Abstract
Context: Based on its antioxidant activity, melatonin was recently found to have a protection effect against photocarcinogenesis.
Objective: This work aimed to develop an innovative sunscreen formulation based on the Pickering emulsions concept, stabilized by physical UV filters, modified starch and natural oils associated to melatonin as a key strategy for prevention against UV-induced skin damage.
Materials and methods: For this purpose, melatonin was incorporated in Pickering emulsions that were characterized using physicochemical, in vitro and in vivo testing. Physicochemical studies included physical and chemical stability by a thorough pharmaceutical control. The possible protective effects of melatonin against UV-induced cell damage in HaCaT cell lines were investigated in vitro. The safety assessment and the in vivo biological properties of the final formulations, including Human Repeat Insult Patch Test and sunscreen water resistance tests were also evaluated.
Results and discussion: These studies demonstrated that melatonin sunscreen Pickering emulsion was beneficial and presented a powerful protection against UVB-induced damage in HaCat cells, including inhibition of apoptosis. The inclusion of zinc oxide, titanium dioxide, green coffee oil and starch ensured a high SPF (50+) against UVA and UVB.
Conclusion: The combination of melatonin, multifunctional solid particles and green coffee oil, contributed to achieve a stable, effective and innovative sunscreen with a meaningful synergistic protection against oxidative stress.
Introduction
Skin cancer is one of the most common types of cancer worldwide with an increasing incidence, mainly the non-melanoma skin cancer (NMSC). In addition, there is an arising group of high-risk patients submitted to surgeries, chemotherapy and radiotherapy, which have an increased risk of developing NMSC, either as basal cell carcinoma or squamous cell carcinoma (Hofbauer et al., Citation2010; Rangwala & Tsai, Citation2011). Ultraviolet radiation (UVR) from sun exposure is the main etiological agent for skin cancer (Hadshiew et al., Citation2000; Yaar & Gilchrest, Citation2001) and the UV-induced cell damage occurs by two mechanisms: (a) development of cyclobutane–pyrimidine dimers (Ahmed et al., Citation1999); (b) generation of reactive oxygen species (ROS), which are also involved in mutagenesis and carcinogenesis events. Therefore, avoiding sun exposure, covering the skin and applying sunscreens with a high degree of protection are the leading strategies recommended to prevent UV-induced cell damage.
Melatonin (N-acetyl-5-methoxytryptamine, AMK) is a well-known neuroendocrine mediator discovered by Lerner (Citation1960), and mostly produced by the pineal gland that follows a circadian light-dependent rhythm of secretion (Lerner et al., Citation1960; Marshall et al., Citation1996; Papagiannidou et al., Citation2014). This hormone can be also produced by retina, bone marrow, gastrointestinal tract, gonads, immune system and skin (Rezzani et al., Citation2014). In skin, melatonin can be synthesized by normal and malignant keratinocytes (stratum corneum), melanocytes (epidermis) and fibroblasts (dermis). This synthesis may be induced or modified by UVB irradiation (Kleszczynski & Fischer, Citation2012; Rezzani et al., Citation2014; Kim et al., Citation2015a). Melatonin receptors are G protein-coupled receptors, and there are two subtypes in humans (MT1, MT2). MT1 receptors expression has been detected by immunocytochemistry in skin (stratum granulosum, stratum spinosum, upper and inner epithelial root sheath, eccrine sweat gland and blood vessel endothelium). The MT2 receptors expression has been detected in the inner epithelial root sheath, eccrine sweat gland and blood vessel endothelium (Slominski et al., Citation2002, 2012b; Kim et al., Citation2015b).
The melatonin-biosynthesis pathway in skin, similar to what happens in other organs, is divided into four stages, initiated by the uptake of the essential amino acid L-tryptophan by pineal parenchymal cells (Slominski et al., Citation2008, 2014a). After this, L-tryptophan is converted to another amino acid, 5-hydroxytryptophan due the action of tryptophan hydroxylase enzyme (TPH), which is dependent on (6R) 5,6,7,8-tetrahydrobiopterin (6-BH4). There are two isoforms of tryptophan hydroxylase identified as TPH1 and TPH2. The first one is expressed in many peripheral tissues including the skin, whereas TPH2 is expressed predominantly in the central nervous system (Zhang et al., Citation2004). Thus, TPH1 is the responsible enzyme for the production of melatonin at skin level (Slominski et al., Citation2005a).
Melatonin is metabolized through enzymatic as well as chemical reactions in a total of three major metabolic pathways: classic, indolic and kynuric (Slominski et al., Citation2014a,b). All these pathways are operative in skin (Kim et al., Citation2015b). The main metabolites are 5-methoxytryptamine (5-MTT), Cyclic 3-hydroxymelatonin (3-OHM), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), AMK, 6-hydroxymelatonin sulfate (epidermis major metabolite), 2-hydroxymelatonin, 1-nitromelatonin and nitrosomelatonin. Due to its biological significance, three of these metabolites are more widely studied, namely, 3-OHM, AFMK and AMK (Fischer et al., Citation2006; Galano et al., Citation2015). Moreover, the metabolism of melatonin is cell-type dependent and expressed in all three main cell populations of human skin – keratinocytes, melanocytes and fibroblasts. The skin cell type-specific metabolism, which determines the biological effects of melatonin, has not been established up to the Kim et al. work. The main conclusions of this multidimensional experimental study were as follows: (a) identification of 6(OH)M and AFMK as the main metabolites of the melatonin degradation; (b) identification of the indolic pathway as the predominant route of melatonin metabolism; (c) importance of the kyrunic pathway in organs exposed to environmental stress such as skin, in contrast to the systemic metabolism, in which AFMK may be below or at the limit of detection; (d) differences in the rate of melatonin metabolism dependent on cell type (Kim et al., Citation2013).
Probably, this metabolization exhibits species-, site- and tissue compartment-dependent differences which are modulated by several factors, such as environmental UVR or local adrenergic stimulation levels and hemeproteins (Slominski et al., Citation2012a).
As it is involved in several physiological processes related to circadian rhythms, seasonal reproduction, retinal function, etc melatonin also seems to participate in seasonal and non-seasonal skin changes, hair growth and pigmentation (Papagiannidou et al., Citation2014; Rezzani et al., Citation2014). It acts as an immunomodulator, anti-inflammatory and potent antioxidant agent by down-regulating several pro-inflammatory cytokines and up-regulating antioxidant enzymes, besides being an extra- and intracellular free radical scavenger of ROS and RNS, able to quench the hydroxyl radical, among others (Fischer et al., Citation1999; Rodriguez et al., Citation2004; Fischer et al., Citation2013). The ability of melatonin to act as an antioxidant, by itself and through its active metabolites, makes it particularly efficient either in vitro or in vivo, even at a low concentration (Kostoglou-Athanassiou, Citation2013; Janjetovic et al., Citation2014; Slominski et al., Citation2014a). Several reports in the literature emphasize the superior antioxidant activity of this molecule, when compared to other antioxidants (e.g. ascorbic acid or vitamin C; vitamin E, etc.) (Martín et al., Citation2000; Yalcinkaya et al., Citation2009). Moreover, it also inhibits the cAMP signal transduction pathway when it binds to the MT1A receptor and activates phospholipase C, potentiating the release of arachidonate (Slominski et al., Citation2012b; Singh & Jadhav, Citation2014). By activating cytoprotective pathways, counteracting oxidative stress and suppressing the damaged cells proliferation, melatonin has been shown to provide cellular protection against UVR damage. In fact, numerous studies have recognized the photoprotective effects of melatonin after topical application (Reiter et al., Citation1997; Slominski et al., Citation2005b; Kleszczynski & Fischer, Citation2012), including reduction of UV-induced erythema, protection against oxidative damage caused by UVA rays and prevention of both photoageing and photocarcinogenesis (Papagiannidou et al., Citation2014).
