Abstract
Electro-phonophoresis (EP) has been used in various clinical fields. The objective of present study is to evaluate the skin permeability of isoniazid (INH) and rifampicin (RIF) in patients with tuberculous lymphadenitis with the aid of EP to validate the clinical applications of this transdermal delivery system for the treatment of superficial extrapulmonary tuberculosis. INH and RIF solutions were delivered transdermally, with or without EP, in the surrounding tissue of the lesion for 0.5 h. Local pyogenic fluids or necrotic tissue samples from the infection sites in patients were collected at 1 h after dosing. Drug concentrations in samples were evaluated by high performance liquid chromatography. The median INH and RIF intra-lesional concentrations were 0.365 (interquartile range [IQR] 0.185–1.775) μg/mL and 1.231 (IQR 0.304–1.836) μg/mL in oral group; 2.964 (IQR 0.193–7.325) μg/mL and 2.646 (IQR 1.211–3.753) μg/mL in INH- and RIF-transdermal plus EP group. Drug concentrations in the local sites of patients receiving INH or RIF through EP transdermal delivery were statistically higher than those observed in patients only taking INH and RIF orally. However, this enhancement was not observed in the transdermal delivery of INH or RIF without EP in contrast to the oral administrations of drugs. EP can effectively enhance the skin permeability of INH and RIF in patients with tuberculous lymphadenitis. The increase in drug concentrations in the lesions could help eradication of the germs; shorten the treatment course and increase the cure rate of patients with tuberculous lymphadenitis.
Introduction
Superficial lymph node tuberculosis is among the most common types of extrapulmonary tuberculosis (TB) and accounts for about 80% of the lymphatic system diseases. The six month-administration of first line anti-TB drugs recommended for lymphadenitis, caused by drug-susceptible TB, includes two months of four-drug antibiotic therapy (isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA) and ethambutol (EMB)), followed by another four months of two-drug antibiotic therapy (INH and RIF) (American Thoracic et al., Citation2003). However, the unsatisfactory therapeutic outcomes of this traditional administration approach frequently elongate the treatment course for lymphadenitis. In China, the antibiotic treatment for lymphadenitis tuberculous is generally extended to 12 months or more. In some patients, the standardized treatment stage often produces enlarging nodes, new nodes or a new draining sinus on the basis of intumescent lymph node. Surgical excision has been applied for lymphadenitis as an adjunct to antibiotic therapy (Fontanilla et al., Citation2011), but recommended only for a few lymphatic TB patients who are at risk of worsening of the symptoms during treatment. However, no controlled studies have confirmed if the excision plus antibiotic treatment is superior to antibiotic treatment alone (Fontanilla et al., Citation2011).
Transdermal drug delivery has been applied as a substitute or complementary approach to oral administration and intravenous drug injections (Prausnitz & Langer, Citation2008). During the past 30 years, it has been adopted for treatment of various diseases e.g. tumor, hypertension and angina (Prausnitz et al., Citation2004; Wiedersberg & Guy, Citation2014). The development of various approaches, with enhanced transdermal permeability of drug, would greatly expand the application of this system.
Electro-phonophoresis (EP) is a new combinatorial transdermal drug delivery system based on electroporation and ultrasound. Both of them have been proved to enhance the transdermal drug deliveries but with different mechanisms. Electroporation uses electric pulses to transiently create aqueous pores in phospholipid bilayers of cell membranes to enhance the skin permeability for multiple types of molecules with different lipophilicity and sizes (Prausnitz et al., Citation1993; Zorec et al., Citation2013). On the contrary, ultrasounds enhance the skin permeability by cavitation which induces disorganization of the stratum corneum lipid bilayers and the occurrence of convective transport (Polat et al., Citation2011). Since the mechanisms by which electroporation and ultrasound enhance transdermal delivery are different, synergistic effect of both of these have been well established and described in previous studies (Kost et al., Citation1996). Some types of extrapulmonary TB are superficial, which is a favorable condition for EP-based treatment, but the applications of EP in treatment of extrapulmonary TB are yet to be evaluated.
