Abstract
Purpose: The skin permeation enhancement of local anesthetics by newer innovative nanotechnologies has been an appealing field recently. However, which nanocarrier is better for drug loading and has better stability? Therefore, the aim of our study was to compare two kinds of nanocarriers: liposomes and lipid–polymer hybrid nanoparticles (LPNs) for lidocaine (LA) delivery.
Methods: LA-loaded liposomes (LA-LPs) and LPNs (LA-LPNs) were prepared. Two kinds of nanocarriers were characterized in terms of particle size, zeta potential, drug encapsulation efficiency (EE), drug release, and stability. Their in vitro skin permeation was studied using a Franz diffusion cell mounted with depilated mouse skin in vitro. In vivo local anesthetic effects of LA containing formulations were evaluated by tail flick latency (TFL) test using a tail-flick measuring device.
Results: Compared with LA-LPs, LA-LPNs showed significantly better in vitro skin permeation ability and in vivo local anesthetic effects.
Conclusion: The results demonstrated that LPNs could improve the efficacy of drugs to higher levels than LPs and free drugs, thus could serve as an effective drug system for LA loading for local anesthetic therapy.
Introduction
Drug delivery of anesthetics to or via the skin presents both unique opportunities and obstacles due to skin structure, physiology and barrier properties (Pathak et al., Citation2009). Poor permeability due to skin barrier such as the stratum corneum is one of the most obstacles have significantly limited their clinical application (Alexander et al., Citation2012). While, compared to the injection, the benefits for local anesthetics such as improving patient compliance, avoiding systemic toxicity, and providing continuous drug delivery makes them the appealing field for pharmaceutical researchers (Wang et al., Citation2013). Lidocaine (LA) is local anesthetics often used to alleviate pain after surgery, trauma, or medical procedures (Puglia et al., Citation2011). Their action is characterized by a rapid but short effect, compared with the potential duration of pain. Thus, it is urgent to develop effective and prolonged local transdermal anesthetics.
Optimization of local anesthesia applications for higher permeation and bioavailability includes considerations of interactions between the formulation and the molecular components of the skin barrier (Brown et al., Citation2006). Nowadays, the development of local anesthetics skin-delivery systems using liposomes (LPs) or lipid nanoparticles have been explored as alternatives to commercial formulations such as lidocaine and prilocaine cream (EMLA®, Astra Zeneca), lidocaine tape (Penles®, Wyeth) or lidocaine gel path (Lidoderm®, Endo Pharmaceuticals), modifying the release rate of drugs, increasing bioadhesive properties and reducing toxicity, resulting in an improved therapeutic efficacy (Houck et al., Citation2005; de Araújo et al., Citation2013).
LPs, which are spherical vesicles composed of a bilayer of phospholipids, are similar to cell membranes in the body, and are most commonly used in local anesthesia (Elsaie et al., Citation2008; Lokajová et al., Citation2011). However, the poor stability of LPs can lead to rapid leakage of incorporated drugs (Takeuchi et al., Citation2001). To address the limitations of LPs and polymeric nanoparticles, a new generation delivery vehicle of therapeutics termed lipid–polymer hybrid nanoparticles (LPNs) has been developed (Zhang et al., Citation2008; Hadinoto et al., Citation2013).
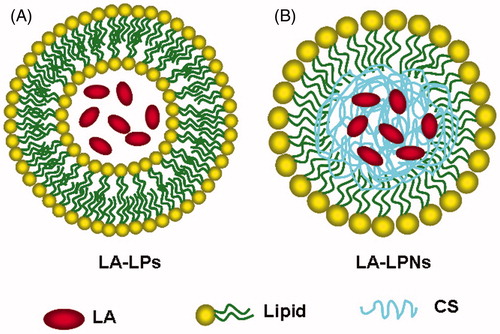
LPNs, which combine the characteristics of both polymeric nanoparticles and LPs, are core-shell nanoparticle structures comprising polymer cores and lipid shells. Therefore, the special structure of LPNs makes them exhibit high structural integrity, stability during storage, controlled release, and high biocompatibility and bioavailability owed to the lipid layers (Chan et al., Citation2009). Chitosan (CS) belongs to the class of cationic bioadhesive polymers. CS hydrogels are non-cytotoxic and potential for extended drug release, making them promising local anesthetic delivery vehicles (Pignatello et al., Citation2009; Foley et al., Citation2013). In this study, we designed lidocaine-loaded chitosan nanoparticle core, and coated by the lipid shell to combine the advantages of both chitosan nanoparticle and LPs for improving therapeutic efficacy.