In addition, melatonin may function as an antimutagenic and anticarcinogenic agent, capable of suppressing all three stages of carcinogenesis (initiation, promotion and progression) (Pugazhenthi et al., Citation2008; Papagiannidou et al., Citation2014; Rezzani et al., Citation2014). Therefore, these properties suggest that melatonin may be a promising candidate as a new and effective sun protective agent especially for immunosuppressed patients (Scheuer et al., Citation2014).
However, the short half-life (1 h) and chemical instability of melatonin may limit its therapeutic use. Consequently, melatonin is a challenging drug for stabilization in a suitable prolonged-release sunscreen formulation. In this context, Pickering emulsions are excellent candidates to stabilize this radical scavenger on a safe and effective manner. Pickering emulsions, i.e. surfactant-free liquid or semi-solid systems stabilized by solid particles (SP) present important advantages over the classic surfactant-based emulsions, such as a higher tolerability, and easy production (Laredj-Bourezg et al., Citation2012).
Therefore, the major aim of this research study was to develop and characterize a melatonin-based innovative sunscreen formulation based on semi-solid Pickering emulsions stabilized by physical UV filters and natural oils associated to melatonin as a key strategy to prevent UV-induced skin damage. Physicochemical characterizations as well as in vitro and in vivo testing were performed. Studies included physical testing and chemical stability of melatonin by a thorough pharmaceutical control. The Sun Protection Factor (SPF) was determined and the skin permeation and retention was evaluated in vitro using newborn pig skin. The ability of melatonin-containing sunscreens to decrease ROS production was assessed in vitro, using cell cultures. In vivo biological properties of the final formulations, including the Human Repeat Insult Patch Test (HRIPT) and sunscreen water resistance were also evaluated.
Material and methods
Materials
Melatonin was purchased from Alfa Aesar (Ward Hill, MA). Green coffee oil (GCO) was supplied by Cooxupé – Cooperativa de Cafeicultores de Gauxupé (Minas Gerais, Brazil). Triethoxycaprylylsilane titanium dioxide (mTiO2) (Unipure White LC 987) was a gift from Sensient (Milwaukee, WI). Aluminum starch octenylsuccinate (ASt) (DryFlo® Plus) was obtained from AkzoNobel (Amsterdam, Netherlands). Zinc oxide (ZnO) (Tego® Sun Z 500) was obtained from Evonik Industries AG (Essen, Germany). Ethanol was obtained from Merck® (Kenilworth, NJ). All other reagents were HPLC grade. Purified water was obtained by reverse osmosis (Millipore, Elix 3, Millipore Corporation, Billerica, MA).
Methods
Sunscreen formulations – PhotoMel 1 (PM1) and PhotoMel 2 (PM2)
Preparation of the PM1 and PM2
According to the pre-formulation studies (results not shown), two final melatonin-containing formulations were selected () based on macroscopic appearance, stability and SPF value. The continuous oil phase (Phase A) consisted of green coffee oil, and the aqueous phase (Phase B) was composed by purified water, ethanol and melatonin. Solid particles – mTiO2, ZnO and ASt were firstly dispersed in the oil phase. The oil and aqueous phases were then mixed using a high-speed homogenizer (UltraTurrax®, IKA-Werke GmbH & Co. KG, Germany) at room temperature (cold process). Two batches of PM1 and PM 2 emulsions were stored during 3 months at room temperature (25 ± 2 °C/60 ± 5% RH) and under accelerated conditions (40 ± 2 °C/75 ± 5% RH).
Table 1. Qualitative and quantitative composition of the final melatonin-containing formulations.
Efficacy of the sunscreen formulations
In vitro SPF determination
The SPF was assessed using the Optometrics SPF-290S Analyzer (Optometrics Corporation, Essex, UK). Samples were prepared by spreading 110 mg of each formulation over a Transpore® tape (70.7 × 70.7 mm) to obtain a film of 2 mg/cm2, as specified by the European legislation (EC, Citation2009). Each sample was exposed to a xenon arc solar simulator, and the analyzer performed scans in 6 different spots on the Transpore® tape substrate. Each scan takes a transmittance (T) measurement every 2 nm from a wavelength ranging from 290 to 400 nm. The Monochromatic Protection Factor (MPF) was determined for the selected wavelengths using EquationEquation (1)(1) . The SPF value was calculated using EquationEquation (2)
(2) .
(1)
(2)
where E is the spectral irradiance of terrestrial sunlight under controlled conditions and B is the erythema effectiveness (Kale et al., Citation2010).
Stability of sunscreen formulations
Two batches of PM1 and PM 2 emulsions were stored during 3 months at room temperature (25 ± 2 °C) and under accelerated conditions (40 ± 2 °C). Samples were analyzed for chemical, physical (melatonin assay, macroscopic appearance, pH by potentiometry, droplet size distribution) and microbiological stability before the storage period and after 14 days, 1 and 3 months.
Melatonin chemical stability
Melatonin assay was performed by liquid chromatography using a HPLC system (Shimadzu®, Japan) with a SPD-10A UV-VIS detector at 300 nm according to a previously validated method reported in the literature (Zhang et al., Citation2012). Briefly, chromatographic separation was obtained using a reverse-phase chromatography column (Lichrospher 100 RP-18, 250 × 4 mm, Merck®, Germany) with a flow rate of 1.0 mL/min and an injection volume of 10 μL. The run time was approximately 10 min. The mobile phase for melatonin assay consisted of methanol:acetonitrile:0.5% acetic acid solution (40:10:50, v/v/v).
Droplet size distribution
The emulsions were observed using an optical microscope (Olympus CX40, Japan) equipped with a video camera. One drop of each emulsion was added to a glass slide without covering glass, and diluted with two drops of green coffee oil. The droplet size was determined using the image analysis software Olympus Stream Essentials®. The size data was expressed in terms of relative size distribution of particles, and given as diameter values corresponding to percentiles of 50% (BS, Citation1993).
Microbiological stability
The microbiological stability was performed according to the ISO 16212:2008, ISO 21149:2006 and ISO 21148:2005 (ISO Standard, Citation2005, 2006, 2008).
UV degradation studies
Three UVB lamps (Sankyo Denki G8T5E, Kanagawa, Japan) with a peak emission at 312 nm were used as the UVB source, and measured with a VLX 312 radiometer equipped with a UVB sensor (Vilber Lourmat, Marne-la-Vallée Cedex, France). The formulations were exposed to a UV-B radiation dose (26 mJ/cm2) for 2 h to simulate the solar radiation exposure (Gonzalez et al., Citation2007). Non-irradiated PM2 and melatonin solution (water:ethanol, 50:50) (Mel Sol) formulations were used as negative controls of the irradiated group. Following UVB irradiation, the samples were analyzed at pre-determined time points (15, 30, 60 and 120 min) using HPLC.