In the current study, we examined whether EP can effectively increase the penetration of the frequently used anti-TB drugs through skin in tuberculous lymphadenitis patients. The two most important first-line anti-TB drugs i.e. INH and RIF, one of which is a hydrophilic drug while the other is a lipophilic drug, were evaluated. We verified that application of EP significantly enhances both INH and RIF penetration through skin, thus our approach provides reliable evidence for applying this method in clinical practice.
Material and methods
Ethnic statement
A prospective study was designed and the applied protocols were approved by the Ethics Committee of Beijing Chest Hospital. The use of human samples and the experiments conducted in this study were in accordance with the institutional guidelines approved by the Medical Ethics Committee of Beijing Tuberculosis and Thoracic Tumor Research Institute. Written consent was obtained from each recruited patient.
Patients and drugs
A total of 41 patients agreed and signed the informed contents before participating in this study. INH injection solution (Tianjin Jin Yao Amino Acid Co., Ltd, China) and RIF injection solution (Shenyang Shuang Ding Pharmaceutical Co., LTD, China) with a concentration of 50 mg/mL and 60 mg/mL, respectively, were employed to prepare drug patches for transdermal delivery assay.
Sample preparation
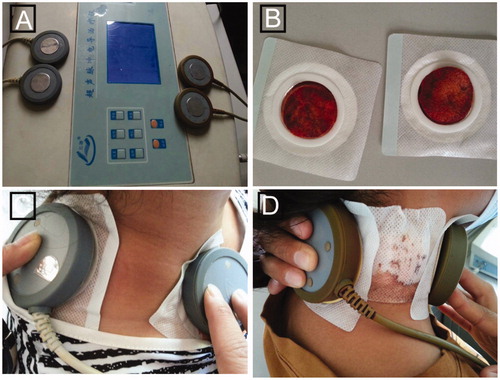
Patients were treated with INH, RIF, PZA and EMB orally. For transdermal drug administration, the patients were given two patches (each of a 4 × 4 cm single piece of cotton) immersed with a single dose of 4 mL INH injection solution or 5 mL RIF injection solution with or without EP for 30 min. EP was conducted using ultrasonic conductometric instrument (Beijing Noah Tongzhou Medical Technology Co. Ltd., Beijing, China). The setting parameters were 1 MHz and intensity of 75 mW/cm2. The ultrasonic probes were placed next to the lesion with the prepared transdermal patches while the lesion was kept as midpoint between the two probes (). The drug patches were kept in place for an additional 1 h to achieve best absorption. The pus or necrotic tissue samples from local infection sites were taken via drainage following the transdermal administration. Pusor necrotic tissue samples without transdermal administration served as a control. All of the collected samples were homogenized in a FastPrep-24 Instrument (MP Biomedicals Europe) for 45 s at 4 m/s by MP Bio FASTPREP-24. Homogenates were then centrifuged and supernatant was collected. Three volumes of methanol was added to the supernatant collected from the homogenates and mixed thoroughly. Following the centrifugation, supernatant was transferred to glass injection vials for HPLC analysis.
Figure 1. Transdermal delivery with EP. (A) Ultrasonic conductometric instrument used in this study. Each ultrasonic conductometric instrument has two pairs of probes that can be used for two patients at the same time. (B) Two patches immersed with RIF solution. (C) and (D) Patients with intumescent or ruptured lymph nodes were given RIF-transdermal patches through EP.
