Abstract
Context: Doxorubicin (DOX)-loaded folate-targeted poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) [P(HB-HO)] nanoparticles [DOX/FA-PEG-P(HB-HO) NPs] have potential application in clinical treatments for cervical cancer due to specific affinity of folate and folate receptor in HeLa cells.
Objective: The aim of this study was to develop an optimized formulation for DOX/FA-PEG-P(HB-HO) NPs, and investigate the targeting and efficacies of the nanoparticles.
Materials and methods: DOX/FA-PEG-P(HB-HO) NPs were prepared by W1/O/W2 solvent extraction/evaporation method, and an orthogonal experimental design [L9 (34)] was applied to establish the optimum conditions. The physico–chemical characteristics, microscopic observation and in vivo antitumor study of the nanoparticles were evaluated.
Results: The optimum formulation was obtained with DOX 10% (w/v), FA-PEG-P(HB-HO) 6.5% (w/v), PVA 3%(w/v) and oil phase/internal water phase volume ratio of 3/1. The size distribution, drug loading and encapsulation efficiency of the optimized nanoparticles were 150–350 nm, 29.6 ± 2.9% and 83.5 ± 5.7%, respectively. In vitro release study demonstrated that 80% of the drug could release from the nanoparticles within 11 days. Furthermore, in vitro microscopic observation and in vivo antitumor study showed that DOX/FA-PEG-P(HB-HO) NPs could inhibit HeLa cells effectively, and the tumor inhibition rate (TIR) in vivo was 76.91%.
Discussion and conclusions: DOX/FA-PEG-P(HB-HO) NPs have been successfully developed and optimized. In vitro drug release study suggested a sustained release profile. Moreover, DOX/FA-PEG-P(HB-HO) NPs could effectively inhibit HeLa cells with satisfying targeting, and reduce side effects and toxicity to normal tissues. DOX/FA-PEG-P(HB-HO) NPs were superior in terms of inhibiting HeLa tumor over non-targeted formulations therapy.
Introduction
Human cervical cancer is one of the most common types of cancer in females worldwide and is the leading cause of cancer deaths among women of reproductive age in developing countries (Joo & Kim, Citation2002). According to the International Agency for Research on Cancer, certain risk factors account for the development of cervical cancer, including an inordinate sexual behavior, certain virus infection, weakened immune system function and smoking and so on (Song et al., Citation2015). In the currently clinical treatments for cervical cancer, chemotherapy drugs play very important roles in killing cancer cells. However, these drugs often bring cancer patients with great pain owing to their drastic side effects (Zhao et al., Citation2012). Thus, it is necessary to design and develop a novel anticancer drug formulation.
Doxorubicin (DOX), an anticancer agent, is isolated from a soil bacterium namely Streptomyces peucetius (Danhier et al., Citation2014). It is widely used against cervical cancer, breast cancer, acute lymphoblastic leukemia, lymphoma, etc. (Zhang et al., Citation2007). DOX has been shown to induce double-strand DNA breaking and cell death (Forrest et al., Citation2012; Danhier et al., Citation2014). However, DOX exhibits cardiotoxicity, myelosuppression and immunosuppression, and limits its clinical application (Yi et al., Citation2006; Kim et al., Citation2009; Wu et al., Citation2013). Various formulations are available to reduce these side effects and improve therapeutic efficacy, e.g. using of microspheres, micelles, nanoparticles or targeted nanoparticles (Yoo & Park, Citation2004; Tan et al., Citation2005; Sadighian et al., Citation2014; Joseph et al., Citation2015). Polymers may also play an important role in preparing various drug formulations. Poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) [P(HB-HO)] possessing good physical–chemical and biological properties is derived from Sinorhizobium fredii (Zhao et al., Citation2006), and it can be utilized as drug carrier (Zhang et al., Citation2013).
The aim of the present study was to develop an optimized formulation for preparation of DOX/FA-PEG-P(HB-HO) NPs, and evaluate characterizations of the nanoparticles. Furthermore, the targeting and efficacies of the nanoparticles were investigated by in vitro microscopic observation and in vivo antitumor activity.
