination of sertaconazole (EE%) in nPEVs
The amount of sertaconazole entrapped in nPEVs was calculated after disruption of the dialyzed nPEVs using methanol. An aliquot of nPEVs was mixed with an appropriate volume of methanol to obtain a clear solution, which was then covered with a parafilm to prevent methanol evaporation. The concentration of sertaconazole was determined spectrophotometrically (model 1800, Shimadzu Tokyo, Japan) at 302 nm after appropriate dilution (Bsieso et al., Citation2015). No interference was found from blank nPEVs at this wavelength. The EE% was calculated through the following equation (Nasr et al., Citation2008b):
Determination of the particle size and zeta potential of nPEVs
The size, polydispersity index and zeta potential of sertaconazole nPEVs were determined using Zetasizer nanoZS (model ZS3600, Malvern Instruments Ltd., Worcestershire, UK) after appropriate dilution (Bsieso et al., Citation2015).
Determination of the elasticity of sertaconazole nPEVs
Determination of nPEVs elasticity was performed via extrusion using a locally fabricated and validated stainless steel pressure filter holder. The vesicles were extruded through membrane filter with a pore size 50 nm at a constant pressure 0.17 MPa. Elasticity of nPEVs was expressed in terms of deformability index, which was calculated according to the following equation (Salama et al., Citation2012):
where D is the deformability index (ml/sec), j is the amount of vesicular dispersion extruded in ml, t is the time of extrusion in second, rv is the size of vesicles after extrusion (nm), rp is the pore size of the filter (nm).
Determination of the viscosity of nPEVs
The viscosity of nPEVs was determined using Anton Paar rheometer (model Physica MCR 301, 2.5 cm parallel plate, Austria) connected to spindle no PP25, and measured using 0.5 rpm at 37 °C (Lim et al., Citation2008).
Physical stability study of sertaconazole nPEVs
The selected nPEVs formulation was stored in tightly closed vials at 4 °C for 90 days, and re-evaluated for its particle size, polydispersity index and zeta potential (Bsieso et al., Citation2015). The change in EE% upon storage was tested as well.
Quantification of sertaconazole using high performance liquid chromatography
Determination of sertaconazole was achieved using a validated high-performance liquid chromatography (HPLC) method (model LC-10AD, Shimadzu, Kyoto, Japan) adopted from a previously reported work (Wang et al., Citation2009). The mobile phase was a mixture of methanol: water containing 0.2% formic acid (70:30). The flow rate of mobile phase was 1 ml/min and the injection volume was 10 µl. Samples were injected into a C18 column (Agilent Eclipse XDB, 5um, 4.6 × 250 mm) and the column effluent was monitored at 260 nm.
Determination of the morphology of nPEVs using transmission electron microscope (TEM)
Transmission electron microscopic (TEM) analysis (model JEM- 100 S, Joel, Tokyo, Japan) was carried out on the selected nPEVs formula in order to characterize the shape and ultrastructure of the vesicles (Caddeo et al., Citation2013), after negative staining with 1% uranyl acetate.
Nail hydration/transungual drug uptake of sertaconazole nPEVs
Nail clippings were obtained from healthy human volunteers (males and females, aged 25–50 years) using nail clippers. Only middle, index and ring finger nails were used for the current study, which are commonly used as an in vitro model for evaluation of transungual delivery (Shivakumar et al., Citation2012). Nail clippings were cleaned exhaustively by rinsing with distilled water for five times, wiping with tissue paper, and drying at 37 °C for 24 hours, then storage in air tight containers till further use (Pal et al., Citation2015).