Drug concentration detection by HPLC
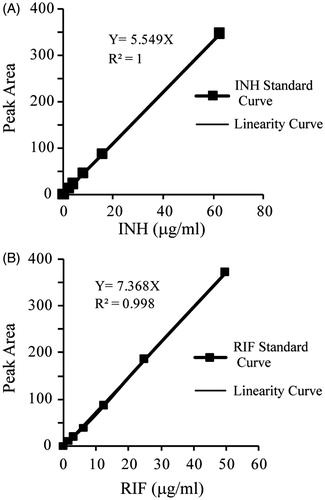
Ten microliters of the supernatant from each sample preparation was analyzed by HPLC (Agilent 1200, Palo Alto, CA) using Agilent ZORBAX Eclipse Plus C18 column (4.6 mm × 150 mm, 5 μm) guarded by a ZORBAX SB-Aqcolumn (2.1 mm × 12.5 mm, 5 μm) and detected by a UV2000 ultraviolet detector (with wavelengths of 264 nm for INH and 340 nm for RIF). For INH analysis, the mobile phase consisted of 0.02 M heptanesulfonic acid sodium salt:methanol:acetonitrile (78:17:5, v/v/v) and the column was kept at 25 °C. For RIF analysis, the mobile phase consisted of 0.01 M phosphate buffer:methanol (30:70, v/v) and the column was kept at 25 °C. Standard curves were constructed by dissolving known concentrations of INH (0–62.5 μg/mL) or RIF (0–50 μg/mL) into control homogenates and underwent the same treatment that was given to the experimental samples. Six different concentrations were used for developing a standard curve. The concentrations of INH and RIF in each tested samples were calculated on the basis of calibration curve.
Statistical analysis
Statistical analysis of the in vivo data obtained after the transdermal application of the patches was performed by the Mann–Whitney test using SPSS Statistics 21. A “p” value of less than 0.05 was considered to be statistically significant.
Results
Forty-one patients with tuberculous lymphadenitis were enrolled in our study. The mean age of the patients was 32.0 years old; the largest number of patients fell into the 21–40 years’ group, accounting for 65.9% (27/41); the number of female patients is comparable to that of male patients, with the ratio of 1.05. A total of 122 samples from 41 patients with different treatment were collected ().
Table 1. Experiment design.
For the HPLC assay, the total run times for INH and RIF were 12 and 10 min, respectively. The retention times for INH and RIF were 6.23 ± 0.07 and 3.13 ± 0.01 min, respectively. The calibration curves for INH and RIF ranged from 1.95 to 62.5 μg/mL and 1.56 to 50 μg/mL, respectively (). The standard curve correlation coefficient was 1.0 and 0.998 for INH and RIF (in methanol), respectively. The detection limit of the assay was 0.25 μg/mL for INH and whereas 0.125 μg/mL for RIF, while the estimated limits of quantitation for the samples were 1.0 μg/mL and 0.5 μg/mL for INH and RIF, respectively.
From 41 patients, 122 pyogenic fluid samples were available for measurement of INH and RIF. Among the orally administered group, 20.0% (INH (5/25)) and 16.7% (RIF (7/42)) samples had the intralesional concentrations lower than the detection limit. After transdermal delivery of both drugs, the number of samples with concentrations above minimum detection limit increased, especially in the RIF-transdermal plus EP group.
The median INH intralesional concentrations were 0.365 (interquartile range [IQR] 0.185–1.775) μg/mL in the orally administered group while 0.701 (IQR 0.170–4.342) μg/mL in INH-transdermal only group and 2.964 (IQR 0.193–7.325) μg/mL in INH-transdermal patches plus EP group. In the INH-transdermal plus EP group, the highest level of INH in pyogenic fluid sample was 27.620 μg/mL. Various statistically significant INH concentrations were observed after dosing between the INH-transdermal plus EP group and patients who did not receive the INH patches by transdermal delivery (p < 0.05). Furthermore, the INH concentrations in local abscess site of the patients dosed by INH-transdermal only were comparable with the oral group, some of which could hardly be detected due to much lower concentrations (). Some samples showed an increased INH concentration after receiving transdermal patches only, but no apparent improvement was found.