Materials and methods
Materials
Poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) [P(HB-HO)] (Mw 1.85 × 105) containing 10 mol% of 3-hydroxyoctanoate units was fermented by S. fredii. DOX was purchased from Wanle pharmaceuticals Inc. (Shenzhen, China). Folic acid (FA) was product of Sigma-Aldrich (St. Louis, MO). The targeting material [FA-PEG-P(HB-HO)] was synthesized as described previously (Zhang et al., Citation2010). Fetal bovine serum (FBS) was purchased from Gibco (Life Technologies AG, Basel, Switzerland). Other chemicals were of analytical grade. Millipore water was produced by the Milli-Q Plus System (Millipore Corporation, Breford, MA). HeLa cells (a human cervical carcinoma cell line) with overexpressed folate receptors on the cell surfaces were purchased from Cell Bank of Chinese Academy of Sciences (Beijing, China).
Female BALB/c nude mice (4–6 weeks of age) were provided by Chinese Academy of Medical Sciences (Beijing, China) and maintained under a pathogen-free condition.
Methods
Preparation of DOX/FA-PEG-P(HB-HO) NPs
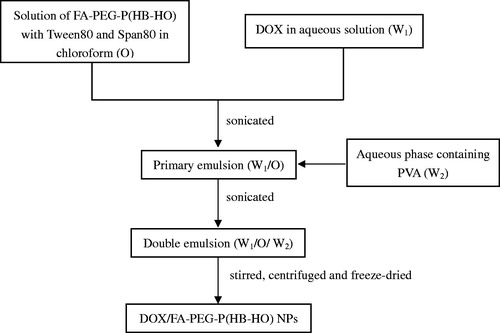
DOX/FA-PEG-P(HB-HO) NPs were prepared by W1/O/W2 solvent extraction/evaporation method (Zhang et al., Citation2010). A schematic representation of the process of DOX/FA-PEG-P(HB-HO) NPs preparation is shown in . Briefly, FA-PEG-P(HB-HO) was dissolved in chloroform containing emulsifiers. DOX in aqueous solution (W1) was added to the FA-PEG-P(HB-HO) solution (O), and the mixture was sonicated using a sonicator. This primary emulsion (W1/O) was then added to an aqueous phase containing an emulsifier (W2), and the mixture was sonicated to form the W1/O/W2 emulsion. The resulting double emulsion was stirred and centrifuged. Finally, the sample was washed and freeze-dried to obtain the NPs powder.
Orthogonal experiments designing
Orthogonal experimental design was applied to evaluate the influence of main variables on the nanoparticles properties. Four main variables, containing DOX concentration (A, %), volume ratio of O/W1 (B, %), PVA concentration (C, %), and FA-PEG-P(HB-HO) concentration (D, %), are presented in . The design was composed of four variables set at three-levels each, where the encapsulation efficiency (EE) was the dependent variables. Nine groups of experiments were designed by orthogonal design, as shown in .
Table 1. Factors and levels of the orthogonal design L9 (34).
Table 2. Design of orthogonal design L9 (34).
Physico-chemical characterizations of DOX/FA-PEG-P (HB-HO) NPs
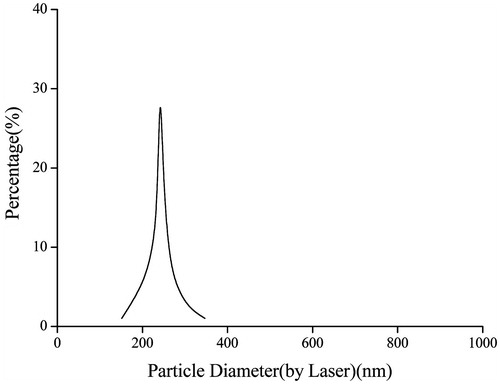
The in vitro physico-chemical characterizations of DOX/FA-PEG-P(HB-HO) NPs, including size distribution, drug loading (DL), EE and in vitro release study, were determined by methods as follows. Briefly, the size distribution of the NPs was measured by a laser particle size analyzer (Eye Tech, Ankersmid Ltd., Nijverdal, Holland).
DOX contents encapsulated in the nanoparticles were determined by measuring the UV absorbance using a UV–Vis spectrophotometer (U-2010 spectrophotometer, Hitachi, Tokyo, Japan) at 498.5 nm. The DL and EE were measured after extracting DOX from the nanoparticles. A known quantity of DOX/FA-PEG-P(HB-HO) NPs was dissolved in a mixed solution of chloroform and methanol (v/v 3:1). The DL and EE are expressed as the amount of DOX loaded in a unit weight of DOX/FA-PEG-P(HB-HO) NPs and the ratio of calculated and theoretical contents of DOX, respectively.