For the nail hydration experiment, 50 mg nail clippings were placed in separate glass vials. Three groups were set for this experiment; group 1 (control group) in which the pre-weighed nail clippings were placed in 1 ml deionized water (pH 7.04), group 2 in which the nail clippings were placed in 1 ml of the selected nPEVs formula, and group 3 in which the nail clippings were immersed in 1 g of the marketed Dermofix® cream, Cairo, Egypt. The glass vials were sealed and incubated at room temperature for 24 hours and then the nail clippings were re-weighed after thorough tissue paper wiping to calculate weight gain (Chouhan & Saini, Citation2012). The hydration enhancement factor after 24 hours (HE24) was calculated according to the following equation:
For the transungual drug uptake experiment, quantification of the amount of sertaconazole which penetrated into the nail clippings was determined using HPLC. The nail clippings of groups 2 and 3 were washed three times with methanol to remove any traces of drug on the surface, then they were dissolved in 1 M sodium hydroxide (1 ml) by constant overnight stirring (Chouhan & Saini, Citation2012; Palliyil et al., Citation2013). After complete digestion of the nail clippings, the solutions were filtered by 0.22 µm syringe filter, and an aliquot was taken and diluted with methanol followed by HPLC analysis. The enhancement factor (EFnail); denoting the improvement of sertaconazole penetration into the nail clippings from nPEVs compared to Dermofix® cream was calculated according to the following equation (Chouhan & Saini, Citation2012; Palliyil et al., Citation2013):
Microbiological efficacy of nPEVs for the treatment of onychomycosis
The selected nPEVs formula and its blank counterpart prepared without the drug (control) and the commercial preparation (Dermofix® cream) were assayed for their antifungal activity against the fungal strain Trichophyton rubrum (RCMB Code 08325), grown on Sabouraud’s agar plate at 25 °C. After fungal growth, 0.1 ml of the fungal culture suspension was mixed in 9.9 ml liquid broth (without agar) and was inoculated for 24 hours in an incubator at 25 °C (Function line CO2, Heraeus company, Hanau, Germany).
To the sterile petri dishes in which solidified agar growth medium was placed, 1 ml of inoculated liquid broth containing fungal culture suspension was added, and the inoculum was spread uniformly over the solid agar surface by rotating the plate in clockwise and anticlockwise direction. Three wells were made in the middle of the plates with the help of sterile cork-borer, each well (6 mm internal diameter) was accurately filled with either 0.1 ml of the nPEVs formula, 0.1 ml of the control formula or an amount of Dermofix® cream equivalent to the sertaconazole dose in the 0.1 ml nPEVs formula. The plates were then incubated at 25 °C for three days in the incubator to allow fungal growth (Barot et al., Citation2012; Sahoo et al., Citation2014).
The antifungal activity was evaluated by measuring zones of inhibition of fungal growth surrounding the formulations. They were measured with scale in mm and the complete antifungal analysis was carried out under strict aseptic conditions. (Devi et al., Citation2011; Shahin et al., Citation2011; Verma et al., Citation2014).
Statistical analysis of data
All data were expressed as mean ± standard deviation, in which experiments were conducted in triplicate. One way ANOVA followed by the Tukey–Kramer post-test were carried out using Graphpad® Instat software (version 3.06) (La Jolla, CA).
Results and discussion
Preparation of sertaconazole nPEVs
Sertaconazole nPEVs were successfully prepared using the thin film hydration technique. The concentration of nail penetration enhancers was restricted to a maximum of 5%, since higher concentrations were reported to significantly soften the nail to a clinically undesirable level (Palliyil et al., Citation2013).
The utilized enhancers for the preparation for nPEVs were transcutol, N-acetyl-l-cysteine, thiourea, thioglycolic acid and ethanol. Transcutol was chosen as the main penetration enhancer for nPEVs based on a previously conducted work by the same authors, concluding that transcutol-based PEVs displayed the best storage properties compared to labrasol and oleic acid PEVs (Bsieso et al., Citation2015). In addition, transcutol was reported to increase the permeation through bovine hooves membrane which is structurally similar to the human nails, through the induction of conformational changes in the keratin structure (Shivakumar et al., Citation2012). The chosen nail penetration enhancers (N-acetyl-L-cysteine, thiourea and thioglycolic acid) were reported to increase the flux across the nail plate through reduction of disulphide linkages in the keratin of the nail, associated with pore formation and consequent swelling and softening of the nail plate, leading to reduction of nail barrier integrity (Brown et al., Citation2009; Nogueiras-Nieto et al., Citation2013; Palliyil et al., Citation2013; Miron et al., Citation2014). Ethanol was reported to be a nail uptake enhancer as well (Gupta & Paquet, Citation2013). The positive charge inducer stearylamine was also included to augment transungual permeation, owing to the negatively charged nature of the nails at pH 7.4 (Hao and Li, Citation2008).