Figure 3. Local drug concentrations in patients receiving transdermal patches with or without EP after dose administration. INH (A) and RIF (B) concentrations were measured in pyogenic fluids from subjects receiving transdermal patches with EP (INH, n=20; RIF, n=30) and subjects receiving transdermal patches-only (INH, n=13; RIF, n=17). Basically the systemic treatment group was taken as the control group. Horizontal lines represent the median for each group. The statistical significance was determined by the Mann–Whitney U-test. *p < 0.05; **P < 0.01; ***P < 0.001, significantly different from the oral group or the transdermal only group.
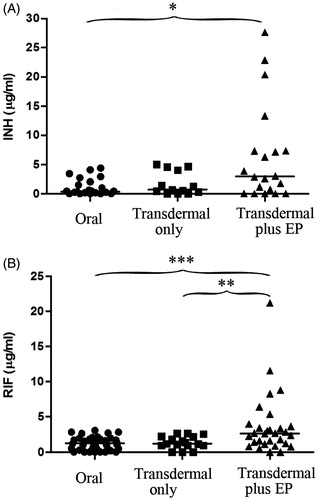
RIF concentrations in pyogenic fluids, measured by HPLC, were significantly higher in samples with RIF-transdermal plus EP group than both oral group (p < 0.001) and the RIF-transdermal only group (p < 0.01) (). The median intralesional concentrations of RIF were 1.231 (IQR 0.304–1.836) μg/mL in samples without transdermal delivery, 1.225 (IQR 0.961–2.207) μg/mL in the RIF-transdermal patches-only group and 2.646 (IQR 1.211–3.753) μg/mL in the RIF-transdermal plus EP group. Although RIF concentrations increased after transdermal delivery, no statistically comparable RIF concentrations were observed after dosing between the patients taking oral drugs only and the group of patients receiving the RIF patches only.
Discussion
TB has been the most common cause of cervical lymphadenopathy, causing about 35% of the cases (Olu-eddo & Omoti, Citation2011). The therapy for extrapulmonary TB often takes a long time, which, according to previous studies might lead to the acquisition of drug-resistance and higher mortality (Elliott et al., Citation1995; Pusch et al., Citation2014). Therefore, new treatment approaches with high efficacy against the tuberculous lymphadenitis are immediately required (Thomas & Schudel, Citation2015).
The ultrasonic conductometer induces the aqueous pores between the skin and cell membrane by electroporation and cavitation under ultrasonic conditions. Through these artificially formed channels, a drug can directly reach the lesion in specific tissues and organs. The rapid development of a local, high infiltration area promotes the uptake of drugs into the cytosol. The major advantages of this approach include the ease of use, direct effects caused on the focal lesions reduced first-pass drug-degradation, improved drug transportation into the cells and its bioavailability, reduced side effects due to limited circulation via blood, higher efficiency for a long time course, and its utility for various of drugs without any cross infection (Paudel et al., Citation2010; Prausnitz et al., Citation2004). The clinical use of this method has verified that the therapeutic doses of ultrasound are safe to human body (Mitragotri, Citation2005; Schoellhammer et al., Citation2015; Wiedersberg & Guy, Citation2014). A series of experiments proved that ultrasonic penetration is effective for many drugs, such as liposomal lidocaine and 5-Aminolevulinate (used for actinic keratosis and other non-melanoma skin cancers) (Becker et al., Citation2005; Krishnan et al., Citation2013; Mitragotri, Citation2005; Polat et al., Citation2011; Prausnitz & Langer, Citation2008; Skarbek-Borowska et al., Citation2006). Using the novel second- and third-generation enhancement strategies, including microneedles, thermal ablation, microdermabrasion, electroporation and cavitational ultrasound methods, transdermal delivery is poised to significantly increase its impact on medicine (Brown et al., Citation2006; Donnelly et al., Citation2010).