Topical delivery studies of sunscreen formulations
In vitro skin permeation
The skin permeation of sunscreen formulations was measured using Franz diffusion cells and newborn pig skin obtained from a local slaughterhouse. The entire skin was cut into sections (1 cm2 permeation area). Ethanol/water (1:1) was used as the receptor phase that assured perfect sink conditions during all experiment period. The cells were immersed in a bath system at 37 ± 2 °C under stirring (200 rpm). The formulations samples were applied (0.2–0.4 g, an infinite dose) on the skin surface in the donor compartment further sealed by Parafilm® in order to prevent the water evaporation (occlusive conditions). Samples were collected from the receptor fluid at pre-determined time points – 2, 4, 8, 12 and 24 h and replaced with an equivalent amount (200 μL) of fresh receptor medium. The melatonin content in the withdrawn samples was analyzed by HPLC. The Mel Sol was used as a control. Six replicates for each sample were used (n = 6). The cumulative amount of melatonin permeated (Qt) through excised newborn pig skin was plotted as function of time and determined based on the following equation:
(3)
where Ct is the drug concentration of the receiver solution at each sampling time, Ci is the melatonin concentration of the sample applied on the donor compartment, Vr and Vs are the volumes of the receiver solution and the sample, respectively and S is the skin surface area (1 cm2).
In vitro skin retention
In vitro skin retention or penetration study was performed by tape stripping according to the method recommended by OECD Guideline 428 (OECD, Citation2004). Both formulations (0.2–0.4 g) were spread over the newborn pig skin (1 cm2) in contact with 4 mL of receptor phase as described before. After 24 h, the skin samples were rinsed to remove the excess formulation and dried with filter paper. After the skin samples had been attached and fixed on a smooth surface, the stratum corneum (SC) was removed using 20 adhesive tapes (Scotch® 3M, UK). In order to improve the reproducibility of the tape stripping technique, a cylinder (2 kg) on foam and an acrylic disk were used and the pressure was applied for 10 s for each tape. All the tapes (excluding the first one) with the SC removed and the remaining skin (viable epidermis and dermis, ED) were cut into small pieces used for the extraction process previously validated. In this extraction process, 3 mL of mobile phase for melatonin assay was added to the SC tapes and ED pieces. Both samples were vigorously stirred for 2 min in a vertical mixer (Kinematica AG), and sonicated for 20 min to obtain the cell lysis. The final solution was centrifuged (30 000 rpm, 10 min) and the supernatant was filtered (0.2 μm) and injected in HPLC to quantify the amount (% w/w) of melatonin retained in these skin layers (SC + ED).
In vitro studies of sunscreen formulations
The cytotoxicity was assessed in HaCaT cells (CLS, Germany), a spontaneously immortalized human keratinocyte cell line, using a general cell viability endpoint MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) reduction assay (Cadete et al., Citation2012; Lopes et al., Citation2012). Inhibitory concentrations (IC50) were calculated using GraphPad Prism software v5.0 (GraphPad Software, Inc., La Jolla, CA) by the sigmoidal curve fitting method.
The HaCaT cells were seeded (2 × 105 cells/mL) in sterile flat bottom 96 well tissue culture plates (Greiner, Germany) with RPMI 1640 culture medium (Life Technologies, UK) supplemented with 10% Fetal serum bovine, 100 units of penicillin G (sodium salt) and 100 μg of streptomycin sulfate and 2 mM L-glutamine (Life Technologies, UK). Cells were incubated at 37 °C and 5% CO2. The medium was replaced (after 24 h) by fresh medium containing PM1, PM2 and Mel solution at various concentrations. Cells were incubated for another 48 h period. After cells exposure, the medium was replaced by medium containing 0.5 mg/mL MTT, and the cells were further incubated for 3 h. After that time, the medium was removed and the intracellular formazan crystals were solubilized with dimethylsulfoxide (DMSO). After 15 min at room temperature, the absorbance was measured at 570 nm in Microplate Reader (FLUOstar Omega, BMGLabtech, Germany). The relative cell viability (%) was compared to control cells (culture medium and sodium dodecyl sulfate (SDS) at 1 mg/mL as negative and positive controls, respectively).
Measurement of ROS production
The HaCaT sub-confluent cells were incubated for 30 min with 20 μM 2,7’dichlorodihydrofluorescein diacetate (H2-DCFDA, Life Technologies, UK) in the dark at 37 °C, with 5% CO2. Fresh medium was added to the cells before exposure to PM1, PM2 and melatonin solutions (1% w/v) and ascorbic acid (1% w/v) for 1 h. After hydrogen peroxide (H2O2, 500 μM) exposure, ROS levels were determined at 485 nm (excitation) and 520 nm (emission) wavelengths using a fluorescence microplate reader (FLUOstar BMGLabtech, Germany) (Seida et al., Citation1984). Data from six replicates were reported as relative fluorescence units (RFU) percentage and expressed as mean fluorescence ratio (fluorescence of exposed cells/fluorescence of unexposed control from the same experiment).
UV-irradiation dose and measurement of caspase-3 activity
Three UV-B lamps (Sankyo Denki G8T5E, Kanagawa, Japan) with a peak emission at 312 nm were be used as the UV-B source, and measured with a VLX 312 radiometer equipped with a UV-B sensor (Vilber Lourmat, Marne-la-Vallée Cedex, France). Twenty-four hours after seeding cell culture (∼300 000 cells/2 mL well), the cells were exposed to formulations and controls for 2 h and then irradiated with a UV-B single dose of 26 mJ/cm2. The caspase-3 fluorimetric assay was based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-ValAsp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) by caspase-3, resulting in the release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety. As caspase-7 shares the same substrate sequence as caspase-3, this assay also detects caspase-7 activity.
After the incubation period, cells were washed with 10 mM PBS at pH 7.4, and 100 μl of lysing buffer (50 mM HEPES, pH 7.4, with 5 mM CHAPS and 5 mM DTT) were added to each well and cells incubated for 15 min on ice. After lysis, the samples were removed and added to white 96-well plate and 100 μl of assay buffer containing Ac-DEVD-AMC substrate (20 mM HEPES, pH 7.4, with 2 mM EDTA, 0.1% CHAPS, 5 mM DTT and 20 mM Ac-DEVD-AMC) was added to each well. The reaction was followed by reading the fluorescence in a kinetic mode every minute for 60 min at 37 °C in a fluorescence microplate reader (FLUOstar Omega, BMGLabtech GmbH, Germany). Results are presented as the rate of enzyme activity measured in relative fluorescence unites (RFU) by minute and normalized to the protein content. Protein concentration was assayed by the BCA kit (Micro BCA Kit, Applichem, Germany) using bovine serum albumin (BSA) as the standard.
Safety assessment of PM emulsions
The safety assessment of the PM emulsions was accomplished according to the Scientific Committee on Consumer Safety’s (SCCS) Notes of Guidance for Testing of Cosmetic Ingredients and their Safety Evaluation (SCCS/1416/11, Citation2011). The information for each ingredient was obtained from the respective supplier.
Hazard identification
The results of the in vitro and in vivo tests, clinical studies, physical, chemical and toxicological properties of each ingredient were considered to recognize if the ingredient has the potential to damage human health.
Dose–response assessment
The dose–response assessment describes the change in effect on an organism caused by different levels of exposure to a chemical after a certain exposure time. In the case of an effect with a threshold, the dosage at which No Observed Adverse Effect Level (NOAEL), is determined (SCCS/1416/11, Citation2011).
Exposure assessment
The amount and the frequency of human exposure to the PM emulsions were determined, using the systemic exposure dose (SED) that was calculated for each ingredient, according to the EquationEquation (4)(4) .
(4)
where E is the amount expected to enter the blood stream per kg body weight and per day, C is the concentration of the ingredient in the PM emulsions, DA is the dermal absorption reported as a percentage of the test dose to be applied under conditions simulating those of real-life.
Risk characterization
The probability that the substances under study cause damage to human health was considered. In the case of a threshold effect, the margin of safety (MoS) was calculated according to the EquationEquation (5)(5) .