Upon preparation of nPEVs, formulae N7-N12 prepared using thioglycolic acid showed instant aggregation, similar to what was reported with Palliyil et al., (Citation2013) when encapsulating the drug ciclopirox olamine, hence, they were excluded from further experiments. Furthermore, formulae N13-N18 prepared using thiourea displayed an overall micrometer sized aggregates, hence they were excluded from further experiments as well.
Determination of sertaconazole (EE%) in nPEVs
shows the EE% results for sertaconazole nPEVs prepared using N-acetyl-l-cysteine as nail penetration enhancer. The EE% of sertaconazole in nPEVs ranged from 77 to 95%. These high EE% values could be attributed to the lipophilicity of sertaconazole (log P = 6.2), leading to its effective incorporation within lipid bilayers, in addition to the presence of solubilizers in the differently prepared formulations.
Table 2. Characterization of sertaconazole nPEVs prepared using N-acetyl-l-cysteine as nail penetration enhancer.
As evident in , the increase in the concentration of N-acetyl-l-cysteine from 2 to 5% led to an overall decrease in EE% of sertaconazole in nPEVs, which might be attributed to its possible displacement from the phospholipid bilayers by the N-acetyl-l-cysteine, which was reported to have some sort of affinity to lipid materials (Khattar et al., Citation2012).
By further inspection of , it was evident that the increase in ethanol concentration from 0–20% led to a decrease in EE% of sertaconazole, which might be attributed to the leakiness of the vesicles (Verma & Pathak, Citation2012). However, further increase in ethanol concentration to 40% led to an increase in the EE% of sertaconazole, which might be attributed to the concomitant increase in the particle size of formulae N3 and N6.
Determination of the particle size and zeta potential of nPEVs
As shown in , the particle size of N-acetyl-l-cysteine nPEVs ranged from 38 to 538 nm. A significant increase in particle size (p < 0.05) was observed upon increasing the ethanol concentration from 0 to 40%. This could be ascribed to the gel forming potential of phospholipid-ethanol-aqueous mixtures at high ethanol concentration and the induced interdigitation of phospholipid bilayers by ethanol (McIntosh et al., Citation2001; Polozova et al., Citation2005).
As also shown in , the PDI values of nPEVs did not exceed 0.4 (except for N6), suggesting a homogenous and monodisperse population. The zeta potential values ranged from +48 to +72 mV owing to the presence of the positive charge inducer stearylamine. The high magnitude of charge is indicative of good stability against vesicle aggregation and fusion.
Determination of the elasticity of sertaconazole nPEVs
Formulae N1, N2 and N5 were not tested for their deformability since their particle size was smaller than the utilized membrane (50 nm). As shown from the results in , formula N4 was the most deformable one. The deformability index of formula N4 was almost double a control formula prepared without transcutol and N-acetyl-l-cysteine (0.93 ml/s).
Determination of the viscosity of nPEVs
As shown in , the viscosity of nPEVs ranged from 1.31 to 277 cp. The nPEVs dispersions displayed higher viscosity values than water owing to the existence of vesicular lamellar structures occupying high hydrodynamic volume (Bsieso et al., Citation2015). The significantly higher viscosity (p < 0.05) encountered with nPEVs prepared using 40% ethanol might be attributed to the ethanolic induced interdigitation of the phospholipid bilayer (McIntosh et al., Citation2001).
Physical stability study of sertaconazole nPEVs
Formula N4 was selected for stability and further characterization studies, owing to its relatively higher deformability than the other nPEVs and its high content of N-acetyl-l-cysteine (5%). Upon storage, only a slight increase in particle size and PDI was observed (73.37 ± 2.2 nm and 0.37 ± 0.04 respectively). Furthermore, only a slight decrease in zeta potential to +55.1 ± 2.4 mV occurred. The EE% of sertaconazole after storage was the same as before storage (92 ± 2.83). This suggests good physical stability of nPEVs upon refrigeration storage.