The aim of the present study is the development and evaluation of LA-loaded LPs (LA-LPs) and lipid–polymer hybrid nanoparticles (LA-LPNs) as carrier systems respectively for topical delivery of LA aiming to produce a fast acting and long lasting topical formulation. LA-LPs and LA-LPNs were prepared and their particle size, entrapment efficacy and zeta potential were evaluated. In vitro release study and in vitro skin permeation were studied; in vivo evaluation of local anesthetic effects were performed on animal models.
Materials and methods
Materials
Lidocaine hydrochloride (LA), 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), chitosan (CS), cholesterol, and dichloromethane (DCM), and cetyltrimethyl ammonium bromide (CTAB) were purchased from Sigma Aldrich (St. Louis, MO). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) was provided by Lipoid GmbH (Ludwigshafen, Germany). Hyaluronic acid was provided by Shandong Freda Biochem Co., Ltd. (Shangdong, China). All other reagents and solvents were of analytical or high-performance liquid chromatography (HPLC) grade.
Animals
Sprague-Dawley rats (SD rats) weighing 350–400 g (10–12 weeks old) were purchased from the Medical Animal Test Center of Shandong University. The rats were maintained under controlled conditions (temperature, 20 ± 1 °C; humidity, 60 ± 5%; 12/12 h light/dark cycle). All animal experiments complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China.
Fabrication of LA-LPs
LA-LPs were prepared using a thin lipid film method () (Bartneck et al., Citation2015). Briefly, a mix of chloroform (10 mL) and methanol (1 mL) containing DLPC (20 mg), DSPE-PEG2000 (2 mg), and cholesterol (10 mg) was prepared. The organic phase was evaporated with a rotavapor, followed by nitrogen flushing for the removal of residual organic solvent. LA (10 mg) in PBS (1 mL) was mixed with the LPs solution (50 mL) and incubated at 60 °C for 2 h (Chiang et al., Citation2014). Non-encapsulated free LA was removed by dialysis the LPs against PBS (pH 7.4) for 12 h at room temperature (Li et al., Citation2015). Blank LPs without LA were prepared using the same method. LA-LPs were stored at 4 °C before use.
Fabrication of LA-LPNs
LA-LPNs were prepared by a modified solvent extraction/evaporation method () (Mandal et al., Citation2013). Weighed amount of CS (20 mg) and LA (10 mg) was dissolved in DCM to form the oil phase (Liu et al., Citation2010). A mix of DLPC (50 mg), and DSPE-PEG2000 (10 mg) was dispersed in PBS by sonication to form the aqueous phase. The oil phase was then poured into the water phase and the mixture was sonicated by probe ultrasonicator under ice bath. DCM was evaporated from the emulsion by magnetic stirring. The suspension was centrifuged at 10 000 rpm at 4 °C for 15 min, washed three times to collect the LA-LPNs. Blank LPNs without LA were prepared using the same method. LA-LPNs were stored at 4 °C before use.
Shape and surface morphology
LA-LPs and LA-LPNs were prepared respectively by placing a drop of three kinds of nanoparticle suspensions onto a copper grid and air drying, followed by negative staining with one drop of 3% aqueous solution of sodium phosphotungstate for contrast enhancement. The air-dried samples were then directly examined under the transmission electron microscopy (TEM, JEM-1200EX; JEOL Ltd., Tokyo, Japan) (Mishra et al., Citation2015).