The dynamic dialysis method was employed for in vitro release study. In brief, 100 mg of DOX/FA-PEG-P(HB-HO) NPs was added to the pretreated dialysis bag, 10 mL PBS (pH 7.4) was then added. The dialysis bag was kept in beaker flasks filling 100 mL PBS (pH 7.4) and shaken at 100 rpm and 37 ± 1 °C. Five microliters of medium was collected at the pre-determined intervals and the equal volume of fresh PBS was replenished. DOX concentration was determined in triplicate at 480 nm on the UV-Vis spectrophotometer (U-2010 spectrophotometer, Hitachi, Tokyo, Japan).
In vitro microscopic observation of HeLa cells treated with DOX formulations
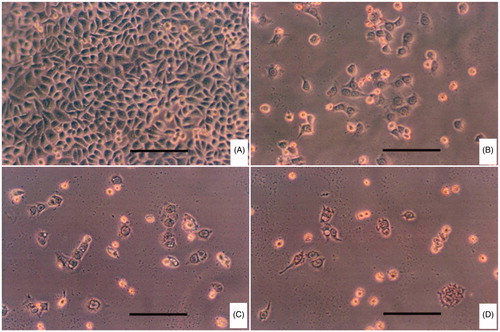
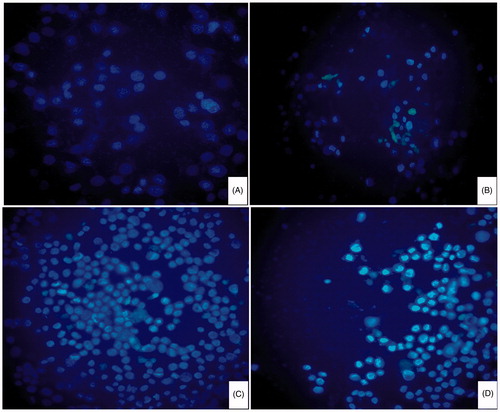
HeLa cells were incubated in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin–streptomycin solution at 37 °C, 5% CO2. The cells harvested in a logarithmic growth phase were seeded on six-well plates at 2 × 104 cells per well. After the cells reached 70–80% confluency, they were maintained in RPMI 1640 medium (without FA) supplemented with 10% FBS in 5% CO2 incubator (Thermo, Waltham, MA) at 37 °C for 24 h. The cells were then incubated with fresh medium containing DOX/FA-PEG-P(HB-HO) NPs, DOX/P(HB-HO) NPs or free DOX for 12 h. After the treatment, the cells were washed, fixed with 10% formalin and stained with DAPI. The apoptotic HeLa cells were visualized by Olympus fluorescence microscope (BX51, Olympus, Tokyo, Japan).
In vivo antitumor activity
Animal experiments were carried out in compliance with the ethical standards required by law and according to the guidelines for the use of experimental animals in China. Female BALB/c nude mice were subcutaneously inoculated with HeLa cells resuspended in normal saline at 1 × 107 cells/20 μL in the right limb armpits and the tumors were allowed to grow. When the tumor volume reached approximately 50 mm3, the mice were randomly divided into four groups, each group having six mice. Various formulations (group I: normal saline; group II: free DOX; group III: DOX/P(HB-HO) NPs; group IV: DOX/FA-PEG-P(HB-HO) NPs) were injected intravenously via the tail vein every two days for a total of four times. The amounts of DOX administered were 5 mg/kg in all of the formulations. The mice were sacrificed on day 7 after the last injection, and then the tumors and organs (heart, liver, spleen, lungs and kidneys) were excised and weighted. The tumor inhibition rate (TIR) and organ/body weight ratio (OBR) were calculated as follows:
(1)
(2)
where Wt is the average tumor weight of the treated group, Wc is the average tumor weight of the control group, Wo and Wb are the organ weight and body weight of the mice after treatment, separately.
Pathological observation
The tumors were fixed in 10% formalin for 48 h and embedded in paraffin, then sliced and placed onto glass slides. The histological analysis was carried out with routine hematoxylin and eosin (H&E) staining, and photographed under an Olympus microscope (BX51, Olympus, Tokyo, Japan).