(5)
In vivo studies of sunscreen formulations
Human Repeat Insult Patch Test
A safety evaluation study was performed on the melatonin-containing sunscreen formulations, using the Marzully & Maibach (Citation1976) Human Repeat Insult Patch Test (HRIPT protocol as described in detail elsewhere (Marto et al., Citation2015). This protocol was approved by the local Ethical Committee and respected the Helsinki Declaration and the AFSSAPS regulations on performed HRIPT studies on cosmetic products. The study was conducted under the supervision of a dermatologist.
In vivo sunscreen water resistance
The water resistance of sunscreens was tested on three subjects (Fitzpatrick skin type II). Panelists cleanse their forearms using a mild cleanser and leave them to air dry for 30 min before starting the test. Initial cross polarized images are taken after the sunscreens application (2 mg/cm2) on the inner forearm (4 cm2).
The amount of each sunscreen formulation left before and after water bath immersion was quantified via cross-polarized imaging by means of the Visia® CA (Canfield Scientific, Faitfield, NJ). Panelists immerse their forearms into a water bath system (29 ± 2 °C) and washed away by the flow of water (150 rpm) during 40 min (Ahn et al., Citation2008). Their forearms were allowed to air dry for 15 min and the amount of sunscreen was measured again. This procedure was repeated and the amount of sunscreen was measured again to calculate the water resistance of the sunscreen formulations.
This method yields a series of three cross-polarized images for each panelist: clean skin (without sunscreen), immediately after sunscreen application and post-water bath. Water resistance information was obtained from the cross-polarized Visia® CA imaging mode in visible light. The RGB color space of the raw bitmap images was converted to relative luminance using ImageJ®. From these images, average L changes for each sunscreen area were obtained from histograms. Skin whiteness was defined as the change in L value before and after water immersion, and the percentage of water resistance retention (%WRR) of the sunscreens was determined according to EquationEquation (6)(6) .
(6)
Statistical analysis
The data were expressed as mean and standard deviation (mean ± SD) of experiments. Statistical evaluation of data was performed using one-way analysis of variance (ANOVA). Tukey–Kramer multiple comparison test (GraphPad PRISM 5 software) was used to compare the significance of the difference between the groups (p < 0.05).
Results and discussion
Strategy to prevent skin photodamage using melatonin
UV radiation from sun exposure is the main etiological agent for skin cancer, causing DNA damage by increasing the levels of ROS. Hence, increased free radical action can contribute to the development of cutaneous diseases. Thus, new strategies for skin protection comprise the synergistically use of sunscreen and antioxidants to counteract oxidative stress.
Sunscreens are usually composed of synthetic chemical filters with a high capacity to absorb sun light at the region of UVB (320–290 nm) and UVA (400–320 nm) spectrum. The reduction of filter concentration in sunscreen formulations is a strategy to improve quality without affecting their properties as well as to reduce the adverse effects (e.g. estrogenic effects, disruption of human endocrine activity, etc.). In addition, the inclusion of natural products with antioxidant activity (e.g. GCO and melatonin) may also improve the photoprotective activity of sunscreen formulations. In fact, these natural products present several advantages, such as the bioactivity, relative safety and obtainment from renewable sources, low cost, besides the feasibility for application in a wide range of health care products. In this context, melatonin was associated to GCO, a rich source of antioxidants and polyphenols, which has arisen as a potential candidate to replace the chemical filters in sunscreen PM formulations (Ribeiro et al., Citation2013). As reviewed by Scheuer et al. (Citation2014), topical application of melatonin conduced to a dose-dependent decrease of erythema degree and a formulation containing 1% (w/w) of melatonin proved to be effective in preventing UV-induced erythema. However, it is important to note that the ideal concentration of melatonin is still to be defined, so further studies will be needed to assess the required dose to achieve an effective photoprotection. Thus, attending to pre-formulation studies (data not shown), two formulations (PM1 and PM2) were characterized and selected for further assessment. Concerning the composition, the only difference between these two emulsions was the presence (PM2) or absence (PM1) of 1% (w/w) of melatonin. Based on the results, in , both emulsions showed high values of SPF with a suitable UVA/UVB ratio, assuring the water resistance claim (p > 0.05).
Table 2. In vitro and in vivo efficacy tests of the PM emulsions.
Improvement of long-term stability of melatonin
In previous studies, the degradation profile of melatonin has already been evaluated (data not shown). These results confirmed the high degradation tendency of melatonin, particularly in the presence of an oxidizing agent. Therefore, Pickering emulsions were formulated to deliver this radical scavenger on a safe and effective manner. The stability of these emulsions in terms of melatonin content, pH and macroscopic characteristics was assessed for 3 months at room temperature (25 ± 2 °C) and under accelerated conditions (40 ± 2 °C; relative humidity 75 ± 5%). After this period, both PM1 and PM2 emulsions remained white with a creamy and homogeneous aspect for both storage conditions. In fact, no instability-related processes, such as creaming (or sedimentation), flocculation, coalescence or phase inversion were observed. The pH values (pH ∼ 5) did not significantly vary over time as well as the droplet size (). Melatonin assay remained within the pre-established limits (90–110%) throughout the study.
Table 3. Droplet size distribution of the PM1 and PM2 emulsions (n = 625; mean ± SD) and percentage of melatonin recovered in batches 1 and 2 (mean ± SD; n = 3) stored at 25 and 40 °C during 90 days.
Microbiological testing revealed that PM formulations were stable for 3 months as the total aerobic microbial, yeast and mold count presented accepted values following the established criteria (<10 cfu/g). Thus, Pickering emulsions showed to be an effective vehicle to stabilize melatonin.
Enhancement of melatonin photostability
The photo-instability of melatonin is well documented. As a result of its absorption at 254 nm, this compound is quite vulnerable to UV light, originating N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) as its main photodegradation product (Fischer et al., Citation2006; Bromme et al., Citation2008). Melatonin can also be attacked by the ˙OH radical, which interacts similarly with AFMK. The concentrations of these photodegradation products are directly proportional to UVR-dose and melatonin content, and their accumulation are time-dependent (Andrisano et al., Citation2000).
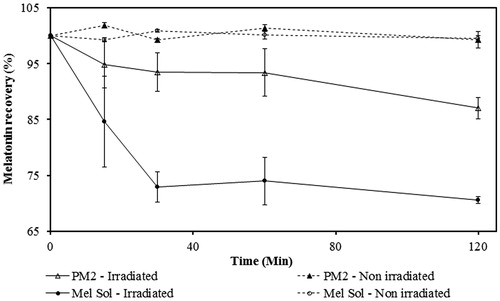
Thus, photodegradation studies were performed using an UVB lamp (wavelength that mostly affects the melatonin stability). An acute exposure was simulated for 2 h correspondent to the reapplication time of sunscreen, as recommended (FDA, Citation2012). The melatonin solution (Mel Sol) presented a significant decrease (p < 0.05) of the melatonin content to values below the inferior limit of the 90–110% specification after 15 min of UVB exposure (). In contrast, PM2 remained within the established range until the end of exposure time (120 min).
Comparing the photodegradation profiles of PM2 and Mel Sol, it is possible to conclude that this novel sunscreen formulation is suitable for the protection of melatonin.