Particle size, size distribution, and zeta potential
The particle size, size distribution, and zeta potential of the LA-LPs and LA-LPNs was measured by dynamic laser scattering (DLS, Malvern zetasizer 3000, Malvern Instruments, Worceshtire, UK). The dispersion of LA-LPs and LA-LPNs was diluted by ultrapure water according to the mass concentration and completely sonicated before measurement. The zeta potentials of the nanocarriers were measured based on electrophoretic mobility under an electric field (Pokharkar et al., Citation2015). The stability of LA-LPs and LA-LPNs over 30 days was investigated by monitored the size variation (Rao et al., Citation2014). Changes in the size were used to evaluate stability.
Drug encapsulation efficiency and drug-loading content
The drug encapsulation efficiency (EE) and drug-loading efficiency (DL) of the LA-LPs and LA-LPNs was measured by gel filtration method using Sephadex G50 Column (GE Healthcare Bio-Sciences, Pittsburgh, PA) (Vitorino et al., Citation2013). The free LA was separated from the LA-LPs and LA-LPNs, the content of free drug was measured by using high performance liquid chromatography (HPLC), performed on an Agilent 1100 series with a zorbax RP-select B colum, and a G1315A Diode-array detector (Agilent Technologies, Santa Clara, CA). The mobile phase was a mixture of 0.01 M sodium dihydrogen phosphate:methanol (25:75, v/v). The flow rate was 1 mL/min. The EE and DL were calculated using the following equations:
where Wtotal LA is the total amount of LA determined in the whole system, Wfree LA is the amount of free LA determined in the aqueous phase after separation of the nanocarriers, and Wlipid is the weight of the lipid phase.
In vitro LA release
The release of LA from LA-LPs and LA-LPNs was determined in vitro using static Franz diffusion cells with a diffusion area of 0.75 cm2 and a receptor compartment of 4.5 mL (Vitorino et al., Citation2013; Lee et al., Citation2014). The receptor phase was filled with distilled water containing 5% Tween 80 as an acceptor medium to achieve sink conditions during the experiment, and water-jacketed at 37 °C. LA-LPs or LA-LPNs dispersion (100 μL) was applied to the donor compartment. Aliquots (300 μL) were withdrawn from the receptor compartment at predetermined time intervals for 48 hours and diluted 2.5-fold with water. Separately, an LA containing solution was prepared with Tween 80 (5% v/v) as a control, and the release of LA from LA-LPs and LA-LPNs was quantified by using HPLC analysis as described in the “drug encapsulation and loading efficiency” section.
In vitro skin permeation experiments
In vitro skin permeation studies were performed using Franz diffusion cells (Pathak et al., Citation2009; Pignatello et al., Citation2009). SD rats were sacrificed by dislocation. The fur on the abdominal area of the rats was carefully removed by an electrical shaver to avoid damage to stratum corneum. The skin from the abdominal surface was excised and the adherent fat and subcutaneous tissue was removed. The in vitro skin permeation studies of LA from different formulations was carried out using Franz glass diffusion cell maintained at 37 °C under non-occlusive conditions. The skin permeation study was performed using 6 diffusion cells and represented as an average of 6 cells. The skin samples were mounted on Franz diffusion cell with a surface area of 0.75 cm2 and a receptor volume of 4.5 mL. Accurately weighed LA containing formulations corresponding to 5 mg of LA was applied on the donor compartment side of the skin. The donor compartment was covered with an aluminum foil to avoid evaporation. The temperature was maintained at 37 ± 0.5 °C. 0.5 mL of receptor medium was withdrawn at time intervals of 0, 0.5, 1, 2, 4, 6, 8, 10, 12, 15, 18, and 24 h and was replaced by the same volume of fresh buffer to maintain the sink condition. The samples were quantified by using HPLC analysis as described in the “Drug encapsulation and loading efficiency” section.