Results and discussion
Formulation optimization using orthogonal experimental design
Based on previous experiments of univariate analysis (data not shown), an orthogonal experimental design at four factors and three levels [L9 (34)] was performed to optimize the formulation compositions. The formulation composition factors and their levels are given in . The dependent variable considered was EE. The results of an orthogonal test are presented in . The range of EE observed from the orthogonal experimental runs was 48.48–84.62%. However, we could not select the best formulation composition based only on these results in , and a further orthogonal analysis was conducted. Thus, the X (X1, X2, X3) and S values were calculated. In , the factors influencing the EE are listed in decreasing order as follows A > D > B > C according to the S value. In addition, the influences on the EE at individual levels within each factor can be ranked as: A: 1 > 2 > 3; B: 2 > 1 > 3; C: 2 > 3 > 1; D: 2 > 1 > 3. The optimum formulation should be A1D2B2C2 [the concentrations of DOX, FA-PEG-P(HB-HO) and PVA were 10%, 6.5% and 3%, the volume ratio of O/W1 was 3:1].
Based on previous univariate analysis and orthogonal experimental design, the final optimal conditions of preparing nanoparticles were summarized. The oil phase (O) was prepared by dissolving FA-PEG-P(HB-HO) (6.5%) in chloroform containing Tween80 (5%) and Span80 (1%). The internal water phase (W1) was prepared by dissolving DOX (10%) in distilled water. The O and W1 phase were mixed and soni-cated (200 W, 180 s) to make a primary emulsion (W1/O). The W1/O emulsion was then added to an external aqueous phase (W2) containing PVA (3%), and the mixture was sonicated (200 W, 180 s) to form the W1/O/W2 emulsion. The final emulsion was stirred, centrifuged (15 000 rpm for 10 min) and freeze-dried.
Physico-chemical characterizations of DOX/FA-PEG-P(HB-HO) NPs
The size distribution of the optimized nanoparticles was found to be in the range of 150–350 nm (). It can be suggested that the nanoparticles with such size are safe and stable for intravenous injection. The DL and EE were 29.6 ± 2.9% and 83.5 ± 5.7%, respectively. The high DL and EE may increase the treatment efficacy at the targeted sites.
The in vitro release study of DOX/FA-PEG-P(HB-HO) NPs is shown in . During the first day, the DOX in the nanoparticles exhibited a small burst release, which accounted for less than 20% of the total drug encapsulated. The initial burst is caused by the rapid release of DOX adhered on the surface of DOX/FA-PEG-P(HB-HO) NPs. Thereafter, the cumulative release amount was increased to nearly 50% on the fifth day. Within 11 days, 80% of the loaded drugs released from the nanoparticles (). The drug release behavior might be closely related to the encapsulation manner and the distribution of DOX in the nanoparticles. The data obtained from in vitro release studies of DOX/FA-PEG-P(HB-HO) NPs were fitted to various kinetic equations such as Higuchi, zero-order and first-order models (). The Higuchi equation was best fitted for DOX release from DOX/FA-PEG-P(HB-HO) NPs as indicated by a higher correlation coefficient (R2 = 0.9938) compared with zero-order (R2 = 0.8263) and first-order models (R2 = 0.9780) (). DOX/FA-PEG-P(HB-HO) NPs showed a desirable sustained release property, suggesting that the drug incorporated into the nanoparticles is retained for a certain amount of time, which allows for slow release into the medium (Wei et al., Citation2012). Therefore, DOX in the nanoparticles could be released in a sustained manner as shown in .
Figure 3. In vitro release study of DOX/FA-PEG-P(HB-HO) NPs. (A) In vitro cumulative release of DOX from DOX/FA-PEG-P(HB-HO) NPs in pH 7.4 PBS, (B) Higuchi model of DOX/FA-PEG-P(HB-HO) NPs, (C) zero-order model of DOX/FA-PEG-P(HB-HO) NPs and (D) first-order model of DOX/FA-PEG-P(HB-HO) NPs.
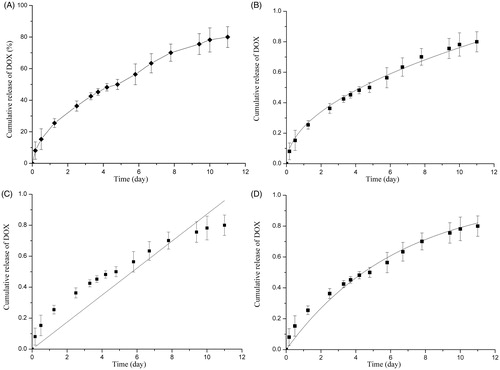
Table 3. Fitting of drug release data of optimized formulation according to various models.