Determination of the morphology of nPEVs using transmission electron microscope (TEM)
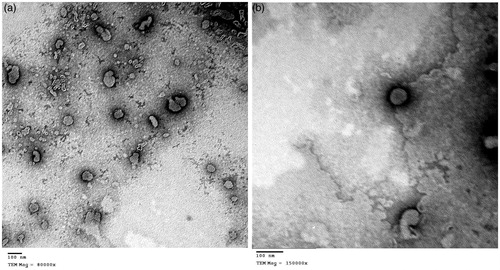
shows the outline and the core of well identified vesicular structures displaying spherical morphology, similar to the morphology of sertaconazole PEVs prepared by the same authors (Bsieso et al., Citation2015).
Figure 1. (a) Negative stain transmission electron micrograph of formula N4 at a magnification of 80 000× showing the outline and core of spherical vesicular structures. (b) Negative stain transmission electron micrograph of formula N4 at a magnification of 150 000×, showing a perfect spherical structure of nPEV vesicle.
Nail hydration/transungual drug uptake of sertaconazole nPEVs
Regarding the nail hydration experiment, the average weight gain for groups 1 (control), group 2 (formula N4) and group 3 (Dermofix® cream) was 34.43 ± 8.2 53.91 ± 6.1 and 37.22 ± 8.2 mg; corresponding to hydration enhancement factor HE24 values of 1.57 and 1.08 for N4 and the marketed cream respectively. The significantly higher weight gain encountered with formula N4 compared to Dermofix® cream is probably attributed to the aqueous nature of the former. This was an advantage in our case; since water was reported to be the best nail plasticizer, leading to an increased flux of drugs across the nails (Gunt & Kasting, Citation2007; Hafeez et al., Citation2013).
Regarding the transungual uptake of sertaconazole, effective partitioning of the drug into the nail clippings was observed, in which the amount of sertaconazole uptaken by the nail clippings exposed to formula N4 and Dermofix® cream were 95.5 ± 3.9% and 69.4 ± 5.4%, respectively, corresponding to nail uptake enhancement factor EFnail for N4 of 1.38 compared to the cream. This demonstrates the high affinity of sertaconazole to the nail clippings, in which nPEVs allowed the drug to penetrate through the nail in significant amounts, capable of treatment of the deeply seated natured disease onychomycosis.
Microbiological efficacy of nPEVs for the treatment of onychomycosis
Trichophyton rubrum was selected for the in vitro antifungal activity test, as it is considered the main causative dermatophyte for onychomycosis (Sigurgeirsson & Ghannoum, Citation2012; Miron et al., Citation2014). Sertaconazole was reported to exhibit both fungistatic and fungicidal activities on the aforementioned species (Carrillo-Munoz et al., Citation2011).
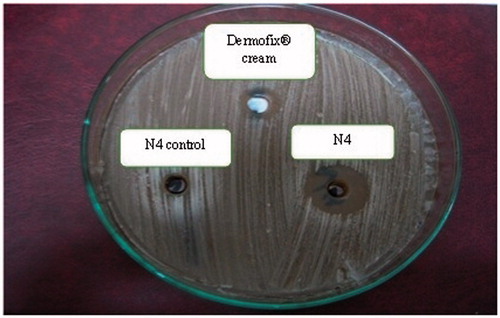
The in vitro antifungal activity of the formulations, i.e. vesicular dispersion (N4), plain unmediated formula (control) and commercial product (Dermofix® cream) was investigated through the agar diffusion technique (Devi et al., Citation2011). The clear rings appearing around the dishes are the “zones of inhibition”. The larger the zone of inhibition, the more effective the formulation is.
Interestingly, the plain unmedicated formula (control) showed a mean zone of inhibition (5.3 ± 0.58 mm) against Trichophyton rubrum. This may be explained by the fact that cysteine and its derivatives (N-acetyl-l-cysteine) were reported to exhibit a therapeutic potential against fungal infections (Galgóczy et al., Citation2009).
Formula N4 showed a mean zone of inhibition (20.9 ± 0.25 mm) which was significantly greater than the mean zone of inhibition exhibited by the commercial preparation Dermofix® cream (11.6 ± 0.44 mm) (p < 0.05) as shown in . This may be caused by the higher release and diffusion potential of sertaconazole from formula N4, coupled with the antifungal potential of N-acetyl-l-cysteine in comparison with the commercial preparation, and hence greater partitioning of sertaconazole from the preparation (Shahin et al., Citation2011).