Improvement of melatonin’s efficacy during sun exposure
Skin permeation and retention
In vitro studies with Franz diffusion cells are a useful tool to determine the drug flow before performing in vivo assays (Gujjar & Banga, Citation2014). Skin permeation occurs via two main routes: the transappendageal (through the sweat glands and across the hair follicles associated with the sebaceous glands) and the transepidermal (intercellular route through intercellular lipid domains and transcellular pathway through the keratinocytes). The relative importance of which route will be followed depends on the molecules physicochemical characteristics.
shows the permeation profiles of melatonin from PM2 and solution (Mel Sol) through newborn pig skin, considering the cumulative amount of melatonin permeated to the acceptor compartment as a function of time for 24 h. There was a significant increased permeation of melatonin with time for PM2 and Mel Sol (p > 0.05). However, permeation profiles showed that, only at 12 h PM2 is statistically different from Mel Sol (p < 0.05). These profiles are typical of infinite dose experiments where the applied dose is so high that the depletion of the permeant in the donor chamber caused by evaporation or diffusion into and through the barrier is negligibly low (Selzer et al., Citation2013). The high permeation through the skin was also due to the small molecular size (232.3 Da) and to the octanol-water partition coefficient (logP) of melatonin. Although melatonin is usually considered an amphiphilic molecule, its log P (≃1.4) is relatively close to that of amphoteric molecules (log P ≥ 1.7). Finally, the presence of ethanol in the tested formulations also justifies the permeation enhancement. In fact, low molecular weight alkanols, like ethanol, act by enhancing the solubility of the drug in the SC lipid matrix by extraction of hydrophobic alcohols, disrupting its integrity (Kikwai et al., Citation2002; Marto et al., Citation2014). Besides lowering the skin barrier function, ethanol also increased the melatonin solubility in the vehicle.
Figure 2. Permeation profile of melatonin from PM2 and Mel Solution (Sol) through newborn pig skin (mean ± SD, n = 6).
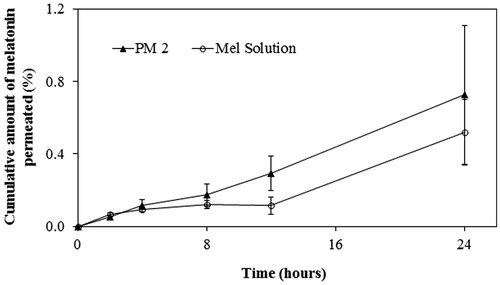
However, it should be noted that this data obtained using newborn pig skin cannot be translated to in vivo delivery in humans, as other factors significantly alter its permeation profile, such as cutaneous microvasculature, which prevents the accumulation of melatonin in the skin, and the cutaneous metabolism of fatty alcohols (Kikwai et al., Citation2002).
The tape stripping method is well-suited to determine the dermatopharmacokinetics of topically applied substances especially if all procedures are standardized and validated, as it was previously performed in this work.
Melatonin extraction from the skin by tape stripping was performed for PM2 and Mel Sol ().
Figure 3. Penetration of PM2 and melatonin solution (Mel Sol) in the SC (from tape stripping, TS) and viable skin layers (epidermis and dermis) after 24 h. Results are expressed as mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA with Tukey’s post-hoc test (p < 0.05).
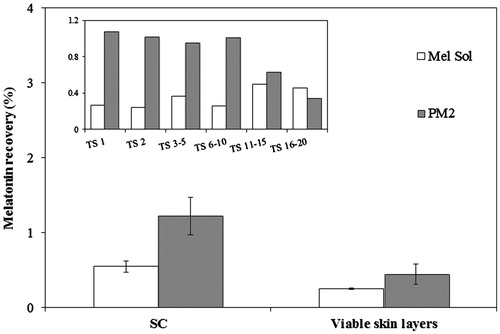
The amount of melatonin extracted from the SC was significantly higher than from viable skin layers (epidermis and dermis) for all tested formulations, as expected attending that SC is the first main skin barrier. This is a required outcome, as the target activity of sunscreens is in the outermost layers of the skin, reflecting UVR in order to reduce penetration to deeper viable skin layers where damage may occur.
The statistical analysis indicated that the amounts of melatonin extracted from the SC and viable skin layers are significantly lower when using Mel Sol compared to PM2 (p < 0.05). However, PM2 probably due its composition, i.e. w/o emulsions, could be more retained due to the lipophilicity of the upper skin layers, suggesting that w/o emulsions may provide advantages with respect to low penetration rates and high substantively to the SC (Benson et al., Citation2005).
Although PM2 is a sunscreen formulation usually considered as cosmetic products, in this case it was also incorporated an active molecule (melatonin) to offer DNA protection against UVR damage. Attending to the fact that melatonin receptors are located in epidermis layer, inner epithelial root sheath, sweat glands and blood vessel endothelium (Slominski et al., Citation2012b), the skin permeation and penetration of these formulations were desirable. Fischer et al. (Citation2004) also related that topical melatonin had a depot effect in the SC being continuously released to the rest of the skin and dermal vasculature.
Melatonin improves sunscreen photoprotection against UV-induced reactive oxygen species
Keratinocytes represent the major population in the skin and UVR causes damage to these cells. Thus, the possible protective effect of melatonin against oxidative damage in HaCaT cell lines was investigated in vitro.
To investigate the potential cytotoxicity of the melatonin, the cell viability was evaluated using HaCaT cell lines in a MTT assay. Melatonin is not cytotoxic in HaCaT cells on the range of concentrations tested, 1–0.015% w/v.
Effect of melatonin on generation of ROS
The intracellular production of reactive oxygen species (ROS) within cells was assessed with a fluorimetric technique using 2,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA, Life Technologies, UK). H2-DCFDA is non-fluorescent molecule that is hydrolyzed by intracellular esterases to non-fluorescent 2,7′-dichlorodihydrofluorescein (H2-DCF), which is oxidized in the presence of H2O2, hydroxyl radicals and diverse peroxides (Soh, Citation2006) to a highly fluorescent compound (DCF) (Wardman, Citation2007). Ascorbic acid was used as a positive control due to its potent antioxidant properties.
refers to the difference between ROS generation in ethanol/water sample (vehicle) and in the tested formulations. It is clear that all formulations decreased the formation of ROS, particularly the melatonin loaded emulsions due to its antioxidant activity.
Figure 4. Relative ROS determination of HaCat cell line measured by the H2-DCFDA assay. Melatonin concentration is 1% w/w in all cases. Results are expressed as mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA with Tukey’s post-hoc test (*p < 0.05).
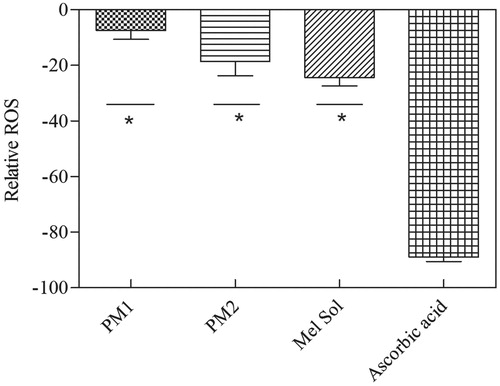
All formulations and melatonin solution led to an increase of ROS formation when compared to ascorbic acid and the statistical analysis indicated that the Mel Sol provides a significant better protection than PM2. This phenomenon can be explained probably due to the presence of ethanol, once ethanol may induce ROS production per se and due to the incomplete release of melatonin from PM2 (Helkin et al., Citation2010; Ma et al., Citation2014). Furthermore, some studies refer the cytotoxicity of ZnO nanoparticles, which may be associated not only with its action as an antibacterial, disinfecting and drying agent but also with its capacity to enhance ROS production (Ma et al., Citation2014). This suggests that the excipients of these formulations could have an oxidant activity, and that by themselves may induce ROS formation. On the other hand, ascorbic acid (vitamin C) was used as a positive control due to its potent antioxidant properties and because it possesses a variety of other topical advantages including photoprotection from UVR, inhibition of melanogenesis and improvement of a variety of inflammatory skin disorders. However, despite of its potential as a topical antioxidant, the instability of this water-soluble vitamin, together with difficulties associated with its topical delivery, proved ascorbic acid unattractive for topical application (Murray et al., Citation2008).