In vitro microscopic observation of HeLa cells treated with DOX formulations
In order to explore the effects of three DOX formulations on cellular morphologies, HeLa cells were stained (or not stained) with DAPI 12 h after exposure to different formulations and then viewed under microscope ( and ). The cells without any treatments were used as control. There was almost no apoptotic evidence in the control group, and the nuclei were smooth. Cells in other three treated groups exhibited nuclear condensation (smaller in size) and fragmentation (irregular shape), or cytoplasmic vacuolization and cell shrinkage in varying degrees, all of which are characteristics of cells undergoing apoptotic death (Zhang et al., Citation2011). In the DOX/FA-PEG-P(HB-HO) NPs group, the typical apoptotic morphology was more evident than that of cells treated with DOX or DOX/P(HB-HO) NPs. The results demonstrated that DOX/FA-PEG-P(HB-HO) NPs could induce apoptosis and inhibition of HeLa cells effectively. To further establish the inhibition of DOX/FA-PEG-P(HB-HO) NPs to HeLa cells, in vivo antitumor study has been conducted.
In vivo antitumor activity and pathological observation
The therapeutic efficacy of DOX/FA-PEG-P(HB-HO) NPs was investigated in HeLa tumor-bearing mice. shows the tumor weights and TIRs of the four groups. All of the treated groups (II, III, IV) had statistical differences in tumor weights when compared to the normal saline group (p < 0.05). A significant difference (p < 0.01) was found in the tumor growth between the control group and DOX/FA-PEG-P(HB-HO) NPs group. These results showed that the TIR of DOX/FA-PEG-P(HB-HO) NPs group (76.91%) was the smallest among treated groups, followed by DOX/P(HB-HO) NPs group (53.44%) and free DOX group (31.35%). The higher antitumor efficacy of DOX/FA-PEG-P(HB-HO) NPs might be due to enhanced permeability and retention effect, and high binding affinity of folate in the nanoparticles and folate receptor in HeLa cells (Ruan et al., Citation2015; Yang et al., Citation2015), resulting in higher concentration and accumulation of the folate-targeted nanoparticles in HeLa tumors. In , free DOX group exhibited lower OBR than the control group (p < 0.05) due to its toxic side effects (Meng et al., Citation2011). However, there was no significant difference (p > 0.05) in the OBR of control group and nanoparticles-treated groups (III and IV). No mice died during the treatment. The results demonstrated that the side effects and toxicity of DOX to normal tissues might be reduced by the formulation of nanoparticles, especially the folate-targeted nanoparticles.
Table 4. The results of in vivo antitumor activity.
Table 5. Organ/body weight ratios of different groups.
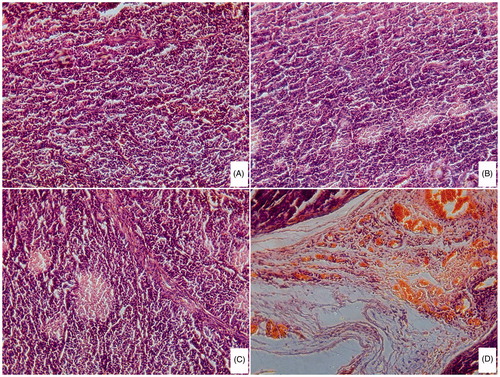
In H&E staining, biological samples are stained with two dyes: hematoxylin is a basic purple-blue dye that binds to basophilic structures (such as cell nucleus) and eosin is an acidic bright pink dye that binds to eosinophilic structures (such as cytoplasmatic components) (Yuan et al., Citation2008). Necrosis areas after treatment were colored pink, which indicated the effectiveness of different DOX formulations. presents the results of histological examinations using H&E staining of tumors subjected to saline, free DOX, DOX/P(HB-HO) NPs and DOX/FA-PEG-P(HB-HO) NPs, respectively. Different degrees of necrosis areas were observed in these sections, in the following order: DOX/FA-PEG-P(HB-HO) NPs group > DOX/P(HB-HO) NPs group > free DOX group > nomal saline group. The tumor cells in DOX/FA-PEG-P(HB-HO) NPs-treated group reduced greatly and scattered. However, cytoplasmic vacuolization was found in some cells, meaning that some apoptotic cells presented. The results further confirm that the folate-targeted nanoparticles possess a greater therapeutic ability than non-targeted nanoparticles and free DOX. This is also in accordance with the increased TIR in antitumor study.