The flux of drug from each formulation was calculated (Junyaprasert et al., Citation2008). The cumulative drug permeation (Qt) was calculated as follows:
where Ct is the drug concentration of the receptor fluid at each sampling time; Ci is the drug concentration of the i-th sample; Vr and Vs are the volumes of the receptor fluid.
Data were expressed as the cumulative drug permeation per unit of skin surface area, Qt/S (S = 0.75 cm2). The steady-state fluxes (JSS) were calculated by linear regression interpolation of the experimental data at steady state (△T) as shown in the following equation:
In vivo evaluation of local anesthetic effects
Local anesthetic effects of LA containing formulations were evaluated by tail flick latency (TFL) test using a tail-flick measuring device (Ugo Basile, Varese, italy) (Ouchi et al., Citation2013; Caffarel-Salvador et al., Citation2015). Animals were randomly divided into different experimental groups, and 50 mg of different formulations was applied on the midline of the tail to cover approximately 2–2.5 cm. A noxious heat stimulus was applied via a focused, radiant heat light source to the dorsal surface of the tail (Puglia et al., Citation2011). Briefly, anesthetic effect was determined by measuring the duration taken for the rat’s tail to react (flicking of the tail) in response to an infrared light stimulus. TFL duration stops when animals feel pain, hence, reflecting the response to pain threshold was converted to represent the maximum possible effect (MPE) according to the following equation:
The predrug latency was calculated as the mean of three different measurements taken at 10-min intervals. A maximum cutoff latency of 10 s was set to avoid tissue damage in analgesic animals.
Statistical analysis
All studies were repeated at least three times. Results were reported as means ± standard deviation. Statistical significance was tested by two-tailed Student’s t-test. Differences between experimental groups were considered significant when the p value was less than 0.05 (p < 0.05).
Results
Surface morphology, particle size, and size distribution
Surface morphology of LA-LPs and LA-LPNs were visualized by TEM images (). According to the 100 nm bars in the images, the LA-LPs (Figure 2-1) were larger in size compared to the LA-LPNs ().
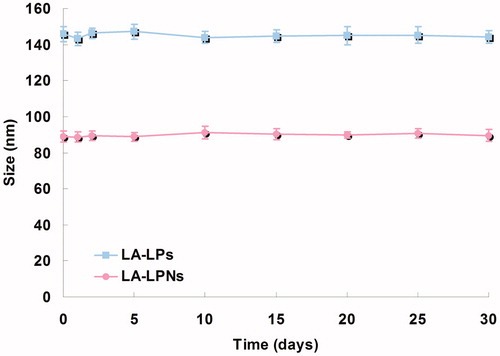
The size of LA-LPs and LA-LPNs was around 145.6 and 88.6 nm, with a size distribution of 0.19 and 0.12, respectively (). The size of LA-LPs and LA-LPNs was larger than blank LPs and LPNs, which indicated the loading of LA result in enlarging the size of the nanocarriers.
Table 1. Characterization of LA-LPs and LA-LPNs.
Zeta potential, EE, and DL
summarizes the zeta potentials of LA-LPs and LA-LPNs. The zeta potential of blank LPs was +6.8 mV, with the loading of negatively charged LA, the zeta potential of LA-LPs decreased to -4.6 mV. CS is a cationic polysaccharide. The using of CS made blank LPNs had the zeta potential of +32.7 mV. The zeta potential of LA-LPNs decreased to +13.6 mV. EE of LA-LPs and LA-LPNs was 78.6% and 85.2%; DL of LA-LPs and LA-LPNs was 1.3% and 2.5%, respectively.
Table 2. Skin permeation steady-state fluxes (JSS) of the LA-LPs and LA-LPNs.
Stability
illustrates the average diameter of LA-LPs and LA-LPNs remained stable during 30 days after formation. The high stability of LA-LPs and LA-LPNs provides support for protecting and delivering the drug for the further therapeutic application.