Figure 2. Microbiological activity of the selected nPEVs formula N4, the marketed Dermofix® cream and the blank N4 against Tricophyton rubrum using agar diffusion technique.
Worthy to note that there were two successful attempts for the use of vesicles in the topical treatment of onychomycosis using transfersomal, liposomal and ethosomal terbinafine (Sigurgeirsson & Ghannoum, Citation2012; Tanriverdi & Ozer, Citation2013). Our findings confirm that nail penetration enhancer containing nanovesicles (nPEVs) present a very promising ungual delivery system, worthy of clinical experimentation on onychomycotic patients.
Conclusion
The incorporation of the penetration enhancer transcutol, with the nail penetration enhancer N-acetyl-l-cysteine and the positive-charge inducer stearylamine within aqueous deformable-natured nanovesicles (nPEVs) proved to be a promising combination for enhancing the transungual delivery of sertaconazole. The described nPEVs open a new approach for topical treatment of nail-related diseases such as onychomycosis. Clinical studies to assess the efficacy of sertaconazole nPEVs in onychomycotic patients are currently in progress.
Acknowledgements
The authors would like to thank Ferrer international company (Spain), Cargill texturizing solutions company (Germany) and Gattefosse' company (France) for their kind supply of chemicals.
Declaration of interest
The authors report no conflicts of interest.
References
- Baran R, Kaoukhov A. (2005). Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venereol 19:21–9
- Barot BS, Parejiya PB, Patel HK, et al. (2012). Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. AAPS PharmSciTech 13:184–92
- Brown MB, Khengar RH, Turner RB, et al. (2009). Overcoming the nail barrier: a systematic investigation of ungual chemical penetration enhancement. Int J Pharm 370:61–7
- Bseiso EA, Nasr M, Sammour O, Abd El Gawad NA. (2015). Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol 81:457–63
- Bsieso EA, Nasr M, Moftah NH, et al. (2015). Could nanovesicles containing a penetration enhancer clinically improve the therapeutic outcome in skin fungal diseases? Nanomedicine (Lond) 10:2017–31
- Caddeo C, Diez-Sales O, Pons R, et al. (2014). Topical anti-inflammatory potential of quercetin in lipid-based nanosystems: in vivo and in vitro evaluation. Pharm Res 31:959–68
- Caddeo C, Manconi M, Fadda AM, et al. (2013). Nanocarriers for antioxidant resveratrol: formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf B Biointerfaces 111:327–32
- Carrillo-Munoz AJ, Tur-Tur C, Cardenes DC, et al. (2011). Sertaconazole nitrate shows fungicidal and fungistatic activities against Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum, causative agents of tinea pedis. Antimicrob Agents chemother 55:4420–1
- Chouhan P, Saini TR. (2012). Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm 436:179–82
- Devi M, Kumar SM, Mahadevan N. (2011). Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Rec Adv Pharm Res 4:37–46
- Galgóczy L, Kovács L, Krizsán K, et al. (2009). Inhibitory effects of cysteine and cysteine derivatives on germination of sporangiospores and hyphal growth of different Zygomycetes. Mycopathologia 168:125–34
- Gunt HB, Kasting GB. (2007). Effect of hydration on the permeation of ketoconazole through human nail plate in vitro. Eur J Pharm Sci 32:254–60
- Gupta AK, Paquet M. (2013). Improved efficacy in onychomycosis therapy. Clin Dermatol 31:555–63
- Hafeez F, Hui X, Chiang A, et al. (2013). Transungual delivery of ketoconazole using novel lacquer formulation. Int J Pharm 456:357–61
- Hall M, Monka C, Krupp P, O’Sulllivan D. (1997). Safety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patients. Arch Dermatol 133:1213–19
- Hao J, Li SK. (2008). Mechanistic study of electroosmotic transport across hydrated nail plates: effects of pH and ionic strength. J Pharm Sci 97:5186–97
- Khattar H, Singh S, Murthy RSR. (2012). Formulation and characterization of nano-lipid carrier dry powder inhaler containing ciprofloxacin hydrochloride and N-acetyl cysteine. Int J Drug Deliv 4:316–25