Conclusions
DOX/FA-PEG-P(HB-HO) NPs have been successfully developed in the present study. The optimal parameters in preparation of DOX/FA-PEG-P(HB-HO) NPs were identified by an orthogonal experimental design. The optimum formulation was as follows: 10% DOX, 6.5% FA-PEG-P(HB-HO), 3% PVA and 3:1 (volume ratio) of O/W1. The results of in vitro drug release suggested a sustained release profile, and the Higuchi equation was best fitted for DOX release from DOX/FA-PEG-P(HB-HO) NPs. Moreover, in vitro microscopic observation and in vivo antitumor study with DOX/FA-PEG-P(HB-HO) NPs showed reduced side effects and significantly improved targeting and antitumor efficacy compared to free DOX and non-targeted P(HB-HO) nanoparticles. Based on these results, it can be concluded that the folate-targeted P(HB-HO) nanoparticles developed in this work demonstrate great potential for anticancer in clinical applications.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the Doctorate Science Funds of Taiyuan University of Science and Technology [20122007]; and the Natural Science Foundation for Young Scientists of Shanxi Province, China [2013021011-7].
References
- Danhier F, Kouhé TTB, Duhem N, et al. (2014). Vitamin E-based micelles enhance the anticancer activity of doxorubicin. Int J Pharm 476:9–15
- Forrest RA, Swift LP, Rephaeli A, et al. (2012). Activation of DNA damage response pathways as a consequence of anthracycline–DNA adduct formation. Biochem Pharmacol 83:1602–12
- Joo SY, Kim JS. (2002). Enhancement of gene transfer to cervical cancer cells using transferrin-conjugated liposome. Drug Dev Ind Pharm 28:1023–31
- Joseph MM, Aravind SR, George SK, et al. (2015). Anticancer activity of galactoxyloglucan polysaccharide-conjugated doxorubicin nanoparticles: mechanistic insights and interactome analysis. Eur J Pharm Biopharm 93:183–95
- Kim JH, Sung NY, Raghavendran HB, et al. (2009). Gamma irradiation reduces the immunological toxicity of doxorubicin, anticancer drug. Radiat Phys Chem 78:425–8
- Meng H, Xue M, Xia T, et al. (2011). Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano 5:4131–44
- Ruan S, Cao X, Cun X, et al. (2015). Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials 60:100–10
- Sadighian S, Rostamizadeh K, Hosseini-Monfared H, et al. (2014). Doxorubicin-conjugated core-shell magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf B 117:406–13
- Song D, Li H, Li H, et al. (2015). Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer (Review). Oncol Lett 10:600–6
- Tan EC, Lin R, Wang CH. (2005). Fabrication of double-walled microspheres for the sustained release of doxorubicin. J Colloid Interface Sci 291:135–43
- Wei LJ, Marasini N, Li G, et al. (2012). Development of ligustrazine-loaded lipid emulsion: formulation optimization, characterization and biodistribution. Int J Pharm 437:203–12
- Wu Y, Lu CT, Li WF, et al. (2013). Preparation and antitumor activity of bFGF-mediated active targeting doxorubicin microbubbles. Drug Dev Ind Pharm 39:1712–19
- Yang Y, Li N, Nie Y, et al. (2015). Folate-modified poly (malic acid) graft polymeric nanoparticles for targeted delivery of doxorubicin: synthesis, characterization and folate receptor expressed cell specificity. J Biomed Nanotechnol 11:1628–39
- Yi X, Bekeredjian R, DeFilippis NJ, et al. (2006). Transcriptional analysis of doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol 290:H1098–102
- Yoo HS, Park TG. (2004). Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release 96:273–83
- Yuan F, Qin X, Zhou D, et al. (2008). In vitro cytotoxicity, in vivo biodistribution and antitumor activity of HPMA copolymer-5-fluorouracil conjugates. Eur J Pharm Biopharm 70:770–6
- Zhang HJ, Chen BA, Jiang H, et al. (2011). A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials 32:1906–14
- Zhang C, Dong YF, Zhao LQ. (2013). Preparation and characterization of novel microparticles based on poly(3-hydroxybutyrate-co-3-hydroxyoctanoate). J Microencapsul 31:9–15
- Zhang Z, Lee SH, Feng SS. (2007). Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials 28:1889–99
- Zhang C, Zhao LQ, Dong YF, et al. (2010). Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur J Pharm Biopharm 76:10–16
- Zhao YZ, Dai DD, Lu CT, et al. (2012). Using acoustic cavitation to enhance chemotherapy of DOX liposomes: experiment in vitro and in vivo. Drug Dev Ind Pharm 38:1090–8
- Zhao LQ, Xiao JF, Feng T, et al. (2006). Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) by a Sinorhizobium fredii strain. Lett Appl Microbiol 42:344–9