INH is a small water-soluble molecular compound which is listed among the preferred anti-TB drugs due to effective killing of Mycobacterium tuberculosis, low toxicity and cost. It is applicable to all types of TB, such as lung, lymph node, bone, kidney, intestine TB, tuberculous meningitis, pleurisy, and peritonitis. Another clinically important anti-TB drug, RIF, is a semisynthetic antibiotic derived from rifamycin B and can be used for the treatment of TB, enterococcus infection, etc. by the inhibition of bacterial DNA transcription (Maggi et al., Citation1966). Studies have shown that the drug’s anti-TB effects can be improved by increasing the drug concentrations (van Crevel et al., Citation2002; Zhang et al., Citation2014). However, we found that the INH and RIF concentrations in local infection sites were generally low with a respective median of 0.365 and 1.231 μg/mL. Our results were consistent with the observation of low intralesional drug levels in local lesions in previous reports (Jutte et al., Citation2004; Kumar, Citation1992). The development of auxiliary methods is necessary for increasing the intralesional drug concentrations. The present study evaluates the efficacy of transdermal drug delivery by EP in treating tuberculous lymphadenitis. The enhanced skin penetration of the most commonly used anti-TB drugs by EP was analyzed in patients with tuberculous lymphadenitis using ultrasonic conductometer. Although the drug concentrations increased in some samples after transdermal delivery by EP, the local skin absorption of INH with and without EP were similar (p > 0.05), whereas significantly increased for RIF with EP (p < 0.01). Thus, EP has a great effect on the skin permeation of RIF which is a lipophilic drug.
Intralesional drug concentrations from the EP treatment group demonstrated great deviations for different patients. These outcomes may be have been caused by non-homogenous concentrations throughout the lesion due to different density of lymph node and high fluid viscosity in lesion, adjacent degree of ultrasonic probes and the skin, or by the deviation between penetration direction and lesion. The approximate time for local treatment in this study was about 1.5 h, however due to variations in actual operation; the time required for application of local patches can be different. The overall time for local lesions administration varied from 1.5 to 2 h and still it remained unclear whether the local administration with extended time can improve the drug concentrations of lesions or not. We also observed that the liquid on the patches had either been largely absorbed or naturally evaporated 1.5–2 h later. Therefore, we speculate that extending the duration of drug penetration will not have a significant improvement in the effect. The local drug concentration usually remained very low after oral administration regardless of the drug administration time. We did not assess the effect of oral drugs on the local drug concentrations, hence the temporal-relation between oral drugs administration and transdermal delivery was not considered.
Our study has several limitations. First, the experimental sample size is not big enough. Second, bacteriology and clinical studies of the germs in the investigated samples were not conducted. Third, because the separation of pyogenic fluids was very difficult, the pharmacokinetic studies of the change of drug concentrations in pyogenic fluids over an extended period of time were not taken into account in our study. Finally, due to the unwillingness of the patient and the fixed ultrasonic parameters used by the equipment, the optimal ultrasonic intensity, time and dosage could not be discussed in the present study and would be addressed in the future.
Conclusions
Very low intralesional INH and RIF concentrations were found in the majority of patients that were given the systemic treatment as recommended by the WHO. The drug sat such low concentrations were inadequate to either restrain the bacterial growth at the local infection site or to kill the germs. These outcomes justified the longer treatment durations for tuberculous lymphadenitis. The results of the present studies support the use of the EP to administer RIF for lymphoid TB treatment, since a significantly higher drug concentrations were achieved in the local lesion and its surroundings. Since the ultrasonic conductometric instrument is easy to use, low priced, the EP transdermal delivery system which has stronger practicability can be used to treat patients with tuberculous lymphadenitis or other superficial TB e.g. TB in bone and joints, pleura.
This is the first attempt to evaluate the efficacy of EP transdermal delivery system as an additional therapeutic approach for tuberculous lymphadenitis. Our findings have important implications for the treatment of superficial extrapulmonary TB with the most common anti-TB drugs using EP transdermal delivery system.
Declaration of interest
The authors report no conflicts of interest. The work was supported by the research funding from Infectious Diseases Special Project, Minister of Health of China (2012ZX10003002-009) and by Collaborative Innovation Center of Infectious Diseases (PXM2015_014226_000058).