Effect of melatonin on apoptosis
This study investigates the effects of a broad-spectrum melatonin-containing sunscreen formulation on the ability of sunscreen filters and melatonin to protect HaCaT from UVR.
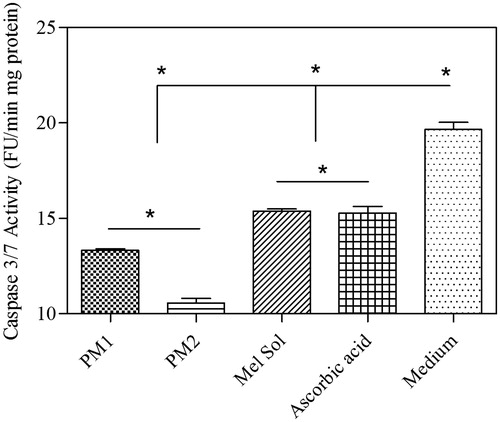
To confirm the inhibitory effect of melatonin on ROS synthesis and apoptotic cell death, the activity of caspase-3/7 using Ac-DEVD-pNa was performed. The levels of the activity of the two enzymes caspase3/7 decreased in melatonin-treated HaCaT cells after UVB irradiation compared to those of UVB-treated HaCaT cells without melatonin treatment (). The decrease of the activity of these enzymes indicates that melatonin reduces the generation of ROS, thereby reducing oxidative stress in UVB-irradiated HaCaT cells and, finally, decreasing apoptotic cell death. Also, caspase 3/7 activity was very low for PM2. Thus, topical application of an efficiently broad-spectrum melatonin-containing sunscreen formulation almost completely protect against photodamage.
Safety assessment of PM formulations
Hazard identification
Many variables affect the skin penetration and permeation, including the physicochemical parameters of each ingredient (). The chemical structures and physical properties of the ingredients used in the PM emulsions, namely, the emollient and modified solid particles are complex, hindering to predict interactions between them. However, it is recognized that the safety of a final product is determined based on the theoretical safety assessment of its ingredients. Molecules must be in the liquid form, because chemicals in the solid state and with a molecular weight greater than 500 Da do not penetrate the skin. In addition, if a chemical is excessively hydrophilic or too strongly lipophilic, it will not partition or it will partition more readily into the SC, respectively. In general, a Log P of between 1 and 3 is considered to be ideal for skin penetration. Thus, when no permeation data is available, the value considered is 100% according to OCDE, however in case molecular weight > 500 Da and log P < −1 or > 4, the value of 10% for dermal absorption is considered (Potts & Guy, Citation1992; Bos & Meinardi, Citation2000; Brain & Chilcott, Citation2008).
Table 4. Chemical properties of the ingredients presented in the PM formulations.
Assessment of the safety for human health of the finished product shall take into consideration the toxicological profile of each ingredient, its chemical structure and its levels of exposure (). In vitro and in vivo studies make it possible to investigate the toxicological profile of a cosmetic ingredient, namely, the cytotoxicity, sensitization and irritation or intracutaneous reactivity, the risk of carcinogenicity, mutagenicity or teratogenicity based on period of exposure of the cosmetic products (SCCS/1416/11, Citation2011).
Table 5. Summary of the biological safety of the ingredients.
The main ingredient present in PM formulations is green coffee oil. Due to the absence of data in the literature for this emollient, it was included information about the fatty acids present in these oils and the safety profile of each ingredient was assessed. Vegetable extracts are widely used is cosmetic products due to their moisturizing, occlusive and emollient properties. Moreover, it is known that after topical application of one product only a very small amount is able to penetrate the skin due to the SC properties. There are several mechanisms of hydration but the majority of the oils act by occlusion, i.e. they avoid the evaporation of endogenous water, decreasing the transepidermal water loss. The green coffee is considered food oil and thus it is considered safe after topical application (Boekschoten et al., Citation2004).
Despite the high concentration of mTiO2 and ZnO, the toxicological profile of these ingredients does not give rise to concern in human use, since the substance is not absorbed through the skin. The ingredients in the solid state and particle size greater than 100 nm are not able to penetrate the SC if not solubilized. mTiO2 and ZnO are not soluble in water neither in oils. Thus, they are in suspension and they cannot penetrate through the skin. Given the negligible, dermal penetration of mTiO2 and ZnO when applied on skin, and in consideration of the low toxicity observed, the calculation of a margin of safety (MoS) is not relevant for this assessment (SCCNFP/0649/03, Citation2003; SCCS/1516/13, Citation2013).
Concerning the ASt, it was demonstrated that it has unimportant acute toxicity in animals, and no toxicity was reported following chronic administration. This ingredient was not recognized as ocular irritant in rabbits. Oral studies using ASt produced no adverse systemic, reproductive or developmental effects. ASt may produce minor skin irritation, but in general this ingredient is not irritating at concentrations used in cosmetics, and neither sensitizer nor photosensitizer. Based on these data, the author concluded that this ingredient is safe as cosmetic ingredient in the current concentration (Nair & Yamarik, Citation2002).
In the particular case of ethanol, Pendlington et al. (Citation2001) described the only study in the literature about serum ethanol levels in humans after using a body deodorant spray. Despite the high concentration of ethanol and the high exposure to large surfaces, the concentration of ethanol was 0.4 mg/l. Overall it is concluded that ethanol poses no risk for the consumer in the normal and reasonably foreseeable use of this product (Lachenmeier, Citation2008).
Considering the molecular weight and the Log P values (when available), the ingredient that most probably penetrates into the SC is melatonin, because PM emulsions contain potent skin enhancers (ethanol and fatty acids).
Exposure assessment
The PM emulsions are proposed for use on intact skin of adults and can be used as a sunscreen. These emulsions are leave-on cosmetic products intended to stay in prolonged contact with the skin and should be applied generously and repeatedly before sun bathing, especially after spending time in water.
According to the SCCS opinion, the exposed skin surface area for a sunscreen is 17 500 cm2. The estimated daily amount applied for a sunscreen is 70.0 g/day and the frequency of application is twice a day which is converted in a daily exposure of 1166.7 mg/kg bw/day. Applying the EquationEquation (4)(4) , the SED values were calculated for each ingredient () (SCCS/1416/11, Citation2011). Note that both worst scenarios were used: highest skin surface and highest amount applied.
Table 6. Exposure data of formulation ingredients.
shows the estimated SED from the ingredients present in the PM emulsions. In the lack of dermal absorption studies, the worst-case scenario of 100% of dermal penetration should be taken into consideration (SCCS/1416/11, Citation2011). In addition, a large number of studies suggest that solid particles do not penetrate healthy or sun burnt human skin deep enough to reach live cells of the epidermis, as a result, the dermal absorption considered for mTiO2, ZnO and Ast was 0%.
Dose–response assessment
The NOAEL is an important part of the non-clinical risk assessment. The NOAEL values found out for mTiO2, ZnO and melatonin were 62.5, 53.5 and 200 mg/kg/day, respectively.
Risk characterization
The MoS is used to extrapolate from a group of test animals to an average human being, and subsequently from average humans to sensitive subpopulations. The WHO proposes a value of 100, and it is usually recognized that the MoS should at least be 100 to claim an ingredient safe for human use. The value of 100 consists of a factor 10 for the extrapolation from animal to man and another factor 10 taking into account the inter-individual variations within the human population. The MoS for melatonin was calculated according to EquationEquation (5)(5) , and the value obtained was 2500, which is above the threshold value of 100, hence the ingredient may be considered safe.