In vitro LA release
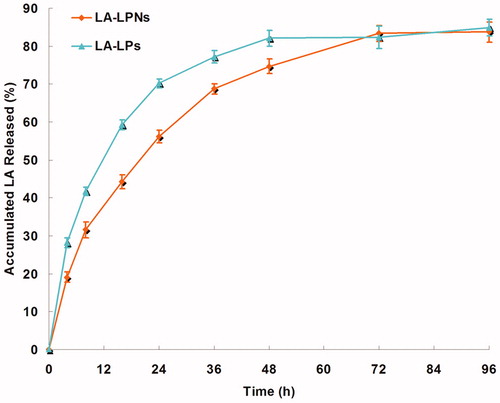
The in vitro LA release profiles of LA-LPs and LA-LPNs are illustrated in . The drug release of LA-LPNs was obviously slower than that of LA-LPs, complete release of drugs were achieved at 72 and 48 h, respectively. The drug release profile exhibits the biphasic patterns, initial about 40% burst release of drug in 8 h, and later sustained drug release up to 72 h.
In vitro skin permeation
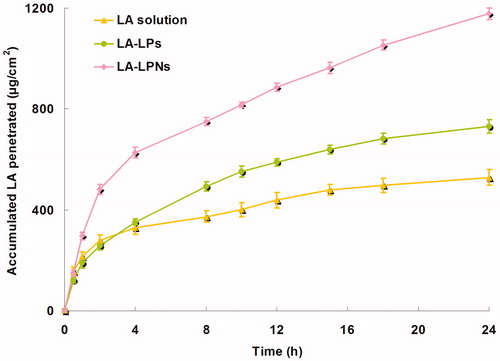
illustrates the calculation of the in vitro permeation profiles of LA from LA-LPs and LA-LPNs. The steady-state fluxes (JSS) are summarized in . Data show that the permeation of LA in LA-LPs and LA-LPNs was much more sufficient than LA solutions (p < 0.05). Better skin permeation capacity was gain by LA-LPNs. The JSS of CS containing LA-LPNs (65.4 μg/h/cm2) was significantly higher than LA-LPs (40.6 μg/h/cm2) (p < 0.05). This finding could be explained by the lipid surface and CS core of LPNs acting as a drug carrier provided the higher skin permeation enhancement than their LPs counterparts.
In vivo local anesthetic effects
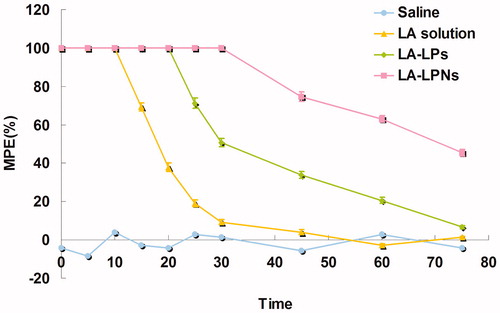
reports the MPE results of TFL test for the evaluation of the in vivo local anesthetic effects of LA-LPs and LA-LPNs. Firstly, all the LA containing formulations increased the TFL significantly than the saline control group (p < 0.05). There were no significant differences in baseline TFL between the control group and other groups. LA solution formulation showed a rapid but short-lasting anesthetic effect, with a peak effect seen within 10 min from the application. While the LA-LPs and LA-LPNs have a longer effect than the solution formulation. LA-LPNs induce a pronounced anesthetic effect than LA-LPs after the application (p < 0.05).
Discussion
The development of local anesthetics skin-delivery systems using LPs or LPNs have been identified as effective drug carriers. The aim of the present study is to construct LA-LPs and LA-LPNs as carrier systems respectively for topical delivery of LA aiming to produce a fast acting and long lasting topical formulation. The comparison of these two vectors is throughout the paper and the overall subject is to determine which one is better.