References
- American Thoracic CDC, Infectious Diseases Society of America. (2003). Treatment of tuberculosis. MMWR Recomm Rep 52:1–77
- Becker BM, Helfrich S, Baker E, et al. (2005). Ultrasound with topical anesthetic rapidly decreases pain of intravenous cannulation. Acad Emerg Med 12:289–95
- Brown MB, Martin GP, Jones SA, Akomeah FK. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–87
- Donnelly RF, Singh TRR, Woolfson AD. (2010). Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv 17:187–207
- Elliott AM, Berning SE, Iseman MD, Peloquin CA. (1995). Failure of drug penetration and acquisition of drug resistance in chronic tuberculous empyema. Tuber Lung Dis 76:463–7
- Fontanilla JM, Barnes A, von Reyn CF. (2011). Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis 53:555–62
- Jutte PC, Rutgers SR, Van Altena R, et al. (2004). Penetration of isoniazid, rifampicin and pyrazinamide in tuberculous pleural effusion and psoas abscess. Int J Tuberc Lung Dis 8:1368–72
- Kost J, Pliquett U, Mitragotri S, et al. (1996). Synergistic effect of electric field and ultrasound on transdermal transport. Pharm Res 13:633–8
- Krishnan G, Grice JE, Roberts MS, et al. (2013). Enhanced sonophoretic delivery of 5-aminolevulinic acid: preliminary human ex vivo permeation data. Skin Res Technol 19:e283–9
- Kumar K. (1992). The penetration of drugs into the lesions of spinal tuberculosis. Int Orthop 16:67–8
- Maggi N, Pasqualucci CR, Ballotta R, Sensi P. (1966). Rifampicin: a new orally active rifamycin. Chemotherapy 11:285–92
- Mitragotri S. (2005). Innovation: healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov 4:255–60
- Olu-eddo AN, Omoti CE. (2011). Diagnostic evaluation of primary cervical adenopathies in a developing country. Pan Afr Med J 10:52
- Paudel KS, Milewski M, Swadley CL, et al. (2010). Challenges and opportunities in dermal/transdermal delivery. Ther Deliv 1:109–131
- Polat BE, Hart D, Langer R, Blankschtein D. (2011). Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release 152:330–48
- Prausnitz MR, Bose VG, Langer R, Weaver JC. (1993). Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci USA 90:10504–8
- Prausnitz MR, Langer R. (2008). Transdermal drug delivery. Nat Biotechnol 26:1261–8
- Prausnitz MR, Mitragotri S, Langer R. (2004). Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3:115–24
- Pusch T, Pasipanodya JG, Hall RG 2nd, Gumbo T. (2014). Therapy duration and long-term outcomes in extra-pulmonary tuberculosis. BMC Infect Dis 14:115
- Schoellhammer CM, Srinivasan S, Barman R, et al. (2015). Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J Control Release 202:93–100
- Skarbek-Borowska S, Becker BM, Lovgren K, et al. (2006). Brief focal ultrasound with topical anesthetic decreases the pain of intravenous placement in children. Pediatr Emerg Care 22:339–45
- Thomas SN, Schudel A. (2015). Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr Opin Chem Eng 7:65–74
- van Crevel R, Alisjahbana B, de Lange WC, et al. (2002). Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int J Tuberc Lung Dis 6:497–502
- Wiedersberg S, Guy RH. (2014). Transdermal drug delivery: 30+ years of war and still fighting! J Control Release 190:150–6
- Zhang Z, Dai F, Luo F, et al. (2014). Could high-concentration rifampicin kill rifampicin-resistant M. tuberculosis? Rifampicin MIC test in rifampicin-resistant isolates from patients with osteoarticular tuberculosis. J Orthop Surg Res 9:124. doi: 10.1186/s13018-014-0124-1
- Zorec B, Becker S, Rebersek M, et al. (2013). Skin electroporation for transdermal drug delivery: the influence of the order of different square wave electric pulses. Int J Pharm 457:214–23