In vivo safety studies
Safety testing can be used to generate product claims. Thus, HRIPT was conducted to justify the claim “dermatological tested”. During the HRIPT study, no reactions or skin sensitization/irritation were observed in the initial 3 weeks contact and even after the final challenge contact. Thus, very good skin compatibility was obtained for these sunscreen formulations.
Water resistance performance of sunscreens
After ensuring in vivo safety of these formulations, the WRR was evaluated in humans. Human testing is considered to be the most acceptable and definitive method for claiming WRR. Although this method is a new in vivo screening approach to measure WRR, it does not allow determining the exact SPF before and after immersion. It rather evaluates the sunscreen loss due to the action of water immersion. The value of 90% lower unilateral confidence limit must be %WRR ≥ 50%. The PM1 and PM2 emulsions possess 84.44 and 80.62% of lightness distribution (L50) without water exposure, respectively. However, when exposed to water for 40 min, both products showed lower values of L50, 76.36% for PM1 and 73.85% for PM2, as a consequence of the water immersion. Nevertheless, both products showed quite similar amounts of L50 on skin with a satisfactory mineral distribution along the irregular skin surface, which means that the presence of melatonin did not significantly influence the water resistance performance (). The combination of the multifunctional particles with a suitable cosmetic vehicle usually affords the acceptable distribution of the physical sunscreens onto skin.
Figure 6. Skin whiteness resulting from sunscreens applied on dry skin and cross-polarized images of two sunscreens (PM1 and PM2) applied to the volar forearm of a subject. Fresh sunscreen applications with 30 min air drying (FPM) and sunscreen after 40 min water immersion (PMaI).
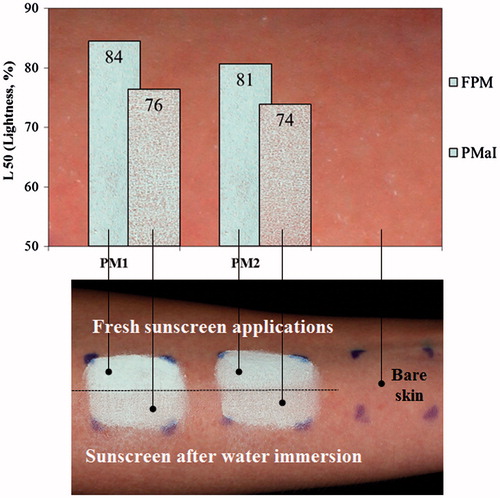
Therefore, based on the WRR values (), it is possible to claim water resistance of this product. Thus, w/o emulsions possess a higher degree of water repellency needed to avoid products to coalesce in contact with water drops, remaining on wet skin.
Conclusions
This study arose from the necessity to fill the gap in the photoprotection whose attention is frequently more focused on cosmetic issues rather than maximum protection against both UVA and B radiation as well as cellular protection. Thus, a novel sunscreen formulation with a high UVB/A protection, biological activity and better tolerability was designed to stabilize and deliver melatonin, based on the Pickering emulsions concept.
As previously outlined, melatonin may be beneficial for sun protection. Based on its free radical scavenger and antioxidant activity, the advantage of including melatonin in PM emulsions became obvious.
Regarding the advantages of melatonin over other antioxidants, these are related to its several mechanisms of action such as immunomodulatory and anti-inflammatory besides potent antioxidant actions, suppressing all three stages of carcinogenesis. Moreover, melatonin can be produced in different skin cells from dermis and epidermis layers which may determine a strong effect on both NMSC and MSC as well as photoaging processes. Pickering emulsions proved to be a promising solution not only for the sunscreen development but also for the photostability of drugs. To the best of our knowledge, this is the first successful formulation able to protect melatonin from photodegradation. All results revealed an excellent compromise between stability, UV protection, topical delivery, efficacy, safety and cosmeticity.
Our findings combined with data reported so far thus enrich existing knowledge about the potent anti-oxidant action of melatonin and highlight that melatonin-containing Pickering emulsion sunscreens could be one of the most promising segments in the personal care industry.
Acknowledgements
The authors would like to thank to Carla Eleutério for performing HPLC analysis and Doctor Bruna Chiari-Andréo and Professor Vera Isaac for SPF determinations.
Declaration of interest
This work was supported by the Fundação para a Ciêcia e a Tecnologia, Portugal (UID/DTP/04138/2013 to iMed. ULisboa and grant SFRH/BDE/51599/2011) and Laboratórios Atral S.A., Portugal.
References
- Ahmed NU, Ueda M, Nikaido O, et al. (1999). High levels of 8-hydroxy-2′-deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation. Br J Dermatol 140:226–31
- Ahn S, Yang H, Lee H, et al. (2008). Alternative evaluation method in vitro for the water-resistant effect of sunscreen products. Skin Res Technol 14:187–91
- Andrisano V, Bertucci C, Battaglia A, Cavrini V. (2000). Photostability of drugs: photodegradation of melatonin and its determination in commercial formulations. J Pharm Biomed Anal 23:15–23
- Benson HAE, Sarveiya V, Risk S, Roberts MS. (2005). Influence of anatomical site and topical formulation on skin penetration of sunscreens. Ther Clin Risk Manag 1:209–18
- Boekschoten MV, Schouten EG, Katan MB. (2004). Coffee bean extracts rich and poor in kahweol both give rise to elevation of liver enzymes in healthy volunteers. Nutr J 3:7
- Bos JD, Meinardi MM. (2000). The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol 9:165–9
- Brain KR, Chilcott RP. (2008). Physicochemical factors affecting skin absorption. In: Chilcott RP, Price S, eds. Principles and practice of skin toxicology. Chichester (UK): John Wiley & Sons, Ltd, 83–92
- Bromme HJ, Peschke E, Israel G. (2008). Photo-degradation of melatonin: influence of argon, hydrogenperoxide, and ethanol. J Pineal Res 44:366–72
- British Standards (BS). (1993). Methods for determination of particle size distribution. Part 4, Guide to microscope and image analysis methods. British Standards 3406-4:1993
- Cadete A, Figueiredo L, Lopes R, et al. (2012). Development and characterization of a new plasmid delivery system based on chitosan-sodium deoxycholate nanoparticles. Eur J Pharm Sci 45:451–8
- EC. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal of the European Union
- FDA. (2012). Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use – small entity compliance guide. Silver Spring (MD): FDA
- Fischer T, Wigger-Alberti W, Elsner P. (1999). Melatonin in der dermatologie experimentelle und klinische aspekte. Der Hautarzt 50:5–11
- Fischer TW, Greif C, Fluhr JW, et al. (2004). Percutaneous penetration of topically applied melatonin in a cream and an alcoholic solution. Skin Pharmacol Physiol 17:190–4
- Fischer TW, Kleszczyński K, Hardkop LH, et al. (2013). Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR‐induced depletion, and protects against the formation of DNA damage (8‐hydroxy‐2'‐deoxyguanosine) in ex vivo human skin. J Pineal Res 54:303–12
- Fischer TW, Sweatman TW, Semak I, et al. (2006). Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J 20:1564–6
- Galano A, Medina ME, Tan DX, Reiter RJ. (2015). Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J Pineal Res 58:107–16
- Gonzalez H, Tarras-Wahlberg N, Stromdahl B, et al. (2007). Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatol 7:1
- Gujjar M, Banga AK. (2014). Vehicle influence on permeation through intact and compromised skin. Int J Pharm 472:362–8
- Hadshiew IM, Eller MS, Gilchrest BA. (2000). Skin aging and photoaging: the role of DNA damage and repair. Am J Contact Dermatitis 11:19–25