The investigation started with the fabrication of LA-LPs and LA-LPNs. Surface morphology of LA-LPs and LA-LPNs were visualized by TEM images (). Both LA-LPs and LA-LPNs had spherical or ellipsoidal shapes. The LA-LPs were larger in size compared to the LA-LPNs according to the 100 nm bars. The size of LA-LPs and LA-LPNs was around 145.6 and 88.6 nm, with a size distribution of 0.19 and 0.12, respectively (). The LA-LPs were larger in size compared to the LA-LPNs. The size of LA-LPs and LA-LPNs was larger than blank LPs and LPNs, which indicated the loading of LA result in enlarging the size of the nanocarriers. The size has a great impact on the delivery efficiency of the carriers, including decreased uptake by the liver, prolonged blood circulation time, and improved bioavailability (Huang et al., Citation2015). The small particle size of delivery systems may enhance the bioavailability of bioactive compounds, adhesion with biological cells, and cellular uptake of delivery systems (Chinaeke et al., Citation2015). It has been reported that decreasing the particle size results in an occlusive effect on the skin layer which leads to a long duration of action of the drug (Pathak et al., Citation2009). summarizes the zeta potentials of LA-LPs and LA-LPNs. Surface charge is an important indication for the stability of nanoparticle system (Jiang et al., Citation2015). Cationic surface charge may augment in absorption across the skin barrier which could enhance the potential of nano-sized delivery system (Yang et al., Citation2015). The zeta potential of blank LPs was +6.8 mV, with the loading of negatively charged LA, the zeta potential of LA-LPs decreased to −4.6 mV. CS is a cationic polysaccharide. It belongs to the class of cationic bioadhesive polymers (Pignatello et al., Citation2009). Biodegradability and absence of toxicity increase the use of CS and derivatives in the pharmaceutical technology. Moreover, it can form hydrogels and flms, is hydrophilic, and can be sterilized at high temperatures. The using of CS made blank LPNs had the zeta potential of +32.7 mV. The zeta potential of LA-LPNs decreased to +13.6 mV. The positive charge surface of LA-LPNs could make the carriers easier bind toward the negatively charged cell surfaces than LA-LPs. EE of LA-LPs and LA-LPNs was 78.6% and 85.2%, respectively. The EE is a key effect of drug therapeutic effect (Ghanbarzadeh et al., Citation2015). The high EE achieved by the LA-LPs and LA-LPNs could be the evidence that the preparation methods and the excipients added were suitable for the loading of LA into the carriers.
The in vitro LA release profiles of LA-LPs and LA-LPNs illustrated that the LA released from both LPs and LPNs in sustained manners (). The in vitro release profiles revealed that AE achieved sustained release by loading into SLNs. The sustained-release behavior is an important prerequisite for successful drug delivery (Chen et al., Citation2015). This phenomenon could be explained by the entrapment of the drugs by the lipid matrix which can slow down the release of the drugs. The drug release of LA-LPNs was obviously slower than that of LA-LPs. Complete release of drugs were achieved at 72 and 48 h, respectively. This could contribute to the using of CS which incorporated LA and let it release in a more sustained rate (Khalkhali et al., Citation2015). The drug release profile exhibit the biphasic patterns, initial about 40% burst release of drug in 8 h might be attributed to that of surface bound and superficial embedded drug in CS nanoparticles, and later sustained drug release up to 72 h was observed due to strong cross-linking complex of CS resulting into denser particle which retards LA release due to swelling of CS which lowers the membrane permeability for LA (Shirsat et al., 2015).