- Lim EH, Kim HR, Park YO, et al. (2014). Toenail onychomycosis treated with a fractional carbon-dioxide laser and topical antifungal cream. J Am Acad Dermatol 70:918–23
- Lim GJ, Ishiuji Y, Dawn A, et al. (2008). In vitro and in vivo characterization of a novel liposomal butorphanol formulation for treatment of pruritis. Acta Derm Venereol 88:327–30
- Manca ML, Manconi M, Falchi AM, et al. (2013). Close-packed vesicles for diclofenac skin delivery and fibroblast targeting. Colloids Surf B Biointerfaces 111:609–17
- Manconi M, Caddeo C, Sinico C, et al. (2012). Penetration enhancer-containing vesicles: composition dependence of structural features and skin penetration ability. Eur J Pharm Biopharm 82:352–9
- Manconi M, Sinico C, Caddeo C, et al. (2011). Penetration enhancer containing vesicles as carriers for dermal delivery of tretinoin. Int J Pharm 412:37–46
- McIntosh TJ, Lin H, Li S, Huang C. (2001). The effect of ethanol on the phase transition temperature and the phase structure of monounsaturated phosphatidylcholines. Biochim Biophys Acta 1510:219–30
- Miron D, Cornelio R, Troleis J, et al. (2014). Influence of penetration enhancers and molecular weight in antifungals permeation through bovine hoof membranes and prediction of efficiency in human nails. Eur J Pharm Sci 51:20–5
- Nasr M, Mansour S, Mortada ND, Elshamy AA. (2008a). Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul 25:499–512
- Nasr M, Mansour S, Mortada ND, El Shamy AA. (2008b). Lipospheres as carriers for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. AAPS PharmSciTech 9:154–62
- Nogueiras-Nieto L, Begona Delgado-Charro M, Otero-Espinar FJ. (2013). Thermogelling hydrogels of cyclodexterin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: application to the delivery of triamcinolone acetonide and ciclopirox olamine. Eur J Pharm Biopharm 83:370–7
- Pal P, Thakur RS, Ray S, Mazumder B. (2015). Design and development of a safer non-invasive transungual drug delivery system for topical treatment of onychomycosis. Drug Dev Ind Pharm 41:1095–9
- Palliyil B, Lebo DB, Patel PR. (2013). A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS PharmSciTech 14:682–91
- Polozova A, Li X, Shangguan T, et al. (2005). Formation of homogenous unilamellar liposomes from interdigitated matrix. Biochim Biophys Acta 1668:117–25
- Repka MA, O’Haver J, See CH, Gutta K, Munjal M. (2002). Nail morphology studies as assessments for onychomycosis treatment modalities. Int J Pharm 245:25–36
- Sahoo S, Pani NR, Sahoo SK. (2014). Effect of microemulsion in topical sertaconazole hydrogel: in vitro and in vivo study. Drug deliv. [Epub ahead of print]. DOI:10.3109/10717544.2014.914601
- Salama HA, Mahmoud AA, Kamel AO, et al. (2012). Phospholipid based colloidal poloxamer-nanocubic vesicles for brain targeting via the nasal route. Colloids Surf B Bionterfaces 100:146–54
- Shahin M, Hady SA, Hammad M, Mortada N. (2011). Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS PharmSciTech 12:239–47
- Shivakumar HN, Juluri A, Desai BG, Murthy SN. (2012). Ungual and transungual drug delivery. Drug Dev Ind Pharm 38:901–11
- Sigurgeirsson B, Ghannoum M. (2012). Therapeutic potential of TDT067 (terbinafine in Transfersome®): a carrier – based dosage form of terbinafine for onychomycosis. Expert Opin Investing Drugs 21:1549–62
- Tanriverdi ST, Ozer O. (2013). Novel topical formulations of Terbinfaine-HCl for treatment of onychomycosis. Eur J Pharm Sci 48:628–36
- Verma P, Pathak K. (2012). Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine 8:489–96
- Verma S, Bhardwaj A, Vij M, et al. (2014). Oleic acid vesicles: a new approach for topical delivery of antifungal agent. Artif Cells Nanomed Biotechnol 42:95–101
- Wang Y, Pang L, Wu M, Ou N. (2009). A validated LC-MS/MS method for determination of sertaconazole nitrate in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 877:4047–50