- Helkin AW, Nguyen HT, Samara GJ. (2010). Alcohol induces reactive oxygen species and migration in keratinocytes. Laryngoscope 120:S35
- Hofbauer GFL, Bavinck JNB, Euvrard S. (2010). Organ transplantation and skin cancer: basic problems and new perspectives. Exp Dermatol 19:473–82
- ISO Standard. (2005). Microbiology – general instructions for microbiological examination. Geneva: ISO
- ISO Standard. (2006). Microbiology – enumeration and detection of aerobic mesophilic bacteria. Geneva: ISO
- ISO Standard. (2008). Microbiology – enumeration of yeast and mould. Geneva: ISO
- Janjetovic Z, Nahmias ZP, Hanna S, et al. (2014). Melatonin and its metabolites ameliorate ultraviolet B‐induced damage in human epidermal keratinocytes. J Pineal Res 57:90–102
- Kale S, Ghoge P, Ansari A, et al. (2010). Formulation and in-vitro determination of sun protection factor of Nigella sativa Linn. seed oil sunscreen cream. Int J PharmTech Res 2:2194–7
- Kikwai L, Kanikkannan N, Babu RJ, Singh M. (2002). Effect of vehicles on the transdermal delivery of melatonin across porcine skin in vitro. J Control Release 83:307–11
- Kim TK, Kleszczyński K, Janjetovic Z, et al. (2013). Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J 27:2742–55
- Kim TK, Lin Z, Li W, et al. (2015a). N1-Acetyl-5-methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology 156:1630–6
- Kim TK, Lin Z, Tidwell WJ, et al. (2015b). Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol 404:1–8
- Kleszczynski K, Fischer TW. (2012). Melatonin and human skin aging. Dermatoendocrinology 4:245–52
- Kostoglou-Athanassiou I. (2013). Therapeutic applications of melatonin. Ther Adv Endocrinol Metab 4:13–24
- Lachenmeier DW. (2008). Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol 3:26
- Laredj-Bourezg F, Chevalier Y, Boyron O, Bolzinger MA. (2012). Emulsions stabilized with organic solid particles. Colloids Surf A 413:252–9
- Lerner AB, Case JD, Takahashi Y. (1960). Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem 235:1992–7
- Lopes R, Eleuterio CV, Goncalves LM, et al. (2012). Lipid nanoparticles containing oryzalin for the treatment of leishmaniasis. Eur J Pharm Sci 45:442–50
- Ma H, Wallis LK, Diamond S, et al. (2014). Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ Pollut 193:165–72
- Marshall KA, Reiter RJ, Poeggeler B, et al. (1996). Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med 21:307–15
- Martín M, Macías M, Escames G, et al. (2000). Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 14:1677–9
- Marto J, Baltazar D, Duarte A, et al. (2014). Topical gels of etofenamate: in vitro and in vivo evaluation. Pharm Dev Technol 20:710–15
- Marto J, Gouveia LF, Gonçaves L, et al. (2015). Starch pickering emulsion: a safe vehicle for topical drug delivery. Athens J Sci 2:77–87
- Marzulli FN, Maibach HI. (1976). Contact allergy: predictive testing in man. Contact Dermatitis 2:1–17
- Murray JC, Burch JA, Streilein RD, et al. (2008). A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol 59:418–25
- Nair B, Yamarik TA. (2002). Final report on the safety assessment of aluminum starch octenylsuccinate. Int J Toxicol 1:1–7
- OECD. (2004). Test No. 428: Skin absorption: in vitro method. Paris: OECD Publishing
- Papagiannidou E, Skene DJ, Ioannides C. (2014). Potential drug interactions with Melatonin. Physiol Behav 131:17–24
- Pendlington RU, Whittle E, Robinson JA, Howes D. (2001). Fate of ethanol topically applied to skin. Food Chem Toxicol 39:169–74
- Potts RO, Guy RH (1992). Predicting skin permeability. Pharm Res 9:663–9
- Pugazhenthi K, Kapoor M, Clarkson AN, et al. (2008). Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J Pineal Res 44:387–96
- Rangwala S, Tsai KY. (2011). Roles of the immune system in skin cancer. Br J Dermatol 165:953–65
- Reiter RJ, Carneiro RC, Oh CS. (1997). Melatonin in relation to cellular antioxidative defense mechanisms. Horm Metab Res 29:363–72
- Rezzani R, Rodella LF, Favero G, et al. (2014). Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by Melatonin. Br J Dermatol 170:382–91
- Ribeiro H, Marto J, Raposo S, et al. (2013). From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur J Lipid Sci Technol 115:330–6
- Rodriguez C, Mayo JC, Sainz RM, et al. (2004). Regulation of anti-oxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9
- SCCNFP/0649/03. (2003). Evaluation and opinion on: Zinc oxide. The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers, 2–27
- SCCS/1416/11. (2011). The SCCS’s Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation, 7th revision, adopted by the SCCS during the 10th plenary meeting of 22 March 2011
- SCCS/1516/13. (2013). Opinion on Titanium Dioxide (nano form). COLIPA n° S75. Scientific Committee on Consumer Safety, 6–103
- Scheuer C, Pommergaard HC, Rosenberg J, Gogenur I. (2014). Melatonin's protective effect against UV radiation: a systematic review of clinical and experimental studies. Photodermatol Photoimmunol Photomed 30:180–8
- Seida P, Parce JW, Seeds MS, Bass DA. (1984). Flow cytometric quantitation of oxidative produce formation by polymorphonuclear leukocytes during phagocytosis. J Immunol 133:3303–7
- Selzer D, Abdel-Mottaleb MA, Hahn T, et al. (2013). Finite and infinite dosing: difficulties in measurements, evaluations and predictions. Adv Drug Deliv Rev 65:278–94
- Singh M, Jadhav HR. (2014). Melatonin: functions and ligands. Drug Discov Today 19:1410–18
- Slominski A, Pisarchik A, Semak I, et al. (2002). Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 16:896–8
- Slominski A, Tobin DJ, Zmijewski MA, et al. (2008). Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab 19:17–24
- Slominski A, Wortsman J, Tobin DJ. (2005a). The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 19:176–94
- Slominski AFT, Zmijewski MA, Wortsman J, et al. (2005b). On the role of melatonin in skin physiology and pathology. Endocrine 27:137–48
- Slominski AT, Kleszczyński K, Semak I, et al. (2014a). Local melatoninergic system as the protector of skin integrity. Int J Mol Sci 15:17705–32
- Slominski AT, Zmijewski MA, Semak I, et al. (2014b). Cytochromes p450 and skin cancer: role of local endocrine pathways. Anti-Cancer Agents Med Chem 14:77–96
- Slominski AT, Zmijewski MA, Skobowiat C, et al. (2012a). Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anatomy EmbryolCell Biol 212:1–115
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, et al. (2012b). Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–66
- Soh N. (2006). Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem 386:532–43
- Tetko IV, Gasteiger J, Todeschini R, et al. (2005). Virtual computational chemistry laboratory – design and description. J Comput Aided Mol Des 19:453–63
- Wardman P. (2007). Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43:995–1022
- Yaar M, Gilchrest BA. (2001). Ageing and photoageing of keratinocytes and melanocytes. Clin Exp Dermatol 26:583–91
- Yalcinkaya FR, Gokce A, Guven EO, et al. (2009). Protective effect of vitamin e and melatonin against radiation induced damage in testes of rats. J Anim Vet Adv 8:2335–40
- Zhang C, Gao Y, Zhao X, Li X. (2012). A validated HPLC method for determining Melatonin in capsule dosage form. Spatula DD 2:147–51
- Zhang X, Beaulieu JM, Sotnikova TD, et al. (2004). Trypto-phan hydroxylase-2 controls brain serotonin synthesis. Science 305:217