It is generally accepted that the diffusion of a molecule in the skin can be modeled as the inverse square of molecular weight (Pino et al., Citation2014). Due to the lipid-rich environment of the stratum corneum, lipophilic molecules are well suited for translocation across corneum. Charged and water-soluble molecules on the other hand are largely excluded from the lipid transport pathways. LPs and LPNs have lipophilic shell and have a natural affinity for skin lipids. This character allows them to facilitate drug transport by favoring the partitioning of the drug from the vehicle into the skin. In vitro skin permeation profiles of LA from LA-LPs and LA-LPNs calculation are shown in . The JSS values show that the permeation of LA in LA-LPs and LA-LPNs was much more sufficient than LA solutions (p < 0.05). Better skin permeation capacity was gain by LA-LPNs, which may due to their lipid layer similar to skin lipids, and a polymer core in which the therapeutic substances are encapsulated for sustained drug delivery (Hadinoto et al., Citation2013). CS are non-cytotoxic, biocompatible polymers, and can degrade slowly over time into bioresorbable products, making them promising local anesthetic delivery vehicles (Yang et al., Citation2012). The JSS of CS containing LA-LPNs (65.4 μg/h/cm2) was significantly higher than LA-LPs (40.6 μg/h/cm2) (p < 0.05). This finding could be explained by the lipid surface and CS core of LPNs acting as a drug carrier provided the higher skin permeation enhancement than their LPs counterparts.
In vivo local anesthetic effects of LA-LPs and LA-LPNs were evaluated by TFL test using a tail-flick measuring device (Caffarel-Salvador et al., Citation2015). The MPE results of TFL test are shown in . Firstly, all the LA containing formulations increased the TFL significantly than the saline control group (p < 0.05). There were no significant differences in baseline TFL between the control group and other groups. LA solution formulation showed a rapid but short-lasting anesthetic effect, with a peak effect seen within 10 min from the application. While the LA-LPs and LA-LPNs have a longer effect than the solution formulation. LA-LPNs induce a pronounced anesthetic effect than LA-LPs after the application (p < 0.05). The results indicated that the anesthetic activity of LA-LPNs revealed a more interesting rapid anesthetic effect in the first few minutes and also displayed sustained activity compared with the LA-LPs. The sustained anesthetic effect of the LA-LPNs could bring about better local anesthetic therapy effects than the LA-LPs.
Conclusions
LA-LPs and LA-LPNs were constructed and applied for the transporting of LA through the skin to achieve topical anesthesia. In vitro and in vivo evaluation illustrated that LA-LPNs have better anesthetic effect than their LA-LPs counterparts. These results demonstrated that the LPNs could function as promising drug delivery system for overcoming the barrier function of the skin and could deliver anesthetic through the skin with sustained release behavior.
Declaration of interest
The authors report no conflicts of interest.
References
- Alexander A, Dwivedi S, Ajazuddin, et al. (2012). Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release 164:26–40
- Bartneck M, Scheyda KM, Warzecha KT, et al. (2015). Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials 37:367–82
- Brown MB, Martin GP, Jones SA, Akomeah FK. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–87
- Caffarel-Salvador E, Tuan-Mahmood TM, McElnay JC, et al. (2015). Potential of hydrogel-forming and dissolving microneedles for use in paediatric populations. Int J Pharm 489:158–69
- Chan JM, Zhang L, Yuet KP, et al. (2009). PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 30:1627–34
- Chen R, Wang S, Zhang J, et al. (2015). Aloe-emodin loaded solid lipid nanoparticles: formulation design and in vitro anti-cancer study. Drug Deliv 22:666–74
- Chiang YT, Lo CL. (2014). pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 35:5414–24
- Chinaeke EE, Chime SA, Onyishi VI, et al. (2015). Formulation development and evaluation of the anti-malaria properties of sustained release artesunate-loaded solid lipid microparticles based on phytolipids. Drug Deliv 22:652–65
- de Araújo DR, da Silva DC, Barbosa RM, et al. (2013). Strategies for delivering local anesthetics to the skin: focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin Drug Deliv 10:1551–63
- Elsaie ML, Baumann LS. (2008). Evaluation of liposomal delivery system for topical anesthesia. Cutis 81:285–9
- Foley PL, Ulery BD, Kan HM, et al. (2013). A chitosan thermogel for delivery of ropivacaine in regional musculoskeletal anesthesia. Biomaterials 34:2539–46
- Ghanbarzadeh S, Khorrami A, Arami S. (2015). Nonionic surfactant-based vesicular system for transdermal drug delivery. Drug Deliv 22:1071–7
- Hadinoto K, Sundaresan A, Cheow WS. (2013). Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85:427–43
- Houck CS, Sethna NF. (2005). Transdermal analgesia with local anesthetics in children: review, update and future directions. Expert Rev Neurother 5:625–34
- Huang RF, Wei YJ, Inbaraj BS, Chen BH. (2015). Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int J Nanomed 10:2823–46
- Jiang S, Gong X, Zhao X, Zu Y. (2015). Preparation, characterization, and antitumor activities of folate-decorated docetaxel-loaded human serum albumin nanoparticles. Drug Deliv 22:206–13
- Junyaprasert VB, Boonme P, Wurster DE, Rades T. (2008). Aerosol OT microemulsions as carriers for transdermal delivery of hydrophobic and hydrophilic local anesthetics. Drug Deliv 15:323–30
- Khalkhali M, Sadighian S, Rostamizadeh K, et al. (2015). Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. Bioimpacts 5:141–50
- Lee SG, Jeong JH, Lee KM, et al. (2014). Nanostructured lipid carrier-loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. Int J Nanomed 9:289–99
- Li Y, Liu R, Yang J, et al. (2015). Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials 41:1–14
- Liu Y, Pan J, Feng SS. (2010). Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance. Int J Pharm 395:243–50
- Lokajová J, Laine J, Puukilainen E, et al. (2011). Liposomes for entrapping local anesthetics: a liposome electrokinetic chromatographic study. Electrophoresis 31:1540–9
- Mandal B, Bhattacharjee H, Mittal N, et al. (2013). Core-shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 9:474–91
- Mishra BJ, Kaul A, Trivedi P. (2015). L-Cysteine conjugated poly L-lactide nanoparticles containing 5-fluorouracil: formulation, characterization, release and uptake by tissues in vivo. Drug Deliv 22:214–22
- Ouchi K, Sekine J, Koga Y, et al. (2013). Establishment of an animal model of sedation using epidural anesthesia that uses the tail-flick test for evaluating local anesthetic effects in rats. Exp Anim 62:137–44
- Pathak P, Nagarsenker M. (2009). Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech 10:985–92
- Pignatello R, Basile L, Puglisi G. (2009). Chitosan glutamate hydrogels with local anesthetic activity for buccal application. Drug Deliv 16:176–81
- Pino CJ, Scherer MA, Shastri VP. (2014). Investigation of the transdermal transport of charged local anesthetics in the presence of triterpene saponin glycosides. Drug Deliv Transl Res 4:131–8
- Pokharkar V, Patil V, Mandpe L. (2015). Engineering of polymer-surfactant nanoparticles of doxycycline hydrochloride for ocular drug delivery. Drug Deliv 22:955–68
- Puglia C, Sarpietro MG, Bonina F, et al. (2011). Development, characterization, and in vitro and in vivo evaluation of benzocaine and lidocaine-loaded nanostructrured lipid carriers. J Pharm Sci 100:1892–9
- Rao L, Ma Y, Zhuang M, et al. (2014). Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitus. Int J Nanomed 9:4819–28
- Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. (2001). Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release 75:83–91
- Vitorino C, Almeida J, Gonçalves LM, et al. (2013). Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release 167:301–14
- Wang Y, Su W, Li Q, et al. (2013). Preparation and evaluation of lidocaine hydrochloride-loaded TAT-conjugated polymeric liposomes for transdermal delivery. Int J Pharm 441:748–56
- Yang C, Shen Y, Wang J, et al. (2015). Cationic polymer-based micro-emulgel with self-preserving ability for transdermal delivery of diclofenac sodium. Drug Deliv 22:814–22
- Yang JA, Kim ES, Kwon JH, et al. (2012). Transdermal delivery of hyaluronic acid - human growth hormone conjugate. Biomaterials 33:5947–54
- Zhang L, Chan JM, Gu FX, et al. (2008). Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2:1696–702